Abstract
Satiety signals arising from the gastrointestinal (GI) tract and related digestive organs during food ingestion and digestion are conveyed by vagal sensory afferents to the hindbrain nucleus of the solitary tract (NST). Two intermingled but chemically distinct NST neuronal populations have been implicated in meal size control: noradrenergic (NA) neurons that comprise the A2 cell group, and glucagon-like peptide-1 (GLP-1)-positive neurons. Previous results indicate that A2 neurons are activated in a meal size-dependent manner in rats that have been acclimated/entrained to a feeding schedule in order to increase meal size, whereas feeding under the same conditions does not activate GLP-1 neurons. The present study was designed to test the hypothesis that both A2 and GLP-1 neuronal populations are recruited in non-entrained rats after voluntarily first-time intake of an unrestricted, satiating volume of liquid Ensure. DBH-positive neurons within the caudal visceral NST were progressively recruited to express cFos in rats that consumed progressively larger volumes of Ensure. Among these DBH-positive neurons, the prolactin-releasing peptide (PrRP)-positive subset was more sensitive to feeding-induced activation than the PrRP-negative subset. Notably, significant activation of GLP-1-positive neurons occurred only in rats that consumed the largest volumes of Ensure, corresponding to nearly 5% of their BW. We interpret these results as evidence that progressive recruitment of NA neurons within the caudal NST, especially the most caudally-situated PrRP-positive subset, effectively “tracks” the magnitude of GI satiety signals and other meal-related sensory feedback. Conversely, GLP-1 neurons may only be recruited in response to the homeostatic challenge of consuming a very large, unanticipated meal.
Keywords: Glucagon-like peptide-1, prolactin-releasing peptide, cFos, re-feeding, A2 cell group, satiety
1. Introduction
Food intake is the product of meal size and meal number (1). Meal number is regulated by cortical, limbic, striatal, and hypothalamic forebrain circuits, and is modulated by signals that convey information about environmental events, learned associations, reward, and energy status between meals {see (2, 3) for reviews}. Conversely, meal size is controlled primarily at the level of the caudal brainstem (1), and is modulated by satiety signals arising from the gastrointestinal (GI) tract and related digestive organs during the ingestion and digestion of food; these signals are conveyed largely by vagal sensory inputs to the NST (4–10). The NST relays feeding-related visceral sensory signals to the forebrain, as well as to brainstem pattern generators and pre-motor neurons that control the motoric components of feeding (i.e., licking, chewing, and swallowing (11, 12)). Thus, NST neurons are critically involved in receiving and processing GI satiety signals that terminate ingestive consummatory behaviors, thereby limiting meal size (13).
NST neurons that receive and process satiety signals are neurochemically diverse (13). However, recent studies have implicated two intermingled but phenotypically distinct caudal NST neuronal populations in meal size control: noradrenergic (NA) neurons that comprise the A2 cell group, and preproglucagon-expressing neurons that are immunopositive for glucagon-like peptide-1 (GLP-1) (13–15). Based on increased expression of the immediate-early gene product cFos, results in laboratory rats indicate that A2 and GLP-1 neurons are stimulated by experimental treatments that activate GI vagal sensory afferents with synaptic inputs to the caudal NST (16–20). Such treatments include mechanical gastric distension (20) and systemic administration of cholecystokinin octapeptide (CCK) (17, 18, 21, 22). A2 neurons also are activated in a meal size-dependent manner by voluntary food intake in rats that have been acclimated/entrained to a feeding schedule in which repeating cycles of overnight food deprivation are followed by a predictable morning re-feeding period (16). Conversely, the same repeating schedule of food deprivation followed by a large anticipated meal does not activate GLP-1 neurons (17), although GLP-1 neurons are activated in rats after a variety of interoceptive stressors (17). Feeding schedule entrainment is associated with anticipatory physiological adjustments that serve to limit the homeostatic challenge of consuming large meals, thereby reducing the interoceptive stress that would otherwise be produced by the meal, and permitting increased meal size (2, 23). Since multiple lines of evidence support the view that central GLP-1 signaling suppresses food intake (5, 24–26), the lack of GLP-1 neuronal recruitment in meal-entrained rats that consume a large anticipated meal may reflect homeostatic adjustments that minimize interoceptive stress. Indeed, attenuated feeding-induced activation of GLP-1 neurons may contribute to the progressively larger meals consumed by rats during meal entrainment (2, 23). In non-entrained rats, such anticipatory physiological adjustments are absent, and deprivation-induced food intake is more directly limited by GI distension and other sensory feedback generated by the acute homeostatic challenge of consuming a large unanticipated meal.
The present study was designed to test the hypothesis that both A2 and GLP-1 neuronal populations are recruited in non-entrained rats after voluntarily intake of a large, unanticipated meal. To challenge this hypothesis, we examined cFos activation among DBH- and GLP-1-positive caudal NST neurons in rats after deprivation-induced intake of unrestricted or restricted volumes of a palatable liquid diet (i.e., Ensure). We extended our analysis of feeding-activated neurons to include a specific caudal subset of DBH-positive NST neurons that co-express prolactin-releasing peptide (PrRP) along with NA synthetic enzymes (27, 28). Central PrRP signaling is implicated in stress responses and control of energy balance (29–36), and PrRP neurons may participate in meal size regulation (37–39). PrRP-positive neurons within the caudal NST are activated in experimentally naïve rats and mice after overnight food deprivation followed by re-feeding (40), although that report did not examine the potential relationship between the amount of food consumed and the extent of PrRP neuronal recruitment.
2. Material and Methods
2.1 Animals and feeding protocol
Experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Adult male Sprague-Dawley rats (Harlan; n=31; 200–270g BW) were individually housed in hanging wire cages in a temperature-controlled room with lights on from 07:00–19:00 hr. Food (Purina rat chow #5001) and water were available ad libitum, except as noted for each experiment. Rats were acclimated to these conditions for at least 48 hr before experiments were initiated. The test diet was liquid Ensure (Creamy Milk Chocolate or Homemade Vanilla; both 1.06 kcal/g; 14% protein, 64% carbohydrate, 22% fat by kcal; Abbott Nutrition, Columbus, OH). A pilot study revealed no flavor-related differences in 30-min intake by rats (n=12) after overnight food deprivation, consistent with a previous report of similar ad libitum intake of chocolate vs. vanilla Ensure by male Sprague-Dawley rats (41). All rats in the present study were pre-exposed overnight to a ball-tipped drinking spout attached to a graduated cylinder containing 10 ml of Ensure (in addition to chow and water) in order to reduce neophobic responses to the drinking spout and test diet during subsequent deprivation-induced feeding. Every rat consumed all of the available Ensure during this pre-exposure. In some cases, up to 5 ml of the 10 ml provided for overnight pre-exposure was inaccessible, either because the drinking spout leaked (as evidenced by a small volume of dried Ensure found on the pan beneath the wire cage floor the next morning), and/or because the inner metal tube extended a bit higher than the volume remaining at the bottom of the cylinder. Thus, pre-exposure volumes varied somewhat across rats, but did not vary systematically across experimental feeding groups.
Several days after Ensure pre-exposure, chow was removed from cages between 15:00 and 16:00 hr; water was not removed. Twenty-four hours later, food-deprived rats were weighed and then given 30 min home-cage access to Ensure (the same flavor to which they had been pre-exposed) in one of three feeding conditions, or served as food-deprived/non-fed controls, as follows:
unrestricted access to Ensure (i.e., rats were given access to a volume in excess of any rat’s 30 min intake)
30% restricted (i.e., rats were given 70% of the average volume consumed in 30 min by rats in the “unrestricted access” group)
50% restricted (i.e., rats were given 50% of the average volume consumed in 30 min by rats in the “unrestricted access” group)
food deprived/non-fed controls (i.e., no Ensure)
Our pilot study confirmed that most food-deprived rats with unrestricted access to Ensure (n=12) voluntary terminated intake within the first 15 min, whereas some rats consumed a few more mls during the subsequent 15 min period. However, at the end of 30 min, none were still actively consuming Ensure. Thus, rats with 30 min unrestricted access to Ensure were able to feed to satiety (i.e., as evidenced by voluntary termination of intake), whereas rats in the two restricted groups were limited to consuming smaller defined volumes during the same 30 min period. At the end of the 30 min feeding period, the amount of Ensure consumed by each rat was recorded and feeding bottles were removed.
2.2 Perfusion/tissue preparation
Rats were sacrificed one hour after the end of the 30 min feeding period. This time point was selected to capture peak cFos activation in response to ingestion and satiation, since cFos protein immunolabeling peaks 60–90 min after neural stimulation and persists at peak levels for at least 30 additional min (42). Rats were deeply anesthetized with pentobarbital sodium (Fatal Plus; 100 mg/kg BW, i.p., Butler Schein, Columbus, OH) and then transcardially perfused with 50–100 mL saline followed by 100 mL of 2% paraformaldehyde (PF; Sigma, St. Louis, MO) containing 1.5–2.0% acrolein (Polysciences Inc., Warrington, PA), followed by 100 mL of 2% PF. After perfusion, clamps were placed at the distal esophagus and proximal duodenum, stomachs were excised, and gastric contents removed and weighed. Brains were post-fixed overnight in situ in 2% PF at 4°C, then removed from the skull, blocked, cryoprotected in 20% sucrose, frozen and sectioned at 35 μm using a sliding microtome. Sections were collected serially in six sets that each contained a complete rostrocaudal series of sections spaced by 210 μm. Sections were stored at −20°C in cryoproservant solution (43) to await immunohistochemical processing.
2.3 Immunohistochemistry
Tissue sections were removed from cryopreservant, rinsed in 0.1 M phosphate buffer (PB, pH 7.2), pre-treated in 0.5% sodium borohydride (Sigma) solution for 20 min, rinsed in PB, immersed in 0.5% H2O2 for 10 min, and rinsed again in PB. Primary and secondary antisera were diluted in PB containing 0.3% Triton X (Sigma), 1% donkey serum (Jackson ImmunoResearch, West Grove, PA), and (when noted) 1% bovine serum albumin (BSA; Sigma).
For dual cFos and GLP-1 immunolabeling, one set of pre-treated sections from each rat was incubated overnight in rabbit anti-GLP-1 at room temperature, using one of two primary antisera that yielded similar GLP-1 neural labeling (Peninsula Laboratories, San Carlos, CA, #T-4057, 1:8000 with BSA, or Peninsula #T-4363, 1:10000, no BSA). After rinsing, sections were incubated in biotinylated donkey anti-rabbit IgG (1:500; Jackson ImmunoResearch) for 1 hr at room temperature, rinsed, then incubated in avidin-biotin complex (Vectastain Elite reagents, Vector Labs, Burlingame, CA) for 1.5 hrs. After rinsing, tissue underwent an H2O2-catalyzed reaction in a solution of diaminobenzidine (DAB; Sigma) in 0.1M Tris buffer to produce a brown cytoplasmic GLP-1 peroxidase reaction product. GLP-1-labeled tissue was then incubated overnight at room temperature or for 48–72 hr at 4°C in one of two primary rabbit antisera raised against cFos protein: one was provided by Drs. Philip Larsen and Jens Mikkelsen, Panum Institute, Denmark (1:5000), and the second was purchased from EMD Chemicals (PC38, San Diego, CA; 1:2000). Pilot studies confirmed that both cFos antisera produced similar results, including similar within-animal cFos activation counts for GLP-1 neurons. To visualize nuclear cFos labeling, sections were rinsed, incubated overnight in CY3-conjugated donkey anti-rabbit IgG (1:300, Jackson Immunochemicals), and rinsed again to produce an immunofluorescent signal.
For triple immunolabeling of cFos, dopamine beta hydroxylase (DBH; to identify all A2 and C2 neurons within the caudal NST; (14)), and PrRP, an adjacent set of pre-treated tissue sections from each rat was incubated in rabbit anti-cFos (1:20,000; EMD Chemicals) followed by donkey anti-rabbit IgG and avidin-biotin reagents, as described above. After rinsing, tissue underwent an H2O2-catalyzed reaction in a solution of 0.1M sodium acetate buffer containing DAB and 2.5% nickel sulfate (Sigma) to produce a blue/black nuclear cFos peroxidase reaction product. Reacted sections were then rinsed and incubated in a cocktail of rabbit anti-PrRP (#H-008-52, 1:1000, Phoenix, Burlingame, CA) and mouse anti-DBH (#MAB308, 1:5000, Millipore, Billerica, MA) primary antisera overnight at room temperature. After rinsing, sections were incubated overnight at 4°C in a cocktail of CY3-conjugated donkey anti-rabbit IgG and Alexa 488-conjugated donkey anti-mouse IgG (both at 1:300, Jackson ImmunoResearch).
After immunocytochemical processing, labeled tissue sections were rinsed in PB and mounted onto adhesion Superfrost Plus Microscope Slides (Brain Research Laboratories, Waltham, MA), allowed to dry, then dehydrated and defatted in a series of graded ethanols followed by xylene. Slides were coverslipped with Cytoseal 60 mounting medium (Fisher Scientific, Pittsburgh, PA) and stored at room temperature in covered boxes.
2.4 Quantification of feeding-induced neural activation
2.4.1. GLP-1 neural activation
GLP-1 immunoperoxidase and cFos immunofluorescent labeling was visualized using an Olympus BX51 microscope equipped with brightfield and epifluorescence (X-Cite 120) optics. In each rat, all GLP-1 neurons within the caudal NST were photographed in sections (spaced by 210 μm) from the upper cervical spinal cord through the mid-level of the area postrema using a 20X objective and a Hamamatsu camera. GLP-1 neurons within the medullary reticular formation were not included in cell counts. Photographs of GLP-1 neurons were uploaded into Adobe Photoshop, and were identified visually on a computer screen based on brown cytoplasmic peroxidase labeling that was clearly perikaryal, rather than dendritic or axonal. By overlaying the brightfield channel (containing visible GLP-1 neurons) and the fluorescent channel, GLP-1 neurons were further classified as cFos-positive if their nucleus contained visible fluorescent cFos immunolabeling, regardless of intensity (see Figure 1B). GLP-1 neuronal activation was quantified as the proportion (percent) of GLP-1-positive NST neurons that also were cFos-positive.
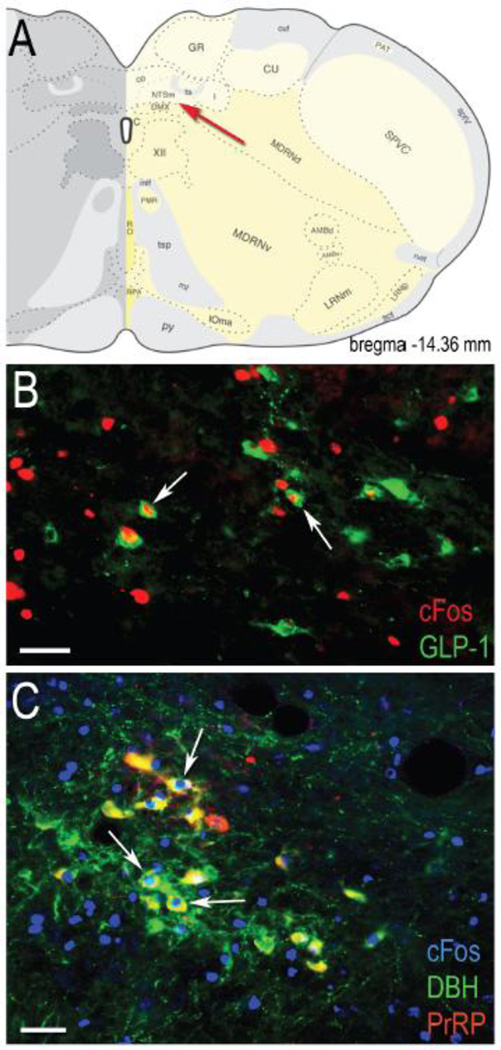
Fig. 1.
GLP-1-positive (B) and DBH+PrRP-positive (C) neurons within the caudal NST (region indicated by red arrow in panel A) are activated to express cFos in representative rats that consumed an unrestricted (B) or 30% restricted (C) Ensure meal after food deprivation. White arrows point out some of the double-labeled neurons visible within each photomicrograph, in which nuclear cFos immunolabeling is co-localized with cytoplasmic GLP-1 or DBH+PrRP immunolabeling. Brain schematic image in panel A adapted from (45). Scale bars in B and C = 50 μm.
2.4.2. Activation of DBH+PrRP-positive and DBH+PrRP-negative neurons
DBH and PrRP dual immunofluorescence and cFos immunoperoxidase labeling was visualized using epifluorescence and brightfield optics. In photographs from each animal, DBH-positive neurons were identified and counted within the caudal NST from the upper cervical spinal cord through sections just rostral to the area postrema, where the NST remains immediately adjacent to the wall of the 4th ventricle. DBH-positive neurons were further classified as PrRP-positive or PrRP-negative based on the presence or absence of dual DBH+PrRP immunofluorescent labeling, and were identified as cFos-positive when their nucleus contained visible blue-black immunoperoxidase labeling, regardless of intensity (see Figure 1C). As previously reported (27), all PrRP-positive NST neurons also were DBH-positive, whereas many DBH-positive NST neurons were PrRP-negative, especially in sections through and rostral to the rostral area postrema.
2.5 Data Analysis
Analyzed data included the amount of Ensure consumed by each rat (expressed as volume, and also as weight, with the latter converted to %BW), the postmortem weight of gastric contents, the percentage of the consumed amount and the total volume of Ensure that emptied from the stomach before gastric contents were collected post-perfusion, and the proportions of identified neurons (i.e., GLP-1, DBH+PrRP-positive, and DBH+PrRP-negative) activated to express cFos. Data are presented in graphs and tables as group mean ± SE. Statistically significant effects of feeding group on experimental outcomes were identified using ANOVA, with feeding group as the independent variable, followed by post-hoc LSD T-tests corrected for multiple comparisons to detect differences between individual feeding groups. Differences were considered statistically significant when p < 0.05. Correlational analyses also were performed to examine relationships between Ensure intake and cFos activation of GLP-1 and/or DBH+PrRP-positive or -negative neurons.
3. Results
The number of rats per feeding condition group is indicated in Table 1. ANOVA confirmed no significant between-group differences in post-deprivation, pre-meal BWs (Table 1).
Table 1.
Bodyweights, meal-related data and gastric content data for each of the four feeding condition groups.
| Feeding condition |
Pre-meal BW (g) |
Meal size (mL) |
Meal size (%BW) |
Caloric value of meal (kcal) |
Post- mortem weight of gastric contents (g) |
% of meal retained in stomach at time of perfusion |
Amount of Ensure emptied from stomach by time of perfusion (mL) |
|---|---|---|---|---|---|---|---|
| Unrestricted (n=10) | 230.10 ± 6.49a | 9.00 ± .84a | 4.74 ± .50a | 9.54 ± .89a | 6.47 ± .79a | 66.98 ± 3.43a | 2.98 ± .20a |
| 30% restricted (n=6) | 217.67 ± 4.40a | 7.00 ± .13b | 3.15 ± .06b | 7.42 ± .14b | 4.38 ± .24b | 62.04 ± 4.74a | 2.62 ± .34a |
| 50% restricted (n=8) | 232.13 ± 4.58a | 4.28 ± .48c | 2.13 ± .15b | 4.50 ± .49c | 1.34 ± .27c | 30.63 ± 4.00b | 2.95 ± .37a |
| Non-fed (n=7) | 216.86 ± 5.89a | 0.00d | 0.00c | 0.00d | 0.00c | - | - |
Values represent group mean ± SE. Different superscript letters (i.e., a, b, c) indicate significant difference between groups within a column as determined by ANOVA and post-hoc T-tests (p < .05).
3.1 Meal sizes and post-mortem gastric contents
Rats within the unrestricted feeding group consumed approximately 4.7% of their BW within 30 min, whereas rats in the 30% and 50% restricted feeding groups consumed approximately 3.2% and 2.1% of their BW, respectively (Table 1). These intake amounts corresponded to approximately 9.5 kcal in the unrestricted group, 7.4 kcal in the 30% restricted group, and 4.5 kcal in the 50% restricted group (Table 1). Rats did not consume water during the Ensure re-feeding period. There were no flavor-related (i.e., chocolate vs. vanilla) differences in intake within any group. All rats in the unrestricted group had excess available but unconsumed Ensure remaining after the end of the 30 min feeding period, whereas all rats in the two restricted feeding groups consumed the entire volume of available Ensure. The post-mortem weight of gastric contents (collected 1 hr after the end of the 30-min feeding period) ranged from approximately 1.3 to 6.5 g (Table 1). Only ~31% of Ensure consumed by rats in the 50% restricted feeding group remained within the stomach post-perfusion, whereas ~62% of the consumed amount remained in the 30% restricted feeding group, and ~67% remained in the unrestricted feeding group. However, the absolute volume of Ensure that emptied from the stomach was similar across feeding groups (~2.6–3.0 ml, Table 1), as expected based on previous research(44). Thus, post-gastric nutrient signals were similar across feeding groups.
3.2 Feeding-induced neuronal activation
Relatively few GLP-1 or DBH+ neurons were cFos-positive in unfed control rats, whereas cFos labeling increased within both neuronal populations in rats that were allowed to feed (see Figure 1). ANOVA revealed a significant main effect of feeding condition on the proportion of GLP-1-positive neurons expressing cFos [F(3, 27) = 12.02, p < 0.001], the proportion of DBH+PrRP-positive neurons expressing cFos [F(3, 25) = 40.70, p < 0.001], and the proportion of DBH+PrRP-negative neurons expressing cFos [F(3, 25) = 23.54, p < 0.001].
3.3 GLP-1 neuronal activation
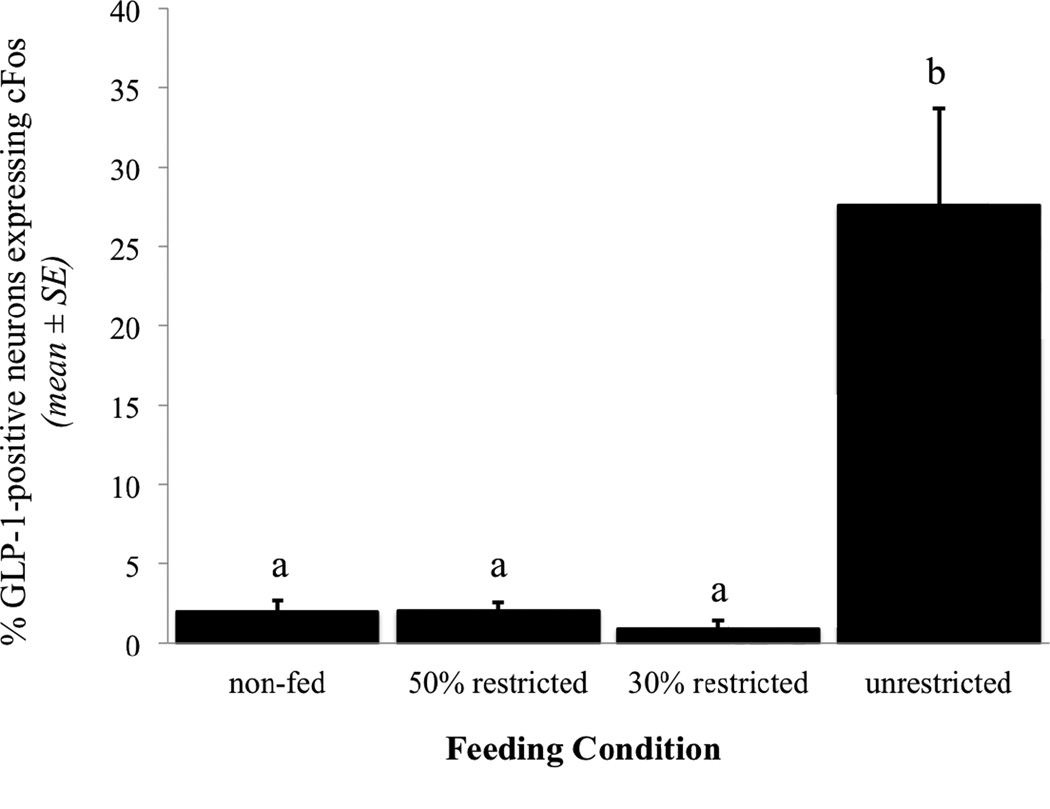
The proportion of GLP-1-positive neurons expressing cFos within each feeding condition group is presented in Figure 2. There was no main effect of feeding condition on the number of identified GLP-1-positive neurons per section ([F(3, 27) = 1.52, p = 0.23). Very few GLP-1 neurons were cFos-positive in non-fed control rats (n=7), or in rats that were allowed to consume either 50% restricted (n=8) or 30% restricted volumes of Ensure (n=6; Fig. 2). Conversely, approximately 28% of GLP-1 neurons were c-Fos-positive in rats that consumed unrestricted volumes of Ensure (n=10; Figs. 1B, 2). Post-hoc analyses revealed no between-group differences in GLP-1 cFos activation among non-fed controls (1.95% ± 0.75), 30% restricted (0.90% ± 0.52), and 50% restricted (2.00% ± 0.58%) groups. The proportion of GLP-1 neurons expressing cFos in rats consuming unrestricted volumes of Ensure (27.57% ± 6.11) was significantly greater than in each of the other three groups (p < 0.001 for each comparison). Thus, intake of only a satiating, unrestricted volume of Ensure was sufficient to activate GLP-1 neurons above levels quantified in non-fed control rats, even though rats in the 50% and 30% restricted feeding condition groups consumed 2–3% of their BW during the 30-min feeding period, and even though similar volumes of Ensure had emptied from the stomach into the small intestine in rats from all three feeding groups (Table 1).
Fig. 2.
Percentage of GLP-1-immunopositive NST neurons that express cFos immunoreactivity in response to meals of increasing size (see Table 1). Different letters indicate statistically significant differences in cFos activation between feeding condition groups (p < .05).
3.4 Activation of DBH+PrRP-positive and DBH+PrRP-negative neurons
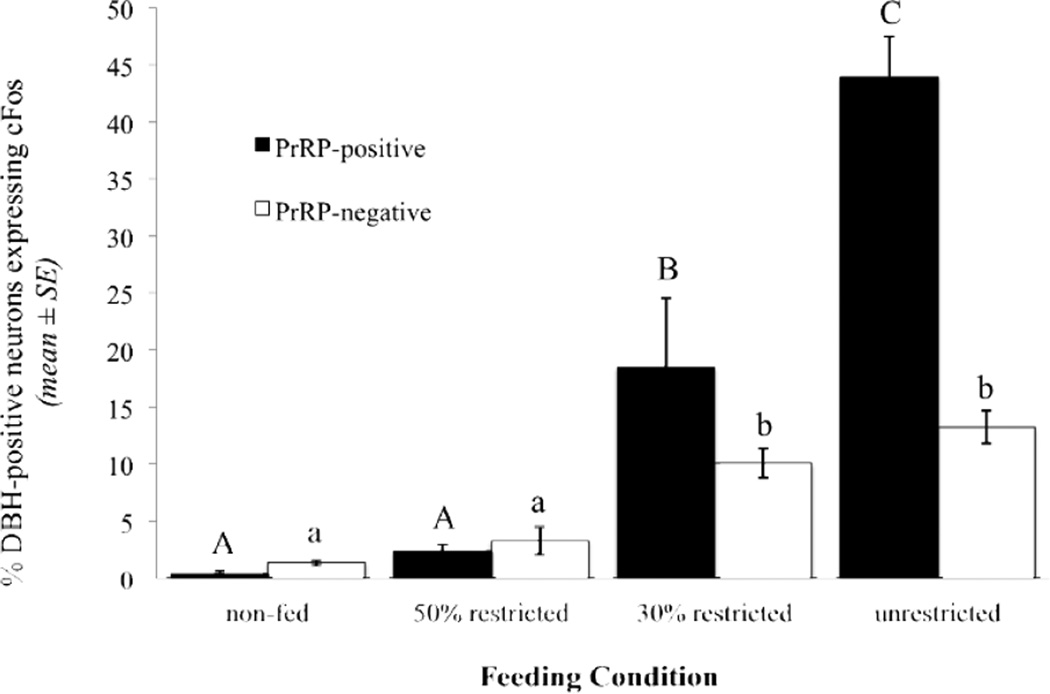
Tissue sections were damaged in two rats (one from the 50% restricted group, one from the unrestricted group) that were included in GLP-1 activation analyses, and thus these two rats did not contribute data for analysis of DBH-positive neurons. There was no main effect of feeding condition on the number of identified DBH-positive neurons per section that were PrRP-positive ([F(3, 24) = 0.72, p = 0.55) or PrRP-negative ([F(3, 24) = 2.73, p = 0.07). In non-fed control rats (n=7), DBH-positive neurons expressed very little cFos regardless of PrRP phenotype (0.37% ± 0.28 of DBH+PrRP-positive neurons and 1.36% ± 0.23 of DBH+PrRP-negative neurons; Fig. 3). Similarly low activation of DBH-positive neurons was observed in the 50% restricted intake group (2.36% ± 1.70 of DBH+PrRP-positive and 3.29% ± 1.19 of DBH+PrRP-negative neurons; n=7). However, unlike the negligible GLP-1 activation observed in the 30% restricted feeding group, both phenotypic subpopulations of DBH-positive neurons displayed significantly increased cFos expression in rats consuming a 30% restricted volume of Ensure (n=6; 18.48% ± 6.06 of DBH+PrRP-positive neurons, and 10.06% ± 1.27 of DBH+PrRP-negative neurons; p < 0.01 compared to activation of each DBH population in 50% restricted or non-fed control rats; Fig. 3). Feeding-induced activation of DBH+PrRP-positive neurons was more markedly increased in the unrestricted feeding condition group (n=9; 43.92% ± 10.68; Figs. 1C, 3), and was significantly greater in the unrestricted feeding group compared to each other feeding group (p < 0.01 for each comparison). Among DBH+PrRP-negative neurons, cFos activation in unrestricted rats (13.24% ± 1.42) was similar to activation of the same phenotypic neural population in rats consuming a 30% restricted meal (Fig. 3).
Fig. 3.
Percentage of DBH-immunopositive neurons, either PrRP-positive or PrRP-negative, that express cFos immunoreactivity in response to meals of increasing size (see Table 1). Within each phenotypic subgroup of DBH-positive neurons, different letters indicate significant differences in cFos activation between feeding condition groups (p < .05).
3.5 Correlational analyses
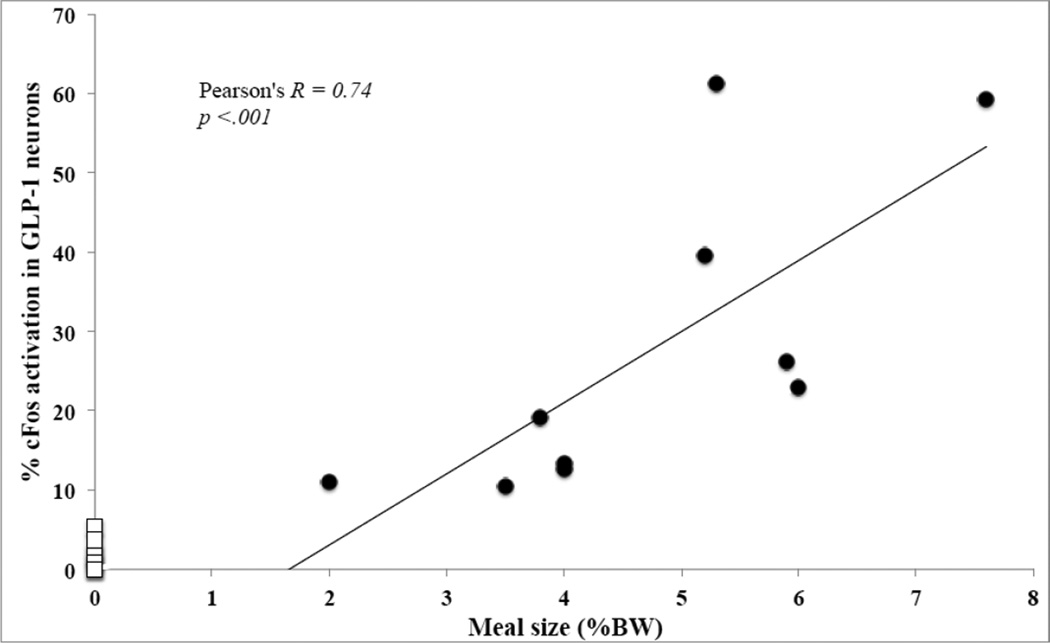
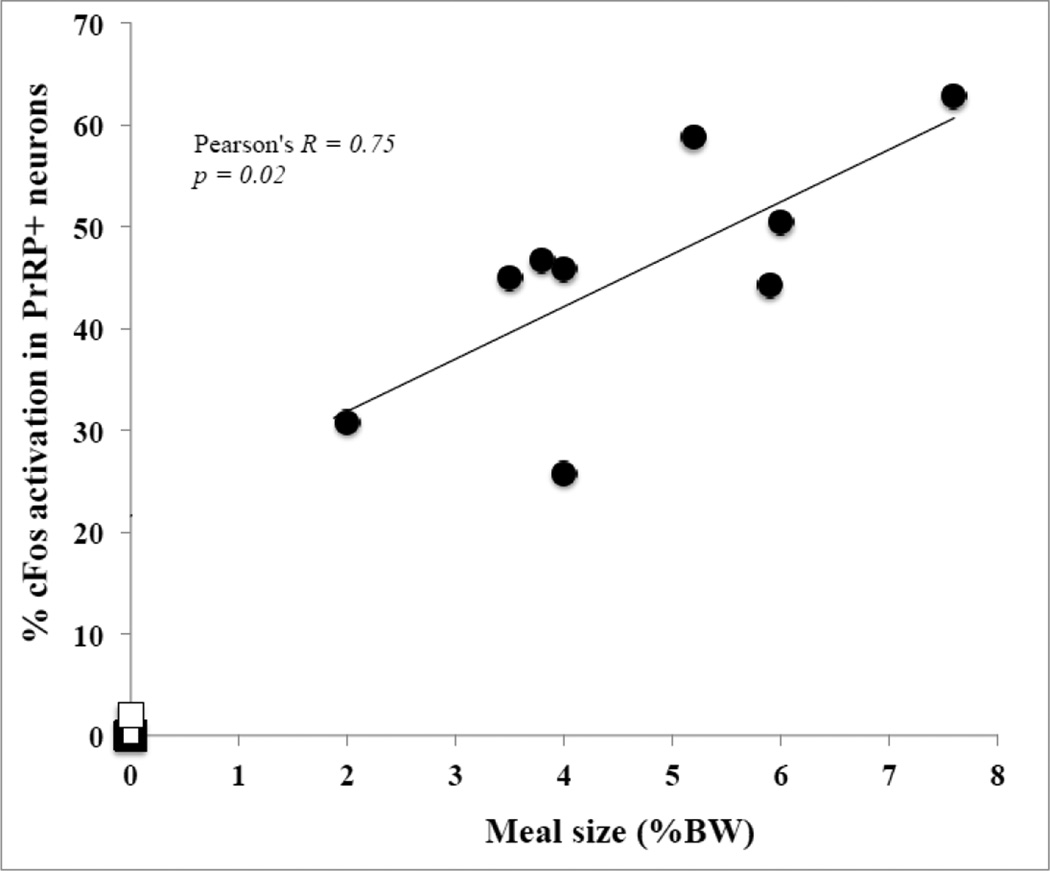
The amount of Ensure consumed by individual rats (expressed as % BW) across all three feeding condition groups (i.e., excluding non-fed controls) was positively and significantly correlated with the proportion of GLP-1 neurons expressing cFos (Pearson’s R = 0.83; p < 0.001), with the proportion of DBH+PrRP-positive neurons expressing cFos (Pearson’s R = 0.81; p < 0.001), and with the proportion of DBH+PrRP-negative neurons expressing cFos (Pearson’s R = 0.61, p = 0.002). In addition, the proportion of GLP-1-positive neurons that expressed cFos was significantly correlated with the proportion of DBH+PrRP-positive neurons that expressed cFos across all three feeding conditions (Pearson’s R = 0.71; p < 0.001). Since the Ensure volumes consumed by rats in the 50% and 30% restricted feeding condition groups were artificially limited to a predetermined amount, additional correlations between Ensure volumes consumed and cFos activation were performed separately for rats within the unrestricted group alone, whose intake volumes were larger but also more variable within the group (Figs. 4 and 5). Once again, strong positive correlations were observed between the amount of Ensure consumed by each rat and the corresponding activation of GLP-1 neurons (Fig. 4; Pearson’s R = 0.74; p < .001) and DBH+PrRP-positive neurons (Fig. 5; Pearson's R = 0.75; p = 0.02). The correlation between amount of Ensure consumed and activation of DBH+PrRP-negative neurons was not significant (Pearson's R = 0.12; p = 0.76), nor was the correlation between activation of DBH+PrRP-positive neurons and activation of GLP-1 neurons within the unrestricted feeding group alone (Pearson's R = 0.58; p = 0.10).
Fig. 4.
Relationship between meal size and percentage of GLP-1-positive neurons activated to express cFos in rats that consumed unrestricted, satiating volumes of Ensure (black dots). Data points representing non-fed controls (n=7) are added for comparison (open squares). Each symbol represents one animal. Best-fit line and correlation statistics refer only to rats in the unrestricted feeding group.
Fig. 5.
Relationship between meal size and percentage of PrRP-positive neurons expressing cFos in rats that consumed unrestricted, satiating volumes of Ensure (black dots). Data points representing non-fed controls (n=7) are added for comparison (open squares). Each symbol represents one animal. Best-fit line and correlation statistics refer only to rats in the unrestricted feeding group.
4. Discussion
Results from the present study demonstrate that DBH-positive neurons within the caudal visceral NST (including primarily the A2 cell group, but also the caudal portion of the C2 cell group) are progressively recruited to express cFos in rats that consume progressively larger volumes of palatable liquid Ensure after first-time food deprivation. Among DBH-positive neurons examined in our study, the PrRP-positive subset was most sensitive to feeding-induced activation, although smaller proportions of DBH+PrRP-negative neurons also were recruited when rats consumed large volumes of Ensure. The only previous report on feeding-induced activation of PrRP-positive NST neurons (40) did not examine the relationship between amount consumed and neuronal recruitment. In the present study, food-deprived rats had to consume at least 3.1% of their BW within a 30 min period in order to significantly increase cFos expression by DBH-positive caudal NST neurons. Notably, however, significant feeding-induced activation of GLP-1 neurons occurred only in the unrestricted feeding group, in which rats consumed very large volumes of Ensure that corresponded to nearly 5% of their BW. Maximal activation of the PrRP-positive subset of DBH neurons also was achieved in the unrestricted feeding group. We interpret these results as evidence that progressive recruitment of A2/C2 neurons within the caudal NST, especially the most caudally-situated PrRP-positive subset of A2 neurons, effectively “tracks” the magnitude of GI satiety signals and other meal-related sensory feedback. Conversely, only the largest meals are sufficient to activate GLP-1 neurons. Based on these and other published results (discussed further, below), we posit that GLP-1 neurons are relatively insensitive to normal, progressively increasing GI satiety signals during food intake, but are ultimately recruited in response to interoceptive stress generated by the homeostatic challenge of consuming a very large, unanticipated meal. Our findings are consistent with several "loss-of-function" studies that have implicated both GLP-1 and DBH/PrRP neuronal populations in meal size control (5, 39, 40, 45, 46). However, many cFos-positive caudal NST neurons in the present study were not immunopositive for either GLP-1 or DBH/PrRP. We do not discount the potential contributions of these phenotypically unidentified neurons to meal termination.
Previous reports from our laboratory and others indicate that NA and GLP-1 neurons are activated to express cFos in rats after a variety of treatments that stimulate vagal sensory inputs to the caudal NST (16–20). In one of these studies, we examined the ability of food intake to activate tyrosine-hydroxylase (TH)-positive catecholamine neurons (which include but are not limited to NA neurons) within the caudal NST (16). However, that study employed a different experimental paradigm, in which rats were acclimated for 5 days to a feeding schedule that included repeating cycles of overnight food deprivation followed by daily 1 hr access to unrestricted amounts of solid or liquid diet. On day 5, rats were killed 30 min after the end of the 1 hr feeding period, during which they consumed unrestricted or rationed amounts of food (16). Despite the different feeding paradigms utilized in that study and the present one, feeding-induced activation of TH-positive NST neurons in the earlier study was similar to feeding-induced activation of DBH-positive neurons in the present study: progressively larger proportions of TH-positive NST neurons expressed cFos in rats that consumed progressively larger amounts of food, with peak activation of TH neurons reaching ~30–40% in rats that consumed an unrestricted (satiating) amount of food (16). Conversely, in a follow-up study (17) using tissue sections from the same meal-entrained rats, we found that GLP-1 neurons were not activated after scheduled feeding, even in rats that consumed liquid diet volumes that were 2–3 times greater than those consumed by non-entrained rats in the present study. GLP-1 neurons were, however, activated in rats after several “interceptive stress” treatments, including i.p. injection of lithium chloride, lipopolysaccharide, or a high dose of CCK (17).
Considered together with the present results in rats that were not entrained to consume large meals, these findings support the view that GLP-1 neurons are recruited by significant homeostatic challenges, including the challenge generated by consuming a large unanticipated meal (2, 23, 47). However, in the present study, the average proportion of GLP-1 neurons expressing cFos in rats that consumed unrestricted meals after 24 hr food deprivation (~28%) is only slightly elevated above the ~20% of GLP-1 neurons that express cFos under “baseline”, ad libitum feeding conditions (18). Thus, our results could be interpreted as evidence that after food deprivation, only large satiating meals of Ensure are sufficient to restore GLP-1 activation to "baseline" levels observed in rats maintained under ad libitum feeding conditions.
We can only speculate as to what feature of the consumed meal serves as the primary stimulus for NA/PrRP and GLP-1 neural activation in the present study. Although rats in each feeding group consumed different volumes of Ensure, and thus different amounts of fat, protein, and carbohydrate calories, statistically similar volumes of consumed Ensure (~ 3 ml) had emptied from the stomachs in all three feeding groups by the time of perfusion. Therefore, differences in intestinal and post-absorptive nutrient signaling are unlikely to contribute to between-group differences in cFos activation. Most of this cFos activation was contained within the medial and commissural subdivisions of the caudal NST, where distension-sensitive gastric and intestinal vagal afferents terminate (48, 49). Thus, we posit that between-group differences in DBH/PrRP and GLP-1 neuronal activation reflect different degrees of GI distension coded by vagal sensory inputs to the caudal NST, where the majority of DBH-positive NA neurons (A2 cell group) are PrRP-positive. Since rats within each feeding group consumed different volumes of Ensure, we cannot rule out potential contributions of differential magnitude or duration of oral sensory and motor stimuli to between-group differences in cFos activation. However, our previous study (17) demonstrated very low activation of GLP-1-positive neurons and similar activation of DbH-positive neurons in food-entrained rats that consumed liquid meals that were much larger than those in the present study, with more concurrent oral sensory and motor stimulation. Therefore, the contribution of oral sensory and motor signals to cFos expression within the caudal NST is likely minimal in the present study.
In more rostral sections through the rostral area postrema, increasing numbers of DBH+PrRP-negative neurons likely include C2 neurons that are intermingled among A2 neurons at this level, and rostral to the area postrema, the large majority of DBH-positive neurons are C2 neurons that are PrRP-negative (27, 50)]. Given the extreme gastric distension produced by intake of unrestricted Ensure meals, recruitment of visceronociceptive spinal afferents might contribute to increased activation of A2 and GLP-1 neurons within the caudal NST. Indeed, some of the NST cFos expression induced by inflation of a gastric balloon is attributable to spinal sensory afferents (51), and the spinosolitary tract conveys viscerosensory activation from the spinal dorsal horn to the caudal NST (52, 53).
Previous anatomical and functional reports demonstrate an important role for brainstem circuits in generating and modulating the motoric aspects of feeding (e.g., licking, chewing, swallowing) and accompanying vagal autonomic adjustments of GI motility and secretion (49, 54–58). Decerebrate rats that lack brainstem-forebrain communication still exhibit satiation (6, 7, 45), demonstrating that isolated brainstem circuits are sufficient for meal termination. Our own unpublished data identify PrRP- and GLP-1-immunoreactive fibers in brainstem regions that control somatic motor and autonomic aspects of feeding, including the dorsal vagal complex, parabrachial nucleus, more rostral (taste) regions of the NST, and the medullary and pontine reticular formation. It currently is unknown whether GLP-1 or PrRP-immunoreactive terminals are synaptically linked to brainstem neurons that control feeding behavior, but this question is ripe for experimental analysis.
5. Conclusions
Our results demonstrate that large satiating meals of palatable liquid Ensure recruit NA (especially PrRP-positive) and GLP-1-positive neurons within the caudal NST in rats that have not been acclimated/entrained to a feeding schedule. Conversely, we previously reported that large meals activate similar proportions of NA neurons but do not activate GLP-1 neurons in meal-entrained rats (16, 17). Considered together, these findings support the conclusion that PrRP-positive A2 neurons are progressively recruited during feeding as a function of meal size, regardless of prior meal entrainment history, whereas GLP-1 neurons are recruited only by very large meals consumed by rats that have not been acclimated to scheduled feeding.
Highlights.
Feeding-induced cFos activation was examined in non-meal-entrained rats.
Liquid meals of 2–3% BW activated hindbrain NA neurons, especially the PrRP subset.
Liquid meals of ~5% BW were required to activate GLP-1 neurons.
NA activation increases proportionally with meal size.
GLP-1 activation occurs only when rats consume a very large, unanticipated meal.
Acknowledgements
This manuscript is based on work presented during the 2013 Annual Meeting of the Society for the Study of Ingestive Behavior held in New Orleans, LA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith GP. The direct and indirect controls of meal size. Neuroscience and Biobehavioral Reviews. 1996;20:41. doi: 10.1016/0149-7634(95)00038-g. [DOI] [PubMed] [Google Scholar]
- 2.Woods SC, Ramsay DS. Pavlovian influences over food and drug intake. Behav Brain Res. 2000 Jun 1;110:175. doi: 10.1016/s0166-4328(99)00194-1. [DOI] [PubMed] [Google Scholar]
- 3.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006 Sep 21;443:289. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 4.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012 Sep 5;16:296. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009 Jun;150:2654. doi: 10.1210/en.2008-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grill HJ, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 1978 Jul 21;201:267. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- 7.Grill HJ, Smith GP. Cholecystokinin decreases sucrose intake in chronic decerebrate rats. Am J Physiol. 1988 Jun;254:R853. doi: 10.1152/ajpregu.1988.254.6.R853. [DOI] [PubMed] [Google Scholar]
- 8.Appleyard SM, et al. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J Neurosci. 2007 Nov 28;27:13292. doi: 10.1523/JNEUROSCI.3502-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hisadome K, Reimann F, Gribble FM, Trapp S. CCK stimulation of GLP-1 neurons involves alpha1-adrenoceptor-mediated increase in glutamatergic synaptic inputs. Diabetes. 2011 Nov;60:2701. doi: 10.2337/db11-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grill HJ. Leptin and the systems neuroscience of meal size control. Front Neuroendocrinol. 2010 Jan;31:61. doi: 10.1016/j.yfrne.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith GP. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition. 2000 Oct;16:814. doi: 10.1016/s0899-9007(00)00457-3. [DOI] [PubMed] [Google Scholar]
- 12.Travers JB, Rinaman L. Identification of lingual motor control circuits using two strains of pseudorabies virus. Neuroscience. 2002;115:1139. doi: 10.1016/s0306-4522(02)00489-x. [DOI] [PubMed] [Google Scholar]
- 13.Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010 Sep 2;1350:18. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 2011;300:R222. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vrang N, Larsen PJ. Preproglucagon derived peptides GLP-1, GLP-2 and oxyntomodulin in the CNS: role of peripherally secreted and centrally produced peptides. Progress In Neurobiology. 2010;92:442. doi: 10.1016/j.pneurobio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Rinaman L, Baker EA, Hoffman GE, Stricker EM, Verbalis JG. Medullary c-Fos activation in rats after ingestion of a satiating meal. Am J Physiol. 1998 Jul;275:R262. doi: 10.1152/ajpregu.1998.275.1.R262. [DOI] [PubMed] [Google Scholar]
- 17.Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol. 1999 Aug;277:R582. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- 18.Maniscalco JW, Rinaman L. Overnight food deprivation markedly attenuates hindbrain noradrenergic, glucagon-like peptide-1, and hypothalamic neural responses to exogenous cholecystokinin in male rats. Physiol Behav. 2013 Feb 4; doi: 10.1016/j.physbeh.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaykema RP, et al. Immune challenge and satiety-related activation of both distinct and overlapping neuronal populations in the brainstem indicate parallel pathways for viscerosensory signaling. Brain Res. 2009 Oct 19;1294:61. doi: 10.1016/j.brainres.2009.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol. 2003 Aug;285:R470. doi: 10.1152/ajpregu.00732.2002. [DOI] [PubMed] [Google Scholar]
- 21.Rinaman L, Verbalis JG, Stricker EM, Hoffman GE. Distribution and neurochemical phenotypes of caudal medullary neurons activated to express cFos following peripheral administration of cholecystokinin. J Comp Neurol. 1993 Dec 22;338:475. doi: 10.1002/cne.903380402. [DOI] [PubMed] [Google Scholar]
- 22.Rinaman L, et al. Cholecystokinin activates catecholaminergic neurons in the caudal medulla that innervate the paraventricular nucleus of the hypothalamus in rats. The Journal of Comparative Neurology. 1995;360:246. doi: 10.1002/cne.903600204. [DOI] [PubMed] [Google Scholar]
- 23.Woods SC. The eating paradox: how we tolerate food. Psychol Rev. 1991 Oct;98:488. doi: 10.1037/0033-295x.98.4.488. [DOI] [PubMed] [Google Scholar]
- 24.Zhao S, Kanoski SE, Yan J, Grill HJ, Hayes MR. Hindbrain leptin and glucagonlike- peptide-1 receptor signaling interact to suppress food intake in an additive manner. Int J Obes (Lond) 2012 Jan 17; doi: 10.1038/ijo.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrera JG, et al. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci. 2011 Mar 9;31:3904. doi: 10.1523/JNEUROSCI.2212-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asarian L, Corp ES, Hrupka B, Geary N. Intracerebroventricular glucagon-like peptide-1 (7–36) amide inhibits sham feeding in rats without eliciting satiety. Physiol Behav. 1998 Jun 1;64:367. doi: 10.1016/s0031-9384(98)00089-4. [DOI] [PubMed] [Google Scholar]
- 27.Chen C, Dun SL, Dun NJ, Chang JK. Prolactin-releasing peptide-immunoreactivity in A1 and A2 noradrenergic neurons of the rat medulla. Brain Res. 1999 Mar 20;822:276. doi: 10.1016/s0006-8993(99)01153-1. [DOI] [PubMed] [Google Scholar]
- 28.Iijima N, et al. Cytochemical study of prolactin-releasing peptide (PrRP) in the rat brain. Neuroreport. 1999 Jun 3;10:1713. doi: 10.1097/00001756-199906030-00016. [DOI] [PubMed] [Google Scholar]
- 29.Maruyama M, et al. Prolactin-releasing peptide as a novel stress mediator in the central nervous system. Endocrinology. 2001 May;142:2032. doi: 10.1210/endo.142.5.8118. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto H, et al. Stimulation of corticotropin-releasing hormone-mediated adrenocorticotropin secretion by central administration of prolactin-releasing peptide in rats. Neurosci Lett. 2000 May 19;285:234. doi: 10.1016/s0304-3940(00)01077-6. [DOI] [PubMed] [Google Scholar]
- 31.Mochiduki A, Takeda T, Kaga S, Inoue K. Stress response of prolactin-releasing peptide knockout mice as to glucocorticoid secretion. J Neuroendocrinol. 2010 Jun;22:576. doi: 10.1111/j.1365-2826.2010.01993.x. [DOI] [PubMed] [Google Scholar]
- 32.Ohiwa N, et al. Possible inhibitory role of prolactin-releasing peptide for ACTH release associated with running stress. Am J Physiol Regul Integr Comp Physiol. 2007 Jan;292:R497. doi: 10.1152/ajpregu.00345.2006. [DOI] [PubMed] [Google Scholar]
- 33.Onaka T, Takayanagi Y, Leng G. Metabolic and stress-related roles of prolactinreleasing peptide. Trends Endocrinol Metab. 2010 May;21:287. doi: 10.1016/j.tem.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Samson WK, et al. Prolactin-releasing peptide and its homolog RFRP-1 act in hypothalamus but not in anterior pituitary gland to stimulate stress hormone secretion. Endocrine. 2003 Feb-Mar;20:59. doi: 10.1385/ENDO:20:1-2:59. [DOI] [PubMed] [Google Scholar]
- 35.Toth ZE, et al. Chronic repeated restraint stress increases prolactin-releasing peptide/tyrosine-hydroxylase ratio with gender-related differences in the rat brain. J Neurochem. 2008 Feb;104:653. doi: 10.1111/j.1471-4159.2007.05069.x. [DOI] [PubMed] [Google Scholar]
- 36.Gu W, Geddes BJ, Zhang C, Foley KP, Stricker-Krongrad A. The prolactinreleasing peptide receptor (GPR10) regulates body weight homeostasis in mice. J Mol Neurosci. 2004;22:93. doi: 10.1385/JMN:22:1-2:93. [DOI] [PubMed] [Google Scholar]
- 37.Lawrence CB, Celsi F, Brennand J, Luckman SM. Alternative role for prolactinreleasing peptide in the regulation of food intake. Nat Neurosci. 2000 Jul;3:645. doi: 10.1038/76597. [DOI] [PubMed] [Google Scholar]
- 38.Lawrence CB, Ellacott KL, Luckman SM. PRL-releasing peptide reduces food intake and may mediate satiety signaling. Endocrinology. 2002 Feb;143:360. doi: 10.1210/endo.143.2.8609. [DOI] [PubMed] [Google Scholar]
- 39.Bechtold DA, Luckman SM. Prolactin-releasing Peptide mediates cholecystokinin-induced satiety in mice. Endocrinology. 2006 Oct;147:4723. doi: 10.1210/en.2006-0753. [DOI] [PubMed] [Google Scholar]
- 40.Takayanagi Y, et al. Endogenous prolactin-releasing peptide regulates food intake in rodents. J Clin Invest. 2008 Dec;118:4014. doi: 10.1172/JCI34682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Archer ZA, Brown YA, Rayner DV, Stubbs RJ, Mercer JG. Effect of flavour of liquid Ensure diet supplement on energy intake in male SD rats. Physiol Behav. 2006 Oct 30;89:414. doi: 10.1016/j.physbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Chaudhuri A, Zangenehpour S, Rahbar-Dehgan F, Ye F. Molecular maps of neural activity and quiescence. Acta Neurobiol Exp (Wars) 2000;60:403. doi: 10.55782/ane-2000-1359. [DOI] [PubMed] [Google Scholar]
- 43.Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986 Jan-Feb;7:155. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 44.Kalogeris TJ, Reidelberger RD, Mendel VE. Effect of nutrient density and composition of liquid meals on gastric emptying in feeding rats. Am J Physiol. 1983 Jun;244:R865. doi: 10.1152/ajpregu.1983.244.6.R865. [DOI] [PubMed] [Google Scholar]
- 45.Grill HJ, Kaplan JM. Sham feeding in intact and chronic decerebrate rats. Am J Physiol. 1992 Jun;262:R1070. doi: 10.1152/ajpregu.1992.262.6.R1070. [DOI] [PubMed] [Google Scholar]
- 46.Barrera JG, et al. Hyperphagia and Increased Fat Accumulation in Two Models of Chronic CNS Glucagon-Like Peptide-1 Loss of Function. Journal of Neuroscience. 2011 Mar 9;31:3904. doi: 10.1523/JNEUROSCI.2212-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shiraishi I, Honma K, Honma S, Hiroshige T. Ethosecretogram: relation of behavior to plasma corticosterone in freely moving rats. Am J Physiol. 1984 Jul;247:R40. doi: 10.1152/ajpregu.1984.247.1.R40. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Fogel R, Renehan WE. Relationships between the morphology and function of gastric- and intestine-sensitive neurons in the nucleus of the solitary tract. J Comp Neurol. 1995 Dec 4;363:37. doi: 10.1002/cne.903630105. [DOI] [PubMed] [Google Scholar]
- 49.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roland BL, et al. Anatomical distribution of prolactin-releasing peptide and its receptor suggests additional functions in the central nervous system and periphery. Endocrinology. 1999 Dec;140:5736. doi: 10.1210/endo.140.12.7211. [DOI] [PubMed] [Google Scholar]
- 51.Traub RJ, Sengupta JN, Gebhart GF. Differential c-fos expression in the nucleus of the solitary tract and spinal cord following noxious gastric distention in the rat. Neuroscience. 1996 Oct;74:873. doi: 10.1016/0306-4522(96)00173-x. [DOI] [PubMed] [Google Scholar]
- 52.Menétrey D, Basbaum AI. Spinal and trigeminal projections to the nucleus of the solitary tract: A possible substrate for somatovisceral and viscerovisceral reflex activaiton. J Comp Neurol. 1987;255:439. doi: 10.1002/cne.902550310. [DOI] [PubMed] [Google Scholar]
- 53.Menétrey D, de Pommery J. Origins of spinal ascending pathways that reach central areas involved in visceroception and visceronociception in the rat. European Journal of Neuroscience. 1990;3:249. doi: 10.1111/j.1460-9568.1991.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 54.Llewellyn-Smith IJ, Gnanamanickam GJ, Reimann F, Gribble FM, Trapp S. Preproglucagon (PPG) neurons innervate neurochemically identified autonomic neurons in the mouse brainstem. Neuroscience. 2013 Jan 15;229:130. doi: 10.1016/j.neuroscience.2012.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pearson RJ, Gatti PJ, Sahibzada N, Massari VJ, Gillis RA. Ultrastructural evidence for selective noradrenergic innervation of CNS vagal projections to the fundus of the rat. Auton Neurosci. 2007 Oct 30;136:31. doi: 10.1016/j.autneu.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hermann GE, Nasse JS, Rogers RC. Alpha-1 adrenergic input to solitary nucleus neurones: calcium oscillations, excitation and gastric reflex control. J Physiol. 2005 Jan 15;562:553. doi: 10.1113/jphysiol.2004.076919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol Regul Integr Comp Physiol. 2003 Aug;285:R479. doi: 10.1152/ajpregu.00155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 2011 Feb;300:R222. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]