Abstract
Background
Randomized clinical trials comparing coronary artery bypass grafting (CABG) with percutaneous coronary intervention (PCI) have largely excluded patients with chronic kidney disease (CKD), leading to uncertainty about the optimal coronary revascularization strategy. We sought to test the hypothesis that an initial strategy of CABG would be associated with lower risks of long-term mortality and cardiovascular morbidity compared with PCI for the treatment of multivessel coronary heart disease in the setting of CKD.
Methods
We created a propensity score–matched cohort of patients aged ≥30 years with no prior dialysis or renal transplant who received multivessel coronary revascularization between 1996 and 2008 within a large integrated health care delivery system in northern California. We used extended Cox regression to examine death from any cause, acute coronary syndrome, and repeat revascularization.
Results
Coronary artery bypass grafting was associated with a significantly lower adjusted rate of death than PCI across all strata of estimated glomerular filtration rate (eGFR) (in mL/min per 1.73 m2): the adjusted hazard ratio (HR) was 0.81, 95% CI 0.68 to 1.00 for patients with eGFR ≥60; HR 0.73 (CI 0.56–0.95) for eGFR of 45 to 59; and HR 0.87 (CI 0.67–1.14) for eGFR <45. Coronary artery bypass grafting was also associated with significantly lower rates of acute coronary syndrome and repeat revascularization at all levels of eGFR compared with PCI.
Conclusions
Among adults with and without CKD, multivessel CABG was associated with lower risks of death and coronary events compared with multivessel PCI.
Cardiovascular disease is the leading cause of death among patients with chronic kidney disease (CKD).1,2 Up to two-thirds of patients with CKD have coronary heart disease (CHD),1 which usually involves multiple coronary arteries.3 Given the high burden of CHD in patients with CKD and associated poor prognosis,4,5 it is important to determine the optimal method of coronary revascularization for this high-risk patient population.
There are large knowledge gaps regarding the optimal coronary revascularization strategy in patients with CKD. Randomized trials of coronary artery bypass grafting (CABG) versus percutaneous coronary intervention (PCI) have largely excluded patients with CKD or have not reported outcomes by level of preprocedural kidney function, limiting the generalizability of the results to patients with CKD.6–15 Observational studies comparing CABG and PCI in patients with CKD have yielded conflicting results, with some reporting lower mortality associated with CABG16,17 and others reporting no significant differences.18–20 However, these studies were limited by relatively small sample sizes, varying definitions of CKD and limited spectra of CKD severity. Moreover, many of these studies included patients with both single and multivessel CHD, which, without confirmation of left main or proximal left anterior descending artery disease, may not necessarily represent fair comparison groups.21
To address these issues, we compared the effectiveness of CABG with PCI for multivessel CHD within a large, diverse, contemporary cohort of real-world patients. We hypothesized that an initial strategy of CABG would be associated with lower risks of long-term mortality, acute coronary syndrome, and repeat revascularization compared with PCI for the treatment of multivessel CHD in the setting of CKD.
Methods
Source population and study cohort
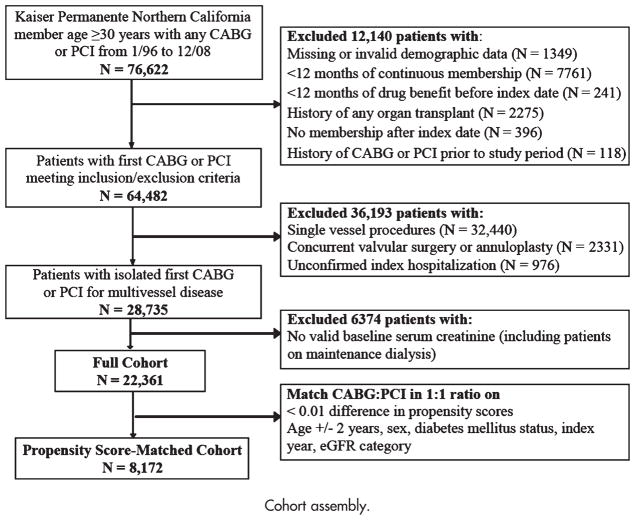
Kaiser Permanente Northern California is a large, integrated health care delivery system caring for >3.2 million persons who are broadly representative of the local and statewide population.22 To construct a cohort of patients with isolated CABG or PCI as the initial revascularization strategy for multivessel CHD (Figure 1), we identified all health plan members aged ≥30 years who received a multivessel (≥2 vessels) CABG or PCI between January 1, 1996, and December 31, 2008, using International Classification of Diseases, Ninth Edition (ICD-9), and Current Procedure Terminology, Fourth Edition, codes (online Appendix Supplemental Table I). We chose these inclusion dates because there was a systematic change to the database in 1995, and at the time of planning of the analysis, 2008 represented the most up-to-date information available. The index date was the date of the revascularization procedure.
Figure 1.
Cohort assembly.
As we sought to compare revascularization strategies for multivessel CHD, we excluded patients in whom the number of vessels revascularized was one or unknown. Because of a change in the ICD-9 coding system in 2005, the proportion of PCI patients in whom we were not able to identify the number of revascularized vessels increased from 0.3% for patients revascularized between 1996 and 2004 to 25.5% between 2005 and 2008. Patients with an unknown number of revascularized vessels by PCI had similar mean age, proportion of women, and prevalence of cardiovascular disease but were more often persons of color compared with patients in whom we were able to identify the coded number of revascularized coronary vessels (data not shown).
To ascertain information on comorbid conditions in a uniform manner, we restricted the analysis to patients with complete demographic data and at least 12 months of continuous membership and pharmacy benefit before the index revascularization procedure.23,24 We excluded patients with prior coronary revascularization and history of organ transplantation and patients who underwent concurrent valvular surgery or annuloplasty at the time of their CABG. Finally, we excluded patients on maintenance dialysis because these patients have very different physiology and risk factors for cardiovascular disease compared with patients with non–dialysis-dependent kidney disease.25–27
We used propensity score matching to create the final analytical cohort. We calculated a propensity score using the variables listed in Table I as predictors of receiving CABG or PCI. We matched each patient who received a PCI with 1 patient who received a CABG using a greedy matching algorithm to within ≥0.01 level of the propensity score. We required that each patient match within 2 years of the index age and match exactly on sex, index year, diabetes mellitus status, and category of estimated glomerular filtration rate (eGFR) (see below) at the time of the revascularization.
Table I.
Baseline characteristics in the matched cohort, stratified by baseline eGFR category and initial revascularization with multivessel CABG versus PCI
| eGFR ≥60
|
eGFR 45–59
|
eGFR <45
|
||||
|---|---|---|---|---|---|---|
| CABG
|
PCI
|
CABG
|
PCI
|
CABG
|
PCI
|
|
| n = 2628 | n = 2628 | n = 941 | n = 941 | n = 517 | n = 517 | |
| Demographics | ||||||
| Mean (SD) age, y | 62.3 (9.4) | 62.3 (9.4) | 70.6 (7.9) | 70.7 (8.0) | 74.5 (7.5) | 74.4 (7.5) |
| Women, % | 18.8 | 18.8 | 25.2 | 25.2 | 41.0 | 41.0 |
| Race, % | ||||||
| White | 73.4 | 73.7 | 80.7 | 81.4 | 77.6 | 80.1 |
| Black | 6.0 | 5.1 | 3.3 | 3.6 | 4.6 | 6.2 |
| Asian | 11.2 | 11.7 | 9.0 | 8.0 | 10.3 | 8.1 |
| Other/unknown | 9.5 | 9.6 | 7.0 | 7.0 | 7.5 | 5.6 |
| Hispanic ethnicity, % | 13.1 | 13.1 | 11.2 | 10.3 | 13.7 | 12.6 |
| Comorbid conditions, % | ||||||
| Current or former smoker | 35.9 | 35.0 | 31.8 | 33.2 | 33.8 | 33.3 |
| MI on index presentation | 24.0 | 29.0 | 22.6 | 26.8 | 29.0 | 30.9 |
| Cardiovascular history | ||||||
| Myocardial infarction | 32.5 | 35.3 | 32.4 | 36.9 | 44.5 | 41.8 |
| Unstable angina | 25.9 | 24.4 | 24.7 | 25.3 | 23.4 | 25.7 |
| Heart failure | 5.4 | 3.5 | 8.5 | 11.3 | 16.6 | 19.3 |
| Cerebrovascular disease | 10.3 | 9.1 | 13.8 | 17.2 | 22.1 | 22.2 |
| Stroke/transient ischemic attack | 2.1 | 2.1 | 3.6 | 3.8 | 6.6 | 5.4 |
| Peripheral arterial disease | 3.1 | 2.7 | 5.0 | 4.7 | 9.1 | 7.2 |
| Mitral and/or aortic valve disease | 3.8 | 2.9 | 6.2 | 7.7 | 7.4 | 9.3 |
| Rheumatic heart disease | 0.1 | 0.1 | 0.0 | 0.1 | 0.4 | 0.4 |
| Atrial fibrillation/flutter | 5.3 | 4.8 | 9.5 | 10.4 | 11.4 | 13.2 |
| Ventricular fibrillation/tachycardia | 1.0 | 1.3 | 1.7 | 2.1 | 1.7 | 2.3 |
| Other medical history, % | ||||||
| Diabetes mellitus | 34.6 | 34.6 | 30.8 | 30.8 | 44.3 | 44.3 |
| Hypertension | 55.7 | 55.9 | 68.0 | 68.7 | 83.8 | 80.5 |
| Dyslipidemia | 87.8 | 88.1 | 88.8 | 87.8 | 88.0 | 89.0 |
| Dementia | 0.5 | 0.3 | 1.0 | 1.2 | 1.5 | 1.2 |
| Depression | 9.7 | 10.7 | 11.8 | 12.0 | 10.8 | 15.9 |
| Chronic liver disease | 1.5 | 1.5 | 1.0 | 1.4 | 0.2 | 1.0 |
| Chronic lung disease | 16.9 | 17.0 | 18.6 | 19.7 | 18.0 | 25.5 |
| Hyperthyroidism | 0.6 | 0.5 | 0.4 | 0.4 | 0.0 | 1.0 |
| Hypothyroidism | 8.5 | 8.4 | 13.9 | 14.0 | 15.7 | 20.9 |
| Systemic cancer | 7.4 | 8.2 | 13.2 | 12.3 | 14.3 | 15.1 |
| Hospitalized bleed | 1.6 | 1.1 | 2.4 | 2.2 | 3.1 | 3.5 |
| Arthritis | 0.4 | 0.4 | 0.7 | 0.7 | 0.2 | 1.4 |
| Laboratory values, % | ||||||
| Urinary dipstick protein excretion | ||||||
| None | 95.2 | 95.5 | 93.3 | 94.2 | 81.8 | 80.6 |
| +1 | 2.8 | 2.7 | 4.7 | 3.1 | 7.9 | 7.2 |
| +2 | 1.5 | 1.4 | 1.4 | 2.1 | 5.8 | 5.8 |
| +3 or +4 | 0.5 | 0.4 | 0.6 | 0.6 | 4.4 | 6.4 |
| Median (IQR) HDL, mg/dL, | 41 (36–49) | 42 (36–49) | 42 (37–51) | 42 (36–50) | 42 (35–51) | 42 (35–50) |
| Median (IQR) LDL, mg/dL, | 119 (94–149) | 115 (93–145) | 109 (90–139) | 111 (88–139) | 105 (85–131) | 104 (82–130) |
| Hemoglobin, g/L, Median (IQR) | 14.5 (13.5–15.3) | 14.5 (13.5–15.3) | 14.0 (13.0–14.9) | 14.2 (13.0–15.0) | 13.0 (11.8–14.2) | 12.9 (11.8–14.1) |
| Baseline medication use | ||||||
| Angiotensin-converting enzyme inhibitor | 35.8 | 34.6 | 40.8 | 40.4 | 48.2 | 49.3 |
| Angiotensin II receptor blocker | 5.8 | 5.4 | 8.2 | 8.2 | 7.4 | 14.1 |
| Aldosterone receptor antagonist | 0.7 | 0.6 | 1.8 | 1.6 | 2.3 | 4.8 |
| β-Blocker | 53.9 | 52.0 | 59.6 | 57.9 | 65.8 | 64.8 |
| Calcium-channel blocker | 19.1 | 18.9 | 27.3 | 28.4 | 42.0 | 40.6 |
| Diuretic | 26.8 | 26.5 | 40.4 | 41.1 | 62.5 | 63.2 |
| Hydralazine | 0.9 | 0.5 | 2.4 | 1.9 | 7.9 | 7.4 |
| Nitrates | 24.8 | 23.3 | 30.9 | 30.1 | 34.2 | 31.7 |
| α-Adrenergic receptor antagonist | 8.7 | 8.9 | 13.5 | 15.5 | 22.6 | 19.9 |
| Digoxin | 3.5 | 2.7 | 5.8 | 6.2 | 7.9 | 8.5 |
| Statins | 52.0 | 49.6 | 56.2 | 54.1 | 61.7 | 61.5 |
| Other lipid-lowering agent | 5.9 | 6.0 | 6.7 | 6.2 | 8.7 | 6.2 |
| Anti-inflammatory agent | 18.2 | 19.7 | 18.5 | 20.9 | 13.9 | 18.6 |
| Aspirin | 6.3 | 5.8 | 7.3 | 6.7 | 9.1 | 8.3 |
| Other antiplatelet agent | 4.0 | 4.6 | 5.5 | 3.9 | 7.7 | 7.7 |
| Diabetic therapy | 27.1 | 27.4 | 24.5 | 24.2 | 37.3 | 36.6 |
All values are percentages, except where otherwise noted.
Measurement of kidney function
Baseline serum creatinine was defined as the most recent outpatient, nonemergency department serum creatinine measured with an isotope dilution mass spectrometry–calibrated assay28 between 7 and 365 days before the index date. For nonurgent revascularization procedures, we also allowed use of a serum creatinine measured on the index date.29 We calculated the eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation30 and categorized patients into the following 3 eGFR groups in milliliters per minute per 1.73 m2: ≥60, 45 to 59 (CKD stage 3A) and <45 (CKD stage 3B+). Patients with an eGFR <60 mL/min per 1.73 m2 were considered to have CKD.
Follow-up and outcomes
We followed patients through December 31, 2008, censoring at the time of health plan disenrollment (defined as >90-day gap in membership) or at the end of follow-up. The primary outcome was death from any cause identified from health plan databases, annual California state death certificate files, and quarterly updated Social Security Administration vital status files.31,32 Secondary outcomes were subsequent acute coronary syndrome and repeat coronary revascularization. We used hospitalization databases to ascertain subsequent acute coronary syndrome based on primary discharge diagnoses of either acute myocardial infarction (MI) or unstable angina (online Appendix Supplemental Table I). Coronary artery bypass grafting procedures occurring within 14 days of the initial revascularization and PCIs occurring within 3 days of the initial revascularization were considered part of the index procedure and not as repeat revascularizations. Patients who had an initial PCI and subsequent CABG were analyzed in the PCI group.
Covariates
We obtained data on age, sex, self-reported race/ethnicity, and treatment facility. The following comorbid conditions were ascertained up to 4 years before the index date and throughout the duration of follow-up based on diagnosis and procedure codes: smoking status and history of heart failure, ventricular fibrillation/tachycardia, ischemic stroke/transient ischemic attack, peripheral arterial disease, valvular heart disease, rheumatic heart disease, atrial fibrillation/flutter, diabetes mellitus, hypertension, dyslipidemia, arthritis, dementia, chronic liver disease, chronic lung disease, diagnosed depression, hyperthyroidism, hypothyroidism, systemic cancer, and hospitalized bleed.24,33 We also collected data on urine dipstick proteinuria and serum hemoglobin, high-density lipoprotein cholesterol (HDL), and low-density lipoprotein cholesterol (LDL) up to 1 year before or on the index date and throughout follow-up.
We ascertained baseline and longitudinal exposure to the following medications based on pharmacy records34,35: α-adrenergic antagonists, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β-blockers, diuretics, aldosterone receptor antagonists, hydralazine, nitrates, digoxin, calcium-channel blockers, statins, nonstatin lipid-lowering agents, anti-inflammatory agents, antiplatelet agents, diabetes medications, and aspirin.
Statistical analysis
We stratified all analyses by pre-revascularization eGFR category. We compared baseline characteristics of patients by CKD status and revascularization type using the Student t tests, Wilcoxon rank sum tests, and χ2 tests where appropriate. We used extended Cox regression to evaluate the independent association of CABG versus PCI for each outcome of interest, after adjustment for baseline covariates (sex, race, Hispanic ethnicity, and history of acute MI or unstable angina) and time-varying covariates (age, cardiovascular medication use, smoking status, comorbid conditions, proteinuria, hemoglobin, HDL, and LDL). We adjusted for index year to account for secular trends over time and local health care facility to account for potential unmeasured cluster effects at that level. We conducted additional analysis stratifying the subgroups by diabetes mellitus status, given the evidence from previous studies suggesting that it may modify the effectiveness of the revascularization strategy.6,36
Sensitivity analyses
We conducted several additional sensitivity analyses. First, we repeated the analysis using the full cohort, adjusting for the baseline and longitudinal covariates as listed above. Second, because the change in the billing codes in 2005 resulted in an increase in the proportion of PCI patients in whom we could not identify the number vessels revascularized, we repeated the analysis restricting the full cohort to patients who had their revascularization between 1996 and 2004. Third, because of the rapid adoption of drug-eluting stents after their introduction in mid 2003, we performed a stratified analysis on the full cohort by stent-era: 1996 to 2003 (bare metal stents) and 2004 to 2008 (primarily drug-eluting stents). Fourth, because 7.6% of CABG patients went on to receive a PCI and 5.8% of PCI patients went on to receive a CABG, we performed an analysis using the full cohort, which accounted for these crossover procedures in a time-varying fashion for the outcomes of death and acute coronary syndrome.
The institutional review boards of the Kaiser Foundation Research Institute and Stanford University approved the study. A waiver of informed consent was obtained due to the nature of the study. All analyses were performed using SAS version 9.1.3 (Cary, NC). The work was supported by a grant from the American Heart Association (0875162N). Dr Go had full access to and control of the data and takes full responsibility for the study results. The authors are solely responsible for the design and conduct of this study, all analyses, the drafting and editing of the manuscript, and its final contents.
Results
We identified 22,361 patients who underwent initial multivessel coronary revascularization between January 1996 and December 2008 (Figure 1). The proportion of patients receiving CABG as initial revascularization decreased steadily from 94% in 1996 to a nadir of 49% in 2005, and these proportions were consistent across eGFR categories (data not shown). The proportion of PCI patients receiving a drug-eluting stent rose sharply in late 2003, with >88% of all PCI patients receiving ≥1 drug eluting stents between 2004 and 2008, consistent across eGFR categories. In patients receiving CABG, 40%, 39%, and 22% had 2, 3, or ≥4 vessels revascularized, respectively. In patients receiving PCI, the exact number of vessels revascularized could be determined in only 26% of patients (with the remainder identified solely as having had a multivessel procedure); of these, 70% had 2 vessels, 22% had 3 vessels, and 8% had ≥4 vessels revascularized.
In our propensity score-matched cohort (C-statistic 0.791), we matched 67% of patients who received a PCI (n = 4,086) to a patient who received a CABG (Figure 1). Overall, 60% of patients had a baseline eGFR ≥60 mL/min per 1.73 m2, 25% had an eGFR 45 to 59 mL/min per 1.73 m2, and 15% had an eGFR <45 mL/min per 1.73 m2. Baseline variables were well balanced in the matched cohort (Table I). In the full cohort, PCI patients were more likely to be female, and to have a history of MI, hypertension, dyslipidemia, depression, liver disease, and lung disease, these patients were also more likely to use angiotensin II receptor blockers, statins, and an antiplatelet agent, whereas CABG patients were more likely to use nitrates at baseline (online Appendix Supplemental Table II).
Primary outcome: all-cause mortality
Median follow-up of surviving CABG patients was 3.9 years (interquartile range [IQR] 2.0–6.5 years); median follow-up of surviving PCI patients was 3.8 years (IQR 2.0–6.4 years). A total of 1202 patients died in the matched cohort during follow-up. Patients with lower baseline eGFR had higher unadjusted 5-year rates of death than patients with preserved eGFR, regardless of the revascularization type (Table II). Compared with PCI, CABG patients had a lower relative hazard of death across levels of baseline eGFR even after adjustment for potential confounders and longitudinal use of postrevascularization cardiovascular medications, although the CI included 1.0 for patients in the lowest eGFR category (Figure 2). Our results were similar in patients with and without diabetes mellitus (Table III).
Table II.
Unadjusted 5-year event rates per 100 patient-years for specified clinical outcomes by initial revascularization type in adults with multivessel coronary disease, stratified by preprocedural kidney function
| 5-y event rate per 100 patient-years (95% CI)
|
||
|---|---|---|
| CABG | PCI | |
| Death | ||
| eGFR ≥60 | 2.09 (1.85–2.37) | 2.19 (1.95–2.47) |
| eGFR 45–59 | 3.81 (3.26–4.44) | 4.75 (4.12–5.47) |
| eGFR <45 | 8.87 (7.62–10.33) | 9.13 (7.85–10.62) |
| Acute coronary syndrome | ||
| eGFR ≥60 | 2.94 (2.65–3.27) | 7.53 (7.06–8.04) |
| eGFR 45–59 | 2.06 (1.67–2.54) | 9.47 (8.56–10.47) |
| eGFR <45 | 4.86 (3.96–5.97) | 14.32 (12.70–16.15) |
| Repeat revascularization | ||
| eGFR ≥60 | 2.59 (2.32–2.90) | 7.63 (7.16–8.14) |
| eGFR 45–59 | 1.77 (1.41–2.22) | 7.75 (6.93–8.66) |
| eGFR <45 | 1.76 (1.25–2.48) | 8.05 (6.86–9.45) |
Figure 2.
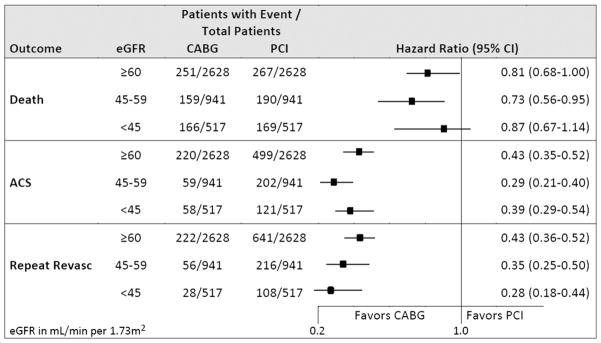
Multivariable-adjusted HRs for specified outcomes comparing CABG to PCI as initial treatment for multivessel coronary artery disease by eGFR category in the matched cohort. Models adjusted for age, sex, race, Hispanic ethnicity, baseline history of acute MI or unstable angina, medications, smoking status, comorbid conditions, dipstick proteinuria, hemoglobin, HDL, LDL, index year, and local health care facility.
Table III.
Adjusted HRs and 95% CIs for CABG compared with PCI in the matched cohort, stratified by baseline kidney function and diabetes mellitus status
| HR (95% CI)
|
||
|---|---|---|
| Diabetes | No diabetes | |
| Death | ||
| eGFR ≥60 | 0.71 (0.51–0.99) | 0.81 (0.61–1.09) |
| eGFR 45–59 | 0.75 (0.46–1.22) | 0.67 (0.46–0.96) |
| eGFR <45 | 0.76 (0.45–1.27) | 0.85 (0.58–1.23) |
| Acute coronary syndrome | ||
| eGFR ≥60 | 0.34 (0.25–0.44) | 0.49 (0.38–0.63) |
| eGFR 45–59 | 0.23 (0.14–0.37) | 0.29 (0.20–0.44) |
| eGFR <45 | 0.31 (0.20–0.51) | 0.37 (0.22–0.61) |
| Repeat revascularization | ||
| eGFR ≥60 | 0.42 (0.32–0.55) | 0.43 (0.34–0.53) |
| eGFR 45–59 | 0.41 (0.24–0.69) | 0.35 (0.23–0.54) |
| eGFR <45 | 0.25 (0.13–0.45) | 0.16 (0.08–0.34) |
Secondary outcomes: acute coronary syndrome and repeat revascularization
A total of 1159 patients in the matched cohort were hospitalized during follow-up for acute coronary syndrome, and patients with eGFR <45 mL/min per 1.73 m2 had the highest unadjusted rates (Table II). A total of 1271 patients had a repeat revascularization event during follow-up, but despite having the highest rates of hospitalized acute coronary syndrome, patients in the lowest eGFR category did not have higher unadjusted rates of repeat revascularization (Table II).
Compared with PCI, CABG was associated with significantly lower adjusted risks of acute coronary syndrome and repeat revascularization for patients across all categories of baseline eGFR (Figure 2). Results were similar in patients with and without diabetes mellitus (Table III).
Sensitivity analyses
Analyses using the full cohort yielded similar results: for the primary outcome of all-cause mortality, the hazard ratio (HR) was 0.85 (95% CI 0.74–1.00) for eGFR ≥60 mL/min per 1.73 m2; HR 0.82 (CI 0.68–0.98) for eGFR 45–59 mL/min per 1.73 m2; and HR 0.76 (CI 0.64–0.90) for eGFR <45 mL/min per 1.73 m2. Results did not materially change in the other sensitivity analyses, which limited the analysis to patients revascularized between 1996 and 2004, stratified by stent-era, or included a time-varying adjustment for subsequent revascularization procedures (data not shown).
Discussion
Within this large, diverse cohort of patients, initial multivessel coronary revascularization with CABG rather than PCI in patients with CKD was associated with a 15% to 24% lower adjusted rate of death, a 56% to 62% lower adjusted rate of acute coronary syndrome, and a 59% to 71% lower adjusted rate of repeat revascularization. The associations in patients with CKD were similar to patients with preserved kidney function and in patients with and without diabetes mellitus. Forty percent of our cohort had a baseline eGFR <60 mL/min per 1.73 m2, confirming the high prevalence of concomitant CKD among patients with multivessel CHD.
Our results confirm and extend the results of the recently published ASCERT study, which compared CABG with PCI in Medicare recipients using linked data from a cardiac surgery registry and a coronary catheterization registry.37 In that study, Weintraub et al37 found a 17% to 25% lower risk of long-term death associated with CABG compared with PCI across strata of eGFR, consistent with our findings. Importantly, our analysis goes beyond the findings of ASCERT, which was limited to a predominantly white, older Medicare population, to address a younger and more racially diverse patient population.
Our results contrast with findings from several smaller studies of coronary revascularization that reported no significant differences in the mortality of patients with non–dialysis-dependent CKD after CABG or PCI.19,20 In a single center study of 725 patients with CKD receiving revascularization of multivessel CHD, CABG was associated with a 30% lower risk of death compared with PCI, but the association was not statistically significant (P = .2).20 Similarly, in a series of 1,069 consecutive patients with CKD and multivessel CHD at a single center in China, CABG was associated with a 44% lower risk of death compared with PCI, but the results were not statistically significant (P = .2).19 Lopes et al38 reanalyzed data from 611 patients with multivessel CHD randomly assigned to medical therapy, CABG, or PCI in Medicine, Angioplasty, or Surgery Study and found that, in the 150 patients with eGFR <60, CABG was associated with a 63% lower risk of death compared with PCI (P = .06). Finally, a post hoc analyses of Arterial Revascularization Therapies Study13 found no significant difference in mortality (HR 0.98, CI 0.40–2.42, P = .97) in the 290 subjects with baseline CKD randomized to CABG or PCI,5 but the small sample size and low event rates led to limited power.
Our study confirms that CKD is associated with higher mortality rates compared with preserved renal function among patients with multivessel CHD. Given this higher baseline risk, patients with CKD had a greater absolute reduction in mortality from CABG, although the relative risk reduction was similar to that of patients with preserved kidney function. Of note, patients with the lowest levels of baseline eGFR (<45 mL/min per 1.73 m2) had higher rates of acute coronary syndrome than patients with preserved renal function but did not have higher rates of repeat revascularization. The reason for this apparent discrepancy is uncertain but may stem from possible concerns about higher procedural risks or the presence of more extensive and diffuse CHD that is less amenable to additional revascularization.
Our study has several strengths, including its large sample size, diverse cohort of real-world patients, and inclusion of patients being treated with multivessel coronary disease only, allowing us to address some of the limitations of previous observational studies. Importantly, a substantial proportion of patients in our cohort had baseline eGFR <45 mL/min per 1.73 m2, a high-risk patient population that is often excluded from randomized clinical trials.39 We also had longitudinal information on postrevascularization medication use, comorbidities, and laboratory values, which allowed us to account for these potential confounders in a time-varying fashion.
As an observational study of outcomes associated with treatments delivered outside a randomized trial, we cannot fully exclude residual selection bias and confounding despite adjustment for a large number of covariates and findings that were robust to several sensitivity analyses. Second, we did not have data on details of the coronary anatomy that may influence revascularization type (eg, bifurcation disease, small caliber vessels, or chronic total occlusions). Third, because of a change in 2005 in the ICD-9 codes, we were unable to identify the number of revascularized vessels during PCI in up to one quarter of patients receiving PCI between 2005 and 2008. However, the differences in baseline characteristics between PCI patients with identifiable versus unidentifiable number of revascularized vessels were minimal. Moreover, because those data were missing as a result of a systematic change in coding practices that was unrelated to practice patterns or outcomes, this would be expected to bias our results toward the null. The results of the sensitivity analysis restricted to procedures performed before the change in coding practices also did not materially differ from the results of the primary analysis. Fourth, we did not have information on left ventricular systolic function in 85% of the cohort, which can influence the choice of revascularization procedures40,41 and also affects postrevascularization outcomes. Finally, given the lack of consensus on the definition of a periprocedural MI for CABG or PCI during the study period, along with the lack of complete periprocedural data on cardiac enzymes (eg, cardiac troponin I), clinical symptoms and electrocardiographic findings, we were unable to assess for differences in periprocedural MI.
The prevalence of CKD among patients with multi-vessel CHD is high, and these patients are among the highest risk for death and cardiovascular morbidity. Although PCI is being used with increasing frequency as initial treatment for multivessel CHD, our findings suggest a significantly lower adjusted risk of death, acute coronary syndrome, and repeat revascularization with CABG in patients with multivessel CHD and CKD. We hypothesize that the apparent advantage of CABG compared with PCI could stem from more frequent achievement of complete revascularization with CABG. Alternatively, PCI could be less effective in patients with CKD due to increased inflammation, uremia, or anemia.42 Our findings support conducting adequately powered randomized clinical trials comparing CABG versus PCI as the initial coronary revascularization strategy in patients with multivessel CHD complicated by CKD.
Supplementary Material
Acknowledgments
Funding: American Heart Association (grant no.: 0875162N).
An abstract based on these data was presented as a poster at the American Heart Association Quality of Care and Outcomes Research Scientific Sessions in Atlanta, GA, on May 11, 2012.
Footnotes
Disclosures
The authors have no relevant disclosures.
References
- 1.U.S. Renal Data System; National Institutes of Health NIoDaDaKD, editor. USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: 2011. [Google Scholar]
- 2. [Accessed May 25, 2010];KDOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. 2002 at http://www.kidney.org/professionals/KDOQI/guidelines_bp/index.htm. [PubMed]
- 3.Joki N, Hase H, Nakamura R, et al. Onset of coronary artery disease prior to initiation of haemodialysis in patients with end-stage renal disease. Nephrol Dial Transplant. 1997;12:718–23. doi: 10.1093/ndt/12.4.718. [DOI] [PubMed] [Google Scholar]
- 4.Szczech LA, Best PJ, Crowley E, et al. Outcomes of patients with chronic renal insufficiency in the bypass angioplasty revascularization investigation. Circulation. 2002;105:2253–8. doi: 10.1161/01.cir.0000016051.33225.33. [DOI] [PubMed] [Google Scholar]
- 5.Ix JH, Mercado N, Shlipak MG, et al. Association of chronic kidney disease with clinical outcomes after coronary revascularization: the arterial revascularization therapies study (ARTS) Am Heart J. 2005;149:512–9. doi: 10.1016/j.ahj.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 6.BARI Investigators. The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol. 2007;49:1600–6. doi: 10.1016/j.jacc.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 7.CABRI Trial Participants. First-year results of CABRI (Coronary Angioplasty versus Bypass Revascularisation Investigation) Lancet. 1995;346:1179–84. [PubMed] [Google Scholar]
- 8.King SB, III, Kosinski AS, Guyton RA, et al. Eight-year mortality in the Emory Angioplasty versus Surgery Trial (EAST) J Am Coll Cardiol. 2000;35:1116–21. doi: 10.1016/s0735-1097(00)00546-5. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez AE, Baldi J, Fernandez Pereira C, et al. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II) J Am Coll Cardiol. 2005;46:582–8. doi: 10.1016/j.jacc.2004.12.081. [DOI] [PubMed] [Google Scholar]
- 10.Hueb W, Lopes NH, Gersh BJ, et al. Five-year follow-up of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation. 2007;115:1082–9. doi: 10.1161/CIRCULATIONAHA.106.625475. [DOI] [PubMed] [Google Scholar]
- 11.Henderson RA, Pocock SJ, Sharp SJ, et al. Long-term results of RITA-1 trial: clinical and cost comparisons of coronary angioplasty and coronary-artery bypass grafting. Randomised Intervention Treatment of Angina. Lancet. 1998;352:1419–25. doi: 10.1016/s0140-6736(98)03358-3. [DOI] [PubMed] [Google Scholar]
- 12.Booth J, Clayton T, Pepper J, et al. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS) Circulation. 2008;118:381–8. doi: 10.1161/CIRCULATIONAHA.107.739144. [DOI] [PubMed] [Google Scholar]
- 13.Serruys PW, Unger F, Sousa JE, et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med. 2001;344:1117–24. doi: 10.1056/NEJM200104123441502. [DOI] [PubMed] [Google Scholar]
- 14.Kaehler J, Koester R, Billmann W, et al. 13-year follow-up of the German angioplasty bypass surgery investigation. Eur Heart J. 2005;26:2148–53. doi: 10.1093/eurheartj/ehi385. [DOI] [PubMed] [Google Scholar]
- 15.Carrie D, Elbaz M, Puel J, et al. Five-year outcome after coronary angioplasty versus bypass surgery in multivessel coronary artery disease: results from the French Monocentric Study. Circulation. 1997;96:II-1–6. [PubMed] [Google Scholar]
- 16.Hemmelgarn BR, Southern D, Culleton BF, et al. Survival after coronary revascularization among patients with kidney disease. Circulation. 2004;110:1890–5. doi: 10.1161/01.CIR.0000143629.55725.D9. [DOI] [PubMed] [Google Scholar]
- 17.Reddan DN, Szczech LA, Tuttle RH, et al. Chronic kidney disease, mortality, and treatment strategies among patients with clinically significant coronary artery disease. J Am Soc Nephrol. 2003;14:2373–80. doi: 10.1097/01.asn.0000083900.92829.f5. [DOI] [PubMed] [Google Scholar]
- 18.Szczech LA, Reddan DN, Owen WF, et al. Differential survival after coronary revascularization procedures among patients with renal insufficiency. Kidney Int. 2001;60:292–9. doi: 10.1046/j.1523-1755.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang ZJ, Zhou YJ, Liu YY, et al. Comparison of drug-eluting stents and coronary artery bypass grafting for the treatment of multivessel coronary artery disease in patients with chronic kidney disease. Circ J. 2009;73:1228–34. doi: 10.1253/circj.cj-08-1091. [DOI] [PubMed] [Google Scholar]
- 20.Ashrith G, Lee VV, Elayda MA, et al. Short- and long-term outcomes of coronary artery bypass grafting or drug-eluting stent implantation for multivessel coronary artery disease in patients with chronic kidney disease. Am J Cardiol. 2010;106:348–53. doi: 10.1016/j.amjcard.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 21.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: Executive Summary. Circulation. 2011;124(23):2574–609. doi: 10.1161/CIR.0b013e31823a5596. [DOI] [PubMed] [Google Scholar]
- 22.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fireman BH, Fehrenbacher L, Gruskin EP, et al. Cost of care for patients in cancer clinical trials. J Natl Cancer Inst. 2000;92:136–42. doi: 10.1093/jnci/92.2.136. [DOI] [PubMed] [Google Scholar]
- 24.Selby JV, Ray GT, Zhang D, et al. Excess costs of medical care for patients with diabetes in a managed care population. Diabetes Care. 1997;20:1396–402. doi: 10.2337/diacare.20.9.1396. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Lacson E, Jr, Lowrie EG, et al. The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis. 2006;48:606–15. doi: 10.1053/j.ajkd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Fellström BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 27.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–92. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–72. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 29.Go AS, Parikh CR, Ikizler TA, et al. The assessment, serial evaluation, and subsequent sequelae of acute kidney injury (ASSESS-AKI) study: design and methods. BMC Nephrol. 2010;11:22. doi: 10.1186/1471-2369-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman TB, Brown AN. Use of commercial record linkage software and vital statistics to identify patient deaths. J Am Med Inform Assoc. 1997;4:233–7. doi: 10.1136/jamia.1997.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arellano MG, Petersen GR, Petitti DB, et al. The California Automated Mortality Linkage System (CAMLIS) Am J Public Health. 1984;74:1324–30. doi: 10.2105/ajph.74.12.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 34.Go AS, Yang J, Gurwitz JH, et al. Comparative effectiveness of different beta-adrenergic antagonists on mortality among adults with heart failure in clinical practice. Arch Intern Med. 2008;168:2415–21. doi: 10.1001/archinternmed.2008.506. [DOI] [PubMed] [Google Scholar]
- 35.Go AS, Yang J, Ackerson LM, et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113:2713–23. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 36.Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet. 2009;373:1190–7. doi: 10.1016/S0140-6736(09)60552-3. [DOI] [PubMed] [Google Scholar]
- 37.Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366:1467–76. doi: 10.1056/NEJMoa1110717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopes NH, da Silva Paulitsch F, Pereira A, et al. Mild chronic kidney dysfunction and treatment strategies for stable coronary artery disease. J Thorac and Cardiovasc Surg. 2009;137:1443–9. doi: 10.1016/j.jtcvs.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 39.Coca SG, Krumholz HM, Garg AX, et al. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA. 2006;296:1377–84. doi: 10.1001/jama.296.11.1377. [DOI] [PubMed] [Google Scholar]
- 40.Levine GN, Bates ER, et al. Writing Committee Members. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: Executive Summary. Circulation. 2011;124:2574–609. doi: 10.1161/CIR.0b013e31823a5596. [DOI] [PubMed] [Google Scholar]
- 41.Hillis LD, Smith PK, et al. Writing Committee Members. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: Executive Summary. Circulation. 2011;124:2610–42. doi: 10.1161/CIR.0b013e31823b5fee. [DOI] [PubMed] [Google Scholar]
- 42.Kaysen GA, Eiserich JP. The role of oxidative stress-altered lipoprotein structure and function and microinflammation on cardiovascular risk in patients with minor renal dysfunction. J Am Soc Nephrol. 2004;15:538–48. doi: 10.1097/01.asn.0000111744.00916.e6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.