Abstract.
SHOX haploinsufficiency due to mutations in the coding exons or microdeletions involving the coding exons and/or the enhancer regions accounts for approximately 80% and 2–16% of genetic causes of Leri-Weill dyschondrosteosis and idiopathic short stature, respectively. The most characteristic feature in patients with SHOX deficiency is Madelung deformity, a cluster of anatomical changes in the wrist that can be attributed to premature epiphyseal fusion of the distal radius. Computed tomography of SHOX-deficient patients revealed a thin bone cortex and an enlarged total bone area at the diaphysis of the radius, while histopathological analyses showed a disrupted columnar arrangement of chondrocytes and an expanded hypertrophic layer of the growth plate. Recent studies have suggested that perturbed programmed cell death of hypertrophic chondrocytes may underlie the skeletal changes related to SHOX deficiency. Furthermore, the formation of an aberrant ligament tethering the lunate and radius has been implicated in the development of Madelung deformity. Blood estrogen levels and mutation types have been proposed as phenotypic determinants of SHOX deficiency, although other unknown factors may also affect clinical severity of this entity.
Keywords: chondrocyte, Leri-Weill dyschondrosteosis, Madelung deformity, short stature, Vickers ligament
Introduction
SHOX (NM_000451.3) encodes a transcription factor exclusively expressed in the developing limb and pharyngeal arch in the human embryo (1). Heterozygous mutations of SHOX lead to Leri-Weill dyschondrosteosis characterized by wrist deformity and mesomelic short stature (LWD; OMIM #249700), as well as idiopathic short stature without apparent skeletal malformations (ISS; OMIM # 300582) (2,3,4). Less specific skeletal changes such as high arched palate, short metacarpals, scoliosis, and micrognathia have also been described in patients with SHOX deficiency (5). Previous studies have shown that SHOX deficiency accounts for approximately 80% and 2–16% of genetic causes of LWD and ISS, respectively (5,6,7,8). Furthermore, haploinsufficiency of SHOX represents the major cause of growth failure in patients with Turner syndrome (9). Thus, SHOX deficiency is a clinically important condition, particularly in the field of pediatric endocrinology and orthopedics. This review article introduces our current understanding of the causative mechanisms and phenotypic characteristics of SHOX-associated skeletal malformations.
Molecular Basis of SHOX Deficiency
SHOX resides in the short arm pseudoautosomal region of the X and Y chromosomes (PAR1) and escapes X inactivation (2). Thus, although SHOX is located on the sex chromosomes, molecular defects of SHOX are inherited in an autosomal dominant manner. To date, several point mutations in the SHOX-coding exons and submicroscopic deletions encompassing the coding region and/or the upstream or downstream enhancer regions have been identified in more than 200 patients with LWD or ISS (5,6,7,8,9). Previously reported SHOX mutations are listed in the SHOX Mutation Database (http://hyg-serv-01.hyg.uni-heidelberg.de/lovd/index.php?select_db=SHOX) (10). The precise position of the SHOX enhancers remains to be determined, although the upstream and downstream enhancers have been mapped to a ~300 kb region ~95 kb upstream and an ~30 kb region ~250 kb downstream from the start codon, respectively (11,12,13). Deletions involving the coding and/or downstream enhancer regions account for about 60% of Japanese LWD patients (14), and those affecting the downstream enhancer regions are the major genetic causes in Spanish patients (15). High recombination frequency and the abundant presence of repeat sequences in PAR1 likely play a role in the development of submicroscopic genomic rearrangements involving SHOX (14).
Skeletal Deformity in Patients with SHOX Deficiency
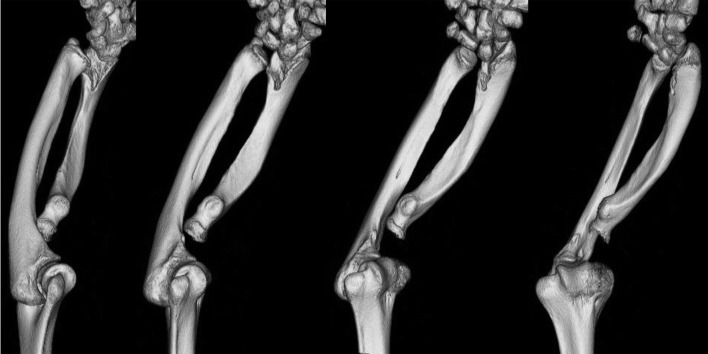
The most characteristic feature in patients with SHOX deficiency is Madelung deformity, a cluster of anatomical changes in the forearm including bowing and shortening of the radius, prominence of the ulnar head and palmar and ulnar deviation (“pyramidal configuration”) of the carpal bones (Fig. 1 and Fig. 2) (16). Clinical manifestations induced by Madelung deformity include wrist pain, deformation and limited joint motion (16, 17). Radiological findings of Madelung deformity include the absence or narrowing of the ulnar portion of the distal radial physis, anterior bowing of the radial shaft and dorsal subluxation of the ulnar head (17, 18). Several additional skeletal changes such as triangularization of the distal radial epiphysis have been associated with Madelung deformity (19). In patients with severe Madelung deformity, the structural organization of the elbow joint is also disrupted (Fig. 2).
Fig. 1.
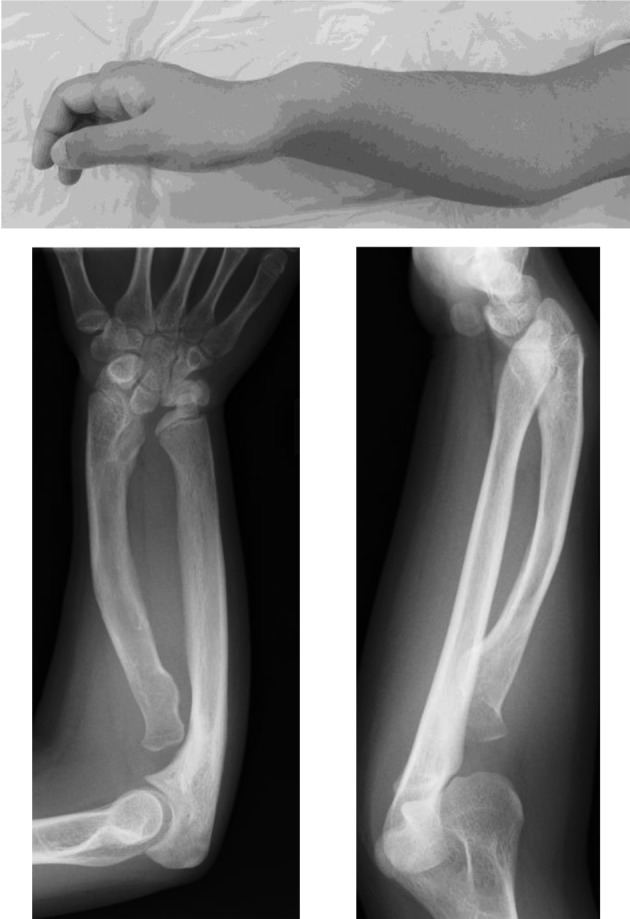
Madelung deformity in a female patient with SHOX deficiency. Upper panel: appearance of the forearm. Prominence of the distal ulna is shown. Lower panel: radiographic findings. Shortening and bowing of the radius and dorsal subluxation of the ulnar head are shown.
Fig. 2.
Forearm three-dimensional computed tomography of a female patient with SHOX deficiency. Significant findings include shortening of the radius, pyramidal configuration of the carpal bones and dorsal subluxation of the ulna in addition to severely disturbed structural organization of the elbow joint.
Previous studies have suggested that the primary lesion of Madelung deformity is the premature epiphyseal fusion at the volar-ulnar portion of the radial growth plate (20). Impaired growth of the radius due to the early epiphyseal fusion, in combination with relatively preserved growth of the ulna, appears to underlie the characteristic deformity (18, 21). The appearance of the wrist varies among patients and probably depends on the fusion position along the anterior-posterior axis of the radial epiphysis (22).
Although Madelung deformity usually occurs as a result of SHOX deficiency, it can also take place as a component of other congenital disorders such as multiple exostoses syndrome, multiple epiphyseal dysplasia and dysostosis multiplex of mucopolysaccharidosis (23). Madelung-like deformity has also been observed in patients with pseudohypoparathyroidism type 1b (24). In addition, Madelung deformity can occur as a change secondary to injury and infection (19). When Madelung deformity is accompanied by short stature and mesomelic shorting of the limbs, it is referred to as LWD. The mesomelic short stature of LWD can be explained as a result of impaired linear growth of the radius, ulna, tibia and fibula. A decreased extremity/trunk ratio with a fairly preserved sitting height and head circumference is a characteristic auxological finding of patients with LWD (5, 25). Rappold et al. developed a phenotype scoring system for screening of individuals with possible SHOX deficiency from patients with short stature (8). They suggested the following eight clinical features as indicators for SHOX deficiency: arm span/height ratio, sitting height/height ratio, body mass index, cubitus valgus, short forearm, bowing of the forearm, muscular hypertrophy and dislocation of the ulna. Although SHOX deficiency is the only condition that has been implicated in LWD, SHOX abnormalities have been detected only in 50–90% of patients with LWD (5). It remains currently unknown whether LWD patients with apparently normal SHOX alleles have mutations in the regulatory regions of SHOX or in a hitherto unidentified gene involved in skeletal development.
Changes in Bone Geometry andBone Mineral Density
Using peripheral quantitative computed tomography of the forearm, Soucek et al. investigated bone mineral density and bone geometry in 10 prepubertal patients with SHOX deficiency and 22 patients with Turner syndrome (26). They found that patients of both groups had a thin bone cortex and an enlarged total bone area at the diaphysis of the radius compared with control individuals. On the other hand, these patients had a normal trabecular bone mineral density and bone strength index. Soucek et al. proposed that the skeletal changes observed in patients with SHOX deficiency are attributable to an adjustment of the long bones with a disrupted cortex to the mechanical loading that aims to increase bone strength.
Histopathological Changes
Munns et al. investigated histopathological findings of the surgically-excised growth plate of the distal radius obtained from two patients with molecularly confirmed SHOX deficiency (22). They found disrupted columnar arrangement of chondrocytes; the normal tandem stacking of mature chondrocytes within columns was replaced by a side-by-side arrangement. Furthermore, the presence of hypertrophic osteoid with micro-enchondromata in the metaphysis suggested aberrant endochondral ossification. Significant expansion of the hypertrophic layer and reduction of the proliferative layer were observed in the growth plate. These data imply that the SHOX protein is required for ordered zonal development of chondrocytes. In this regard, SHOX is strongly expressed in terminally differentiated hypertrophic chondrocytes and less obviously in proliferating and reserve chondrocytes (27). In vitro assays with osteosarcoma cells indicated that SHOX induces oxidative stress and activates the intrinsic apoptotic pathway (27). Thus, it is possible that SHOX plays a critical role in chondrocyte development by regulating the cell cycle and apoptosis of hypertrophic chondrocytes. Indeed, premature epiphyseal fusion in patients with SHOX deficiency may reflect a perturbed cell death process in the growth plate. To date, however, the precise mechanism by which SHOX exerts its effect on chondrocyte development remains unknown. Although in vivo and in vitro assays have indicated that several proteins such as BNP, FGFR3, SOX5, and SOX6 can interact with SHOX (28, 29), the function of SHOX in human tissues has yet to be elucidated.
Formation of an Abnormal Ligament
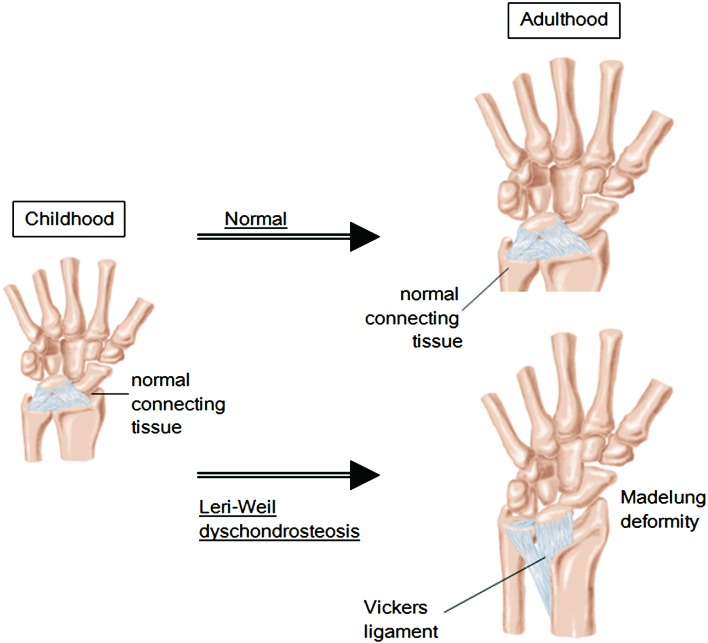
Vickers and Nielsen identified an abnormal ligament in patients with Madelung deformity (30). The “Vickers ligament” tethers the lunate to the distal portion of the radius and can have a diameter as large as 8 mm (Fig. 3) (22). Histological analysis demonstrated that the Vickers ligament is a morphologically normal ligament consisting of collagen and elastin fibers (22). This ligament is predicted to promote pyramidal configuration of the carpal bones by disturbing the physiological migration of these bones during growth (22). Furthermore, this ligament may exert an inhibitory effect on linear growth of the radius by compressing its distal epiphysis (18). Therefore, it is possible that the Vickers ligament constitutes an essential factor in the development of Madelung deformity. Although the process by which the Vickers ligament forms has yet to be clarified, this ligament is regarded as a secondary change of the forearm deformity (30, 31). Actually, the ligament seems to consist of hypertrophied connective tissues that form under a mechanical force that arises from asymmetrical growth of the radius and ulna.
Fig. 3.
Schematic representation of the Vickers ligament that tethers the lunate to the distal portion of the radius. This ligament seems to consist of hypertrophied connective tissues that form under the mechanical force that arises from asymmetrical growth of the radius and ulna.
Recent advancements in high-resolution magnetic resonance imaging have enabled early detection of the Vickers ligament (31). Previous studies have indicated that surgical removal of the Vickers ligament in combination with dome osteotomy is beneficial to patients with Madelung deformity; Harley et al. reported that these surgical interventions effectively improved the clinical features of adolescent patients with Madelung deformity (21), while Steinman et al. showed that these interventions can provide long-term correction of the wrist deformity (16). However, since the number of treated patients was small, further studies are necessary to validate these findings.
Phenotypic Determinants
Skeletal changes of SHOX deficiency tend to be more severe in adult females than in children or adult males (5). Obvious Madelung deformity is rare in prepubertal patients, although decreased extremity/trunk ratios and subtle skeletal changes are observed in the majority of children with SHOX deficiency (5, 32, 33). These data can be explained by assuming that estrogens exert a deleterious effect on skeletal formation in patients with SHOX abnormalities. Since estrogens induce physiological skeletal maturation in both sexes (34), they may also enhance premature epiphyseal fusion in patients with SHOX deficiency. Consistent with this, severe Madelung deformity is rarely seen in Turner females in whom ovarian function is frequently impaired (35). Furthermore, a longitudinal study of a female patient with SHOX deficiency and normal ovarian function showed age-appropriate skeletal maturation before puberty and rapidly advanced bone age during puberty (36). On the other hand, since Soucek et al. revealed a significant difference in bone geometry between prepubertal patients with the 46,XX karyotype and prepubertal Turner females, it is likely that some factors other than estrogens may also underlie relatively mild skeletal features in Turner females (26). Soucek et al. suggested karyotype mosaicism as one of the possible candidates for such factors (26). It is known that the karyotype of Turner females is heterogeneous and includes 45,X/46,XX. Since two normal SHOX alleles are present in a certain percentage of cells in females with the 45,X/46,XX karyotype, this may lead to relatively well preserved skeletal structures in such patients.
Mutation types may affect the phenotypic severity of SHOX deficiency. It has been proposed that molecular defects involving only the enhancer regions are associated with broader phenotypic variation than deletions/mutations affecting the coding exons; Chen et al. have described more severe skeletal changes in patients with enhancer deletions than in those with mutations/deletions affecting the coding exons (37), while Rosilio et al. reported relatively mild phenotypes in patients with enhancer deletions (7). On the other hand, no apparent genotype-phenotype correlation has been reported for SHOX intragenic mutations/deletions (8).
Conclusion
Recent studies have indicated that SHOX deficiency leads to premature epiphyseal fusion at the distal radius, possibly by disturbing programmed cell death of hypertrophic chondrocytes. In addition, the formation of an aberrant ligament tethering the lunate and radius appears to play a role in the development of Madelung deformity. Blood estrogen levels and mutation types have been proposed as phenotypic determinants of SHOX deficiency, although other unknown factors may also modify the clinical severity of this condition.
Acknowledgments
This work was supported by a Grant-in-Aid and for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology, by a Grant for Research on Intractable Diseases from the Ministry of Health, Labor and Welfare and by Grants from the National Center for Child Health and Development and from the Takeda foundation.
References
- 1.Clement-Jones M, Schiller S, Rao E, Blaschke RJ, Zuniga A, Zeller R, et al. The short stature homeobox gene SHOX is involved in skeletal abnormalities in Turner syndrome. Hum Mol Genet 2000;9: 695–702. doi: 10.1093/hmg/9.5.695 [DOI] [PubMed] [Google Scholar]
- 2.Rao E, Weiss B, Fukami M, Rump A, Niesler B, Mertz A, et al. Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nat Genet 1997;16: 54–63. doi: 10.1038/ng0597-54 [DOI] [PubMed] [Google Scholar]
- 3.Shears DJ, Vassal HJ, Goodman FR, Palmer RW, Reardon W, Superti-Furga A, et al. Mutation and deletion of the pseudoautosomal gene SHOX cause Leri-Weill dyschondrosteosis. Nat Genet 1998;19: 70–3. doi: 10.1038/ng0198-70 [DOI] [PubMed] [Google Scholar]
- 4.Belin V, Cusin V, Viot G, Girlich D, Toutain A, Moncla A, et al. SHOX mutations in dyschondrosteosis (Leri-Weill syndrome). Nat Genet 1998;19: 67–9. doi: 10.1038/ng0198-67 [DOI] [PubMed] [Google Scholar]
- 5.Binder G. Short stature due to SHOX deficiency: genotype, phenotype, and therapy. Horm Res Paediatr 2011;75: 81–9. doi: 10.1159/000324105 [DOI] [PubMed] [Google Scholar]
- 6.Rappold GA, Fukami M, Niesler B, Schiller S, Zumkeller W, Bettendorf M, et al. Deletions of the homeobox gene SHOX (short stature homeobox) are an important cause of growth failure in children with short stature. J Clin Endocrinol Metab 2002;87: 1402–6. doi: 10.1210/jcem.87.3.8328 [DOI] [PubMed] [Google Scholar]
- 7.Rosilio M, Huber-Lequesne C, Sapin H, Carel JC, Blum WF, Cormier-Daire V. Genotypes and phenotypes of children with SHOX deficiency in France. J Clin Endocrinol Metab 2012;97: E1257–65. doi: 10.1210/jc.2011-3460 [DOI] [PubMed] [Google Scholar]
- 8.Rappold G, Blum WF, Shavrikova EP, Crowe BJ, Roeth R, Quigley CA, et al. Genotypes and phenotypes in children with short stature: clinical indicators of SHOX haploinsufficiency. J Med Genet 2007;44: 306–13. doi: 10.1136/jmg.2006.046581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross JL, Scott C Jr., Marttila P, Kowal K, Nass A, Papenhausen P, et al. Phenotypes Associated with SHOX Deficiency. J Clin Endocrinol Metab 2001;86: 5674–80. doi: 10.1210/jcem.86.12.8125 [DOI] [PubMed] [Google Scholar]
- 10.Niesler B, Röth R, Wilke S, Fujimura F, Fischer C, Rappold G. The novel human SHOX allelic variant database. Hum Mutat 2007;28: 933–8. doi: 10.1002/humu.20542 [DOI] [PubMed] [Google Scholar]
- 11.Benito-Sanz S, Thomas NS, Huber C, Gorbenko del Blanco D, Aza-Carmona M, Crolla JA, et al. A novel class of Pseudoautosomal region 1 deletions downstream of SHOX is associated with Leri-Weill dyschondrosteosis. Am J Hum Genet 2005;77: 533–44. doi: 10.1086/449313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benito-Sanz S, Aza-Carmona M, Rodríguez-Estevez A, Rica-Etxebarria I, Gracia R, Campos-Barros A, et al. Identification of the first PAR1 deletion encompassing upstream SHOX enhancers in a family with idiopathic short stature. Eur J Hum Genet 2012;20: 125–7. doi: 10.1038/ejhg.2011.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukami M, Kato F, Tajima T, Yokoya S, Ogata T. Transactivation function of an approximately 800-bp evolutionarily conserved sequence at the SHOX 3′ region: implication for the downstream enhancer. Am J Hum Genet 2006;78: 167–70. doi: 10.1086/499254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukami M, Dateki S, Kato F, Hasegawa Y, Mochizuki H, Horikawa R, et al. Identification and characterization of cryptic SHOX intragenic deletions in three Japanese patients with Léri-Weill dyschondrosteosis. J Hum Genet 2008;53: 454–9. doi: 10.1007/s10038-008-0269-z [DOI] [PubMed] [Google Scholar]
- 15.Benito-Sanz S, del Blanco DG, Aza-Carmona M, Magano LF, Lapunzina P, Argente J, et al. PAR1 deletions downstream of SHOX are the most frequent defect in a Spanish cohort of Léri-Weill dyschondrosteosis (LWD) probands. Hum Mutat 2006;27: 1062. doi: 10.1002/humu.9456 [DOI] [PubMed] [Google Scholar]
- 16.Steinman S, Oishi S, Mills J, Bush P, Wheeler L, Ezaki M. Volar ligament release and distal radial dome osteotomy for the correction of Madelung deformity: long-term follow-up. J Bone Joint Surg Am 2013;95: 1198–204. doi: 10.2106/JBJS.L.00714 [DOI] [PubMed] [Google Scholar]
- 17.Schmidt-Rohlfing B, Schwöbel B, Pauschert R, Niethard FU. Madelung deformity: clinical features, therapy and results. J Pediatr Orthop B 2001;10: 344–8 [PubMed] [Google Scholar]
- 18.Ghatan AC, Hanel DP. Madelung deformity. J Am Acad Orthop Surg 2013;21: 372–82. doi: 10.5435/JAAOS-21-06-372 [DOI] [PubMed] [Google Scholar]
- 19.Felman AH, Kirkpatrick Jr JA. Radiology 1969;93: 1037–42 [DOI] [PubMed] [Google Scholar]
- 20.Henry A, Thorburn MJ. Madelung’s deformity. A clinical and cytogenetic study. J Bone Joint Surg Br 1967;49: 66–73 [PubMed] [Google Scholar]
- 21.Harley BJ, Brown C, Cummings K, Carter PR, Ezaki M. Volar ligament release and distal radius dome osteotomy for correction of Madelung’s deformity. J Hand Surg Am 2006;31: 1499–506. doi: 10.1016/j.jhsa.2006.07.012 [DOI] [PubMed] [Google Scholar]
- 22.Munns CF, Glass IA, LaBrom R, Hayes M, Flanagan S, Berry M, et al. Histopathological analysis of Leri-Weill dyschondrosteosis: disordered growth plate. Hand Surg 2001;6: 13–23. doi: 10.1142/S0218810401000424 [DOI] [PubMed] [Google Scholar]
- 23.Ty JM, James MA. Failure of differentiation: Part II (arthrogryposis, camptodactyly, clinodactyly, madelung deformity, trigger finger, and trigger thumb). Hand Clin 2009;25: 195–213. doi: 10.1016/j.hcl.2008.12.003 [DOI] [PubMed] [Google Scholar]
- 24.Sanchez J, Perera E, Jan de Beur S, Ding C, Dang A, Berkovitz GD, et al. Madelung-like deformity in pseudohypoparathyroidism type 1b. J Clin Endocrinol Metab 2011;96: E1507–11. doi: 10.1210/jc.2011-1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolters B, Lass N, Wunsch R, Böckmann B, Austrup F, Reinehr T. Short stature before puberty: which children should be screened for SHOX deficiency? Horm Res Paediatr 2013;80: 273–80. doi: 10.1159/000354989 [DOI] [PubMed] [Google Scholar]
- 26.Soucek O, Zapletalova J, Zemkova D, Snajderova M, Novotna D, Hirschfeldova K, et al. Prepubertal girls with Turner syndrome and children with isolated SHOX deficiency have similar bone geometry at the radius. J Clin Endocrinol Metab 2013;98: E1241–7. doi: 10.1210/jc.2013-1113 [DOI] [PubMed] [Google Scholar]
- 27.Hristov G, Marttila T, Durand C, Niesler B, Rappold GA, Marchini A. SHOX triggers the lysosomal pathway of apoptosis via oxidative stress. Hum Mol Genet 2014;23: 1619–30. doi: 10.1093/hmg/ddt552 [DOI] [PubMed] [Google Scholar]
- 28.Rappold GA, Durand C, Decker E, Marchini A, Schneider KU. New roles of SHOX as regulator of target genes. Pediatr Endocrinol Rev 2012;9 (Suppl 2): 733–8 [PubMed] [Google Scholar]
- 29.Aza-Carmona M, Shears DJ, Yuste-Checa P, Barca-Tierno V, Hisado-Oliva A, Belinchón A, et al. SHOX interacts with the chondrogenic transcription factors SOX5 and SOX6 to activate the aggrecan enhancer. Hum Mol Genet 2011;20: 1547–59. doi: 10.1093/hmg/ddr032 [DOI] [PubMed] [Google Scholar]
- 30.Vickers D, Nielsen G. Madelung deformity: surgical prophylaxis (physiolysis) during the late growth period by resection of the dyschondrosteosis lesion. J Hand Surg [Br] 1992;17: 401–7. doi: 10.1016/S0266-7681(05)80262-1 [DOI] [PubMed] [Google Scholar]
- 31.Stehling C, Langer M, Nassenstein I, Bachmann R, Heindel W, Vieth V. High resolution 3.0 Tesla MR imaging findings in patients with bilateral Madelung’s deformity. Surg Radiol Anat 2009;31: 551–7. doi: 10.1007/s00276-009-0476-0 [DOI] [PubMed] [Google Scholar]
- 32.Kosho T, Muroya K, Nagai T, Fujimoto M, Yokoya S, Sakamoto H, et al. Skeletal features and growth patterns in 14 patients with haploinsufficiency of SHOX: implications for the development of Turner syndrome. J Clin Endocrinol Metab 1999;84: 4613–21. doi: 10.1210/jcem.84.12.6289 [DOI] [PubMed] [Google Scholar]
- 33.Ogata T, Matsuo N, Nishimura G. SHOX haploinsufficiency and overdosage: impact of gonadal function status. J Med Genet 2001;38: 1–6. doi: 10.1136/jmg.38.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emons J, Chagin AS, Sävendahl L, Karperien M, Wit JM. Mechanisms of growth plate maturation and epiphyseal fusion. Horm Res Paediatr 2011;75: 383–91. doi: 10.1159/000327788 [DOI] [PubMed] [Google Scholar]
- 35.Binder G, Fritsch H, Schweizer R, Ranke MB. Radiological signs of Leri-Weill dyschondrosteosis in Turner syndrome. Horm Res 2001;55: 71–6. doi: 10.1159/000049973 [DOI] [PubMed] [Google Scholar]
- 36.Fukami M, Matsuo N, Hasegawa T, Sato S, Ogata T. Longitudinal auxological study in a female with SHOX (short stature homeobox containing gene) haploinsufficiency and normal ovarian function. Eur J Endocrinol 2003;149: 337–41. doi: 10.1530/eje.0.1490337 [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Wildhardt G, Zhong Z, Röth R, Weiss B, Steinberger D, et al. Enhancer deletions of the SHOX gene as a frequent cause of short stature: the essential role of a 250 kb downstream regulatory domain. J Med Genet 2009;46: 834–9. doi: 10.1136/jmg.2009.067785 [DOI] [PMC free article] [PubMed] [Google Scholar]