Abstract
The aim of this study was to assess changes in quality of life (QoL) in Japanese children with GH deficiency (GHD) after 12 mo of GH treatment or with idiopathic short stature (ISS) after 12 mo without treatment. Children with GHD were treated with GH after enrollment. Outcome measures included the parent-rated Child Behavior Checklist (CBCL), the Youth Self-Report Form (YSR), and height standard deviation scores (SDS). Total CBCL scores significantly decreased in children with GHD (n = 152, mean change (standard deviation [SD]) = –3.42 [11.21]) and ISS (n = 129, mean change = –4.82 [10.09]) after 12 mo (p < 0.001). Total YSR scores (mean change = –9.21 [14.07]) and height SDS (mean change = 0.35 [0.38]) significantly decreased in children with GHD (p < 0.001), but were unchanged in children with ISS. The change in total YSR score was significantly correlated with the change in height SDS in children with GHD (r = –0.516, p = 0.003). Our findings demonstrate that GH treatment can improve QoL in Japanese children with GHD. The correlation between the changes in total YSR score and height SDS demonstrated that increased height resulted in improved QoL.
Keywords: Child Behavior Checklist, GH deficiency, GH treatment, idiopathic short stature, Youth Self-Report
Introduction
Short stature in childhood, generally defined by a height that is at least two standard deviations below normal (1), may be caused by several different factors (2, 3). One of the reasons causing short stature in childhood is GH deficiency (GHD) (2, 3), although short stature may also be idiopathic (idiopathic short stature, ISS) (4). In addition to having a shorter than normal stature, children with GHD or ISS are presumed to have an associated poorer quality of life (QoL) compared with children of normal stature (4). Growth hormone treatment may be prescribed for children of short stature, and has been reported to promote short-term growth and increase final adult height in children with GHD (5,6,7) and in children with ISS (4, 7, 8). However, the effect of GH treatment on QoL in these children is not well‑defined.
Evidence from several studies suggests that QoL is worse in Caucasian children with GHD or ISS (9, 10) compared with Caucasian children of normal stature, and that GH treatment may improve QoL in children with GHD (10). However, the evidence for improvements in QoL with GH treatment is not conclusive (11, 12), and few studies have been conducted in Japanese children with GHD or ISS. We recently reported that Japanese children with GHD or ISS have a poorer QoL, as determined using the parent-rated Child Behavior Checklist (CBCL), compared with Japanese children without GHD (13). In addition, Abe et al. reported that Japanese children with GHD have depressive tendencies and that GH treatment ameliorates these tendencies (14). To our knowledge, no study has described the effects of GH treatment on QoL in Japanese children with GHD as determined using the CBCL or Youth‑Self Report Form (YSR). Furthermore, no study has described QoL in Japanese children with ISS as determined using the YSR.
The aims of our study were to assess QoL in Japanese children with GHD after 12 mo of treatment with GH and in Japanese children with ISS who were not treated for the same period of time. Two measures of QoL were compared, the parent-rated CBCL and the self-rated YSR. The children with GHD were enrolled in the Genetics and Neuroendocrinology of Short Stature International Study (GeNeSIS), an ongoing, open-label, multicenter, multinational postmarketing surveillance study designed to evaluate the long‑term safety and efficacy of somatropin (Humatrope®, Eli Lilly and Company, Indianapolis, IN, USA) in pediatric patients.
Subjects and Methods
Study population
All children with GHD were enrolled in GeNeSIS at participating Japanese hospitals from July 1999 to June 2007. The eligibility criteria for enrollment in GeNeSIS were as follows: GHD as defined by the Study Group of Hypothalamo-pituitary Disorders, the Japanese Health, Labour, and Welfare Ministry (15); a prescription of GH to enhance growth; and being naive to GH treatment at study entry. GHD was indicated by a peak GH concentration of ≤ 10 ng/mL in two or more provocation tests including insulin, arginine, L‑DOPA, clonidine or glucagon. Children with closed epiphyses were not eligible for enrollment; however, children could remain in the study if epiphyseal closure occurred during the study. All children with ISS were recruited from Japanese hospitals participating in GeNeSIS.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of each participating hospital. GeNeSIS complies with international postmarketing surveillance study guidelines (16) and is registered at www.clinicaltrials.gov (NCT01088412). Written informed consent was provided by a parent or guardian of each enrolled child.
GH treatment
All children with GHD received recombinant GH (0.175 mg/kg/wk: Humatrope®, Eli Lilly and Company) after enrollment in accordance with the Japanese prescribing information (17). Children with ISS were not treated with GH.
Demographics and clinical characteristics at study entry
Body weight, height, height standard deviation scores (SDS), time since diagnosis of GHD or ISS, and parental height were recorded at study entry for each child. Height SDS was also determined after 12 mo.
Quality of life
Quality of life assessments were made at study entry and after 12 mo using Japanese versions of the parent-rated CBCL / 4–18 (18) and the YSR (19).
Child Behavior Checklist: The CBCL consisted of 118 multiple choice questions that were completed by each child’s parent or guardian. Scores were standardized with reference to Japanese child norms (18). Total scores range from 0 to 100, with a lower score indicating better behavioral functioning. Results are presented as a total score, two broad-band scores representing externalization and internalization of behavioral and emotional problems, and eight narrow‑band scores representing withdrawn behavior, somatic complaints, social problems, anxiety / depression, thought problems, attention problems, rule-breaking behavior, and aggressive behavior.
Youth-Self Report form: The YSR (derived from the parent-rated CBCL) consisted of 118 multiple choice questions that were completed by all children ≥ 11 yr of age. Scores were standardized with reference to Japanese child norms (19) and are presented as per CBCL scores.
Statistical analysis
Categorical variables are presented as number (percent), whereas continuous variables are presented as mean ± standard deviation (SD). Gender and age range distributions were compared between the GHD and ISS groups using the Chi-square test. All other study entry clinical variables, QoL scores, and the percent change in QoL scores at 12 mo from study entry were compared between the GHD and ISS groups using the Wilcoxon rank-sum test. Within-group percent changes from study entry in QoL scores at 12 mo were compared using the Wilcoxon signed-rank test. Spearman’s correlations between the changes in height SDS and the changes in QoL scores at 12 mo were determined for both groups. All statistical analyses were performed using SAS version 8.02 (SAS Inc, Cary, NC, USA) and differences were considered to be statistically significant if p < 0.05.
Results
Participant disposition
A total of 181 children with GHD and 136 children with ISS were enrolled in this study. Of these children, 29 with GHD and 7 with ISS were subsequently excluded. The reasons for exclusion were uncollected questionnaires or clinical report forms at baseline (GHD: n = 18), inclusion criteria not met (GHD: n = 10; ISS: n = 7), and identification of coexisting disease (GHD: n = 1). Hence, 152 children with GHD and 129 children with ISS were included in the final analysis.
Demographics and clinical characteristics at study entry
Both groups of children contained more boys than girls (Table 1). Weight, height, and paternal and maternal height were similar in both groups of children. Children with GHD had a significantly lower height SDS and a shorter time since diagnosis than children with ISS (Table 1, p < 0.001). Children with GHD were also significantly older than children with ISS (9.1 ± 3.0 yr vs. 8.3 ± 2.8 yr, p = 0.028). The age distribution was also significantly different between children with GHD and children with ISS (p = 0.031). Specifically, the majority (61%: 92 of 152) of children with GHD were 9 to 15 yr of age, whereas the majority of children with ISS (51%: 66 of 129) were 4 to 8 yr of age.
Table 1. Demographic and clinical characteristics of children with GH deficiency or idiopathic short stature at study entry.
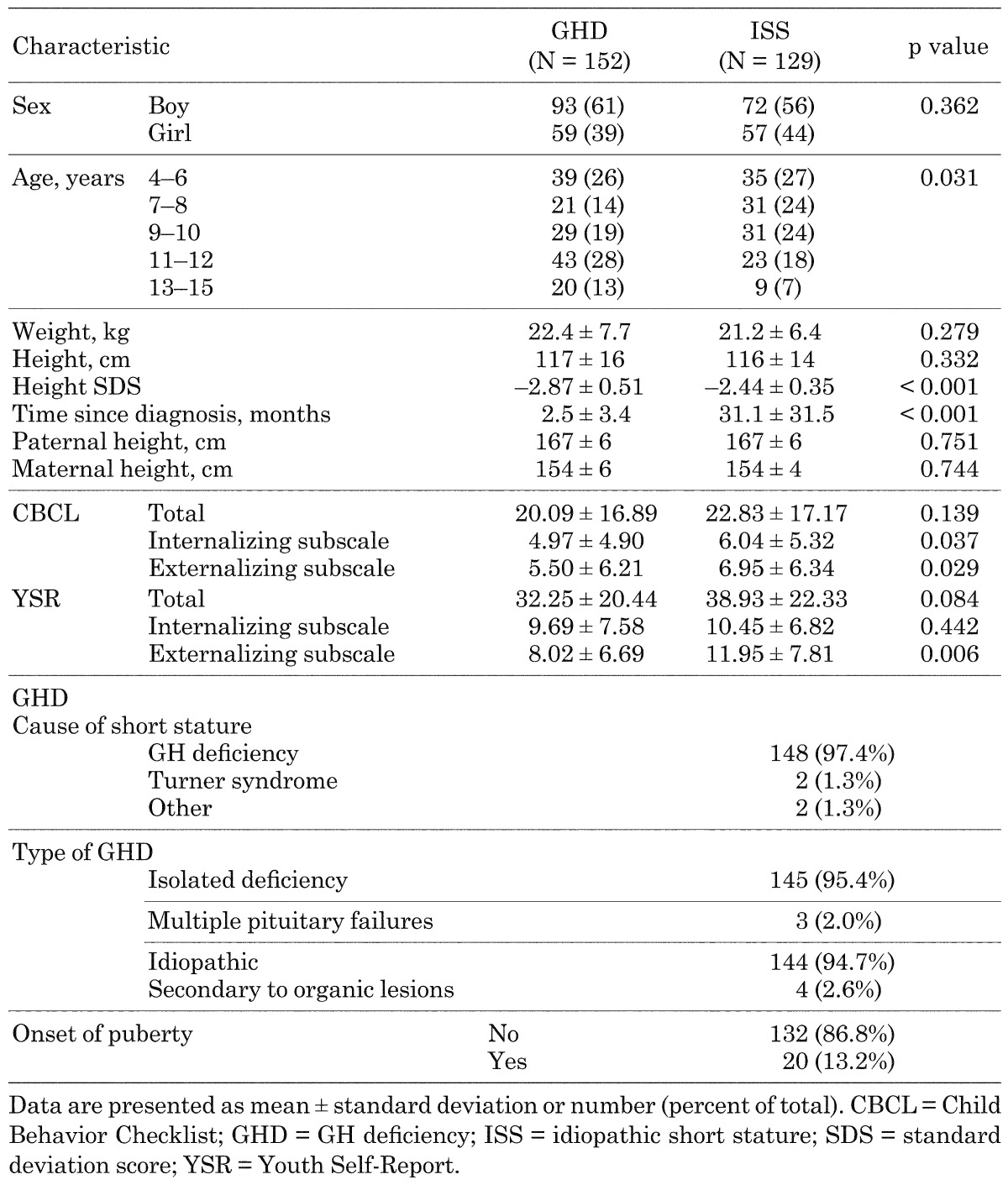
Of the patients with short stature, GHD was the cause in 148 (97.4%) patients and Turner syndrome in 2 patients. Of those patients with GHD, 145 (95.4%) were due to isolated GH deficiency and 3 (2.0%) to multiple pituitary failures. Idiopathic GHD were 144 patients (94.7%), and secondary to organic lesions were 4 patients. Regarding onset of puberty, 132 patients (86.8%) had not begun at baseline and 20 had already started.
At study entry, the total parent-rated CBCL and YSR scores were similar for children with GHD and ISS. However, children with GHD had significantly lower parent‑rated internalizing and externalizing subscale CBCL scores (p = 0.037 and 0.029, respectively), and significantly lower externalizing subscale YSR scores (p = 0.006) than children with ISS.
Quality of life after 12 mo of study
Patients who had both pre- and post-dosing (after 12 mo) measurements were included in the QoL analysis.
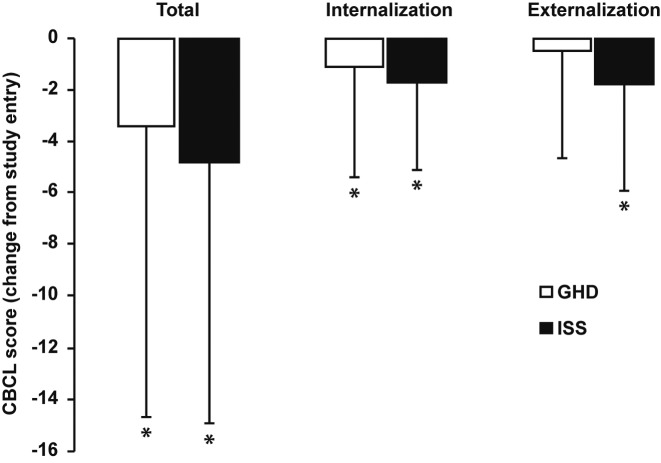
Child Behavior Checklist: Most parent-rated CBCL scores decreased significantly from study entry in both groups of children after 12 mo (Fig. 1). Specifically, mean changes (± SD) from study entry in the GHD group were –3.42 ± 11.21 (p < 0.001), –1.12 ± 4.26 (p = 0.039), and –0.52 ± 4.12 (p = 0.109) for total, internalizing and externalizing subscale CBCL scores, respectively. Corresponding mean changes (± SD) from study entry in the ISS group were –4.82 ± 10.09 (p < 0.001), –1.72 ± 3.32 (p < 0.001), and –1.75 ± 4.18 (p = 0.003) for total, internalizing and externalizing subscale CBCL scores, respectively. There were no significance between-group differences in CBCL scores after 12 mo. Specifically, total CBCL scores (mean ± SD) in the GHD and ISS groups were 17.88 ± 17.70 and 21.92 ± 19.57 (p = 0.132), respectively. Corresponding internalizing and externalizing subscale CBCL scores in the GHD and ISS groups were 4.19 ± 5.02 and 4.89 ± 5.00 (p = 0.212) and 5.04 ± 6.11 and 6.73 ± 7.61 (p = 0.171), respectively.
Fig. 1.
Change from study entry in parent-rated Child Behavior Checklist (CBCL) scores in children with GH deficiency (GHD, n = 66) or idiopathic short stature (ISS, n = 61) after 12 mo. Children with GHD received GH treatment for the entire 12 mo, whereas children with ISS were not treated. Data are shown as mean ± standard deviation. * Indicates a statistically significant change from study entry (p < 0.05).
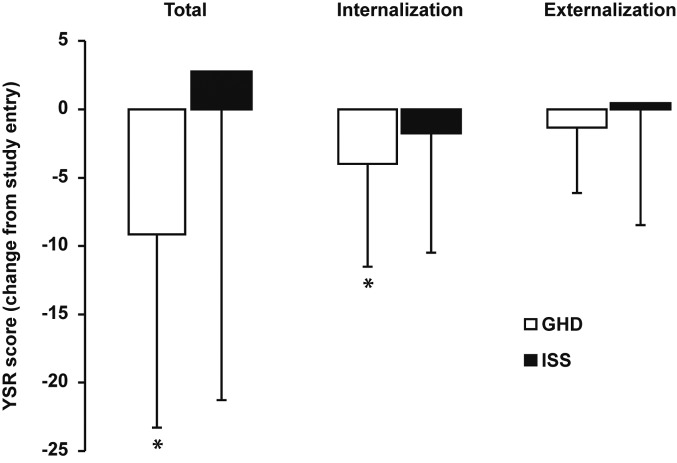
Youth Self-Report form: Most YSR scores decreased significantly from study entry in children with GHD after 12 mo of GH treatment and were unchanged in children with ISS who were not treated (Fig. 2). Specifically, mean changes (± SD) from study entry in the GHD group were –9.21 ± 14.07 (p < 0.001), –4.07 ± 7.47 (p < 0.001), and –1.36 ± 4.86 (p = 0.253) for total, internalizing and externalizing subscale YSR scores, respectively. Corresponding mean changes (± SD) from study entry in the ISS group were 2.69 ± 23.93 (p = 0.800), –1.77 ± 8.76 (p = 0.639) and 0.38 ± 8.86 (p = 0.980) for total, internalizing and externalizing subscale YSR scores, respectively. Children with GHD had significantly lower total and externalizing subscale YSR scores (indicating better QoL) after 12 mo of GH treatment than children with ISS who were not treated (p = 0.023 and p = 0.014, respectively). Specifically, total YSR scores after 12 mo of study (mean ± SD) were 23.97 ± 13.73 and 48.71 ± 33.81 in the GHD and ISS groups, respectively. Corresponding internalizating and externalizing YSR subscale scores in the GHD and ISS groups were 6.76 ± 5.23 and 11.79 ± 10.28, and 6.48 ± 5.26 and 13.93 ± 10.40, respectively.
Fig. 2.
Change from study entry in Youth Self-Report (YSR) scores in children with GH deficiency (GHD, n = 28) or idiopathic short stature (ISS, n = 13) after 12 mo. Children with GHD received GH treatment for the entire 12 mo, whereas children with ISS were not treated. Data are shown as mean ± standard deviation. * Indicates a statistically significant change from study entry (p < 0.05).
Changes in YSR scores were also analysed by patient background: isolated GH deficiency or multiple pituitary failures, idiopathic or secondary GHD, and onset of puberty (Y/N). The decrease in YSR somatic complaint score was significantly larger in the multiple pituitary failures group than in the isolated GHD group (p = 0.022). The decreases in the YSR externalizing subscale, withdrawn, and social problem scores were significantly larger in the group without onset of puberty compared with those in the group of onset of puberty (p = 0.042, 0.041, 0.007, respectively).
Height standard deviation scores
Height SDS significantly increased from study entry in children with GHD after 12 mo of GH treatment (mean ± SD = 0.35 ± 0.38, p < 0.001), but was unchanged in children with ISS who were not treated (mean ± SD = 0.06 ± 0.30, p = 0.114). The change from study entry in height SDS was significantly greater in children with GHD than in children with ISS after 12 mo (p < 0.001)
Quality of life and height standard deviation score correlations
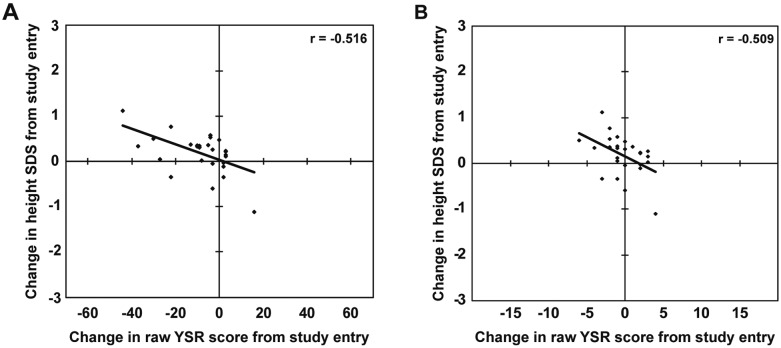
The changes in parent-rated CBCL scores were not correlated with the change in height SDS after 12 mo, whereas several YSR scores were significantly correlated with the change in height SDS (Table 2). Specifically, the changes in total, internalizing subscale, and attention problems YSR scores were significantly correlated with the change in height SDS in children with GHD (p = 0.006, 0.046, and 0.007, respectively) (Fig. 3).
Table 2. Correlation coefficients highlighting the relationships between the change in height standard deviation score and the changes in Child Behavior Checklist and Youth-Self Report scores in children with GH deficiency or idiopathic short stature after 12 mo of study.
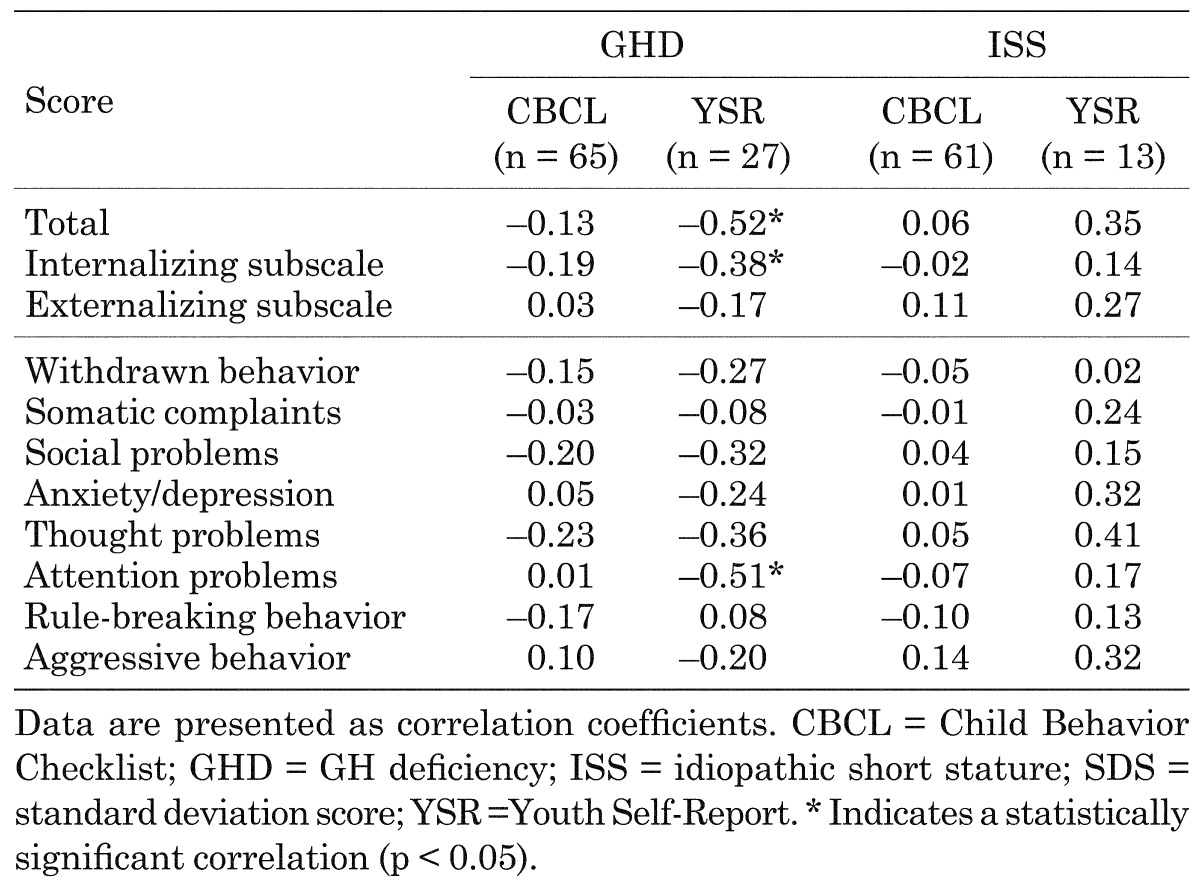
Fig. 3.
Scatter plots showing the correlations between the changes in total (A) and attention problems (B) Youth-Self Report (YSR) scores and the change in height standard deviation scores (SDS) after 12 mo of GH treatment in children with GH deficiency (n = 27).
Discussion
In this study, we examined QoL in Japanese children with GHD who were treated with GH for 12 mo and in Japanese children with ISS who were not treated for the same period of time. From the ethical point of view, we could not conduct a blinded placebo-controlled clinical study in children. We found that parent-rated CBCL scores were decreased (indicating improved QoL) in children with GHD and in children with ISS. In contrast, the self-rated YSR scores were decreased in children with GHD, but remained unchanged in children with ISS. The changes in several YSR scores were significantly correlated with the change in height SDS in children with GHD after 12 mo of GH treatment. Although comparison of children with GHD and ISS was limited because of the differences in patient background and treatment, our findings suggest that GH treatment can improve the QoL of Japanese children with GHD.
In addition to stimulating growth, we found that GH treatment could improve QoL in children with GHD as indicated by significantly decreased parent-rated CBCL and YSR scores. Our finding that GH treatment improved QoL is also consistent with the findings of similar studies conducted in the US (10) and Japan (14) in which QoL was improved after GH treatment as determined using the parent-rated CBCL and the child-rated Depression Self-Rating Scale for Children, respectively. In contrast, Sheppard et al. (20) reported that the QoL of children with idiopathic GHD, assessed using both parent- and child-rated QoL measures, did not significantly change after GH treatment. However, the study conducted by Sheppard et al. included fewer children (n = 8), had a shorter duration of GH treatment (six mo), and used different measures of QoL than in our study. Our findings, and those reported by Abe et al. (14), suggest that GH treatment can enhance the QoL of Japanese children with GHD.
We previously reported that parent-rated CBCL scores in Japanese children with GHD are higher than those of Japanese children without GHD (13). On the other hand, the YSR score decreased significantly from baseline in the children with GHD treated with GH, suggesting that YSR scores provide a sensitive indication of QoL in our cohort of children with GHD.
Evidence suggests that Japanese children with ISS have higher CBCL scores than those of normal stature (13). In the present study, we found that parent-rated CBCL scores were significantly decreased in children with ISS 12 mo after study enrollment, whereas YSR scores were unchanged. This suggests that repeat consultation with a physician might allay the concerns and modify the attitudes of parents of children with ISS, despite the lack GH treatment. In contrast, our finding that YSR scores were unchanged is consistent with the lack of GH treatment and the fact that height SDS did not change in these children. Additionally, in contrast to their parents, children with ISS did not appear to benefit from repeat consultation with a physician in the absence of a change in height. The disparity between the observed changes in parent-rated CBCL and YSR scores clearly demonstrates the importance of assessing QoL from both a parental and child perspective. Given the lack of GH treatment and change in height SDS, we suggest that YSR scores provided a more accurate indication of QoL than CBCL scores in our cohort of children with ISS.
An important finding of our study was that the changes in several YSR scores were significantly correlated with the change in height SDS in children with GHD after 12 mo of treatment with GH. This finding is consistent with the expectation that any psychosocial benefit of GH treatment (leading to improved QoL) is most likely to become apparent with increased height. In keeping with the findings reported by Stabler et al. (10), we found that there were no correlations between the changes in parent-rated CBCL scores and the change in height SDS in children with GHD after 12 mo of treatment with GH. Similarly, we found no correlations between these measures in children with ISS who were not treated. As parent‑rated CBCL scores were decreased after 12 mo in both groups of children, our findings suggest that repeat consultation with a physician may be an important factor leading to improved QoL in children with GHD or ISS from a Japanese parental perspective.
Our study has several limitations that should be acknowledged, since they might negatively affect interpretation of the results. One limitation is that we did not include a normal stature, age-matched control group of children or a group of untreated children with GHD for comparison. A second limitation is that the children were not randomly selected to take part in the study. Our study was, however, conducted in a clinical practice setting and hence provides real world insight into the effects of GH treatment on QoL in children with GHD. Further, our study included a larger number of children compared with previous studies (7, 14, 20) that have examined the effects of GH treatment on QoL in children with GHD. A third limitation is that we used generic measures to assess QoL rather than a measure that was specific to short stature (21). However, both the parent-rated CBCL and the YSR are validated measures, whereas many of the currently available short-stature-specific measures lack validity (22). Our use of parent- and child-rated measures of QoL is also critical given that both provide important information and that there is not always agreement between parental and child measures of QoL (12).
In conclusion, we have reported that 12 mo of GH treatment decreased YSR and parent‑rated CBCL scores in Japanese children with GHD. Over the same period of time, parent-rated CBCL scores also decreased in Japanese children with ISS who were not treated, whereas YSR scores did not change in these children. Our finding that parent‑rated CBCL scores were decreased in children with ISS suggests that, from a parental perspective, regular consultation with a physician can lead to improved QoL in Japanese children with ISS. Importantly, we also found that there were significant correlations between the changes in YSR scores and the change in height SDS in children with GHD after 12 mo of treatment with GH. Although our findings demonstrate that GH treatment can improve the QoL of Japanese children with GHD and that this improvement is related to the associated increase in height, a further follow-up study is warranted.
Acknowledgments
This study was sponsored by Eli Lilly Japan K.K. In compliance with the Uniform Requirements for Manuscripts established by the International Committee of Medical Journal Editors (ICMJE), the sponsor of this study did not impose any impediment, directly or indirectly, on the publication of the study’s results.
In collaboration with the authors, Eli Lilly Japan K.K. was involved in the study design, data collection, data analysis, and preparation of the manuscript. The authors had full access to the data upon request and had the final responsibility for the decision to submit the manuscript for publication.
References
- 1.Lifshitz F, Cervantes CD. Short stature. In: Lifshitz F, editor. Pediatric endocrinology. New York: Marcel Dekker; 1996. p. 1–18. [Google Scholar]
- 2.Krysiak R, Gdula-Dymek A, Bednarska-Czerwińska A, Okopień B. Growth hormone therapy in children and adults. Pharmacol Rep 2007;59: 500–16 [PubMed] [Google Scholar]
- 3.Takeda A, Cooper K, Bird A, Baxter L, Frampton GK, Gospodarevskaya E, et al. Recombinant human growth hormone for the treatment of growth disorders in children: a systematic review and economic evaluation. Health Technol Assess 2010;14: 1–209, iii–iv iii–iv. [DOI] [PubMed] [Google Scholar]
- 4.Bryant J, Baxter L, Cave CB, Milne R. Recombinant growth hormone for idiopathic short stature in children and adolescents. Cochrane Database Syst Rev 2007; CD004440 [DOI] [PubMed] [Google Scholar]
- 5.Guyda HJ. Four decades of growth hormone therapy for short children: what have we achieved? J Clin Endocrinol Metab 1999;84: 4307–16. doi: 10.1210/jcem.84.12.6189 [DOI] [PubMed] [Google Scholar]
- 6.Wetterau L, Cohen P. New paradigms for growth hormone therapy in children. Horm Res 2000;53 (Suppl 3): 31–6. doi: 10.1159/000023530 [DOI] [PubMed] [Google Scholar]
- 7.Högler W, Briody J, Moore B, Lu PW, Cowell CT. Effect of growth hormone therapy and puberty on bone and body composition in children with idiopathic short stature and growth hormone deficiency. Bone 2005;37: 642–50. doi: 10.1016/j.bone.2005.06.012 [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein BS, Imperiale TF, Speroff T, Marrero U, Radcliffe DJ, Cuttler L. Effect of growth hormone therapy on height in children with idiopathic short stature: a meta-analysis. Arch Pediatr Adolesc Med 2002;156: 230–40. doi: 10.1001/archpedi.156.3.230 [DOI] [PubMed] [Google Scholar]
- 9.Stabler B, Clopper RR, Siegel PT, Stoppani C, Compton PG, Underwood LE. Academic achievement and psychological adjustment in short children. The National Cooperative Growth Study. J Dev Behav Pediatr 1994;15: 1–6. doi: 10.1097/00004703-199402000-00001 [DOI] [PubMed] [Google Scholar]
- 10.Stabler B, Siegel PT, Clopper RR, Stoppani CE, Compton PG, Underwood LE. Behavior change after growth hormone treatment of children with short stature. J Pediatr 1998;133: 366–73. doi: 10.1016/S0022-3476(98)70271-9 [DOI] [PubMed] [Google Scholar]
- 11.Sandberg DE, Colsman M. Growth hormone treatment of short stature: status of the quality of life rationale. Horm Res 2005;63: 275–83. doi: 10.1159/000086593 [DOI] [PubMed] [Google Scholar]
- 12.Bullinger M, Kołtowska-Häggström M, Sandberg D, Chaplin J, Wollmann H, Noeker M, et al. Health-related quality of life of children and adolescents with growth hormone deficiency or idiopathic short stature - part 2: available results and future directions. Horm Res 2009;72: 74–81. doi: 10.1159/000232159 [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T, Tai S, Morisaki Y, Tachibana K, Kambayashi Y, Chihara K, et al. Evaluation of Qolity of Life in children with GH deficiency and Idiopathic Short Stature using the Child Behavior Checklist. Clin Pediatr Endocrinol 2009;18: 15–22. doi: 10.1297/cpe.18.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abe S, Okumura A, Mukae T, Nakazawa T, Niijima S, Yamashiro Y, et al. Depressive tendency in children with growth hormone deficiency. J Paediatr Child Health 2009;45: 636–40. doi: 10.1111/j.1440-1754.2009.01586.x [DOI] [PubMed] [Google Scholar]
- 15.Japanese Ministry of Health, Labor, and Welfare. Guideline for diagnosis of children with growth hormone deficiency. http://square.umin.ac.jp/endocrine/tebiki/001/001009.pdf Published 2007. Accessed December 20, 2010.
- 16.Herbold M. International guidelines on post-authorisation research and surveillance. European Economic Community. Pharmacopsychiatry 1997;30 (Suppl): 62–4. doi: 10.1055/s-2007-979519 [DOI] [PubMed] [Google Scholar]
- 17.Humatrope (prescribing information). Kobe, Japan: Eli Lilly Japan K.K. 2013 [Google Scholar]
- 18.Itani T, Kanbayashi Y, Nakata Y, Kita M, Fujii H, Kuramoto H, et al. Standardization of the Japanese version of the Child Behavior Checklist/4-18. Psychiatr Neurol Pediatr Jpn 2001;41: 243–52 [Google Scholar]
- 19.Kuramoto H, Kanbayashi Y, Nakata Y, Fukui T, Mukai T, Negishi T. Standardization of the Japanese version of the Youth Self Report (YSR). Jpn J Child Adolesc Psychiatry 2002;43: 17–32 [Google Scholar]
- 20.Sheppard L, Eiser C, Davies HA, Carney S, Clarke SA, Urquhart T, et al. The effects of growth hormone treatment on health-related quality of life in children. Horm Res 2006;65: 243–9. doi: 10.1159/000092455 [DOI] [PubMed] [Google Scholar]
- 21.Noeker M. Management of idiopathic short stature: psychological endpoints, assessment strategies and cognitive-behavioral intervention. Horm Res 2009;71 (Suppl 1): 75–81. doi: 10.1159/000178044 [DOI] [PubMed] [Google Scholar]
- 22.Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, et al. 2007 ISS Consensus Workshop participants.Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab 2008;93: 4210–7. doi: 10.1210/jc.2008-0509 [DOI] [PubMed] [Google Scholar]