Abstract
Alzheimer disease (AD) is the most common form of dementia among the elderly and is characterized by progressive loss of memory and cognition. Epidemiological data show that the incidence of AD increases with age and doubles every 5 years after 65 years of age. From a neuropathological point of view, amyloid-β-peptide (Aβ) leads to senile plaques, which, together with hyperphosphorylated tau-based neurofibrillary tangles and synapse loss, are the principal pathological hallmarks of AD. Aβ is associated with the formation of reactive oxygen (ROS) and nitrogen (RNS) species, and induces calcium-dependent excitotoxicity, impairment of cellular respiration, and alteration of synaptic functions associated with learning and memory. Oxidative stress was found to be associated with type 2 diabetes mellitus (T2DM), which (i) represents another prevalent disease associated with obesity and often aging, and (ii) is considered to be a risk factor for AD development. T2DM is characterized by high blood glucose levels resulting from increased hepatic glucose production, impaired insulin production and peripheral insulin resistance, which close resemble to the brain insulin resistance observed in AD patients. Furthermore, growing evidence suggest that oxidative stress play a pivotal role in the development of insulin resistance and vice versa. This review article provides molecular aspects and the pharmacological approaches from both preclinical and clinical data and interpreted from the point of view of oxidative stress with the aim to highlight progresses in this field.
Keywords: Alzheimer disease, type-2 diabetes mellitus and insulin resistance, oxidative stress, protein oxidation, heme oxygenase 1 and biliverdin reductase, insulin
1. Introduction
Alzheimer disease (AD) is the most common form of dementia among the elderly and is characterized by progressive loss of memory and cognition [1]. Epidemiological data show the incidence of AD increases with age and doubles every 5 years after 65 years of age with 1275 new cases/100000 persons/year [1, 2]. The Alzheimer Association points out that the financial, emotional, and family costs for care of AD patients are enormous and will increase markedly in the near future in the absence of a therapeutic modality to slow or stop onset. From a neuropathological point of view, amyloid-β-peptide (Aβ) leads to senile plaques, which, together with hyperphosphorylated tau-based neurofibrillary tangles and synapse loss, are the principal pathological hallmarks of AD. Aβ is associated with the formation of reactive oxygen (ROS) and nitrogen (RNS) species, and induces calcium-dependent excitotoxicity, impairment of cellular respiration, and alteration of synaptic functions associated with learning and memory [1].
Like AD, type 2 diabetes mellitus (T2DM) represents another prevalent disease associated with obesity and often aging, and it is estimated that about 24 million people living in the USA show clinical symptoms of T2DM [3]. T2DM is a condition in which high blood glucose levels result from increased hepatic glucose production, impaired insulin production by pancreatic β-cells and “insulin resistance” [inadequate response to insulin by target cells due to a down-regulated expression of the insulin receptor (IR), the IGF-1 receptor (IGF-1R), and the insulin receptor substrate (IRS) proteins] [3]. In different clinical studies, an association of T2DM and neurodegenerative disorders as well as decline in memory has been described. A series of longitudinal studies has shown that glucose intolerance and impairment of insulin secretion are associated with a higher risk to develop dementia or AD [4–6]. Indeed, it was shown that MCI subjects with normoglycemia at baseline had less functional and global cognitive decline, less whole-brain volume loss and lower conversion to AD than subjects with impaired glycemia over 2 years observation [7].
Vascular complications might explain only partially the increased incidence of neurodegeneration observed in patients with T2DM. The causes could be an impaired β-amyloid (Aβ) clearance [8], an up-regulation of the amyloid precursor protein (APP) expression and Aβ deposition [9], or hyperinsulinemia, as is present in T2DM, which may play an important role in the formation of senile plaques [10]. On the other hand, patients with AD more frequently present with an impaired glucose metabolism or T2DM [11]. These observations raise questions thus far unanswered: whether T2DM is a cause, consequence, or compensatory counterregulation to neurodegeneration, and whether neuronal insulin resistance indeed represents a risk factor for AD? Alternative mechanisms might be directly related to insulin/IGF-1 signaling, suggesting a common pathogenic cerebral signaling pathway in T2DM and neurodegeneration, including AD.
On a molecular level, several targets of the insulin machinery with potential influence on the development of neurodegenerative disease have been identified. Insulin and IGF-1 have intense effects in the CNS, regulating key processes such as energy homeostasis, neuronal survival, longevity, learning and memory [12, 13]. Insulin and IGF-1 bind to the tyrosine kinase receptors, IR, and IGF-1R, which share a high degree of identity in their structure and function [12, 13]. IR and IGF-1R are selectively distributed in the brain with a higher density in the olfactory bulb, hypothalamus, as well as in two of the main brain areas affected by AD pathology, i.e., hippocampus and cerebral cortex [12, 13]. Binding of insulin or IGF-1 induces a conformational change of the receptor leading to their auto-phosphorylation on specific tyrosine residues on the β-subunit with the consequent recruitment of the insulin receptor substrate-1 (IRS-1) [12, 13]. This latter, in turn, activates two main signaling pathways: (i) the PI3K pathway, which, among other functions, is involved in the maintenance of synaptic plasticity and memory consolidation [14], Aβ-induced memory loss [15], synthesis of nitric oxide (NO), which in turn plays a role in learning and memory processes [16]; and (ii) the MAPK cascade, which is responsible both for the induction of several genes required for neuronal and synapse growth, maintenance and repair processes, as well as serving as a modulator of hippocampal synaptic plasticity that underlies learning and memory [3]. Importantly, neurons are vulnerable to excitotoxic stress, and with some notable exceptions, there is a slow rate of neurogenesis in the brain. Hence, neurons remain post-mitotic, and any increased stress or reduced repair mechanism can accumulate over time. The impairment of insulin signaling in the brain could well play a role in the development of neurodegenerative disorders, as it leaves neurons more exposed to toxic influences.
2. Insulin resistance: a cross-talk between AD and T2DM
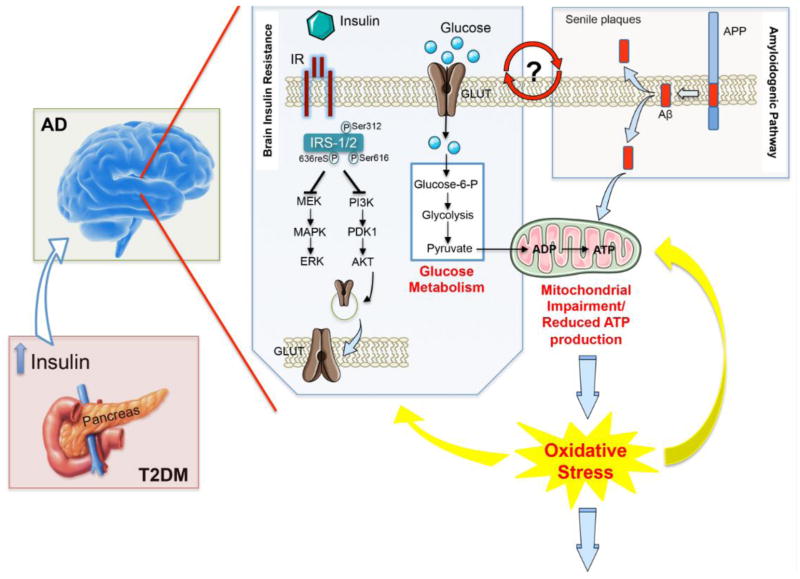
Insulin resistance is clinically defined as the inability of a known quantity of exogenous or endogenous insulin to increase glucose uptake and utilization in an individual as much as it does in a normal population [17]. Historically, insulin was long considered to be a hormone that primarily exerts its influence in the periphery [18]. While in the past years, the signaling mechanism and the biological effects of insulin have been studied mainly in classical insulin target tissues, such as skeletal muscle, fat and liver, with respect to glucose uptake, regulation of cell proliferation, gene expression and the suppression of hepatic glucose production, recently, it has become clear that insulin also produces similar effects in the central nervous system (CNS). Indeed, insulin is a peptide secreted by pancreatic beta cells and is readily transported into the CNS across the blood brain barrier (BBB) by a saturable, receptor-mediated process [19, 20]. Here, insulin binds to and activates the IR (Figure 1), that is, as cited above, widely distributed in the brain [21–23].
Figure 1. Increased oxidative stress levels as a central event driving insulin resistance in Alzheimer disease brain.
Persistently high levels of circulating insulin (as observed in the first phase of T2DM) may exert a negative influence on memory and other cognitive functions by down regulation of insulin receptors (IR) at the blood brain barrier and consequent reduced insulin transport into the brain (as observed in AD), thus leading to insulin resistance. From a molecular point of view, the lack of interaction between insulin and IR is associated with an increase of the inhibitory phosphorylation on insulin receptor substrate-1/2 (IRS1/2) on Ser312, 616 and 636, which, in turn, negatively impacts on the two main arms of insulin-mediated signaling cascade: the PI3K and the MAPK pathways, both involved in the maintenance of synaptic plasticity and cell stress response. Furthermore, turning off insulin signaling results in impaired glucose transport (reduced translocation of the glucose transporter at the plasma membrane) and metabolism thus promoting an alteration of mitochondrial processes involved in energy production. In turn, impairment of mitochondria functions leads to a vicious circle in which reduced energy production is associated with an increase of ROS and RNS responsible for the oxidative/nitrosative damage of mitochondria as well as other cellular components. In addition, increased Aβ production and accumulation, which represents a key feature of AD pathology, also promotes mitochondrial impairment. Moreover, insulin resistance-associated impairments in glucose uptake and utilization are associated with increased endoplasmic reticulum (ER) stress, which deregulate lipid metabolism, causing accumulation of toxic lipids in the brain. All these events contribute to the increased oxidative stress levels responsible of neurodegeneration observed in AD brain. Although insulin resistance and Aβ production can be considered leading causes of the rise of oxidative stress, this latter, in turn, promotes IRS-1/2 Ser-312, -616 and -636 phosphorylation as well as the oxidative damage of protein involved in glycolysis, the Krebs cycle and ATP synthesis that are crucial events in the reduction of glucose metabolism and thus insulin resistance. Finally, because insulin resistance is associated with increased Aβ production and Aβ production is postulated to be responsible for the onset of insulin resistance, it remains to be clarified whether insulin resistance is a cause, consequence, or compensatory response to Aβ-induced neurodegeneration.
In 1978, Havrankova et al. showed that insulin is present in rat brain in high concentration, and it is independent of peripheral insulin levels [24]. Thus, although pancreatic-derived insulin crosses the BBB and reaches the brain, a portion of the insulin in the CNS is locally produced, based on the detection of c-peptide (which is an integral part of the pro-insulin molecule) and insulin mRNA in the brain [25].
Due to the well-known role of insulin in learning and memory processes [25–27], these new lines of evidence make the comprehension of the mechanism(s) responsible for the observed insulin resistance in neurodegenerative disorders more fascinating. Human postmortem studies have convincingly shown that brain insulin resistance with reduced activation of receptors and downstream neuronal survival and plasticity mechanisms are consistent and fundamental abnormalities in AD (Figure 1) [28–30]. Given that, and based on the concept that brain insulin resistance shows a series of similarities with the peripheral insulin resistance, AD was described as a form of T2DM commonly called type 3 diabetes [31, 32]. Notwithstanding, as recently reported by Talbot and Wang, the concept of type 3 diabetes is no longer correct for a couple of reasons including the fact that: (a) hyperglycemia – which is the key diagnostic features of diabetes – is not present in AD CSF: and (b) it is not clearly demonstrated that AD brain is insulin-deficient [33].
Thus, insulin resistance represents the main feature linking T2DM and the risk to develop AD. Indeed, from a pathological point of view, the early stages of T2DM are characterized by hyperinsulinemia (the pancreas makes extra insulin) and insulin resistance (reduced insulin efficiency); however, hepatic glucose production remains normal, with fasting euglycemia and postprandial hyperglycemia relatively mild. In the later stages of T2DM, insulin resistance persists, hepatic glucose production rises, and endogenous insulin production falls, resulting in fasting and postprandial hyperglycemia [34]. While acute hyperinsulinemia may produce beneficial effects on cognition, persistently high levels of circulating insulin (as observed in the first phase of T2DM) may conversely exert a negative influence on memory and other cognitive functions. Indeed, raising peripheral insulin levels acutely elevates brain and cerebrospinal fluid insulin levels, whereas prolonged peripheral hyperinsulinemia down-regulates insulin receptors at the BBB and reduces insulin transport into the brain (Figure 1) [35, 36]. In that regard, patients with moderate-to-severe AD showed elevated true plasma insulin levels, related to healthy older adults, thus suggesting the insulin resistance as a risk factor in AD pathology [34].
Based on both pre-clinical and clinical lines of evidence, it is broadly accepted that AD pathology is driven/worsened by the appearance of the insulin resistance, how brain insulin/IGF-1 resistance and deficiency develop is not completely understood [37].
3. Oxidative stress: is it the driving force for insulin resistance in AD?
Reactive oxygen species/reactive nitrogen species (ROS/RNS) play a dual biological role in living systems since they can be either beneficial or detrimental [38]. Low levels of ROS exert beneficial physiological roles in cellular responses to stress and in the activation of several cellular signaling pathways, such as synaptic signaling, where ROS are messenger molecules in long-term potentiation (LTP) [39, 40]. Moreover, moderate ROS levels are thought to improve peripheral insulin sensitivity [41]. On the other hand, imbalance between the production of ROS/RNS as a consequence of mitochondrial dysfunction and the intracellular antioxidant capacity leads to abnormally elevated ROS levels and a condition known as oxidative stress (OS) that is followed by oxidative damage to cells and, eventually, death (Figure 1) [42]. Increased OS has been implicated in the pathology of several diseases and the accumulation of oxidatively damaged proteins, lipids, and nucleic acids correlate with the onset of age-related cellular alterations, especially in diabetes and AD [43, 44]. Indeed, OS represents a central pathophysiological mediator of diabetes and is deeply involved into the development and progression of neurodegenerative diseases [45]. Due to its elevated levels of peroxidable fatty acids, high requirement for oxygen, relative paucity of antioxidant systems, and richness in iron content, the brain is extremely sensitive to OS [42, 46]. Therefore, neurons are particularly susceptible to oxidative damage and aging, together with age-related diseases, contribute to the disruption of the balance between ROS generation and antioxidant defense resulting in the damage of fundamental biological macromolecules [47].
Oxidative damage to lipids and protein of neuronal membrane affects activities of membrane-bound enzymes, ion channels and receptors. ROS/RNS attack biological component of the cell resulting in the formation of stable adducts with DNA, RNA and can damage cell or organelle membranes directly (e.g., through lipid peroxidation), or reacting with metals, nitrogen or carbon to form intermediates that react with proteins (e.g., through nitration, carbonylation, nitrosylation or reactive alkenals by Michael addition) [48, 49]. Oxidation of amino acids can lead to the formation of advanced glycation end products (AGEs), advanced oxidation protein products (AOPPs), peroxides and carbonyls that can attack other molecules and generate radicals resulting in protein unfolding and in rendering the protein inactive and prone to aggregation [50].
The idea of OS as a link between T2DM and AD is currently under evaluation by several research groups. Both T2DM and AD have common pathogenic factors, and evidence suggests a close link for the presence of cellular OS, mitochondrial abnormalities and paucity in antioxidant defenses [51–53]. Since mitochondria are the central coordinators of energy metabolism and are sources and targets of ROS, their impairment may represent a downstream event of T2DM and/or AD-associated abnormal brain insulin and glucose metabolism [38, 54]. Indeed, has been shown that increased OS lead to the inhibition of cellular energy production and to the reduction of both insulin secretion and sensitivity [55, 56]. In turn, defective insulin signaling makes neurons energy-deficient and more vulnerable to oxidizing insults, which could promote structural and functional alterations of mitochondria (Figure 1)[51, 57].
OS is also believed to modify a number of signaling pathways related to protein unfolding response and protein degradation that can ultimately lead to insulin resistance [58]. In T2DM individuals, resistance to insulin signaling beyond the increase of OS makes neurons energetically defective and susceptible to oxidizing or other metabolic insults impairing synaptic plasticity [59]. Moreover, insulin resistance-associated impairments in glucose uptake and utilization are associated with increased ER stress, which, deregulate lipid metabolism, causing accumulation of toxic lipids in the brain [60]. Moreover, in T2DM subjects the increased production of ROS, as a consequence of elevated glucose levels can be reduced with the administration of antioxidants [58]. Increasing data support the idea that aging-related alterations of mitochondrial function represent the driving force of increased OS in T2DM contributing to the progression and development of AD pathology [51, 55, 56, 60–62].
Several studies were conducted on murine models to demonstrate that mitochondrial failure may represent a functional link between both pathologies. A study on a rat model of sporadic AD generated by the intracerebroventricular (icv) injection of a sub-diabetogenic dose of streptozotocin (STZ) demonstrated that the insulin-resistant brain state is accompanied by the occurrence of mitochondrial abnormalities [63], while STZ-induced T1DM rats showed no significant changes in mitochondrial function and synaptic integrity as result of compensation mechanisms, although reverted by insulin administration [64]. The comparison 3xTg-AD mice with sucrose-treated WT mice reported by Carvalho and colleagues [65] showed a similar impairment of mitochondrial respiratory chain and phosphorylation system, as well as oxidative imbalance and increased Aβ levels, consistent with the notion that mitochondrial metabolic alterations associated with diabetes contribute to the development of AD-like pathologic features. In agreement with this notion, comparison of 11-month-old T2D and AD mice showed, in addition to increased Aβ levels, similar behavioral and cognitive anomalies characterized by increased fear and anxiety and decreased learning and memory abilities [66]. In addition, diabetic and non-diabetic rats infused with Aβ had both profound decreased energy intake, activity and fat oxidation and increased carbohydrate oxidation and energy expenditure; however, these effects were aggravated by 10% to 20% in the diabetic rat group [67].
In a recent study on microvascular endothelial cells from rat (RBMEC) and mice (MBMEC) isolated from adult Sprague-Dawley rats and homozygous db/db (Leprdb/Leprdb) and heterozygous (Dock7m/Leprdb) mice, respectively, and cultured under normal- or hyperglycemic conditions for 7d followed by 24h exposure to Aβ(1–40) showed that high glucose levels increase the susceptibility of brain microvascular endothelial cells to Aβ toxicity, supporting the idea that hyperglycemia is a major risk factor for vascular injury associated with AD [68].
In addition to Aβ pathology, insulin reportedly could regulate Tau phosphorylation in neuronal cells [69, 70], which was confirmed by observations of hyperphosphorylated Tau in mice showing abnormal insulin levels in the brain [71, 72]. Among the kinases able to phosphorylate Tau in vitro, GSK-3β is considered to be one of the major physiological and pathological Tau kinases [73]. However, studies that have addressed GSK-3β activation in diabetic animal models have reported conflicting results [74]. It was recently demonstrated that tau overexpression was influenced by a high fat independently of peripheral hyperglycemia, hyperinsulinemia or insulin resistance in brains of wild-type mice and in mouse models of T2DM and AD [75].
Patients with AD pathology demonstrated the concomitant presence of mitochondrial bioenergetics failure, increased OS and reduced insulin signaling as consequence of increased Aβ and levels of hyperphosphorylated tau (Figure 1) [52, 53]. In brains of subjects with AD, deficits in insulin signaling are due to both insulin deficiency and insulin resistance that is manifested by reduced levels of insulin receptor binding and decreased responsiveness to insulin stimulation (Figure 1) [29, 76]. Hence, AD is considered a brain form of diabetes with features of both insulin resistance and insulin deficiency. The progressive worsening of insulin resistance along stages of AD correlates with the increased OS, DNA damage and protein oxidation demonstrated by 4-hydroxynonenal (HNE), protein carbonyls (PCO) and 3-nytrotyrosine (3NT) accumulation [51]. It was proposed that insulin resistance together with decreased brain insulin levels might lead to accumulation of Aβ and consequently AD (Figure 1) [77]. In addition, insulin administration modulates the cellular clearance of Aβ [78] and reduces Aβ-induced OS, exerting a protective effect on synapses from AD-related damage [79–81].
The mechanism of protection by insulin appears to involve the restoration of the Akt/mTOR/S6K signaling pathway within AD neurons that when aberrantly modulated may increase IRS-1 phosphorylation at Ser312, resulting in IRS-1/2 degradation, thus impairing IR-associated neurotropic and metabolic brain functions [38, 82–84]. A mechanism involving anomalous calcium influx and OS may underlie insulin resistance in AD since chelation of intracellular calcium with BAPTA-AM prevents both oligomer-induced insulin inhibition and OS [41, 85]. Moreover, the presence of a NMDA-R blocker (memantine) attenuated the Aβ oligomer-induced increase in intraneuronal calcium essential in causing neuronal OS; thus, NMDA-R dysfunction might play a role in OS and defective neuronal insulin signaling in AD [86, 87]. These studies suggest a possible physiological feedback between neuronal activity and insulin signaling.
Elevated levels of AGE represent another common pathological marker of both T2DM and AD. Clinical studies have shown that elevated blood glucose levels result in significantly increased accumulation of AGEs in tissues of diabetic subjects [88]. Diabetic patients could have an increased risk of AD via AGE production since the modification of Aβ by AGE accelerates Aβ aggregation and the glycation of tau stabilizes neurofibrillary tangles [52]. High levels of AGE immunoreactivity are present in AD plaques and neurofibrillary tangles [89–92]. Moreover, RAGE has been found to be a receptor for Aβ and mediates Aβ induced microglia activation and subsequent inflammation in AD [93, 94].
3.1 Protein oxidation vs. antioxidant system in T2DM and AD
3.1.1 Protein oxidation
As noted above reduced glucose metabolism occurs both in T2DM and AD and increases the production of free radicals such as ROS/RNS as consequence of mitochondrial failure. In turn, the increased production of ROS/RNS results in protein oxidation and/or lipid peroxidation that contributes to further mitochondrial damage and decreased ATP production (Figure 1). Increased oxidation, including protein bound-HNE, protein carbonyls, protein bound-3NT, in metabolic-related proteins was found in inferior parietal lobule and hippocampus of individuals with AD and mild cognitive impairment compared with healthy controls, indicating that oxidative-related impairment of components of glucose metabolism might occur early in the development of AD [53, 60, 95].
Mounting evidence supports the concept that AD is a metabolic disease in which brain glucose utilization and energy production are impaired. APP and Aβ have been shown to induce mitochondrial activity defects and increased OS, and a number of studies on animal and cell culture models of AD suggested that increased levels of OS are able to impair key players of the glucose metabolic pathway [42, 46, 96]. In parallel, insulin-signaling impairment contributes to AD development by favoring tau hyperphosphorylation, accumulation of Aβ, increased OS and oxidative damage and mitochondrial dysfunction (Figure 1). In this regard, the analysis of oxidation and damage of protein belonging to metabolic pathways (glucose metabolism) might be of interest in understanding the potential molecular mechanisms targeted by OS that trigger common features between T2DM and AD. Redox proteomics studies on AD brain demonstrated the oxidation of alpha-enolase (ENO1), malate dehydrogenase (MDH), fructose bisphosphate aldolase A/C (FBA A/C), ATP synthase, glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Similarly, pyruvate kinase (PK), MDH, ENO1, FBA C, TPI were found increasingly oxidized in amnestic mild cognitive impairment (MCI) and preclinical AD (PCAD) brain [50, 97–102].
Interestingly, redox proteomics data relate with PET studies that demonstrated a reduction of the cerebral metabolic rate of glucose in AD [103] and suggest that the oxidative damage of protein involved in glycolysis, the Krebs cycle and ATP synthesis might be a crucial event in the reduction of glucose metabolism (Figure 1). In addition, the oxidation of the above described proteins, culminating with reduced ATP production, likely contribute to the loss of synapses and synaptic function at nerve terminals merely leading to neurodegenerative processes and cognitive decline (Figure 1) [102, 104]. Since this phenomenon appears early in PCAD and MCI, it conceivably might be the consequence of increased OS caused by insulin resistance, contributing to further alterations of mitochondrial functionality that exacerbate even more oxidative damage. Strong indications suggest that OS occurs before the onset of symptoms in AD and that oxidative damage is found before robust Aβ plaque formation, supporting a causative role of mitochondrial dysfunction and OS in AD [42, 46, 105]. Within this context, data supporting protein oxidation in the presence of insulin resistance and reduced glucose utilization suggest the potential presence of a vicious cycle among these events that eventually culminate with AD pathology (Figure 1). Consistent with this notion, studies on T2DM subjects demonstrated increased levels of PCO AOPPs, AGEs, oxLDL, 8-OHdG, MDA, NOx, and insulin resistance compared with control together with a significant drop in plasma –SH groups, free radical scavenging capacity and deficits in the antioxidant defense enzymes and vitamins [106–110]. Moreover, the plasma GSH/GSSG ratio showed a significant decrease in T2DM as compared to healthy subjects, associated with increased pentosidine, F2-isoprostanes, and HNE levels [111]. In this scenario, OS represents undoubtedly a crucial event in the development of T2DM and AD even if the exact role played by its aberrant increase has not been clarified. However, since OS is shared by both pathologies it might represent the point of intersection between them. The potential oxidative link between diabetes and AD is also supported by studies on DS subjects, which demonstrate reduced glucose utilization, mitochondrial deficit and increased OS early in life that might contribute to the oxidation of metabolic enzymes, such as PK, ENO1, GAPDH and FBA A/C, the build-up of insulin resistance, through the inhibition of IRS1, and the progression to AD-like dementia [84, 112, 113].
3.1.2 The antioxidant response: the role of the heme-oxygenase and its by-products
The heme oxygenase/biliverdin reductase (HO/BVR) system is one of the main and evolutionarily conserved cellular cytoprotectants, whose up-regulation represents an early event in the adaptive response to stress [114]. Despite that the initial attention by the scientific community was focused primarily on the ability of this system to degrade heme to be the main, if not the only, function, quite recently numerous different functions have been elucidated. Humans and rodents have two HO isozymes, namely HO-1 (about 32 kDa, enzyme) and HO-2 (36 kDa). Heme oxygenase-1, also known as heat shock protein (Hsp)-32, is induced by various stimuli, including oxidative and nitrosative stress, ischemia, heat shock, bacterial lipopolysaccharide (LPS), hemin, the neuroprotective agent leteprinim potassium (Neotrofin) [115, 116] and several drugs currently used in the clinic, such as statins, non-steroidal anti-inflammatory drugs, antagonists to the adrenergic β receptor, cyclosporine A etc. [117, 118]. Heme oxygenase-2, the constitutive isoform, is responsive to developmental factors, adrenal glucocorticoids, nitric oxide (NO) [115], atorvastatin [118] and drugs acting on the nervous system, such as morphine and glucocorticoids [117].
HO-1 and HO-2 catalyze the same reaction, namely the transformation of iron-protoporphyrin-IX-alpha (heme) into equimolar amount of ferrous iron [Fe(II)], carbon monoxide (CO), and biliverdin-IX-alpha (BV-alpha) [115, 116]. Both the HO iso-enzymes and their by-products were reported to play a role in the insulin signaling, shedding light on the impairment of the antioxidant response as a central event in the development of the insulin resistance.
First of all, previous studies demonstrated that HO-1 and HO-2 are not highly protective in AD brain due to their oxidative post-translational modifications, which affect their levels and activities. Indeed HO-2 protein levels were found significantly decreased in AD brain [119]. Conversely, because HO-1 is a stress-inducible protein, the increase of OS levels in the hippocampus of AD subjects could lead to an increase in HO-1 protein levels and phosphorylation in order to promote its activity and its interaction with BVR [120]. At the same time, the increased OS could be responsible for the observed increased PC modifications and HNE adducts, already demonstrated for other proteins in AD [121] leading to altered protein structure and function impairment [49, 122–124]. Based on our experimental model (full homogenate of human brain samples), it is difficult to state which post translational modification precedes the other between phosphorylation and oxidative modification. At least two interpretations are conceivable: 1) OS promotes the increase of oxidative damage to HO-1 (e.g., increased PC and HNE adducts on its structure). Consequently, the cell tries to restore the functionality of the protein by increasing Ser residue phosphorylation; 2) OS promotes the increase of Ser-residue phosphorylation in order to activate protein functions, but HO-1 quickly becomes a target for oxidative post-translational modifications, that, in turn, could impair its function [119]. Considering that AD brain is characterized by abnormal levels of oxidative and nitrosative stress [49], it is evident that the functionality of the HO enzymes is compromised, and even in the case that the first interpretation were true, the restoration likely would only be partial, and maybe not enough to promote neuroprotection [125].
With regard to diabetes, no data exist so far about the expression levels and activity of HO in the human brain. The few lines of evidence in mice show that HO-1 protein levels are increased in the brain of db/db mice and that this increase parallels the augmentation of isoprostanes and 8-OHdG, markers of lipid peroxidation and DNA oxidation, respectively [126]. Similarly, it was observed that the ferric nitrilotriacetate (Fe-NTA)-induced diabetes in rats was associated with an increase of HO-1 protein levels in the brain [127]. In addition, in another work, HO-1 mRNA expression in the brain of diabetic rats was unchanged with respect to the controls [128], thus leaving the information about HO-1 in the brain quite vague. Based on these data and our experience, it becomes difficult to argue a unique hypothesis about the role of HO-1 in insulin resistance in the brain because what is still missing with regard to diabetes is the data about HO activity and/or post-translational modifications.
However, considerable information about a possible involvement of HO in insulin resistance could come from HO-derived by-products such as ferrous iron and CO. Accumulating evidence suggests that iron (II) plays a pathogenic role in T2DM and its complications, such as microangiopathy and atherosclerosis [129, 130]. In addition to the induction of OS, iron (II) may also impede insulin extraction in the liver, impair pancreatic insulin secretion, and interfere with insulin action and glucose uptake in adipocytes. Of note, a reduction in iron overload with either phlebotomy or iron chelation therapy has been shown to reverse or improve glycemic control in T2DM [130]. Consistent with the above, ferric nitrilotriacetate (Fe-NTA)-induced diabetes in rats lead to an increased ferrous iron in the cortex and hypothalamus, together with increased HNE [127], thus suggesting that increased HO activity could impact iron production and thus OS-mediated insulin resistance.
With regard to AD, the role of iron in the brain is clearer. Indeed, it was demonstrated that redox-active iron is associated with senile plaques and neurofibrillary tangles, indicating that iron accumulation could be an important contributor toward the oxidative damage of AD [131], thus providing a basis for the future involvement of HO-1 as one of the main source of iron deposition and accumulation. Furthermore, heme-derived ferrous iron may mediate the oxidative modification of mitochondrial lipids, proteins and nucleic acids in these cells. Glial HO-1 hyperactivity may contribute to cellular OS, pathological iron deposition, and bioenergetic failure characteristic of degenerating and inflamed neural tissues as observed in AD [132]. The same group demonstrated that immunoreactive astocytic HO-1 protein was significantly increased in temporal lobe and hippocampal in subjects with MCI and AD, and was associated with global measures of cognitive impairment and specific memory deficits in these individuals. The authors suggested a mechanism favoring early mobilization of free iron, mitochondrial insufficiency and corpora amylacea formation in this neurodegenerative disorder [133].
Experimental studies have indicated that iron deficiency is related to increased insulin sensitivity in animals [134, 135], while epidemiological studies have reported an association between iron overload and peripheral insulin resistance [136]. These observations make the story intriguing because one can hypothesize that the deregulation of iron mobilization and metabolism in AD brain may be responsible, at least in part, for the observed insulin resistance. Thus, in accordance with the hypothesis provided by our group [125], AD progression could be associated with an initial over-activation of the HO-1 with the aim to overcome the raise of the OS levels. Then, the over-production of ferrous iron could participate in the impairment of insulin signaling both directly [134, 135] or indirectly by promoting a further increased OS [41, 137].
To support the idea that the impairment of the HO activity could contribute insulin resistance observed in AD brain, the role of CO has to be analyzed. Under normal physiological conditions, islets of Langerhans produce CO and nitric oxide (NO) to regulate insulin release [138, 139]. While NO negatively modulates glucose-stimulated insulin release, CO stimulates insulin secretion [138, 139]. Moreover, glucose stimulates pancreatic β-cells to produce CO, which in turn triggers insulin release [138, 139]. The critical role of the HO system in insulin release and glucose metabolism was reported in Goto-Kakizaki (GK) rats, a model with defective pancreatic β-cell HO-2 [140]. Since HO-2 is largely responsible for basal HO activity [125] and thus the production CO, the impairment of the HO system in GK rats resulted in reduced CO and insulin insufficiency [140]. Treatment with the HO-inducer, hemin, or CO corrected the defective HO system and enhanced insulin release with improvement of glucose metabolism [140]. Collectively, these studies suggest that reduced CO and/or impaired HO system may lead to dysfunctional glucose metabolism.
Whether CO also exerts these same effects in the brain is not well established and needs further investigation. However, it is conceivable to suppose that, due to the similarity between (i) peripheral insulin resistance observed in T2DM, and (ii) brain insulin resistance as observed in AD, CO could promote the same outcomes. For this reason, the observed decrease of HO-2 and the impairment of HO-1 in AD [119, 125] acquires a new significance in the field of insulin resistance. Given the above, we speculate that the possibility that the elevation of HO-1 in AD brain leads to an overproduction of both ferrous iron [131–133] and CO [125, 141]. However, with the progression of AD pathology, the impairment of HO-1 and the observed dysregulation of the machinery involved in iron metabolism [142] result in the accumulation of ferrous iron together with a decrease of CO levels, which strongly affect insulin signaling. Indeed, the post-mortem analyses showing in human AD brain both insulin resistance and reduced levels of insulin, agree with the paradigm for which iron promotes insulin resistance and CO promotes insulin secretion.
4. Oxidative stress and insulin resistance: therapeutic approaches
As described in more details below, several therapeutic approaches aimed to counteract/slow the effects produced by insulin resistance in AD pathology were recently identified. However, anti-diabetic drugs such as metformin, sulfonylureas, thiazolidinediones (rosiglitazone and pioglitazone) failed to reduce the risk to develop AD or to restore the observed impairment in learning and cognition probably because their: (i) rapid degradation; (ii) poor penetrance of the BBB; and (iii) inability to reduce insulin resistance in vivo. On the other hand, intranasal insulin administration was able to improve delay memory recall associated with changed CSF Aβ42 levels and tau protein-to-Aβ42 ratios [143]. Because a wide literature already has focused on the pro and cons of these drugs, we provide here an analysis of the effects that these drugs have on OS, when known.
4.1 Metformin
Metformin belongs to the class of biguanides, which are agents able to reduce hepatic glucose output and increase uptake of glucose in the periphery, including skeletal muscle. Although it must be used with caution in patients with impaired liver or kidney function, metformin has become the most commonly used agent for type 2 diabetes, and it is the only widely used oral drug that does not cause weight gain [144]. The molecular mechanism of metformin is not completely understood: inhibition of the mitochondrial respiratory chain (complex I), activation of AMP-activated protein kinase (AMPK) and the consequent inhibiton of mTOR activity, inhibition of glucagon-induced elevation of cyclic adenosine monophosphate (cAMP) and consequent activation of protein kinase A (PKA), and an effect on gut microbiota have been proposed as potential mechanisms [145, 146].
In 2009, it was shown that metformin significantly increased the generation of the intracellular and extracellular Aβ species both in vitro (10 mM, N2a695 cells) and in vivo (2 mg/mL per os, C57B6 mice) through the up-regulation of β-secretase (BACE1), which results in an elevated protein level and increased enzymatic activity [147]. In addition, the surface levels of LRP1 (low-density lipoprotein receptor-related protein 1), noted above to be a major efflux transporter for brain Aβ1-42 and found to be oxidatively modified in AD brain [123], were decreased after metformin treatment [147]. Later, other studies showed that metformin induces PP2A activity and reduces tau phosphorylation at PP2A-dependent epitopes in vitro (2.5 mM, different cell types) and in vivo (5 mg/mL per os, WT mice) [148]. Treatment with metformin (1.6 mM, N2A cells) sensitized impaired insulin actions and also prevented the appearance of molecular and pathological characteristics observed in AD such as tau phospshorylation, GSK-3β activation, increased Aβ1-42 production and diminution of acetylcholine esterase activity [149]. In 2012, the first pre-clinical study aimed to study the effects of metformin (200 mg/kg/d, IP) administered to diabetic animals (db/db mice) for a long-time (18 weeks) on AD-like biochemical and cognitive changes in brain was performed. Metformin reportedly attenuated the increase of total tau, phospho-tau and activated c-jun N-terminal kinase (JNK) as well as Aβ levels in the hippocampus, in agreement with previous findings [147, 149]. Despite these changes, metformin did not attenuate the impairments of spatial learning and memory [150]. In another study, high fat-fed animals developed insulin resistance and an impairment in switching task contingency from a matching to a non-matching paradigm, highlighting impaired cognition. Metformin (144 mg/kg in diet for 10 weeks) attenuated insulin resistance and weight gain associated with high-fat feeding, but had no effect on cognitive performance. In addition, no major alterations in proteins associated with insulin signaling or synaptic function, were detected [151]. Unfortunately, the above-cited studies did not evaluate OS levels, given the observed metformin-induced improvement of the AD-like changes suggested a possible positive impact also on the OS levels. Indeed, it was observed in GK rats (a model of diabetes) treated with metformin for 4 weeks a significant decrease in TBARS and MDA levels as well as glutathione peroxidase (GPx) activity together with a significant increase in GSH levels and MnSOD activity. These results suggest that metformin protects against diabetes-associated OS implying that metformin might be an effective neuroprotective agent [152]. Similarly, in high fat-diet fed rats, metformin (15mg/kg BW twice daily, for 21days) significantly attenuated the insulin resistant condition by improving metabolic parameters, decreasing peripheral and brain OS levels, and improving learning behavior, compared to the vehicle-treated group [153]. Furthermore, metformin completely prevented brain mitochondrial dysfunction caused by long-term high fat-diet consumption [153]. In another study, chronic metformin administration prevented memory impairment induced by administration of L-methionine and these changes were associated with a restoration of the antioxidant defenses (significant decrease in GSSG and TBARs, along with elevation in GSH/GSSG ratio and activities of catalase and GPx) [154]. In light of these results, it clearly appears that both dose and time-of-treatment have to be carefully analyzed in order to understand the therapeutic window giving better outcomes. However, with regard to the translation from animals to human, in 2012 and 2013, two population-based case-control studies reported a higher risk of AD in long-term diabetic users of metformin [155, 156], thus questioning the effective protective role for this drug. Consequently, both pre-clinical and clinical of metformin suggest further investigations are necessary to fully determine whether thus mTOR inhibition has promise in treatment AD patients with diabetes.
4.2 Sulfonylureas
Sulphonylureas lower blood glucose by inhibiting the KATP channel of the pancreatic beta cells and thus stimulating insulin secretion and its levels in the blood [157]. Glibenclamide (0.3 mg/kg per os, for 3 weeks) reduced hyper-phosphorylated tau protein levels in i.c.v.-Aβ administered rats [158]. Furthermore, glibenclamide (1mg/kg, i.p.) decreased lipid peroxidation and myeloperoxidase activity accompanied by increased glutathione levels and total antioxidant capacity in the hippocampus of rats that had undergone ischemic-reperfusion injury, highlighting a potential therapeutic utility for this sulphonylurea in brain injury via modulating OS and inflammatory mediators [159]. In another study, glicazide (10 mg/kg, for 21 days) decreased the total oxidant index and increased the total antioxidant defense in the brain of diabetic rats, thus shifting the oxidant/antioxidant balance towards the antioxidant status. This study was the first experimental research demonstrating the effectiveness of gliclazide to protect diabetes-induced OS in brain of diabetic rats [160]. In contrast to metformin, long-term use of sulfonylureas was not associated with an altered risk of developing AD in humans [155].
4.3 Thiazolidinediones
Thiazolidinediones (TZDs), are currently among the two classes of oral hypoglycemic agents – the other is the class of biguanides – that serve as insulin-sensitizers for the treatment of T2DM. While the precise mechanism of action for the broad metabolic effects of TZDs remains unclear, there is a consensus that TZDs depend upon the activation of peroxisomal proliferator-activated receptor gamma (PPARγ) nuclear receptors that are most abundant in adipose tissues but are also present in liver, macrophages and other immune cells, pancreatic beta-cells, bone, and many other tissues [161].
In 2000, it was reported that troglitazone (20–50 μM) prevented both the activation of microglia and the expression of the IL-6, TNF-alpha and those of cycloxygenase-2 following Aβ-stimulated proinflammatory response, thereby attenuating the activation of both microglia and astrocytes and the consequent elaboration of neurotoxins [162]. Notwithstanding that troglitazone was withdrawn due to hepatitis and liver damage risk, this drug represented the first study about the possible neuroprotective role of thiazolidinediones.
With regard the two most prescribed thiazolidinediones, pioglitazone and rosiglitazone, data available in literature are not completely consistent between pre-clinical and clinical outcomes, mainly due to differences in doses and time of the treatment. Pioglitazone (20 mg/kg/d, for 16 weeks) only modestly reduced SDS-soluble Aβ levels and did not affect amyloid plaque burden or microglia activation in 11-month old Tg2576 mice, suggesting that PPARγ activation does not greatly affect Aβ levels [163]. Similarly, in a murine model of AD, pioglitazone (120 mg/g/d, for 2 months) decreased cerebral glucose utilization, thus suggesting that it does not act as a metabolic enhancer in AD [164], but rather, it would increase neurotoxicity in the brain, maybe due, at least in part, to a glucose-dependent generation of ROS [39, 165]. Other studies, in contrast, reported major neuroprotective effects following pioglitazone administration, mainly based on reduced glial activation coupled with Aβ reduction-associated cognitive improvement. In transgenic APP mice, pioglitazone (20 mg/kg/d, for 4 weeks) fully restored cerebrovascular reactivity of isolated cerebral arteries concomitantly with changes in proteins regulating OS, without reducing brain Aβ levels or plaque load [166]. Indeed, pioglitazone significantly attenuated astroglial activation and improved, albeit not significantly, reduced cortical cholinergic innervation [166]. In addition, pioglitazone completely normalized cerebral blood flow and the cerebral glucose utilization responses to increased neuronal activity, but it failed to improve spatial memory [166]. These results were the first to demonstrate that late pharmacological intervention with pioglitazone not only overcomes cerebrovascular dysfunction and altered neurometabolic coupling in aged APP mice, but also counteracts cerebral OS, glial activation, and, partly, cholinergic denervation [166]. Conversely, in 10-month-old APPV717I transgenic mice that received an oral treatment with either standard chow or chow containing pioglitazone (40 mg/kg/d, for 7 days), this latter not only reduced glial activation and BACE1 protein levels, but also Aβ42-positive amyloid deposits and their respective staining intensity as well as soluble Aβ42 in the hippocampus and frontal cortex [167]. Because only ~18% of orally administered pioglitazone crosses the intact blood–brain barrier in mammals [168], it seems likely that drug dosage, rather than treatment duration, is critical to observe drug effects in the brain. Recently, pioglitazone both in APP/PS1 mice (80 mg/kg/d, for 9 days) and in the triple transgenic mouse model of AD with accelerated amyloid-β (Aβ) deposition and tau pathology (3xTg-AD, 18 mg/Kg/d, for 4 months), promoted multiple beneficial effects, including improved learning, reduced serum cholesterol, decreased hippocampal amyloid-β and tau deposits, and enhanced short- and long-term plasticity [169, 170]. Furthermore, pioglitazone (20 and 40 mg/kg, i.p., for 21 days), showed significant dose-dependent improvement in scopolamine-induced dysfunctions in long-term and visuo-spatial memory in passive avoidance and Morris water maze tests, respectively [171]. The same treatment significantly prevented the fall in GSH levels and elevation in brain MDA levels induced by scopolamine. These results demonstrate that pioglitazone offers protection against scopolamine-induced dysfunctions in long-term and visuo-spatial memory, possibly due to its antioxidant action, and potentially could have a therapeutic utility in AD [171].
Reduction of the OS levels was also reported in the hippocampus and cerebral cortex of fructose-drinking insulin resistance rats in which a significant decrease of ROS, TBARS and protein carbonyls levels together with an increase of both GSH levels and SOD, CAT and GPx activities, were observed following pioglitazone administration (10 mg/kg, for 12 weeks) [172]. Decreased OS levels were paralleled by a partial improvement of the cognitive impairment, suggesting that the neuroprotective effects mediated by this drug can be due, at least in part, to an improvement of antioxidant defenses [172]. Consistent with this notion, pioglitazone (20 mg/kg/d) inhibited iron-induced α-synuclein aggregation, elevation in interleukin-1β and interleukin-6 mRNA levels as well as increases in HO-1, COX-2, NOS and ED-1 protein levels, indicators of activated microglia. Moreover, iron-induced DNA laddering as well as activation of ER and mitochondrial pathways were attenuated. In addition, pioglitazone decreased iron-induced elevation in lipid peroxidation in the substantia nigra. Due to the reasons discussed in the previous section, these results emphasize the importance that iron metabolism and HO-1 activity dysregulation have in AD pathology and suggest pioglitazone may be a novel therapeutic approach to investigate with regard to is effects on iron and HO-1.
Studies in humans, reported an overall improvement of cognitive function, whose related molecular mechanism in the brain are not known because the lack of biochemical analyses. Indeed, in a prospective randomized, open-controlled study, MCI and AD patients treated with pioglitazone (15–30 mg/d, for 6 months) showed significantly decreased ADAS-Jcog scores and increased WMS-R logical memory-I scores, thus suggesting an improvement in cognitive function [173]. In another study, AD patients also diagnosed with T2DM treated with pioglitazone (15–30 mg/d, for 6 months) showed improved cognition together with an increase of the plasma Aβ40/Aβ42 ratio, and a decrease in fasting plasma insulin levels, this latter parameter indicating enhanced insulin sensitivity [174].
With regard to rosiglitazone, it was reported an attenuation of progression of insulin resistance in Tg2576 mice with age to the rate occurring in wild-type when treated with this drug (30 mg/kg/d, for 4 months) [175]. In the same animal model, rosiglitazone administration (30 mg/kg/d for 7 months) fostered better spatial learning and memory abilities together with a reduction in: (i) insulin-degrading enzyme (IDE) mRNA and activity; and (ii) Aβ42 levels without affecting amyloid deposition in the brain [176]. The beneficial effects on learning and memory were also observed in another model of AD, the transegenic hAPPSwe-Ind, in which rosiglitazone (5 mg/kg/d, for 10 weeks) reversed memory decline along with an increased expression of the glucocorticoid receptor in the hippocampus [177]. The normalization of glucocorticoid receptors levels by rosiglitazone may contribute to the beneficial effect on cognition of this potent PPARγ agonist by restoring the physiological control of the HPA axis. In the same transegenic hAPPSwe-Ind animal model, rosiglitazone (5 mg/kg/d, for 4 months) ameliorated memory deficits, promoted glial activation, reduced brain Aβ levels, plaque deposition and p-tau aggregates [178]. The observed reduction in amyloid and tau pathology after rosiglitazone could be because of an increase in the brain clearance ability, consistent with a positive effect also on OS markers [178]. Reduction of Aβ levels was also observed in APP/PS1 mice that underwent rosiglitazone treatment (6 mg/kg/d, for 4 weeks) [179]. In contrast to pioglitazone, rosiglitazone induced neuronal mitochondrial biogenesis and improved glucose utilization leading to improved cellular function [180]. However, results in animals do not completely parallel with clinical observations, in particular for cognitive improvement. In a placebo-controlled, double-blind, parallel-group pilot study, subjects receiving rosiglitazone (4 mg/d for 6 months) exhibited better delayed recall and selective attention [181]. Conversely, exploratory analyses in humans suggested that 2, 4 and 8 mg daily had no statistically significant treatment effect on cognition as measured by ADAS-Cog. However, APOE epsilon4 non-carriers exhibited cognitive and functional improvement in response to rosiglitazone, whereas APOE epsilon4 allele carriers showed no improvement and some decline was noted [182]. In a double-blind, randomized, placebo-controlled study, rosiglitazone mono-therapy (2–8 mg/d, for 24 weeks), did not show any improvement in cognition or global functions in mild-to-moderate AD patients [183]. Consequently the utility of TDZs for treatment of AD remains to be proven.
4.4 Insulin
Insulin is a peptide hormone produced by β cells in the pancreas that is central for regulation of carbohydrate and fat metabolism as well as body weight and food intake. However, as cited above, insulin is also produced directly in the brain, thus opening questions about its physiological relevance. Indeed, the role of insulin signaling in the regulation of learning, and memory formation in the human and rodent brain is a matter of controversy. The conflicting information on insulin action in the CNS arises from difficulties in dissecting the direct actions of insulin in the brain from the effects resulting from hypoglycemia after peripheral administration of insulin [34]. Given that IRs are widely expressed in the hippocampus, the most important brain region for learning and memory, it is plausible that decline of IR signaling leads to cognitive impairment [184, 185]. Importantly, high levels of markers of insulin resistance were associated with poor performance on tests of working and episodic memory, independent of levels of β-amyloid and neurofibrillary tangles, suggesting that insulin signaling has a direct effect on cognitive status. This observation may help to explain the finding that adults with either amnestic MCI or non-amnestic MCI (the latter group being presumed not to have prodromal AD) showed increased basal levels of IRS-1 serine kinases [143]. Based on the observation of defective insulin signaling in the brain, it was proposed that insulin treatment could rescue insulin resistance and improves cognitive function. Among the possible mechanisms involved in the neuroprotective effects of insulin, decreasing OS levels received great attention. A number of studies highlighted this aspect and for this reason we will limit our analysis to the major achievements obtained with regard to AD. In 2005 Moreira et al. showed that in the brain of streptozotocin (STZ)-treated rats, insulin (5–20 UN/kg, for 4 weeks) prevented the decline in mitochondrial oxidative phosphorylation efficiency and prevented increased OS, improving or preserving the function of neurons under adverse conditions, such as AD [186]. In addition, insulin (0.1 and 10 microM) prevented the decrease in neuronal viability mediated by OS, decreasing both necrotic and apoptotic cell death [187]. Indeed, insulin inhibited ascorbate/Fe2+-mediated lipid and protein oxidation as well as decline of the GSH/GSSG levels. Interestingly, increased HNE adducts on GLUT3 glucose transporters upon exposure to ascorbate/Fe2+ were also prevented by insulin, suggesting that this peptide can interfere with glucose metabolism. Inhibition of PI3K or MEK reversed the effect of insulin on the GSH/GSSG ratio, suggesting the activation of insulin-mediated signaling pathways [187]. The same authors showed that in rat primary cortical neurons insulin neuroprotection against OS-mediated damage involves: 1) stimulation of glucose uptake and metabolism, increasing energy levels and intracellular adenosine and, ultimately, uric acid formation; and 2) a decrease in extracellular adenosine, which may reduce the facilitatory activity of adenosine receptors [188]. Further, it was shown that insulin (0.1–10 μM) prevented OS-induced increased GPx-1 and decreased hexokinase-II expression in rat primary cortical neurons, supporting previous findings of changes in glutathione redox cycle and glycolysis [189]. Moreover, insulin precluded Bcl-2 decreased expression and caspase-3 increased expression. Consistent with these results, insulin abolished caspase-3 activity and DNA fragmentation caused by OS [189]. Thus, insulin-mediated activation of IR/IGF-1R stimulates PI3K/Akt and inhibits GSK-3beta signaling pathways, modifying neuronal antioxidant defense-, glucose metabolism- and anti-apoptotic-associated protein synthesis [189]. In 2009, De Felice et al. reported for the first time that 100 nM insulin partially prevented and 1 μM insulin completely blocked Aβ-derived diffusible ligands-induced loss of dendritic IRs in hippocampal cultures and IR accumulation in the cell body together with a reduction of neuronal ROS [190].
In 2008, low doses of IGF-1 (2.25 μg/100 g/d, for 1 month) restored circulating IGF-1, improved glucose and lipid metabolism, increased testosterone levels and serum total antioxidant capability, and reduced oxidative damage in brain and liver of aging rats together with a normalization of antioxidant enzyme activities and mitochondrial function [191, 192], suggesting that not only insulin but also IGF-1 as therapeutic targets to combat insulin resistance. With regard to the mechanisms involved in insulin/IGF-1-mediated neuroprotection, insight came from transgenic mouse overexpressing IGF-1, which showed reduced levels of oxidized proteins in the brain associated with an activation of the proteasome via processes involving the PI3K/mTOR axis [193]. These data suggest that appropriate levels of IGF-1 may be important for the elimination of oxidized proteins in the brain in a process mediated by activation of the proteasome and are consistent with findings by us and others suggesting the inactivation of proteasome in AD [113, 194].
However, despite a majority of results suggested a neuroprotective role of insulin treatment, some reports highlighted insulin toxicity. Indeed, while effectively attenuating neuronal apoptosis in mouse cortical culture, insulin paradoxically induced neuronal necrosis with 48 h of exposure, and these effects were blocked by an antioxidant [195]. Similarly, insulin (10–100 ng/mL) could act as anti-apoptosis factor and pro-oxidant depending upon the activation of PI3K in cortical neurons [196]. In addition, ascorbate/Fe2+-induced increased TBARSs was similar in synaptosomal fractions obtained from the brain of Wistar and diabetic GK rats and was not reverted by insulin (1 μM), suggesting that other mechanisms, rather than a direct effect on membrane lipid peroxidation may mediate insulin neuroprotection [197].
On the other hand, due to its strong effects on glucose uptake, insulin could promote a drastic decrease in glucose levels, clearly not a desired result. An intriguing study in mice reported that the severity of hypoglycemia induced by intraperitoneal administration of insulin at a dose 24 IU/kg was associated with the levels of MDA, Cu,Zn-SOD and GPx [198]. The results showed that in severe hypoglycemia (serum glucose concentration below 1.0 mM) the lipoperoxidation in brain tissue expressed as the level of MDA was higher in comparison with normoglycemic controls (glycemia around 3.7 mM) as well as in comparison with the levels of MDA during moderate hypoglycemia (glycemia ranging between 1–2 mM). This indicates the enhancement of lipoperoxidation in the brain tissue during severe hypoglycemia [198].
As enhanced brain insulin signaling improves memory processes and possesses neuroprotective properties, increasing brain insulin concentrations in AD patients could be a promising approach to prevent or slow the progression of this devastating disease. In order to avoid the side effects associated with peripheral hypoglycemia, intranasal insulin (INI) has been proposed as a safe treatment for neurodegenerative disorders, including AD [199]. Mechanisms of INI for bypassing the BBB to reach the brain have been reviewed [199]. INI reaches the CSF in 10 min without meaningful absorption into the circulation [200] and reaches the brain in a fully functionally active condition [201]. Craft and co-workers used INI in patients with amnestic MCI and AD to demonstrate improved delay memory recall that was associated with changed CSF Aβ42 levels and tau protein-to-Aβ42 ratios [143]. Compared to placebo controls, INI-treated patients showed improved glucose utilization based on F-18DG PET studies [143]. Whether the INI treatment that was associated with positive effects on cognitive function also was associated with a reduction of OS levels in the brain, is something not yet established, and studies on this topic are currently ongoing in our laboratory.
4.5 GLP-1 agonist and DPP-4 inhibitors
Glucagon-like peptide 1 (GLP-1) is an endogenous insulinotropic hormone that has an important role in the balance between insulin and glucose levels [202]. Indeed, GLP-1 is secreted by intestinal endocrine L-cells in response to ingestion of nutrients, and among its functions GLP-1: (i) stimulates glucose-dependent insulin secretion and insulin biosynthesis in the pancreatic beta-cells; (ii) inhibits glucagon secretion in the pancreatic alpha-cells; and (iii) slows gastric emptying thus prolonging satiety [202]. Interestingly, GLP-1 also is expressed in neurons and acts as a neurotransmitter [203]. Building on this background, GLP-1 mimetics have been developed as a treatment for T2DM, but it became apparent that these drugs possess a number of other physiological properties such as neuroprotective and anti-inflammatory effects, which may be useful to slow AD progression [204]. Because GLP-1 is quickly metabolized by the activity of the serine protease dipeptidyl peptidase 4 (DPP-4), which limits GLP-1 actions [205–208], synthetic GLP-1 analogs such as exendin-4, liraglutide, and lixisenatide were developed to be resistant to DPP-4. Indeed, with regard to the anti-diabetic drugs previously discussed, GLP-1 agonists showed improved passage through the BBB similar to GLP-1 and a great ability to bind to the GLP-1R, thus promoting: (i) reduction of insulin resistance; (ii) neuroprotective effects; (iii) activation of neuronal stem cells; and (iv) improvement of cognitive functions [209].
With regard to the effects on OS of GLP-1 or its analogues, only a few results are available, mainly on the effects mediated by GLP-1 or exedin-4. In 2003 it was reported that both GLP-1 and exedin-4 infused ICV in the brain of db/db mice [GLP-1 (3.3–6.6 ng) and exedin-4 (0.2 ng) for 14d], or administered to primary cortical neurons [GLP-1 (1–20 nM) and exedin-4 (50–500 nM) for 2h] reduced either sAPP or Aβ levels that were correlated with a reduction of Aβ- or iron-induced OS-associated cell death [210].
The neuroprotective effects of exedin-4 were also demonstrated in both primary cortical neurons and SH-SY5Y cells in which exedin-4 (50–500 nM) ameliorated Aβ- and hydrogen peroxide-induced cell death [211]. In another study, both GLP-1 (50–1000 nM) and exedin-4 (50–1000 nM) increased the viability of neuronal cells under either high glucose or oxidative stress conditions in vitro, and in vivo exedin-4 treatment (10 μg/KG, ICV, for 14d) led to improved memory and cognitive performance in rats treated ICV with STZ [212].
Similarly, in vitro results showed that methylglyoxal (MG, 1 mM), known to accumulate following excessive glucose levels and to be associated with AD pathology, induced apoptosis in PC12 cells by promoting the imbalance of GSH/GSSG ratio, whereas GLP-1 (0.33–3.30 μg/ml) protected against this MG-induced apoptosis [213]. Indeed, GLP-1promoted the phosphorylation of PI3K, Akt, and mTOR, as well as the upregulation of GCLc and the restoration of the redox imbalance [213]. Inhibitors of PI3K (LY294002), Akt (Akt-I), and mTOR (rapamycin) reduced the GLP-1-induced GCLc upregulation and its protection against MG-induced PC12 apoptosis [213]. The GLP-1-induced restoration of redox balance was also attenuated by rapamycin [213]. These results suggest that the neuroprotective effects of GLP-1 could be associated with an enhancement of PI3K/Akt/mTOR/GCLc/redox signaling.
Interestingly, geniposide (50 mg/L) – a GLP-1 agonist isolated from the fruits of Gardenia jasminoides ELLIS (Rubiaceae) – increased the expression of anti-apoptotic proteins, including Bcl-2 and HO-1, to antagonize the oxidative damage in PC12 cells induced by hydrogen peroxide [214]. LY294002 (a PI3K inhibitor) inhibited the effect of geniposide by activation of MAPK, MEK and c-Raf phosphorylation in hydrogen peroxide treated PC12 cells [214]. U0126 (a selective inhibitor of MEK) also attenuated the enhancement of geniposide on Bcl-2 levels by inhibiting the phosphorylation of p90RSK in the hydrogen peroxide treated PC12 cells [214]. All these data suggest that geniposide regulates expression of anti-oxidative proteins including HO-1 and Bcl-2 by activating the transcriptor of p90RSK via MAPK signaling pathway in PC12 cells.
Lixisenatide, an effective GLP-1 receptor (GLP-1R) agonist with much longer half life than GLP-1, has been licensed in the EU as a treatment for T2DM. In 2014, Cai et al. reported for the first time the effects of lixisenatide on Aβ-induced impairments in spatial learning and memory of rats, and investigated its electrophysiological and molecular mechanisms [215]. These researchers found that lixisenatide (5 nmol/μl, ICV) treatment: (i) effectively prevented Aβ(25–35)-induced cognitive impairments; and (ii) inhibited Aβ(25–35) injection-induced activation of GSK3β, with a significant increase in the phosphorylation of Ser9 and a significant decrease in the phosphorylation of Tyr216 [215].
With regard to liraglutide, a number of studies reported that this agent (25 nM, IP, administerd with different schemes of treatment): (i) reduced total APP and Aβ levels; (ii) reduced microglia activation; (iii) increased neurogenesis; and (iv) improved cognitive function in APP/PS1 mice [216–218].
Though direct information in the case of lixisenatide or liraglutide is lacking, these results could well be consistent with an hypothesized reduction of OS in the brain of this AD-relevant mouse model. Further investigations are required to clarify this point.
Drugs able to inhibit DPP-4, such as sitagliptin, saxagliptin and vildagliptin have been shown to possess neuroprotective effects against AD-induced pathology, and studies on those molecules are ongoing. DPP-4 inhibitors such as sitagliptin (Januvia) and sitagliptin in combination with metformin (Janumet) are approved in the USA for the treatment of T2DM. Similarly, vildagliptin (Galvus), a potent and selective DPP-4 inhibitor in combination with metformin or thiazolidinedione, is approved by the European Union for the treatment of T2DM [219]. In 2010, the first study on the role of DPP-4 inhibitor in AD from D’Amico et al. showed that sitagliptin (5, 10 and 20 mg/kg, gastric gavage, for 12w): (i) counteracted the memory impairment in a contextual fear conditioning test; (ii) increased the brain levels of GLP-1; (iii) produced significant reductions of nitrosative stress and inflammation hallmarks within the brain; and (iv) led to a significant diminution in the ultimate number and total area of βAPP and Aβ deposits in APP/PS1 mice [220].
Saxagliptin (0.25, 0.5 and 1 mg/kg, for 60d) exerted complete reversal of cognitive deficits that may be attributed to its unique mechanism of lowering amyloid load, tau phosphorylation and inflammation in the brain of STZ-induced AD rats, results consistent with the notion that saxagliptin has neuroprotective properties [221]. In the same animal model, vildagliptin (2.5, 5 and 10 mg/kg, for 30d) promoted a time-dependent improvement in memory retention and a dose-dependent attenuation of Aβ, tau phosphorylation and inflammatory markers and increased GLP-1 levels [222]. As some DPP-4 inhibitors are already in the market for the treatment of T2DM, they are considered safe for chronic use. However, further investigation is required to elucidate the neurochemical and molecular mechanisms involved in the DPP-4-induced neuroprotective effects.
Finally, because all of these results were obtained both in vitro and in vivo pre-clinical studies, it might be interesting to go further with RCTs in order to determine whether such GLP-1 mimetics- and DPP-4 inhibitors-associated effects are applicable in humans. In that regard it would be helpful to keep in mind the results obtained in Parkinson disease that showed, in the first clinical trial designed to analyze the effects of exedin-4 in human brain, exenatide-4-treated patients had a mean improvement at 12 months on the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) of 2.7 points, compared with mean decline of 2.2 points in control patients [223] and that these motor and cognitive advantages persisted 12 months after exenatide exposure [224].
5. Conclusion
Epidemiological studies evince a strong link between T2DM and risk of developing AD. The precise mechanism by which this enhanced risk is conferred remains unclear, but may coalesce around the elevated oxidative stress in both T2DM and AD. The roles of radical damage to key proteins in the insulin signaling pathways and to other key proteins in brain 0may contribute to the elevated risk of T2DM patients developing AD. More research into these topics is warranted given the explosive increase in T2DM in Western cultures, even among younger individuals.
Therapies for T2DM often appear to be beneficial in animal models of diabetes, but do not seem to translate into human trials of AD patients, with perhaps one exception: intranasal insulin. It is our view that further work into INI treatment to prevent or slow progression of amnestic MCI is needed and important to pursue. The role of oxidative stress and its modulation by INI in unique mouse models of AD and T2DM are being investigated in our laboratory.
Table 1.
Main outcomes obtained in both pre-clinical and clinical studies by using anti-diabetic drugs to slow/prevent AD progression
| Classes of Drugs | Active Principle | Main Outcomes in AD |
|---|---|---|
| Metformin | Metformin |
|
| Sulfonylureas | Glibenclamide Glicazide |
|
| Thiazolidinediones | Troglitazone Pioglitazone Rosiglitazone |
|
| Insulin | Insulin IGF-1 |
|
| GLP-1 and GLP-1 mimetics | GLP-1 Exendin-4 Liraglutide Lixisenatide Geniposide |
|
| DPP-4 inhibitors | Sitagliptin Saxagliptin Vildagliptin |
|
HIGHLIGHTS.
Type 2 diabetes mellitus is a major risk factor for Alzheimer disease.
Insulin resistance may be an intersection point between T2DM and AD.
Oxidative stress may be a driving force for insulin resistance in AD.
Several therapeutic approaches target oxidative stress and insulin resistance in AD.
Acknowledgments
This was supported by NIH grant AG-05119 to D.A.B. and funding from the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme (FP7/2007–2013) under REA grant agreement n° 624341 to E.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Mayeux R. Clinical practice. Early Alzheimer’s disease. N Engl J Med. 2010;362:2194–2201. doi: 10.1056/NEJMcp0910236. [DOI] [PubMed] [Google Scholar]
- 3.Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB. Diabetes mellitus and Alzheimer’s disease: shared pathology and treatment? Br J Clin Pharmacol. 2011;71:365–376. doi: 10.1111/j.1365-2125.2010.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ott A, Stolk RP, Hofman A, van Harskamp F, Grobbee DE, Breteler MM. Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia. 1996;39:1392–1397. doi: 10.1007/s001250050588. [DOI] [PubMed] [Google Scholar]
- 5.Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 6.Ronnemaa E, Zethelius B, Sundelof J, Sundstrom J, Degerman-Gunnarsson M, Berne C, Lannfelt L, Kilander L. Impaired insulin secretion increases the risk of Alzheimer disease. Neurology. 2008;71:1065–1071. doi: 10.1212/01.wnl.0000310646.32212.3a. [DOI] [PubMed] [Google Scholar]
- 7.Morris JK, Vidoni ED, Honea RA, Burns JM Alzheimer’s Disease Neuroimaging, I. Impaired glycemia increases disease progression in mild cognitive impairment. Neurobiology of aging. 2014;35:585–589. doi: 10.1016/j.neurobiolaging.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pluta R. Blood-brain barrier dysfunction and amyloid precursor protein accumulation in microvascular compartment following ischemia-reperfusion brain injury with 1-year survival. Acta Neurochir Suppl. 2003;86:117–122. doi: 10.1007/978-3-7091-0651-8_26. [DOI] [PubMed] [Google Scholar]
- 9.Sadowski M, Pankiewicz J, Scholtzova H, Li YS, Quartermain D, Duff K, Wisniewski T. Links between the pathology of Alzheimer’s disease and vascular dementia. Neurochem Res. 2004;29:1257–1266. doi: 10.1023/b:nere.0000023612.66691.e6. [DOI] [PubMed] [Google Scholar]
- 10.de la Monte SM, Wands JR. Review of insulin, insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2005;7:45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- 11.Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 12.Freude S, Schilbach K, Schubert M. The role of IGF-1 receptor and insulin receptor signaling for the pathogenesis of Alzheimer’s disease: from model organisms to human disease. Current Alzheimer research. 2009;6:213–223. doi: 10.2174/156720509788486527. [DOI] [PubMed] [Google Scholar]
- 13.Plum L, Schubert M, Bruning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab. 2005;16:59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Horwood JM, Dufour F, Laroche S, Davis S. Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci. 2006;23:3375–3384. doi: 10.1111/j.1460-9568.2006.04859.x. [DOI] [PubMed] [Google Scholar]
- 15.Chiang HC, Wang L, Xie Z, Yau A, Zhong Y. PI3 kinase signaling is involved in Abeta-induced memory loss in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7060–7065. doi: 10.1073/pnas.0909314107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 17.Lebovitz HE. Insulin resistance: definition, consequences, Experimental and clinical endocrinology & diabetes: official journal. German Society of Endocrinology [and] German Diabetes Association. 2001;109(Suppl 2):S135–148. doi: 10.1055/s-2001-18576. [DOI] [PubMed] [Google Scholar]
- 18.Cholerton B, Baker LD, Craft S. Insulin, cognition, and dementia. European journal of pharmacology. 2013;719:170–179. doi: 10.1016/j.ejphar.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banks WA, Jaspan JB, Kastin AJ. Selective, physiological transport of insulin across the blood-brain barrier: novel demonstration by species-specific radioimmunoassays. Peptides. 1997;18:1257–1262. doi: 10.1016/s0196-9781(97)00198-8. [DOI] [PubMed] [Google Scholar]
- 20.Baskin DG, Figlewicz DP, Woods SC, Porte D, Jr, Dorsa DM. Insulin in the brain. Annual review of physiology. 1987;49:335–347. doi: 10.1146/annurev.ph.49.030187.002003. [DOI] [PubMed] [Google Scholar]
- 21.Hill JM, Lesniak MA, Pert CB, Roth J. Autoradiographic localization of insulin receptors in rat brain: prominence in olfactory and limbic areas. Neuroscience. 1986;17:1127–1138. doi: 10.1016/0306-4522(86)90082-5. [DOI] [PubMed] [Google Scholar]
- 22.Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- 23.Baskin DG, Wilcox BJ, Figlewicz DP, Dorsa DM. Insulin and insulin-like growth factors in the CNS. Trends in neurosciences. 1988;11:107–111. doi: 10.1016/0166-2236(88)90155-5. [DOI] [PubMed] [Google Scholar]
- 24.Havrankova J, Schmechel D, Roth J, Brownstein M. Identification of insulin in rat brain. Proceedings of the National Academy of Sciences of the United States of America. 1978;75:5737–5741. doi: 10.1073/pnas.75.11.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghasemi R, Haeri A, Dargahi L, Mohamed Z, Ahmadiani A. Insulin in the brain: sources, localization and functions. Molecular neurobiology. 2013;47:145–171. doi: 10.1007/s12035-012-8339-9. [DOI] [PubMed] [Google Scholar]
- 26.Freiherr J, Hallschmid M, Frey WH, 2nd, Brunner YF, Chapman CD, Holscher C, Craft S, De Felice FG, Benedict C. Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS drugs. 2013;27:505–514. doi: 10.1007/s40263-013-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghasemi R, Dargahi L, Haeri A, Moosavi M, Mohamed Z, Ahmadiani A. Brain insulin dysregulation: implication for neurological and neuropsychiatric disorders. Molecular neurobiology. 2013;47:1045–1065. doi: 10.1007/s12035-013-8404-z. [DOI] [PubMed] [Google Scholar]
- 28.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. Journal of Alzheimer’s disease: JAD. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 29.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? Journal of Alzheimer’s disease: JAD. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 30.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation and cognitive decline. The Journal of clinical investigation. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoyer S. Is sporadic Alzheimer disease the brain type of non-insulin dependent diabetes mellitus? A challenging hypothesis. Journal of neural transmission. 1998;105:415–422. doi: 10.1007/s007020050067. [DOI] [PubMed] [Google Scholar]
- 32.de la Monte SM. Contributions of brain insulin resistance and deficiency in amyloid-related neurodegeneration in Alzheimer’s disease. Drugs. 2012;72:49–66. doi: 10.2165/11597760-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talbot K, Wang HY. The nature, significance, and glucagon-like peptide-1 analog treatment of brain insulin resistance in Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2014;10:S12–25. doi: 10.1016/j.jalz.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson GS, Craft S. Modulation of memory by insulin and glucose: neuropsychological observations in Alzheimer’s disease. European journal of pharmacology. 2004;490:97–113. doi: 10.1016/j.ejphar.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz MW, Figlewicz DF, Kahn SE, Baskin DG, Greenwood MR, Porte D., Jr Insulin binding to brain capillaries is reduced in genetically obese, hyperinsulinemic Zucker rats. Peptides. 1990;11:467–472. doi: 10.1016/0196-9781(90)90044-6. [DOI] [PubMed] [Google Scholar]
- 36.Wallum BJ, Taborsky GJ, Jr, Porte D, Jr, Figlewicz DP, Jacobson L, Beard JC, Ward WK, Dorsa D. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. The Journal of clinical endocrinology and metabolism. 1987;64:190–194. doi: 10.1210/jcem-64-1-190. [DOI] [PubMed] [Google Scholar]
- 37.de la Monte SM. Relationships Between Diabetes and Cognitive Impairment. Endocrinology and metabolism clinics of North America. 2014;43:245–267. doi: 10.1016/j.ecl.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreira PI. Alzheimer’s disease and diabetes: an integrative view of the role of mitochondria, oxidative stress, and insulin. Journal of Alzheimer’s disease: JAD. 2012;30(Suppl 2):S199–215. doi: 10.3233/JAD-2011-111127. [DOI] [PubMed] [Google Scholar]
- 39.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The international journal of biochemistry & cell biology. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Droge W. Free radicals in the physiological control of cell function. Physiological reviews. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 41.De Felice FG. Alzheimer’s disease and insulin resistance: translating basic science into clinical applications. The Journal of clinical investigation. 2013;123:531–539. doi: 10.1172/JCI64595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butterfield DA. Oxidative stress in neurodegenerative disorders. Antioxidants & redox signaling. 2006;8:1971–1973. doi: 10.1089/ars.2006.8.1971. [DOI] [PubMed] [Google Scholar]
- 43.Di Domenico F, Perluigi M, Butterfield DA, Cornelius C, Calabrese V. Oxidative damage in rat brain during aging: interplay between energy and metabolic key target proteins. Neurochemical research. 2010;35:2184–2192. doi: 10.1007/s11064-010-0295-z. [DOI] [PubMed] [Google Scholar]
- 44.Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends in molecular medicine. 2001;7:548–554. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- 45.Dominguez RO, Marschoff ER, Gonzalez SE, Repetto MG, Serra JA. Type 2 diabetes and/or its treatment leads to less cognitive impairment in Alzheimer’s disease patients. Diabetes research and clinical practice. 2012;98:68–74. doi: 10.1016/j.diabres.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 46.Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free radical biology & medicine. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 47.Butterfield DA, Sultana R. Redox proteomics: understanding oxidative stress in the progression of age-related neurodegenerative disorders. Expert review of proteomics. 2008;5:157–160. doi: 10.1586/14789450.5.2.157. [DOI] [PubMed] [Google Scholar]
- 48.Butterfield DA, Perluigi M, Sultana R. Oxidative stress in Alzheimer’s disease brain: new insights from redox proteomics. European journal of pharmacology. 2006;545:39–50. doi: 10.1016/j.ejphar.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 49.Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free radical biology & medicine. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 50.Butterfield DA, Liqing G, Di Domenico F, Robinson RAS. Mass spectrometry and redox proteomics: Application in disease. Mass Spectrometry Reviews. 2014 doi: 10.1002/mas.21374. [DOI] [PubMed] [Google Scholar]
- 51.de la Monte SM. Insulin resistance and Alzheimer’s disease. BMB reports. 2009;42:475–481. doi: 10.5483/bmbrep.2009.42.8.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao WQ, Townsend M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochimica et biophysica acta. 2009;1792:482–496. doi: 10.1016/j.bbadis.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 53.Craft S. Insulin resistance syndrome and Alzheimer’s disease: age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiology of aging. 2005;26(Suppl 1):65–69. doi: 10.1016/j.neurobiolaging.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 54.Reddy VP, Zhu X, Perry G, Smith MA. Oxidative stress in diabetes and Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2009;16:763–774. doi: 10.3233/JAD-2009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerbitz KD, Gempel K, Brdiczka D. Mitochondria and diabetes. Genetic, biochemical, and clinical implications of the cellular energy circuit. Diabetes. 1996;45:113–126. doi: 10.2337/diab.45.2.113. [DOI] [PubMed] [Google Scholar]
- 56.Moreira PI, Santos MS, Selca R, Oliveira CR. Brain mitochondrial dysfunction as a link between Alzheimer’s disease and diabetes. J Neurol Sci. 2007;257:206–214. doi: 10.1016/j.jns.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 57.Neumann KF, Rojo L, Navarrete LP, Farias G, Reyes P, Maccioni RB. Insulin resistance and Alzheimer’s disease: molecular links & clinical implications. Current Alzheimer research. 2008;5:438–447. doi: 10.2174/156720508785908919. [DOI] [PubMed] [Google Scholar]
- 58.Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007;14:1729–1738. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- 60.de la Monte SM, Tong M. Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochemical pharmacology. 2014;88:548–559. doi: 10.1016/j.bcp.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 62.Orth M, Schapira AH. Mitochondria and degenerative disorders. American journal of medical genetics. 2001;106:27–36. doi: 10.1002/ajmg.1425. [DOI] [PubMed] [Google Scholar]
- 63.Correia SC, Santos RX, Santos MS, Casadesus G, Lamanna JC, Perry G, Smith MA, Moreira PI. Mitochondrial abnormalities in a streptozotocin-induced rat model of sporadic Alzheimer’s disease. Current Alzheimer research. 2013;10:406–419. doi: 10.2174/1567205011310040006. [DOI] [PubMed] [Google Scholar]
- 64.Santos RX, Correia SC, Alves MG, Oliveira PF, Cardoso S, Carvalho C, Duarte AI, Santos MS, Moreira PI. Insulin therapy modulates mitochondrial dynamics and biogenesis, autophagy and tau protein phosphorylation in the brain of type 1 diabetic rats. Biochimica et biophysica acta. 2014;1842:1154–1166. doi: 10.1016/j.bbadis.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 65.Carvalho C, Cardoso S, Correia SC, Santos RX, Santos MS, Baldeiras I, Oliveira CR, Moreira PI. Metabolic alterations induced by sucrose intake and Alzheimer’s disease promote similar brain mitochondrial abnormalities. Diabetes. 2012;61:1234–1242. doi: 10.2337/db11-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carvalho C, Machado N, Mota PC, Correia SC, Cardoso S, Santos RX, Santos MS, Oliveira CR, Moreira PI. Type 2 diabetic and Alzheimer’s disease mice present similar behavioral, cognitive, and vascular anomalies. Journal of Alzheimer’s disease: JAD. 2013;35:623–635. doi: 10.3233/JAD-130005. [DOI] [PubMed] [Google Scholar]
- 67.James D, Kang S, Park S. Injection of beta-amyloid into the hippocampus induces metabolic disturbances and involuntary weight loss which may be early indicators of Alzheimer’s disease. Aging clinical and experimental research. 2014;26:93–98. doi: 10.1007/s40520-013-0181-z. [DOI] [PubMed] [Google Scholar]
- 68.Carvalho C, Katz PS, Dutta S, Katakam PV, Moreira PI, Busija DW. Increased susceptibility to amyloid-beta toxicity in rat brain microvascular endothelial cells under hyperglycemic conditions. Journal of Alzheimer’s disease: JAD. 2014;38:75–83. doi: 10.3233/JAD-130464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lesort M, Johnson GV. Insulin-like growth factor-1 and insulin mediate transient site-selective increases in tau phosphorylation in primary cortical neurons. Neuroscience. 2000;99:305–316. doi: 10.1016/s0306-4522(00)00200-1. [DOI] [PubMed] [Google Scholar]
- 70.Lesort M, Jope RS, Johnson GV. Insulin transiently increases tau phosphorylation: involvement of glycogen synthase kinase-3beta and Fyn tyrosine kinase. Journal of neurochemistry. 1999;72:576–584. doi: 10.1046/j.1471-4159.1999.0720576.x. [DOI] [PubMed] [Google Scholar]
- 71.Schechter R, Beju D, Miller KE. The effect of insulin deficiency on tau and neurofilament in the insulin knockout mouse. Biochemical and biophysical research communications. 2005;334:979–986. doi: 10.1016/j.bbrc.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 72.Schubert M, Brazil DP, Burks DJ, Kushner JA, Ye J, Flint CL, Farhang-Fallah J, Dikkes P, Warot XM, Rio C, Corfas G, White MF. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:7084–7092. doi: 10.1523/JNEUROSCI.23-18-07084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang JZ, Wu Q, Smith A, Grundke-Iqbal I, Iqbal K. Tau is phosphorylated by GSK-3 at several sites found in Alzheimer disease and its biological activity markedly inhibited only after it is prephosphorylated by A-kinase. FEBS letters. 1998;436:28–34. doi: 10.1016/s0014-5793(98)01090-4. [DOI] [PubMed] [Google Scholar]
- 74.El Khoury NB, Gratuze M, Papon MA, Bretteville A, Planel E. Insulin dysfunction and Tau pathology. Frontiers in cellular neuroscience. 2014;8:22. doi: 10.3389/fncel.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takalo M, Haapasalo A, Martiskainen H, Kurkinen KM, Koivisto H, Miettinen P, Khandelwal VK, Kemppainen S, Kaminska D, Makinen P, Leinonen V, Pihlajamaki J, Soininen H, Laakso M, Tanila H, Hiltunen M. High-fat diet increases tau expression in the brain of T2DM and AD mice independently of peripheral metabolic status. The Journal of nutritional biochemistry. 2014;25:634–641. doi: 10.1016/j.jnutbio.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 76.de la Monte SM. Triangulated mal-signaling in Alzheimer’s disease: roles of neurotoxic ceramides, ER stress, and insulin resistance reviewed. Journal of Alzheimer’s disease: JAD. 2012;30(Suppl 2):S231–249. doi: 10.3233/JAD-2012-111727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han W, Li C. Linking type 2 diabetes and Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6557–6558. doi: 10.1073/pnas.1002555107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gasparini L, Gouras GK, Wang R, Gross RS, Beal MF, Greengard P, Xu H. Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:2561–2570. doi: 10.1523/JNEUROSCI.21-08-02561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duarte AI, Santos MS, Oliveira CR, Rego AC. Insulin neuroprotection against oxidative stress in cortical neurons - Involvement of uric acid and glutathione antioxidant defenses. Free Radical Bio Med. 2005;39:876–889. doi: 10.1016/j.freeradbiomed.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Hong F, Kwon SJ, Jhun BS, Kim SS, Ha J, Kim SJ, Sohn NW, Kang C, Kang I. Insulin-like growth factor-1 protects H9c2 cardiac myoblasts from oxidative stress-induced apoptosis via phosphatidylinositol 3-kinase and extracellular signal-regulated kinase pathways. Life Sci. 2001;68:1095–1105. doi: 10.1016/s0024-3205(00)01012-2. [DOI] [PubMed] [Google Scholar]
- 81.Rensink AA, Otte-Holler I, de Boer R, Bosch RR, ten Donkelaar HJ, de Waal RM, Verbeek MM, Kremer B. Insulin inhibits amyloid beta-induced cell death in cultured human brain pericytes. Neurobiology of aging. 2004;25:93–103. doi: 10.1016/s0197-4580(03)00039-3. [DOI] [PubMed] [Google Scholar]
- 82.Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiology of aging. 2010;31:224–243. doi: 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 83.O’Neill C. PI3-kinase/Akt/mTOR signaling: impaired on/off switches in aging, cognitive decline and Alzheimer’s disease. Exp Gerontol. 2013;48:647–653. doi: 10.1016/j.exger.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 84.Perluigi M, Pupo G, Tramutola A, Cini C, Coccia R, Barone E, Head E, Butterfield DA, Di Domenico F. Neuropathological role of PI3K/Akt/mTOR axis in Down syndrome brain. Biochimica et biophysica acta. 2014 doi: 10.1016/j.bbadis.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paula-Lima AC, Adasme T, SanMartin C, Sebollela A, Hetz C, Carrasco MA, Ferreira ST, Hidalgo C. Amyloid beta-Peptide Oligomers Stimulate RyR-Mediated Ca2+ Release Inducing Mitochondrial Fragmentation in Hippocampal Neurons and Prevent RyR-Mediated Dendritic Spine Remodeling Produced by BDNF. Antioxidants & redox signaling. 2011;14:1209–1223. doi: 10.1089/ars.2010.3287. [DOI] [PubMed] [Google Scholar]
- 86.De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. A beta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 87.Zhao WQ, De Felice FG, Fernandez S, Chen H, Lambert MP, Quon MJ, Krafft GA, Klein WL. Amyloid beta oligomers induce impairment of neuronal insulin receptors. Faseb J. 2008;22:246–260. doi: 10.1096/fj.06-7703com. [DOI] [PubMed] [Google Scholar]
- 88.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annual review of medicine. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 89.Smith MA, Monnier VM, Sayre LM, Perry G. Amyloidosis, advanced glycation end products and Alzheimer disease. Neuroreport. 1995;6:1595–1596. doi: 10.1097/00001756-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 90.Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Bucala R, Manogue K, Cerami A. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:4766–4770. doi: 10.1073/pnas.91.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ko LW, Ko EC, Nacharaju P, Liu WK, Chang E, Kenessey A, Yen SH. An immunochemical study on tau glycation in paired helical filaments. Brain research. 1999;830:301–313. doi: 10.1016/s0006-8993(99)01415-8. [DOI] [PubMed] [Google Scholar]
- 92.Li JJ, Surini M, Catsicas S, Kawashima E, Bouras C. Age-dependent accumulation of advanced glycosylation end products in human neurons. Neurobiology of aging. 1995;16:69–76. doi: 10.1016/0197-4580(95)80009-g. [DOI] [PubMed] [Google Scholar]
- 93.Chaney MO, Stine WB, Kokjohn TA, Kuo YM, Esh C, Rahman A, Luehrs DC, Schmidt AM, Stern D, Yan SD, Roher AE. RAGE and amyloid beta interactions: atomic force microscopy and molecular modeling. Biochimica et biophysica acta. 2005;1741:199–205. doi: 10.1016/j.bbadis.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 94.Lue LF, Walker DG, Brachova L, Beach TG, Rogers J, Schmidt AM, Stern DM, Yan SD. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: identification of a cellular activation mechanism. Experimental neurology. 2001;171:29–45. doi: 10.1006/exnr.2001.7732. [DOI] [PubMed] [Google Scholar]
- 95.Hildreth KL, Van Pelt RE, Schwartz RS. Obesity, insulin resistance, and Alzheimer’s disease. Obesity. 2012;20:1549–1557. doi: 10.1038/oby.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blass JP, Gibson GE, Hoyer S. The role of the metabolic lesion in Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2002;4:225–232. doi: 10.3233/jad-2002-4312. [DOI] [PubMed] [Google Scholar]
- 97.Aluise CD, Robinson RA, Cai J, Pierce WM, Markesbery WR, Butterfield DA. Redox proteomics analysis of brains from subjects with amnestic mild cognitive impairment compared to brains from subjects with preclinical Alzheimer’s disease: insights into memory loss in MCI. Journal of Alzheimer’s disease: JAD. 2011;23:257–269. doi: 10.3233/JAD-2010-101083. [DOI] [PubMed] [Google Scholar]
- 98.Butterfield DA, Sultana R. Redox proteomics identification of oxidatively modified brain proteins in Alzheimer’s disease and mild cognitive impairment: insights into the progression of this dementing disorder. Journal of Alzheimer’s disease: JAD. 2007;12:61–72. doi: 10.3233/jad-2007-12107. [DOI] [PubMed] [Google Scholar]
- 99.Butterfield DA, Gnjec A, Poon HF, Castegna A, Pierce WM, Klein JB, Martins RN. Redox proteomics identification of oxidatively modified brain proteins in inherited Alzheimer’s disease: an initial assessment. Journal of Alzheimer’s disease: JAD. 2006;10:391–397. doi: 10.3233/jad-2006-10407. [DOI] [PubMed] [Google Scholar]
- 100.Sultana R, Poon HF, Cai J, Pierce WM, Merchant M, Klein JB, Markesbery WR, Butterfield DA. Identification of nitrated proteins in Alzheimer’s disease brain using a redox proteomics approach. Neurobiology of disease. 2006;22:76–87. doi: 10.1016/j.nbd.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 101.Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Merchant M, Markesbery WR, Butterfield DA. Redox proteomics identification of oxidized proteins in Alzheimer’s disease hippocampus and cerebellum: an approach to understand pathological and biochemical alterations in AD. Neurobiology of aging. 2006;27:1564–1576. doi: 10.1016/j.neurobiolaging.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 102.Butterfield DA, Perluigi M, Reed T, Muharib T, Hughes CP, Robinson RA, Sultana R. Redox proteomics in selected neurodegenerative disorders: from its infancy to future applications. Antioxid Redox Signal. 2012;17:1610–1655. doi: 10.1089/ars.2011.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen Z, Zhong C. Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Progress in neurobiology. 2013;108:21–43. doi: 10.1016/j.pneurobio.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 104.Butterfield DA, Dalle-Donne I. Redox proteomics: from protein modifications to cellular dysfunction and disease. Mass Spectrom Rev. 2014;33:1–6. doi: 10.1002/mas.21404. [DOI] [PubMed] [Google Scholar]
- 105.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropath Exp Neur. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 106.Serra JA, Marschoff ER, Dominguez RO, Guareschi EM, Famulari AL, Pagano MA, de Lustig ES A Collaborative Group for the Study of the Oxidative Stress. Oxidative stress in Alzheimer’s and vascular dementias: masking of the antioxidant profiles by a concomitant Type II diabetes mellitus condition. J Neurol Sci. 2004;218:17–24. doi: 10.1016/j.jns.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 107.Pandey KB, Mishra N, Rizvi SI. Protein oxidation biomarkers in plasma of type 2 diabetic patients. Clinical biochemistry. 2010;43:508–511. doi: 10.1016/j.clinbiochem.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 108.Gradinaru D, Borsa C, Ionescu C, Margina D. Advanced oxidative and glycoxidative protein damage markers in the elderly with type 2 diabetes. Journal of proteomics. 2013;92:313–322. doi: 10.1016/j.jprot.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 109.Pan HZ, Zhang L, Guo MY, Sui H, Li H, Wu WH, Qu NQ, Liang MH, Chang D. The oxidative stress status in diabetes mellitus and diabetic nephropathy. Acta diabetologica. 2010;47(Suppl 1):71–76. doi: 10.1007/s00592-009-0128-1. [DOI] [PubMed] [Google Scholar]
- 110.D’Archivio M, Annuzzi G, Vari R, Filesi C, Giacco R, Scazzocchio B, Santangelo C, Giovannini C, Rivellese AA, Masella R. Predominant role of obesity/insulin resistance in oxidative stress development. European journal of clinical investigation. 2012;42:70–78. doi: 10.1111/j.1365-2362.2011.02558.x. [DOI] [PubMed] [Google Scholar]
- 111.Calabrese V, Cornelius C, Leso V, Trovato-Salinaro A, Ventimiglia B, Cavallaro M, Scuto M, Rizza S, Zanoli L, Neri S, Castellino P. Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes. Biochimica et biophysica acta. 2012;1822:729–736. doi: 10.1016/j.bbadis.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 112.Di Domenico F, Pupo G, Tramutola A, Giorgi A, Eugenia Schinina M, Coccia R, Head E, Allan Butterfield D, Perluigi M. Redox proteomics analysis of HNE-modified proteins in Down syndrome brain: clues for understanding the development of Alzheimer disease. Free radical biology & medicine. 2014 doi: 10.1016/j.freeradbiomed.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Di Domenico F, Coccia R, Cocciolo A, Murphy MP, Cenini G, Head E, Butterfield DA, Giorgi A, Schinina ME, Mancuso C, Cini C, Perluigi M. Impairment of proteostasis network in Down syndrome prior to the development of Alzheimer’s disease neuropathology: redox proteomics analysis of human brain. Biochimica et biophysica acta. 2013;1832:1249–1259. doi: 10.1016/j.bbadis.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Poon HF, Calabrese V, Scapagnini G, Butterfield DA. Free radicals: key to brain aging and heme oxygenase as a cellular response to oxidative stress The journals of gerontology. Series A. Biological sciences and medical sciences. 2004;59:478–493. doi: 10.1093/gerona/59.5.m478. [DOI] [PubMed] [Google Scholar]
- 115.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annual review of pharmacology and toxicology. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 116.Maines MD. The heme oxygenase system and its functions in the brain. Cell Mol Biol (Noisy-le-grand) 2000;46:573–585. [PubMed] [Google Scholar]
- 117.Mancuso C, Barone E. The heme oxygenase/biliverdin reductase pathway in drug research and development. Current drug metabolism. 2009;10:579–594. doi: 10.2174/138920009789375405. [DOI] [PubMed] [Google Scholar]
- 118.Butterfield DA, Barone E, Di Domenico F, Cenini G, Sultana R, Murphy MP, Mancuso C, Head E. Atorvastatin treatment in a dog preclinical model of Alzheimer’s disease leads to up-regulation of haem oxygenase-1 and is associated with reduced oxidative stress in brain. Int J Neuropsychopharmacol. 2012;15:981–987. doi: 10.1017/S1461145711001118. [DOI] [PubMed] [Google Scholar]
- 119.Barone E, Di Domenico F, Sultana R, Coccia R, Mancuso C, Perluigi M, Butterfield DA. Heme oxygenase-1 posttranslational modifications in the brain of subjects with Alzheimer disease and mild cognitive impairment. Free radical biology & medicine. 2012;52:2292–2301. doi: 10.1016/j.freeradbiomed.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salinas M, Wang J, Rosa de Sagarra M, Martin D, Rojo AI, Martin-Perez J, Ortiz de Montellano PR, Cuadrado A. Protein kinase Akt/PKB phosphorylates heme oxygenase-1 in vitro and in vivo. FEBS letters. 2004;578:90–94. doi: 10.1016/j.febslet.2004.10.077. [DOI] [PubMed] [Google Scholar]
- 121.Sultana R, Perluigi M, Butterfield DA. Oxidatively modified proteins in Alzheimer’s disease (AD), mild cognitive impairment and animal models of AD: role of Abeta in pathogenesis. Acta Neuropathol. 2009;118:131–150. doi: 10.1007/s00401-009-0517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Subramaniam R, Roediger F, Jordan B, Mattson MP, Keller JN, Waeg G, Butterfield DA. The lipid peroxidation product, 4-hydroxy-2-trans-nonenal alters the conformation of cortical synaptosomal membrane proteins. J Neurochem. 1997;69:1161–1169. doi: 10.1046/j.1471-4159.1997.69031161.x. [DOI] [PubMed] [Google Scholar]
- 123.Owen JB, Sultana R, Aluise CD, Erickson MA, Price TO, Bu G, Banks WA, Butterfield DA. Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: implications for Abeta accumulation in AD brain. Free radical biology & medicine. 2010;49:1798–1803. doi: 10.1016/j.freeradbiomed.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: the role of Abeta1–42. J Neurochem. 2001;78:413–416. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- 125.Barone E, Di Domenico F, Mancuso C, Butterfield DA. The Janus face of the heme oxygenase/biliverdin reductase system in Alzheimer disease: it’s time for reconciliation. Neurobiology of disease. 2014;62:144–159. doi: 10.1016/j.nbd.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marrazzo G, Bosco P, La Delia F, Scapagnini G, Di Giacomo C, Malaguarnera M, Galvano F, Nicolosi A, Li Volti G. Neuroprotective effect of silibinin in diabetic mice. Neuroscience letters. 2011;504:252–256. doi: 10.1016/j.neulet.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 127.Nakatsuka I, Maeda S, Andoh T, Hayashi Y, Mizuno R, Higuchi H, Miyawaki T. Oxidative changes in the rat brain by intraperitoneal injection of ferric nitrilotriacetate. Redox report: communications in free radical research. 2009;14:109–114. doi: 10.1179/135100009X392575. [DOI] [PubMed] [Google Scholar]
- 128.Lappalainen J, Lappalainen Z, Oksala NK, Laaksonen DE, Khanna S, Sen CK, Atalay M. Alpha-lipoic acid does not alter stress protein response to acute exercise in diabetic brain. Cell biochemistry and function. 2010;28:644–650. doi: 10.1002/cbf.1702. [DOI] [PubMed] [Google Scholar]
- 129.Rajpathak SN, Crandall JP, Wylie-Rosett J, Kabat GC, Rohan TE, Hu FB. The role of iron in type 2 diabetes in humans. Biochimica et biophysica acta. 2009;1790:671–681. doi: 10.1016/j.bbagen.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 130.Swaminathan S, Fonseca VA, Alam MG, Shah SV. The role of iron in diabetes and its complications. Diabetes care. 2007;30:1926–1933. doi: 10.2337/dc06-2625. [DOI] [PubMed] [Google Scholar]
- 131.Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Song W, Su H, Song S, Paudel HK, Schipper HM. Over-expression of heme oxygenase-1 promotes oxidative mitochondrial damage in rat astroglia. Journal of cellular physiology. 2006;206:655–663. doi: 10.1002/jcp.20509. [DOI] [PubMed] [Google Scholar]
- 133.Schipper HM, Bennett DA, Liberman A, Bienias JL, Schneider JA, Kelly J, Arvanitakis Z. Glial heme oxygenase-1 expression in Alzheimer disease and mild cognitive impairment. Neurobiol Aging. 2006;27:252–261. doi: 10.1016/j.neurobiolaging.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 134.Borel MJ, Beard JL, Farrell PA. Hepatic glucose production and insulin sensitivity and responsiveness in iron-deficient anemic rats. The American journal of physiology. 1993;264:E380–390. doi: 10.1152/ajpendo.1993.264.3.E380. [DOI] [PubMed] [Google Scholar]
- 135.Farrell PA, Beard JL, Druckenmiller M. Increased insulin sensitivity in iron-deficient rats. The Journal of nutrition. 1988;118:1104–1109. doi: 10.1093/jn/118.9.1104. [DOI] [PubMed] [Google Scholar]
- 136.Dandona P, Hussain MA, Varghese Z, Politis D, Flynn DM, Hoffbrand AV. Insulin resistance and iron overload. Annals of clinical biochemistry. 1983;20(Pt 2):77–79. doi: 10.1177/000456328302000203. [DOI] [PubMed] [Google Scholar]
- 137.Ferreira ST, Clarke JR, Bomfim TR, De Felice FG. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2014;10:S76–83. doi: 10.1016/j.jalz.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 138.Mosen H, Salehi A, Henningsson R, Lundquist I. Nitric oxide inhibits, and carbon monoxide activates, islet acid alpha-glucoside hydrolase activities in parallel with glucose-stimulated insulin secretion. The Journal of endocrinology. 2006;190:681–693. doi: 10.1677/joe.1.06890. [DOI] [PubMed] [Google Scholar]
- 139.Henningsson R, Alm P, Ekstrom P, Lundquist I. Heme oxygenase and carbon monoxide: regulatory roles in islet hormone release: a biochemical, immunohistochemical, and confocal microscopic study. Diabetes. 1999;48:66–76. doi: 10.2337/diabetes.48.1.66. [DOI] [PubMed] [Google Scholar]
- 140.Mosen H, Salehi A, Alm P, Henningsson R, Jimenez-Feltstrom J, Ostenson CG, Efendic S, Lundquist I. Defective glucose-stimulated insulin release in the diabetic Goto-Kakizaki (GK) rat coincides with reduced activity of the islet carbon monoxide signaling pathway. Endocrinology. 2005;146:1553–1558. doi: 10.1210/en.2004-0851. [DOI] [PubMed] [Google Scholar]
- 141.Schipper HM, Gupta A, Szarek WA. Suppression of glial HO-1 activity as a potential neurotherapeutic intervention in AD. Current Alzheimer research. 2009;6:424–430. doi: 10.2174/156720509789207985. [DOI] [PubMed] [Google Scholar]
- 142.Crichton RR, Dexter DT, Ward RJ. Brain iron metabolism and its perturbation in neurological diseases. Journal of neural transmission. 2011;118:301–314. doi: 10.1007/s00702-010-0470-z. [DOI] [PubMed] [Google Scholar]
- 143.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Irons BK, Minze MG. Drug treatment of type 2 diabetes mellitus in patients for whom metformin is contraindicated. Diabetes, metabolic syndrome and obesity: targets and therapy. 2014;7:15–24. doi: 10.2147/DMSO.S38753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia. 2013;56:1898–1906. doi: 10.1007/s00125-013-2991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Burcelin R. The antidiabetic gutsy role of metformin uncovered? Gut. 2014;63:706–707. doi: 10.1136/gutjnl-2013-305370. [DOI] [PubMed] [Google Scholar]
- 147.Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, Thompson RC, Zhao Y, Smith L, Gasparini L, Luo Z, Xu H, Liao FF. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3907–3912. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kickstein E, Krauss S, Thornhill P, Rutschow D, Zeller R, Sharkey J, Williamson R, Fuchs M, Kohler A, Glossmann H, Schneider R, Sutherland C, Schweiger S. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21830–21835. doi: 10.1073/pnas.0912793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gupta A, Bisht B, Dey CS. Peripheral insulin-sensitizer drug metformin ameliorates neuronal insulin resistance and Alzheimer’s-like changes. Neuropharmacology. 2011;60:910–920. doi: 10.1016/j.neuropharm.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 150.Li J, Deng J, Sheng W, Zuo Z. Metformin attenuates Alzheimer’s disease-like neuropathology in obese, leptin-resistant mice. Pharmacology, biochemistry, and behavior. 2012;101:564–574. doi: 10.1016/j.pbb.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McNeilly AD, Williamson R, Balfour DJ, Stewart CA, Sutherland C. A high-fat-diet-induced cognitive deficit in rats that is not prevented by improving insulin sensitivity with metformin. Diabetologia. 2012;55:3061–3070. doi: 10.1007/s00125-012-2686-y. [DOI] [PubMed] [Google Scholar]
- 152.Correia S, Carvalho C, Santos MS, Proenca T, Nunes E, Duarte AI, Monteiro P, Seica R, Oliveira CR, Moreira PI. Metformin protects the brain against the oxidative imbalance promoted by type 2 diabetes. Medicinal chemistry. 2008;4:358–364. doi: 10.2174/157340608784872299. [DOI] [PubMed] [Google Scholar]
- 153.Pintana H, Apaijai N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life sciences. 2012;91:409–414. doi: 10.1016/j.lfs.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 154.Alzoubi KH, Khabour OF, Al-Azzam SI, Tashtoush MH, Mhaidat NM. Metformin Eased Cognitive Impairment Induced by Chronic L-methionine Administration: Potential Role of Oxidative Stress. Current neuropharmacology. 2014;12:186–192. doi: 10.2174/1570159X11666131120223201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Imfeld P, Bodmer M, Jick SS, Meier CR. Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study. Journal of the American Geriatrics Society. 2012;60:916–921. doi: 10.1111/j.1532-5415.2012.03916.x. [DOI] [PubMed] [Google Scholar]
- 156.Moore EM, Mander AG, Ames D, Kotowicz MA, Carne RP, Brodaty H, Woodward M, Boundy K, Ellis KA, Bush AI, Faux NG, Martins R, Szoeke C, Rowe C, Watters DA, Investigators A. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes care. 2013;36:2981–2987. doi: 10.2337/dc13-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hemmingsen B, Schroll JB, Lund SS, Wetterslev J, Gluud C, Vaag A, Sonne DP, Lundstrom LH, Almdal T. Sulphonylurea monotherapy for patients with type 2 diabetes mellitus. The Cochrane database of systematic reviews. 2013;4:CD009008. doi: 10.1002/14651858.CD009008.pub2. [DOI] [PubMed] [Google Scholar]
- 158.Baraka A, ElGhotny S. Study of the effect of inhibiting galanin in Alzheimer’s disease induced in rats. European journal of pharmacology. 2010;641:123–127. doi: 10.1016/j.ejphar.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 159.Abdallah DM, Nassar NN, Abd-El-Salam RM. Glibenclamide ameliorates ischemia-reperfusion injury via modulating oxidative stress and inflammatory mediators in the rat hippocampus. Brain research. 2011;1385:257–262. doi: 10.1016/j.brainres.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 160.Alp H, Varol S, Celik MM, Altas M, Evliyaoglu O, Tokgoz O, Tanriverdi MH, Uzar E. Protective effects of beta glucan and gliclazide on brain tissue and sciatic nerve of diabetic rats induced by streptozosin. Experimental diabetes research. 2012;2012:230342. doi: 10.1155/2012/230342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yau H, Rivera K, Lomonaco R, Cusi K. The future of thiazolidinedione therapy in the management of type 2 diabetes mellitus. Current diabetes reports. 2013;13:329–341. doi: 10.1007/s11892-013-0378-8. [DOI] [PubMed] [Google Scholar]
- 162.Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, Citron M, Landreth G. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Galea E, Feinstein DL, Lacombe P. Pioglitazone does not increase cerebral glucose utilisation in a murine model of Alzheimer’s disease and decreases it in wild-type mice. Diabetologia. 2006;49:2153–2161. doi: 10.1007/s00125-006-0326-0. [DOI] [PubMed] [Google Scholar]
- 165.Nohl H, Hegner D. Do mitochondria produce oxygen radicals in vivo? European journal of biochemistry/FEBS. 1978;82:563–567. doi: 10.1111/j.1432-1033.1978.tb12051.x. [DOI] [PubMed] [Google Scholar]
- 166.Nicolakakis N, Aboulkassim T, Ongali B, Lecrux C, Fernandes P, Rosa-Neto P, Tong XK, Hamel E. Complete rescue of cerebrovascular function in aged Alzheimer’s disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:9287–9296. doi: 10.1523/JNEUROSCI.3348-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, O’Banion K, Klockgether T, Van Leuven F, Landreth GE. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1–42 levels in APPV717I transgenic mice. Brain: a journal of neurology. 2005;128:1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- 168.Maeshiba Y, Kiyota Y, Yamashita K, Yoshimura Y, Motohashi M, Tanayama S. Disposition of the new antidiabetic agent pioglitazone in rats, dogs, and monkeys. Arzneimittel-Forschung. 1997;47:29–35. [PubMed] [Google Scholar]
- 169.Searcy JL, Phelps JT, Pancani T, Kadish I, Popovic J, Anderson KL, Beckett TL, Murphy MP, Chen KC, Blalock EM, Landfield PW, Porter NM, Thibault O. Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2012;30:943–961. doi: 10.3233/JAD-2012-111661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mandrekar-Colucci S, Karlo JC, Landreth GE. Mechanisms underlying the rapid peroxisome proliferator-activated receptor-gamma-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:10117–10128. doi: 10.1523/JNEUROSCI.5268-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gupta R, Gupta LK. Improvement in long term and visuo-spatial memory following chronic pioglitazone in mouse model of Alzheimer’s disease. Pharmacology, biochemistry, and behavior. 2012;102:184–190. doi: 10.1016/j.pbb.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 172.Yin QQ, Pei JJ, Xu S, Luo DZ, Dong SQ, Sun MH, You L, Sun ZJ, Liu XP. Pioglitazone improves cognitive function via increasing insulin sensitivity and strengthening antioxidant defense system in fructose-drinking insulin resistance rats. PloS one. 2013;8:e59313. doi: 10.1371/journal.pone.0059313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hanyu H, Sato T, Kiuchi A, Sakurai H, Iwamoto T. Pioglitazone improved cognition in a pilot study on patients with Alzheimer’s disease and mild cognitive impairment with diabetes mellitus. Journal of the American Geriatrics Society. 2009;57:177–179. doi: 10.1111/j.1532-5415.2009.02067.x. [DOI] [PubMed] [Google Scholar]
- 174.Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-gamma agonist pioglitazone in mild Alzheimer disease. Neurobiology of aging. 2011;32:1626–1633. doi: 10.1016/j.neurobiolaging.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 175.Pedersen WA, Flynn ER. Insulin resistance contributes to aberrant stress responses in the Tg2576 mouse model of Alzheimer’s disease. Neurobiology of disease. 2004;17:500–506. doi: 10.1016/j.nbd.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 176.Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Experimental neurology. 2006;199:265–273. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 177.Escribano L, Simon AM, Perez-Mediavilla A, Salazar-Colocho P, Del Rio J, Frechilla D. Rosiglitazone reverses memory decline and hippocampal glucocorticoid receptor down-regulation in an Alzheimer’s disease mouse model. Biochemical and biophysical research communications. 2009;379:406–410. doi: 10.1016/j.bbrc.2008.12.071. [DOI] [PubMed] [Google Scholar]
- 178.Escribano L, Simon AM, Gimeno E, Cuadrado-Tejedor M, Lopez de Maturana R, Garcia-Osta A, Ricobaraza A, Perez-Mediavilla A, Del Rio J, Frechilla D. Rosiglitazone rescues memory impairment in Alzheimer’s transgenic mice: mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:1593–1604. doi: 10.1038/npp.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.O’Reilly JA, Lynch M. Rosiglitazone improves spatial memory and decreases insoluble Abeta(1–42) in APP/PS1 mice. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2012;7:140–144. doi: 10.1007/s11481-011-9282-7. [DOI] [PubMed] [Google Scholar]
- 180.Strum JC, Shehee R, Virley D, Richardson J, Mattie M, Selley P, Ghosh S, Nock C, Saunders A, Roses A. Rosiglitazone induces mitochondrial biogenesis in mouse brain. Journal of Alzheimer’s disease: JAD. 2007;11:45–51. doi: 10.3233/jad-2007-11108. [DOI] [PubMed] [Google Scholar]
- 181.Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 182.Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses ADG Rosiglitazone in Alzheimer’s Disease Study, Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. The pharmacogenomics journal. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- 183.Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, Craft S, Landreth G, Linnamagi U, Sawchak S. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dementia and geriatric cognitive disorders. 2010;30:131–146. doi: 10.1159/000318845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Squire LR. Mechanisms of memory. Science. 1986;232:1612–1619. doi: 10.1126/science.3086978. [DOI] [PubMed] [Google Scholar]
- 185.Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet neurology. 2004;3:169–178. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- 186.Moreira PI, Santos MS, Sena C, Seica R, Oliveira CR. Insulin protects against amyloid beta-peptide toxicity in brain mitochondria of diabetic rats. Neurobiology of disease. 2005;18:628–637. doi: 10.1016/j.nbd.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 187.Duarte AI, Santos MS, Oliveira CR, Rego AC. Insulin neuroprotection against oxidative stress in cortical neurons--involvement of uric acid and glutathione antioxidant defenses. Free radical biology & medicine. 2005;39:876–889. doi: 10.1016/j.freeradbiomed.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 188.Duarte AI, Proenca T, Oliveira CR, Santos MS, Rego AC. Insulin restores metabolic function in cultured cortical neurons subjected to oxidative stress. Diabetes. 2006;55:2863–2870. doi: 10.2337/db06-0030. [DOI] [PubMed] [Google Scholar]
- 189.Duarte AI, Santos P, Oliveira CR, Santos MS, Rego AC. Insulin neuroprotection against oxidative stress is mediated by Akt and GSK-3beta signaling pathways and changes in protein expression. Biochimica et biophysica acta. 2008;1783:994–1002. doi: 10.1016/j.bbamcr.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 190.De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ, Ferreira ST, Klein WL. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Garcia-Fernandez M, Delgado G, Puche JE, Gonzalez-Baron S, Castilla Cortazar I. Low doses of insulin-like growth factor I improve insulin resistance, lipid metabolism, and oxidative damage in aging rats. Endocrinology. 2008;149:2433–2442. doi: 10.1210/en.2007-1190. [DOI] [PubMed] [Google Scholar]
- 192.Puche JE, Garcia-Fernandez M, Muntane J, Rioja J, Gonzalez-Baron S, Castilla Cortazar I. Low doses of insulin-like growth factor-I induce mitochondrial protection in aging rats. Endocrinology. 2008;149:2620–2627. doi: 10.1210/en.2007-1563. [DOI] [PubMed] [Google Scholar]
- 193.Crowe E, Sell C, Thomas JD, Johannes GJ, Torres C. Activation of proteasome by insulin-like growth factor-I may enhance clearance of oxidized proteins in the brain. Mechanisms of ageing and development. 2009;130:793–800. doi: 10.1016/j.mad.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Keller JN, Hanni KB, Markesbery WR. Impaired proteasome function in Alzheimer’s disease. Journal of neurochemistry. 2000;75:436–439. doi: 10.1046/j.1471-4159.2000.0750436.x. [DOI] [PubMed] [Google Scholar]
- 195.Noh KM, Lee JC, Ahn YH, Hong SH, Koh JY. Insulin-induced oxidative neuronal injury in cortical culture: mediation by induced N-methyl-D-aspartate receptors. IUBMB life. 1999;48:263–269. doi: 10.1080/713803514. [DOI] [PubMed] [Google Scholar]
- 196.Ryu BR, Ko HW, Jou I, Noh JS, Gwag BJ. Phosphatidylinositol 3-kinase-mediated regulation of neuronal apoptosis and necrosis by insulin and IGF-I. Journal of neurobiology. 1999;39:536–546. [PubMed] [Google Scholar]
- 197.Duarte AI, Santos MS, Seica R, Oliveira CR. Oxidative stress affects synaptosomal gamma-aminobutyric acid and glutamate transport in diabetic rats: the role of insulin. Diabetes. 2004;53:2110–2116. doi: 10.2337/diabetes.53.8.2110. [DOI] [PubMed] [Google Scholar]
- 198.Patockova J, Marhol P, Tumova E, Krsiak M, Rokyta R, Stipek S, Crkovska J, Andel M. Oxidative stress in the brain tissue of laboratory mice with acute post insulin hypoglycemia. Physiological research/Academia Scientiarum Bohemoslovaca. 2003;52:131–135. [PubMed] [Google Scholar]
- 199.Dhuria SV, Hanson LR, Frey WH., 2nd Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99:1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 200.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 201.Hallschmid M, Schultes B, Marshall L, Molle M, Kern W, Bredthauer J, Fehm HL, Born J. Transcortical direct current potential shift reflects immediate signaling of systemic insulin to the human brain. Diabetes. 2004;53:2202–2208. doi: 10.2337/diabetes.53.9.2202. [DOI] [PubMed] [Google Scholar]
- 202.Lund A, Knop FK, Vilsboll T. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: Differences and similarities. European journal of internal medicine. 2014 doi: 10.1016/j.ejim.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 203.Sarkar S, Fekete C, Legradi G, Lechan RM. Glucagon like peptide-1 (7–36) amide (GLP-1) nerve terminals densely innervate corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain research. 2003;985:163–168. doi: 10.1016/s0006-8993(03)03117-2. [DOI] [PubMed] [Google Scholar]
- 204.Holscher C. Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. The Journal of endocrinology. 2014;221:T31–41. doi: 10.1530/JOE-13-0221. [DOI] [PubMed] [Google Scholar]
- 205.Juillerat-Jeanneret L. Dipeptidyl peptidase IV and its inhibitors: therapeutics for type 2 diabetes and what else? Journal of medicinal chemistry. 2014;57:2197–2212. doi: 10.1021/jm400658e. [DOI] [PubMed] [Google Scholar]
- 206.Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- 207.Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes care. 2003;26:2929–2940. doi: 10.2337/diacare.26.10.2929. [DOI] [PubMed] [Google Scholar]
- 208.Drucker DJ. Therapeutic potential of dipeptidyl peptidase IV inhibitors for the treatment of type 2 diabetes. Expert opinion on investigational drugs. 2003;12:87–100. doi: 10.1517/13543784.12.1.87. [DOI] [PubMed] [Google Scholar]
- 209.Holscher C. Incretin analogues that have been developed to treat type 2 diabetes hold promise as a novel treatment strategy for Alzheimer’s disease. Recent patents on CNS drug discovery. 2010;5:109–117. doi: 10.2174/157488910791213130. [DOI] [PubMed] [Google Scholar]
- 210.Perry T, Lahiri DK, Sambamurti K, Chen D, Mattson MP, Egan JM, Greig NH. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. Journal of neuroscience research. 2003;72:603–612. doi: 10.1002/jnr.10611. [DOI] [PubMed] [Google Scholar]
- 211.Li Y, Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, Tweedie D, Perry T, Mattson MP, Kapogiannis D, Sambamurti K, Lahiri DK, Greig NH. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2010;19:1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen S, Liu AR, An FM, Yao WB, Gao XD. Amelioration of neurodegenerative changes in cellular and rat models of diabetes-related Alzheimer’s disease by exendin-4. Age. 2012;34:1211–1224. doi: 10.1007/s11357-011-9303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kimura R, Okouchi M, Fujioka H, Ichiyanagi A, Ryuge F, Mizuno T, Imaeda K, Okayama N, Kamiya Y, Asai K, Joh T. Glucagon-like peptide-1 (GLP-1) protects against methylglyoxal-induced PC12 cell apoptosis through the PI3K/Akt/mTOR/GCLc/redox signaling pathway. Neuroscience. 2009;162:1212–1219. doi: 10.1016/j.neuroscience.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 214.Liu J, Yin F, Zheng X, Jing J, Hu Y. Geniposide, a novel agonist for GLP-1 receptor, prevents PC12 cells from oxidative damage via MAP kinase pathway. Neurochemistry international. 2007;51:361–369. doi: 10.1016/j.neuint.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 215.Cai HY, Holscher C, Yue XH, Zhang SX, Wang XH, Qiao F, Yang W, Qi JS. Lixisenatide rescues spatial memory and synaptic plasticity from amyloid beta protein-induced impairments in rats. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 216.McClean PL, Parthsarathy V, Faivre E, Holscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:6587–6594. doi: 10.1523/JNEUROSCI.0529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.McClean PL, Holscher C. Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology. 2014;76(Pt A):57–67. doi: 10.1016/j.neuropharm.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 218.Parthsarathy V, Holscher C. Chronic treatment with the GLP1 analogue liraglutide increases cell proliferation and differentiation into neurons in an AD mouse model. PloS one. 2013;8:e58784. doi: 10.1371/journal.pone.0058784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mathieu C, Degrande E. Vildagliptin: a new oral treatment for type 2 diabetes mellitus. Vascular health and risk management. 2008;4:1349–1360. doi: 10.2147/vhrm.s3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.D’Amico M, Di Filippo C, Marfella R, Abbatecola AM, Ferraraccio F, Rossi F, Paolisso G. Long-term inhibition of dipeptidyl peptidase-4 in Alzheimer’s prone mice. Experimental gerontology. 2010;45:202–207. doi: 10.1016/j.exger.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 221.Kosaraju J, Gali CC, Khatwal RB, Dubala A, Chinni S, Holsinger RM, Madhunapantula VS, Muthureddy Nataraj SK, Basavan D. Saxagliptin: a dipeptidyl peptidase-4 inhibitor ameliorates streptozotocin induced Alzheimer’s disease. Neuropharmacology. 2013;72:291–300. doi: 10.1016/j.neuropharm.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 222.Kosaraju J, Murthy V, Khatwal RB, Dubala A, Chinni S, Muthureddy Nataraj SK, Basavan D. Vildagliptin: an anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer’s disease. The Journal of pharmacy and pharmacology. 2013;65:1773–1784. doi: 10.1111/jphp.12148. [DOI] [PubMed] [Google Scholar]
- 223.Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whitton P, Wyse R, Isaacs T, Lees A, Limousin P, Foltynie T. Exenatide and the treatment of patients with Parkinson’s disease. The Journal of clinical investigation. 2013;123:2730–2736. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Kahan J, Fmedsci PE, Whitton P, Wyse R, Isaacs T, Lees A, Limousin P, Foltynie T. Motor and Cognitive Advantages Persist 12 Months After Exenatide Exposure in Parkinson’s Disease. Journal of Parkinson’s disease. 2014 doi: 10.3233/JPD-140364. [DOI] [PubMed] [Google Scholar]