Abstract
The α-actinins are an important family of actin-binding proteins with the ability to cross-link actin filaments when in dimer form. Members of the α-actinin family share a domain topology composed of highly conserved actin-binding and EF-hand domains separated by a rod domain composed of spectrin-like repeats. Functional diversity within this family has arisen through exon duplication and the formation of alternate splice isoforms as well as gene duplications during the evolution of vertebrates. In addition to the known functional domains, α-actinins also contain a consensus PDZ-binding site. The completed genome sequence of over 32 invertebrate species has allowed the analysis of gene structure and exon–gene duplication over a diverse range of phyla. Our analysis shows that relative to early branching metazoans, there has been considerable intron loss especially in arthropods with few cases of intron gains. The C-terminal PDZ-binding site is conserved in nearly all invertebrates but is missing in some nematodes and platyhelminths. Alternative splicing in the actin-binding domain is conserved in chordates, arthropods, and some nematodes and platyhelminths. In contrast, alternative splicing of the EF-hand domain is only observed in chordates. Finally, given the prevalence of exon duplications seen in the actin-binding domain, this may act as a significant mechanism in the modification of actin-binding properties.
Keywords: α-actinin, exon duplication, alternate splicing
Introduction
The α-actinins are an ancient family of actin-binding proteins that play a key role in the maintenance and regulation of the cytoskeleton (Blanchard et al. 1989). The α-actinins have homologues in slime mold (Witke et al. 1986), fungi (Wu et al. 2001), and metazoans but, surprisingly, are not present in plants. Among the metazoans, vertebrates possess four α-actinin genes (ACTN 1–4) postulated to arise from a single invertebrate ancestral gene (Virel and Backman 2004). Both of the nonmuscle α-actinins, α-actinins-1 and -4, are ubiquitously expressed and function as cytoskeletal proteins. α-Actinin-4 has unique functions in kidney tissue (Weins et al. 2007) and has been implicated in cancer invasion, whereas α-actinin-1 is highly expressed at focal adhesions and adherens junctions (Honda et al. 1998). The skeletal muscle α-actinins, α-actinin-2, and α-actinin-3, are highly expressed in muscle where they act as major structural components of the contractile apparatus at the Z-line (Beggs et al. 1992).
The α-actinin family belongs to the larger superfamily of spectrin proteins that includes spectrin, dystrophin, and utrophin (Djinovic-Carugo et al. 2002). A general domain topology is conserved within the α-actinin gene family, which includes an actin-binding domain composed of a CH1 and CH2 domain, a rod domain composed of four spectrin-like repeats in metazoans and two EF-hand domains (MacArthur and North 2004). The actin-binding and EF-hand domains are highly conserved, whereas the rod domain is variable in sequence and the number of spectrin-like repeats (Virel and Backman 2007). In addition to the identified functional domains, α-actinin contains a PDZ consensus binding site that mediates interaction with proteins from the PDZ/LIM family (von Nandelstadh et al. 2008).
There are two known sites of alternative splicing within α-actinin that serve to modify the sequence contained in the actin-binding and EF-hand domains, corresponding to human exons 8 and 19, respectively. In each case, the alternate exons are almost identical, suggesting they were created by tandem exon duplication followed by relatively minor sequence divergence. The duplicated exons at each site are either of the same size, allowing for slight variation in protein function through amino acid changes, or of different size, allowing for the inactivation of specific exon functions (Kondrashov and Koonin 2001). In the actin-binding domain, Drosophila is able to create muscle, nonmuscle, and larval muscle tissue isoforms through mutually exclusive splicing (Roulier et al. 1992). The exon involved in creating these variants is orthologous to human exon 8. In humans, alternative splicing in ACTN2 exon 8 creates a brain-specific isoform (Machuca-Tzili et al. 2006). Despite the identification of exon 8 alternative splicing, little is known regarding its functional significance. In contrast, the alternative splicing of unequally sized variants of exon 19 in vertebrates is known to affect the binding of calcium at the EF-hand, thus creating calcium-sensitive and -insensitive isoforms of α-actinins-1 and -4 (Waites et al. 1992), whereas α-actinins-2 and -3 have lost this alternative splicing and only express the calcium-insensitive isoform. This calcium sensitivity regulates the ability of α-actinin to bind to actin by decreasing binding affinity as calcium concentration increases (Noegel et al. 1987). Although the exon 19 splicing is typically mutually exclusive, both exons are included in the rat-brain isoform (MacArthur and North 2004) without causing a frameshift as both exons are multiples of 3nt in length.
The recent genome sequencing of over 32 invertebrate species from the phyla Arthropoda, Chordata, Annelida, Mollusca, Nematoda, Cnidira, Placozoa, and Echinodermata has allowed us to perform an extensive phylogenetic study that includes an analysis of gene structure, sequence conservation, and exon/gene duplications. In addition, this analysis provides the opportunity to determine whether alternative splicing within the highly conserved actin-binding and EF-hand domains is a feature common to all metazoan species.
Methods
Identification and Annotation of α-Actinin Genes
The genes for all 32 invertebrates were obtained by TBlastN (Altschul et al. 1990) searches against their respective databases (table 1). In most cases, the α-actinin gene was fully contained within a supercontig (or scaffold), and segment pairs were in close proximity to each other. The gene spanned over two or three supercontigs for the following species: Apis mellifera, Nasonia vitripennis, Culex pipiens quinquefasciatus, and Acyrthosiphon pisum. The exons and exon boundaries were identified from the TBlastN output as high-scoring segment pairs or gaps within these segment pairs. In addition, exons were checked for correct ordering and strand. Adjacent exons with high pairwise protein identity were classified as tandem exon duplications. All hits were then manually analyzed for splice acceptor and donor sites to ensure the correct exon–intron boundaries. Finally, multiple sequence alignment using MAFFT was performed to ensure there were no gaps.
Table 1.
The α-Actinin Sequences Were Obtained Using TBlastN Searches Across the Genomes of the Various Species Obtained from Their Respective Sources.
| Species | Species Abbreviation | Source |
| Choanoflagellatea | ||
| Monosiga brevicollis | Mbre | DOE JGI |
| Cnidaria | ||
| Nematostella vectensis | Nvec | DOE JGI |
| Placozoa | ||
| Trichoplax adhaerens | Tadh | DOE JGI |
| Annelida | ||
| Capitella sp I | Ccap | DOE JGI |
| Helobdella robusta | Hrob | DOE JGI |
| Mollusca | ||
| Lottia gigantea | Lgig | DOE JGI |
| Aplysia californica | Acal | Broad |
| Platyhelminthes | ||
| Schmidtea mediterranea | Smed | WUGSC |
| Schistosoma mansoni | Sman | Sanger |
| Echinococcus multilocularis | Emul | Sanger |
| Nematoda | ||
| Pristionchus pacificus | Ppac | WUGSC |
| Heterorhabditis bacteriophora | Hbac | WUGSC |
| Trichinella spiralis | Tspi | WUGSC |
| Haemonchus contortus | Hcon | Sanger |
| Strongyloides ratti | Srat | Sanger |
| Brugia malayi | Bmal | TIGR |
| Caenorhabditis elegans | Cele | UCSC |
| Arthropoda | ||
| Daphnia pulex | Dpul | DOE JGI |
| Pediculus humanus corporis | Phum | NCBI WGS |
| Bombyx mori | Bmor | SilkDB |
| Tribolium castaneum | Tcas | NCBI WGS |
| Nasonia vitripennis | Nvit | NCBI WGS |
| Acyrthosiphon pisum | Apis | NCBI WGS |
| Apis mellifera | Amel | NCBI WGS |
| Drosophila melanogaster | Dmel | UCSC |
| Anopheles gambiae | Agam | NCBI WGS |
| Aedes aegypti | Aaeg | NCBI WGS |
| Culex pipiens quinquefasciatus | Cqui | NCBI WGS |
| Rhodnius prolixus | Rpro | WUGSC |
| Echinodermata | ||
| Strongylocentrotus purpuratus | Spur | UCSC |
| Chordata | ||
| Branchiostoma floridae | Bflo | DOE JGI |
| Ciona savignyi | Csav | Broad |
| Saccoglossus kowalevskii | Skow | Baylor |
NOTE.—DOE JGI = Department of Energy Joint Genome Institute, Broad = Broad Institute, WUGSC = Washington University Genome Sequencing Centre, UCSC = University of California Santa Cruz Genome Browser, SilkDB = Silkworm Database, Sanger = Sanger Institute, Baylor = Baylor College of Medicine Human Genome Sequencing Centre, and TIGR = J. Craig Venter Institute.
Multiple Sequence Alignments
The program ClustalW2 (Thompson et al. 1994) with default settings was used to perform multiple sequence alignment to use as input for PHYML and exon 8 alignments.
Building Trees
The phylogenetic trees based on protein sequences were generated using the maximum likelihood method employed by PHYML (Guindon and Gascuel 2003) using a Jones, Taylor and Thornton model with an estimated proportion of invariable sites and bootstrapping (1,000 replicates).
Results
Identification and Annotation of α-Actinin Genes
The α-actinin genes were identified using TBlastN against the corresponding genome sequence using the Drosophila melanogaster (NP_477484) or Caenorhabditis elegans (NP_506127) α-actinin as the query sequence. The metazoan species analyzed included 12 Arthropods, 2 Annelids, 3 Platyhelminths, 7 Nematodes, 3 Chordates, 1 Echinoderm, 2 Molluscs, 1 Cndiarian, and 1 Placozoan (table 1). The choanoflagellate Monosiga brevicollis was used as an outgroup for subsequent phylogenetic analysis. The resulting TBlastN output was then manually annotated using consensus intron–exon splice junction donor and acceptor sequences to correctly identify the exon boundaries. The highly variable exon 1 made the correct identification of its boundaries ambiguous, and this exon was thus excluded from subsequent analyses. Only partial sequences for the following species could be identified; A. mellifera, N. vitripennis, C. p. quinquefasciatus, Schistosoma mansoni, Echinococcus multilocularis, Pristionchus pacificus, Haemonchus contortus, Aplysia californica, and Ciona savignyi.
Phylogenetic Analysis of α-Actinin Genes
The phylogenetic tree generated included all invertebrates in which full sequences (excluding exon 1) could be obtained, and variant 8a was used where applicable. The tree generated reflects in general the phylogenetic relationship between the species (fig. 1). Low bootstrap values (<400) at several branch sites can be explained by branching events that occurred close together relative to the overall divergence time, and therefore, these branching events cannot be estimated with high confidence. Overall, protein-sequence identity and similarity were high displaying at least 63% pairwise similarity. The four spectrin-like repeats between the actin-binding domain and EF-hand domains account for the majority of the protein-sequence variation and long branch lengths observed.
FIG. 1.
Maximum likelihood tree generated from complete α-actinin protein sequences (excluding exon 1). Numbers at the branch points represent the bootstrap values from 1,000 replicates.
Conservation of α-Actinin Introns
Analysis of intron positions within the α-actinin gene revealed that the Choanoflagellate M. brevicollis was intron-poor, whereas the early branching metazoans Nematostella vectensis and Trichoplax adhaerens are intron-rich (fig. 2). Considerable variability in intron loss has dominated in the arthropods, whereas other invertebrate phyla have maintained the majority of the introns present in the early branching metazoans. Overall, there appear to be few intron gains with the silkworm, Bombyx mori, harboring the most.
FIG. 2.
A scale diagram of the intron positions mapped on the α-actinin protein sequence relative to the Dmel sequence. The diagram spans residues 39–844 with the first and last exons omitted. The green triangles represent conserved introns, and red triangles represent intron gains based on parsimony. The human α-actinin-1 was abbreviated to Hsap. The first and last triangles on Hsap represent first and last introns, respectively.
Conservation of Alternatively Spliced Exons
The known splice variants in α-actinin can be split in two groups, those generated by mutually exclusive alternate splicing of exons orthologous to the human α-actinin exon 8 or exon 19. Variants were labeled based on their position relative to surrounding exons; variant “a” is closer to the preceding exon, whereas variant “b” is closer to the following exon. Exons 8a and 8b are conserved in all arthropods but are not conserved in all nematodes and platyhelminths. These exons differ in two conserved sites (fig. 3a), position 19, which is a cysteine in 8a and serine in 8b, and positions 28 or 29, which creates a charge change between 8a and 8b. Interestingly Ciona savignyi has three variants created by duplicating exon 8 twice instead of just once. In addition, the 8a and 8b variants are conserved in vertebrate ACTN4, and a pattern of species-specific loss was observed for ACTN2 and ACTN1 (fig. 5). The switch between cysteine and serine has been conserved in α-actinin-4; however, compared with arthropods, the exon order in ACTN4 appears to be switched. This suggests that either the ACTN4 exon-8 duplication was independent of the arthropod duplication or a small chromosome translocation involving exon 8 had occurred during vertebrate evolution. In contrast to arthropods, α-actinin-2 switches between positively and negatively charged residues at the C-terminal end (fig. 3c).
FIG. 3.

Multiple sequence alignment of variants a and b of exon 8. (A) Alignment of Arthropod exon 8 showing conserved differences at positions 19 and 28–29. (B) Alignment of non-Arthropod invertebrate exon 8. (C) Alignment of human exon 8 variants and α-actinin paralogues (ACTN1–4).
FIG. 5.
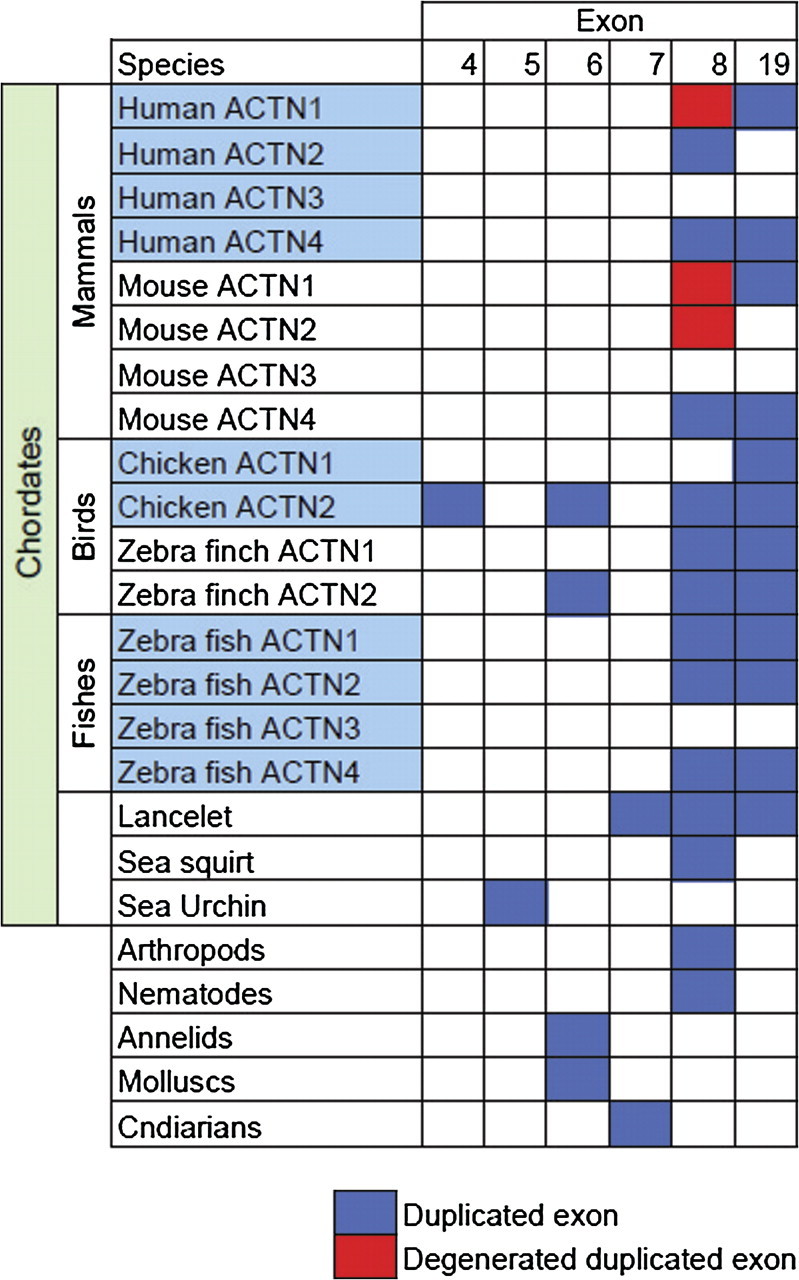
Tandem exon duplication across animal α-actinin genes. Duplication within the actin-binding domain (exons 1–8) is present in all animals, whereas duplication of exon 19 within the EF-hand domain is restricted to chordates. Duplicated exons were considered degenerated if splicing acceptor and donor sites were lost and/or contained a stop codon.
The 8c variant identified in D. melanogaster could not be identified in any arthropod species apart from the genomes of species within the Drosophila genus (data not shown), whereas the variants 19a and 19b identified in vertebrates are restricted to chordates. In addition to the known variants, the analysis identified exon duplications of exon 4 in birds; exon 5 in Strongylocentrotus purpuratus; exon 6 in molluscs, annelids, and birds; and exon 7 in Branchiostoma floridae (fig. 5). All these exon duplicates have conserved splice acceptor and donor sites and therefore can potentially act as splice variants.
Identification of Three α-Actinin Genes in Helobdella robusta
All invertebrates annotated in this study have only one α-actinin gene except for the annelid H. robusta, which had three α-actinins. A pairwise comparison was performed at the DNA-sequence level to rule out the possibility that the additional paralogues are in fact errors in genome assembly. At the protein level, the three genes are approximately 75% similar, a difference comparable with the vertebrate α-actinin orthologues. Interestingly, an inspection of the exon 8 region of H. robusta, actinin genes revealed residue differences typically seen in variants 8a and 8b common to arthropods and nematodes (fig. 3b). These results suggest that H. robusta has taken the route of gene duplication rather than alternate splicing to increase sequence diversity in its α-actinin gene. In addition, the observation that each gene duplicate can only express one of the exon 8 variants fits well with the subfunctionalization model. The subfunctionalization model involves the degeneration of different functions in each of the duplicates such that both duplicates are required to complement the functions of the preduplicate gene (Force et al. 1999). In H. robusta, degeneration of functions mediated by exon 8a or 8b was observed in α-actinin duplicates. However, Capitella sp I, another annelid, has only one α-actinin gene and no variants created by alternative splicing of exon 8, suggesting that H. robusta may be an exception among annelids. Besides the three actinin genes observed in H. robusta, we were able to identify possible tandem gene duplications in N. vectensis, Daphnia pulex, and B. mori. However, on closer inspection, the gene duplicates from N. vectensis and D. pulex are almost identical at the DNA-sequence level, a finding that could be explained either by genome assembly errors or very recent gene duplication. In contrast, the B. mori gene duplicate has considerable divergence at the DNA-sequence level and therefore is more likely to be due to a gene-duplication event followed by degeneration. In support of this finding, B. mori is known to have more genes compared with Drosophila (Xia et al. 2004).
Loss of PDZ-Domain Consensus Site in Nematodes and Flatworms
An analysis of the C-terminal of actinin revealed a highly conserved class I PDZ-binding site (S/T-X-ψ-COOH), where X is any amino acid and ψ is hydrophobic (Stiffler et al. 2007). The consensus sequence is not present in either yeast or slime mold α-actinin, first emerging prior to the origin of metazoans (based on its presence in M. brevicollis) and subsequently conserved throughout the metazoan lineage. The nematodes, however, have lost the last three amino acids and remain an exception to this conservation. The phylogenetic tree (fig. 4) suggests the loss of this consensus site occurred after the divergence from arthropods. Furthermore, the nematode sequences available suggest this loss is confined to the class Secernentea. Similarly, the loss in platyhelminths also appears to be confined to a class, suggesting the loss of this consensus site occurred independently twice in evolution.
FIG. 4.

Maximum likelihood tree generated from the largest overlapping region (last 156 residues) of partial α-actinin protein sequences from nematodes and platyhelminthes. The arrows indicate where the PDZ-binding site was lost from the C-terminal of α-actinin. Numbers at the branch points represent the bootstrap values from 1,000 replicates.
Discussion
The α-actinin gene of the closest living relative to metazoans, M. brevicollis is intron-poor, whereas the early metazoan, N. vectensis is intron-rich. This suggests that either an ancient eukaryotic ancestor was intron-rich and there was massive intron loss in M. brevicollis or there was a massive intron invasion during early metazoan evolution. Given that the α-actinin genes of M. brevicollis and Dictyostelium discoideum are intron-poor, the most parsimonious scenario is the latter. The majority of the introns in vertebrate α-actinins have been conserved from N. vectensis, whereas massive intron loss is observed in some arthropods. In general, N. vectensis genes showed high conservation of introns with vertebrates, but there was considerable intron loss in Drosophila (Putnam et al. 2007). There has been little intron gain within the α-actinin gene with the largest number observed in the arthropod B. mori. This may be expected as compared with D. melanogaster genes, B. mori genes are larger with more exons, possibly due to an increase in transposable elements (Xia et al. 2004).
The interaction between α-actinin and the PDZ/LIM proteins Actinin-associated LIM protein (ALP) and Z-band alternatively spliced PDZ-motif protein (ZASP) is well characterized in mouse (Pashmforoush et al. 2001; Zhou et al. 2001), C. elegans (Han and Beckerle 2009), and D. melanogaster (Jani and Schock 2007). This interaction is mediated by two interaction sites, between the ZM motif and the α-actinin spectrin repeats and between the PDZ domain and the last three residues of α-actinin (Klaavuniemi et al. 2004; Klaavuniemi and Ylanne 2006). Both interaction sites in isolation can colocalize to α-actinin; however, only mutations in the PDZ domain disrupt localization to α-actinin (Zhou et al. 2001). Ablation of ZASP appears to affect structural integrity and maintenance at the muscle Z-lines (Zhou et al. 2001; Jani and Schock 2007). Surprisingly, yeast two hybrid (Li et al. 2004) and colocalization (Han and Beckerle 2009) result from the nematode C. elegans suggest that the interaction between ALP and α-actinin is still maintained despite the loss of the PDZ-binding site. Therefore, either the ZM motif is maintaining this interaction and/or the PDZ domain is interacting with the spectrin-like repeats that was originally reported (Xia et al. 1997).
The α-actinin variants 8a and 8b are conserved among all arthropods and some nematodes and platyhelminths. In addition, these variants are also conserved in a subset of vertebrate α-actinin paralogues (fig. 5). The identification of variant 8b in ACTN4 is a novel finding and may have therapeutic applications, because mutations in the 8a variant exon cause a rare kidney disease (Kaplan et al. 2000). Therefore, strategies designed to promote replacement of the 8a exon with the 8b variant may act to ameliorate the severity of this disease, assuming that the 8a exon does not encode essential kidney-related functions. In addition, the design of the K255E Actn4 knock-in mouse involved the insertion of a neomycin cassette 400 bp after exon 8a (Yao et al. 2004). This is unlikely to disrupt exon 8b splicing as it lies approximately 1.5 kb after exon 8a, but it nonetheless highlights the importance of identifying possible functional regions in introns before disrupting them to create mouse models. The human ACTN2 8a variant is a brain-specific isoform; however, it is not conserved in all species and in particular mouse and rat (fig. 5). This suggests that studies of α-actinin-2 in cells isolated from rat hippocampus cells (Wyszynski et al. 1997) may not be accurate models for human biology.
The mutually exclusive alternative splice forms of exon 8 (8a and 8b) modify the C-terminal sequence of the CH2 domain and may function as a way of altering actin-binding affinity and regulation. There are several observations that support this hypothesis. First, the CH1 domain is essential for actin binding, whereas the CH2 domain is thought to act as a modifier for actin binding (Gimona et al. 2002). Second, mutations in exon 8 cause a structural kidney disease, focal segmental glomerulosclerosis (FSGS) (Kaplan et al. 2000), suggesting exon 8 is important for normal function. In addition, the Actn4 K255E mutation within exon 8 associated with FSGS results in an increased affinity for actin and loss of calcium-regulated actin binding (Weins et al. 2007). Last, in D. melanogaster, variants 8a and 8b create a muscle and nonmuscle isoform (Roulier et al. 1992). In vertebrate nonmuscle α-actinins, variants 19a and 19b create calcium-sensitive and nonsensitive isoforms, whereas muscle α-actinins only encode the calcium-insensitive variant (Waites et al. 1992). Furthermore, putative alternate splicing of exon 6 in molluscs and annelids and exon 7 in B. floridae also acts to modify the sequence of the CH2 domain. Thus, the alternate splicing within the CH2 domain may allow actinin to vary its actin-binding properties. Human but not mouse brain expresses an alternate ACTN2 exon 8. Based on the observations noted above, this alternate isoform may have functional significance for brain development and function in humans.
The 8c variant identified in D. melanogaster larval muscle tissue (Roulier et al. 1992) could not be identified in any other arthropod species and was only identified in genomes of species within the Drosophila genus. The 8c variant is actually an extension of exon 8a and not another duplication of exon 8. In addition, the correct splicing of exon 8 in D. melanogaster is dependent on muscleblind (Machuca-Tzili et al. 2006). Therefore, it is possible that the 8c variant is an incorrectly spliced exon 8a that has coincidently conserved the reading frame and may be considered as just noise in the splicing machinery (Melamud and Moult 2009).
In summary, there are two known regions of alternate splicing (exon 8 and exon 19) in α-actinin genes that have both been created by exon-duplication events. This is evident by comparing the high protein-sequence similarity and conservation of intron phase between the two exons. In addition, we have identified more regions of exon duplication that have intact splice acceptor and donor sites and are therefore likely to create additional splice variants. Variants 6a and 6b are conserved in molluscs and annelids and in the draft genomes of chicken and zebra finch (fig. 5). Interestingly, all exon duplications discovered among invertebrates are confined to the actin-binding domain, and none were detected in the four spectrin-like repeats. This suggests that exon duplications may act primarily as a mechanism to alter actin-binding properties.
The mechanisms of exon duplication and gene duplication, which result in alternative splicing and expanded gene families, respectively, allow for variation in gene function without compromising existing functions. Our analysis has shown that the invertebrate α-actinins have typically achieved this variation in function through exon duplication within a single α-actinin gene, allowing for tissue-specific alternative splicing. In contrast, vertebrate α-actinins have four gene duplicates and alternate splice isoforms, thus permitting richer variation in gene function and expression.
Acknowledgments
We would like to thank the various sources: US Department of Energy Joint Genome Institute, The Genome Center at Washington University School of Medicine in St Louis, The Wellcome Trust Sanger Institute, The Broad Institute, The Human Genome Sequencing Center at Baylor College of Medicine, and the J. Craig Venter Institute (outlined in table 1) for making unpublished sequence data available for our analysis.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Beggs AH, Byers TJ, Knoll JH, Boyce FM, Bruns GA, Kunkel LM. Cloning and characterization of two human skeletal muscle alpha-actinin genes located on chromosomes 1 and 11. J Biol Chem. 1992;267:9281–9288. [PubMed] [Google Scholar]
- Blanchard A, Ohanian V, Critchley D. The structure and function of alpha-actinin. J Muscle Res Cell Motil. 1989;10:280–289. doi: 10.1007/BF01758424. [DOI] [PubMed] [Google Scholar]
- Djinovic-Carugo K, Gautel M, Ylanne J, Young P. The spectrin repeat: a structural platform for cytoskeletal protein assemblies. FEBS Lett. 2002;513:119–123. doi: 10.1016/s0014-5793(01)03304-x. [DOI] [PubMed] [Google Scholar]
- Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151:1531–1545. doi: 10.1093/genetics/151.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimona M, Djinovic-Carugo K, Kranewitter WJ, Winder SJ. Functional plasticity of CH domains. FEBS Lett. 2002;513:98–106. doi: 10.1016/s0014-5793(01)03240-9. [DOI] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Han HF, Beckerle MC. The ALP-Enigma protein ALP-1 functions in actin filament organization to promote muscle structural integrity in Caenorhabditis elegans. Mol Biol Cell. 2009;20:2361–2370. doi: 10.1091/mbc.E08-06-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Yamada T, Endo R, Ino Y, Gotoh M, Tsuda H, Yamada Y, Chiba H, Hirohashi S. Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J Cell Biol. 1998;140:1383–1393. doi: 10.1083/jcb.140.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jani K, Schock F. Zasp is required for the assembly of functional integrin adhesion sites. J Cell Biol. 2007;179:1583–1597. doi: 10.1083/jcb.200707045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JM, Kim SH, North KN, et al. (11 co-authors) Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24:251–256. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- Klaavuniemi T, Kelloniemi A, Ylanne J. The ZASP-like motif in actinin-associated LIM protein is required for interaction with the alpha-actinin rod and for targeting to the muscle Z-line. J Biol Chem. 2004;279:26402–26410. doi: 10.1074/jbc.M401871200. [DOI] [PubMed] [Google Scholar]
- Klaavuniemi T, Ylanne J. Zasp/Cypher internal ZM-motif containing fragments are sufficient to co-localize with alpha-actinin–analysis of patient mutations. Exp Cell Res. 2006;312:1299–1311. doi: 10.1016/j.yexcr.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Kondrashov FA, Koonin EV. Origin of alternative splicing by tandem exon duplication. Hum Mol Genet. 2001;10:2661–2669. doi: 10.1093/hmg/10.23.2661. [DOI] [PubMed] [Google Scholar]
- Li S, Armstrong CM, Bertin N, et al. (48 co-authors) A map of the interactome network of the metazoan C. elegans. Science. 2004;303:540–543. doi: 10.1126/science.1091403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur DG, North KN. A gene for speed? The evolution and function of alpha-actinin-3. Bioessays. 2004;26:786–795. doi: 10.1002/bies.20061. [DOI] [PubMed] [Google Scholar]
- Machuca-Tzili L, Thorpe H, Robinson TE, Sewry C, Brook JD. Flies deficient in Muscleblind protein model features of myotonic dystrophy with altered splice forms of Z-band associated transcripts. Hum Genet. 2006;120:487–499. doi: 10.1007/s00439-006-0228-8. [DOI] [PubMed] [Google Scholar]
- Melamud E, Moult J. Stochastic noise in splicing machinery. Nucleic Acids Res. 2009;37:4873–4886. doi: 10.1093/nar/gkp471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noegel A, Witke W, Schleicher M. Calcium-sensitive non-muscle alpha-actinin contains EF-hand structures and highly conserved regions. FEBS Lett. 1987;221:391–396. doi: 10.1016/0014-5793(87)80962-6. [DOI] [PubMed] [Google Scholar]
- Pashmforoush M, Pomies P, Peterson KL, Kubalak S, Ross J, Jr., Hefti A, Aebi U, Beckerle MC, Chien KR. Adult mice deficient in actinin-associated LIM-domain protein reveal a developmental pathway for right ventricular cardiomyopathy. Nat Med. 2001;7:591–597. doi: 10.1038/87920. [DOI] [PubMed] [Google Scholar]
- Putnam NH, Srivastava M, Hellsten U, et al. (19 co-authors) Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- Roulier EM, Fyrberg C, Fyrberg E. Perturbations of Drosophila alpha-actinin cause muscle paralysis, weakness, and atrophy but do not confer obvious nonmuscle phenotypes. J Cell Biol. 1992;116:911–922. doi: 10.1083/jcb.116.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler MA, Chen JR, Grantcharova VP, Lei Y, Fuchs D, Allen JE, Zaslavskaia LA, MacBeath G. PDZ domain binding selectivity is optimized across the mouse proteome. Science. 2007;317:364–369. doi: 10.1126/science.1144592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virel A, Backman L. Molecular evolution and structure of alpha-actinin. Mol Biol Evol. 2004;21:1024–1031. doi: 10.1093/molbev/msh094. [DOI] [PubMed] [Google Scholar]
- Virel A, Backman L. A comparative and phylogenetic analysis of the alpha-actinin rod domain. Mol Biol Evol. 2007;24:2254–2265. doi: 10.1093/molbev/msm168. [DOI] [PubMed] [Google Scholar]
- von Nandelstadh P, Ismail M, Gardin C, Suila H, Zara I, Belgrano A, Valle G, Carpen O, Faulkner G. A class III PDZ binding motif in myotilin and FATZ families binds Enigma family proteins—a common link for Z-disc myopathies. Mol Cell Biol. 2008;29:822–834. doi: 10.1128/MCB.01454-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites GT, Graham IR, Jackson P, Millake DB, Patel B, Blanchard AD, Weller PA, Eperon IC, Critchley DR. Mutually exclusive splicing of calcium-binding domain exons in chick alpha-actinin. J Biol Chem. 1992;267:6263–6271. [PubMed] [Google Scholar]
- Weins A, Schlondorff JS, Nakamura F, Denker BM, Hartwig JH, Stossel TP, Pollak MR. Disease-associated mutant alpha-actinin-4 reveals a mechanism for regulating its F-actin-binding affinity. Proc Natl Acad Sci U S A. 2007;104:16080–16085. doi: 10.1073/pnas.0702451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witke W, Schleicher M, Lottspeich F, Noegel A. Studies on the transcription, translation, and structure of alpha-actinin in Dictyostelium discoideum. J Cell Biol. 1986;103:969–975. doi: 10.1083/jcb.103.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JQ, Bahler J, Pringle JR. Roles of a fimbrin and an alpha-actinin-like protein in fission yeast cell polarization and cytokinesis. Mol Biol Cell. 2001;12:1061–1077. doi: 10.1091/mbc.12.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski M, Lin J, Rao A, Nigh E, Beggs AH, Craig AM, Sheng M. Competitive binding of alpha-actinin and calmodulin to the NMDA receptor. Nature. 1997;385:439–442. doi: 10.1038/385439a0. [DOI] [PubMed] [Google Scholar]
- Xia H, Winokur ST, Kuo WL, Altherr MR, Bredt DS. Actinin-associated LIM protein: identification of a domain interaction between PDZ and spectrin-like repeat motifs. J Cell Biol. 1997;139:507–515. doi: 10.1083/jcb.139.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Q, Zhou Z, Lu C, et al. (83 co-authors) A draft sequence for the genome of the domesticated silkworm (Bombyx mori) Science. 2004;306:1937–1940. doi: 10.1126/science.1102210. [DOI] [PubMed] [Google Scholar]
- Yao J, Le TC, Kos CH, Henderson JM, Allen PG, Denker BM, Pollak MR. Alpha-actinin-4-mediated FSGS: an inherited kidney disease caused by an aggregated and rapidly degraded cytoskeletal protein. PLoS Biol. 2004;2:e167. doi: 10.1371/journal.pbio.0020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Chu PH, Huang C, Cheng CF, Martone ME, Knoll G, Shelton GD, Evans S, Chen J. Ablation of Cypher, a PDZ-LIM domain Z-line protein, causes a severe form of congenital myopathy. J Cell Biol. 2001;155:605–612. doi: 10.1083/jcb.200107092. [DOI] [PMC free article] [PubMed] [Google Scholar]




