Abstract
Through the combined use of 18F-fallypride positron emission tomography and magnetic resonance imaging this study examined the neural mechanisms underlying the attentional deficits associated with attention deficit/hyperactivity disorder and their potential reversal with a single therapeutic dose of methylphenidate. Sixteen adult patients with attention deficit/hyperactivity disorder and 16 matched healthy control subjects were positron emission tomography and magnetic resonance imaging scanned and tested on a computerized sustained attention task after oral methylphenidate (0.5 mg/kg) and placebo administration in a within-subject, double-blind, cross-over design. Although patients with attention deficit/hyperactivity disorder as a group showed significant attentional deficits and reduced grey matter volume in fronto-striato-cerebellar and limbic networks, they had equivalent D2/D3 receptor availability and equivalent increases in endogenous dopamine after methylphenidate treatment to that observed in healthy control subjects. However, poor attentional performers drawn from both the attention deficit/hyperactivity disorder and the control groups had significantly reduced left caudate dopamine activity. Methylphenidate significantly increased dopamine levels in all nigro-striatal regions, thereby normalizing dopamine levels in the left caudate in low performers. Behaviourally, methylphenidate improved sustained attention in a baseline performance-dependent manner, irrespective of diagnosis. This finding was accompanied by an equally performance-dependent effect of the drug on dopamine release in the midbrain, whereby low performers showed reduced dopamine release in this region. Collectively, these findings support a dimensional model of attentional deficits and underlying nigro-striatal dopaminergic mechanisms of attention deficit/hyperactivity disorder that extends into the healthy population. Moreover, they confer midbrain dopamine autoreceptors a hitherto neglected role in the therapeutic effects of oral methylphenidate in attention deficit/hyperactivity disorder. The absence of significant case–control differences in D2/D3 receptor availability (despite the observed relationships between dopamine activity and attention) suggests that dopamine dysregulation per se is unlikely to be the primary cause underlying attention deficit/hyperactivity disorder pathology in adults. This conclusion is reinforced by evidence of neuroanatomical changes in the same set of patients with attention deficit/hyperactivity disorder.
Keywords: attention deficit/hyperactivity disorder, 18F-fallypride PET, dopamine, methylphenidate, sustained attention
Introduction
Attention deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by symptoms of inattention and/or hyperactivity/impulsivity. Despite its declining prevalence with age, ADHD symptoms in some affected patients persist into adulthood, affecting ∼2.5% of the adult population (Simon et al., 2009). The clinical effectiveness of stimulants such as methylphenidate in the treatment of ADHD suggests a putative role for both dopamine and noradrenaline in the manifestation of the disorder (Castells et al., 2011). However, despite decades of genetic, clinical and neuroimaging research, the precise neurobiological mechanisms underlying the disorder and its treatment remain poorly understood.
Long-standing cognitive theories of ADHD postulate a deficiency in top–down cognitive control processes underpinning attention and executive function deficits (Barkley, 1997; Nigg, 2000; Bush et al., 2005; Kuntsi et al., 2006; Paloyelis et al., 2007 for review see Del Campo et al., 2012). Providing support to these theories, one of the best replicated findings in ADHD is that of reduced grey matter volume in the catecholamine-rich fronto-striato-cerebellar circuits known to subserve these cognitive functions (Seidman et al., 2005; Nakao et al., 2011). Notwithstanding the growing evidence on fronto-striatal dysfunction and the efficacy of stimulant drugs in the clinical management of patients with ADHD, the precise role of dopamine in the pathophysiology of the disorder is still unclear. Indeed, in vivo nuclear imaging techniques such as PET and single photon emission computed tomography (SPECT) have often yielded inconsistent findings regarding the state of the dopamine system in this patient population (Del Campo et al., 2011a). A recent set of case–control PET studies in adult medication-naïve patients found that ADHD is associated with reduced dopamine transporter and dopamine D2/D3 receptor availability in selected brain regions of the left hemisphere, including the nucleus accumbens, caudate and midbrain (Volkow et al., 2007a, b, 2009). Although these impressive studies had substantial numbers of patients, there was considerable overlap with the control population in terms of both dopamine receptor and transporter availabilities, implying that the diagnostic utility of these markers is limited.
The binding competition between D2/D3 receptor radioligands and endogenous dopamine allows measuring changes in dopamine levels following a pharmacological challenge, for example a psychostimulant. Acute administration of methylphenidate would be expected to increase endogenous dopamine, leading to a reduction of D2/D3 receptor radioligand binding through competitive displacement. To date there are two studies that have used this imaging paradigm to examine the endogenous tone of the dopamine system in patients with ADHD. One study in adolescent patients with ADHD found that oral methylphenidate-induced increases in dopamine concentrations in the right striatum were greater in patients showing poor performance on a computerized continuous performance test relative to high performing patients (Rosa-Neto et al., 2005). A subsequent study of intravenous methylphenidate effects in adult patients with ADHD showed blunted dopamine responses to methylphenidate in the caudate in patients compared with control subjects, although there was no concurrent objective measure of cognitive performance (Volkow et al., 2007b).
Although it is possible that the state of the striatal dopamine system changes with age in ADHD, in this dopamine receptor PET imaging study we sought to resolve the above discrepancy by investigating the effects of oral methylphenidate on both endogenous dopamine levels and sustained attention in adult patients with ADHD compared with healthy matched control subjects using a within-subject, counter-balanced placebo-controlled design. High resolution MRI scans were also acquired and analysed for case–control differences in grey matter volume to allow, for the first time in the ADHD literature, the interpretation of the PET results in the light of potential brain tissue abnormalities. The full set of MRI data, including also diffusion tensor imaging data, will be reported in more detail elsewhere.
As a methodological innovation, which further distinguishes the current study from previous ones, we used the co-registered high resolution magnetic resonance images to accurately localize PET signals within the striatum and the midbrain. We employed a model of functional rather than purely anatomical subdivisions of the striatum (Haber et al., 2000) that has been increasingly used to optimize the analysis of PET studies (Kegeles et al., 2010). This model is based on evidence that cortico-striatal networks follow a ventro-medial to dorsolateral gradient, with activity along this gradient modulating limbic, cognitive and motor processing (Martinez et al., 2003). Through its connections with the striatum, the midbrain operates as an interface for the dynamic processing of striatal function. According to preclinical evidence, stimulant-induced increases in endogenous dopamine levels trigger negative feedback mechanisms that inhibit dopamine neuron firing (Bunney and Achajanian, 1976). These negative feedback mechanisms are mediated by somato-dendritic D2/D3 autoreceptors located on midbrain dopamine neurons, which play a key role in regulating dopamine synthesis and release by acting as potent inhibitors in the presence of high dopamine concentrations (Mercuri et al., 1992, 1997; Sesack et al., 1994; Khan et al., 1998). How the therapeutic effects of methylphenidate might depend on the afferent control of midbrain dopamine neurons has been unexplored to date. This question is particularly relevant because findings for both juvenile and adult ADHD have implicated functional and structural midbrain abnormalities in the pathophysiology of the disorder (Ernst et al., 1999; Jucaite et al., 2005; Volkow et al., 2009; Romanos et al., 2010), also consistent with experimental animal models of ADHD (Leo et al., 2003).
To investigate nigro-striatal mechanisms underlying ADHD and its treatment with methylphenidate, we used PET imaging of 18F-fallypride, which has a higher affinity than 11C-raclopride (the radioligand of choice in all previous dopamine receptor imaging studies in ADHD) and is thus better suited for simultaneous measurement of D2/D3 receptor availability in striatal regions and the midbrain (Riccardi et al., 2006a, b; Cropley et al., 2008; Slifstein et al., 2010). Through estimation of non-displaceable 18F-fallypride binding potential (BPND), the following questions regarding the neurochemical underpinnings of ADHD were addressed.
First, do patients with ADHD have reduced D2/D3 receptor availability in the striatum and the midbrain compared with healthy volunteers? Second, how is D2/D3 receptor availability in these regions associated with inattention, a core feature of the disorder (Biederman et al., 2000; Nigg, 2005)? Third, how does a therapeutic oral dose of methylphenidate affect D2/D3 receptor availability in the striatum and the midbrain?, and fourth, how are the effects on receptor availability related to drug-induced changes on performance on a task of sustained attention previously reported to be sensitive to single oral doses of this drug (Turner et al., 2005)? Finally, are the observed drug effects on receptor availability quantitatively different in patients with ADHD and healthy volunteers?
The latter question is especially topical (Sahakian and Morein-Zamir, 2007) because methylphenidate has been reported to augment several aspects of cognition in healthy subjects (Rapoport et al., 1978, 1980; Strauss et al., 1984; Koelega, 1993; Elliott et al., 1997; Mehta et al., 2000) similar to those improved in ADHD (Mehta et al., 2004; Turner et al., 2005; Clark et al., 2007; Spencer et al., 2007). The effects of methylphenidate and related stimulant drugs in healthy individuals have been shown to be baseline-dependent; i.e. those subjects with relatively low performance levels exhibit greater benefit after the drug (Naylor et al., 1985; Rogers et al., 1999; Mehta et al., 2000; Turner et al., 2003; Clatworthy et al., 2009). These findings can be interpreted in the light of a hypothesized inverted U-shaped function relating dopamine and noradrenaline activity to performance (Robbins and Sahakian, 1979). Of course, it is also possible that the cognitive enhancing effects in patients may similarly be dependent on baseline-dependent considerations.
According to previous reports of altered brain tissue volume and dysregulated dopamine neurotransmission in ADHD, we hypothesized that patients with ADHD would show reduced grey matter volume and D2/D3 receptor availability in selected brain regions involved in dopamine-modulated circuits (Volkow et al., 2007b, 2009). We also hypothesized that methylphenidate would increase endogenous dopamine levels in both ADHD and healthy control subjects, improving attention in patients with ADHD and possibly also in some healthy volunteers as a function of baseline performance. In accordance with previous evidence linking abnormalities in the left caudate with attentional deficits, we expected dopamine dysregulation in this region to be specifically associated with impaired sustained attention on the computerized cognitive test.
Materials and methods
Subjects
Sixteen male adult patients with ADHD and 16 control subjects matched for gender, age and IQ were enrolled in the study after meeting inclusion criteria and providing written informed consent. The study was approved by the Cambridge Research Ethics Committee, The UK Administration of Radioactive Substances Advisory Committee and was formally exempted from clinical trial status by the UK Medicines and Healthcare Regulatory Agency.
Patients were recruited from the Adult ADHD Research Clinic Cambridge and through the Attention Deficit Disorder Information and Support Service (ADDISS), UK. Diagnosis was contingent upon six of nine DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, fourth edition) inattention and/or hyperactivity/impulsivity criteria being met during childhood and the previous 6 months (1994). Clinical assessments were performed by two psychiatrists specialized in adult ADHD (J.D., U.M.) as described previously (Chamberlain et al., 2007). Of the 16 patients, 15 met criteria for ‘combined type’ and one for ‘inattentive type’. Seven patients had a history of prescribed medication with methylphenidate, and one had previously been exposed to atomoxetine through participation in a past research study. The remaining patients (n = 8) were ADHD medication-naïve. Patients on methylphenidate treatment at the time of scanning were asked to discontinue their medication at least 3 days before each PET session.
All participants underwent an extended clinical interview using the Mini International Neuropsychiatric Inventory (MINI) (Sheehan et al., 2010). Exclusion criteria were the presence of axis-I disorders or any major neurological or internal disease, left handedness, verbal IQ <90 [National Adult Reading Test (NART)] (Nelson, 1982) and current cigarette smoking and/or drug abuse, as controlled by urine screening (Euromed, Drug Screen Card DOA-154-731, 5072KAB). Participants were asked to abstain from drinking alcoholic or caffeine-containing drinks for 12 h before each testing session.
Experimental design and cognitive assessment
Participants were enrolled into a randomized, double-blind placebo-controlled cross-over design, consisting of two 18F-fallypride PET scans at least a week apart and one MRI scan. On each PET session, subjects were administered a capsule containing either 0.5 mg/kg of methylphenidate or placebo 75 min before the 18F-fallypride injection (Fig. 1). The dose of 0.5 mg/kg was chosen to be within the therapeutic range used in the clinic: according to the NICE 2008 guidelines regarding the diagnosis and treatment of patients with ADHD, starting methylphenidate doses of 3 × 5 mg or 3 × 10 mg and a dose limit of 100 mg per day are recommended. A dose of 0.5 mg/kg equals 40 mg for an 80 kg patient, representing thus a medium dose for a single dose study. At 0, 2 and 4 h after capsule administration, blood samples were collected for determination of methylphenidate plasma concentrations (Supplementary material) to assess compliance with drug discontinuation in medicated patients and account for between-subject differences in methylphenidate plasma levels. Cardiovascular measures were regularly monitored throughout the study.
Figure 1.
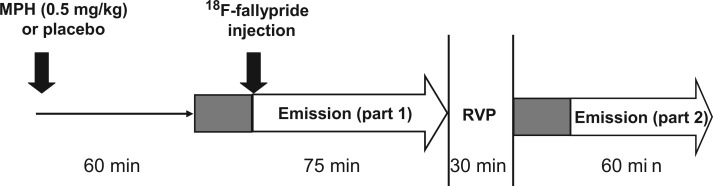
Time line for PET and RVP data acquisition after capsule administration. Grey boxes indicate 15 min transmission scans.
On both PET sessions, participants performed the Rapid Visual Information Processing (RVP) task from CANTAB (Cambridge Automated Neuropsychological Testing Battery, www.camcog.com). RVP primarily measures sustained attention, defined as the ability to maintain attention on a series of numerical stimuli over a period of time, to detect infrequent targets (Coull et al., 1996). The task was administered during a scanning break 2.5 h after capsule intake (Fig. 1), coinciding with the estimated peak effectiveness of methylphenidate (Challman and Lipsky, 2000). Performance on the RVP task was measured by the non-parametric signal detection parameter A’ (discriminative sensitivity) (McNicol, 1972). The selection of RVP A’ to measure attentional performance in this study is supported by evidence that this parameter (i) is a suitable ADHD endophenotype (Gau and Huang, 2013); (ii) is associated with activity in neural networks including frontal and striatal regions (Lawrence et al., 2003); and (iii) is sensitive to the enhancing effects of drugs known to be effective in ADHD, previously reported in both patients with ADHD (Turner et al., 2005) and healthy control subjects (Crockett et al., 2010; Supplementary material). Response latencies were also measured. To minimize practice effects, all subjects performed the task once before their first study session.
All participants completed the adult ADHD self-rating scale (v1.1), a short self-rating test addressing the severity of ADHD symptoms in adults consistent with DSM-IV criteria (Kessler et al., 2005).
Imaging
Magnetic resonance acquisition
Magnetic resonance images were acquired for all subjects on a Siemens Trio 3 T system (Siemens Medical Systems) with gradient coils capable of 45 mT/m and 200 T/m/s slew rate. A 12-channel total-imaging matrix head-coil was used to transmit/receive radio-frequency magnetic resonance signals. T1-weighted anatomical scans were acquired using a 3D MP-RAGE pulse sequence with the following parameters: repetition time/echo time/inversion time = 2300 ms/2.98 ms/900 ms, flip angle 9°, 176 slices, 256 × 256 matrix size, 1 × 1 × 1 mm3 voxel size, echo spacing = 7.1 ms, bandwidth = 240 Hz per pixel. Images were manually aligned to the anterior commissure (AC) – posterior commissure (PC) line. A T2-weighted image was also acquired for each subject (echo time = 104 ms, 27 slices, 0.7 × 0.7 × 4 mm3 voxel size).
Positron emission tomography acquisition
18F-fallypride PET data were acquired in 3D mode on a GE Advance scanner (General Electric Medical Systems). Before 18F-fallypride injection, a 15 min transmission scan was acquired. 18F-fallypride was injected intravenously over 30 s, with a mean activity of 103.5 MBq (range: 52.2–119.8 MBq), and a fallypride mass of 1.3 ± 0.7 nmols.
From the start of the 18F-fallypride injection PET emission data were continuously acquired for 75 min in 55 frames (Fig. 1). After a 30 min break, subjects were repositioned in the scanner and after another 15-min transmission scan, a second set of 18F-fallypride data were acquired over the next hour. Images were reconstructed using the PROMIS 3D filtered back-projection algorithm (Kinahan and Rogers, 1989) into 2.34 × 2.34 × 4.25 mm voxels.
Magnetic resonance imaging preprocessing and grey matter volume analysis
All MPRAGE scans were manually aligned to the AC–PC line (MRAC-PC). Before analysis, volumes were preprocessed, and then spatially normalized and segmented into grey and white matter tissue using the unified segmentation model in SPM5 (Ashburner and Friston, 2005). Statistical case–control differences in tissue volume were assessed through a non-parametric, permutation inference method using Cambridge Brain Analysis v1.3.2 (CAMBA; http://www-bmu.psychiatry.cam.ac.uk/software/) (Bullmore et al., 1999). This methodology confers a number of advantages, notably the enhancement of sensitivity and the control for type I errors. For each between-group comparison, the statistical threshold of β < 0.05 was applied. Results were corrected for multiple comparisons by controlling the family-wise error rate and setting the number of false positive clusters expected under the null hypothesis to <1. Clusters showing significant between-group differences were described in terms of the Automated Anatomical Labelling (AAL) template image (where voxels/region > 20) (Tzourio-Mazoyer et al., 2002).
Positron emission tomography realignment and co-registration to magnetic resonance imaging
Images from each PET scan were realigned and co-registered to the MRAC-PC using SPM2 (www.fil.ion.ucl.ac.uk/spm) as previously described (Del Campo et al., 2011b).
Region of interest delineation
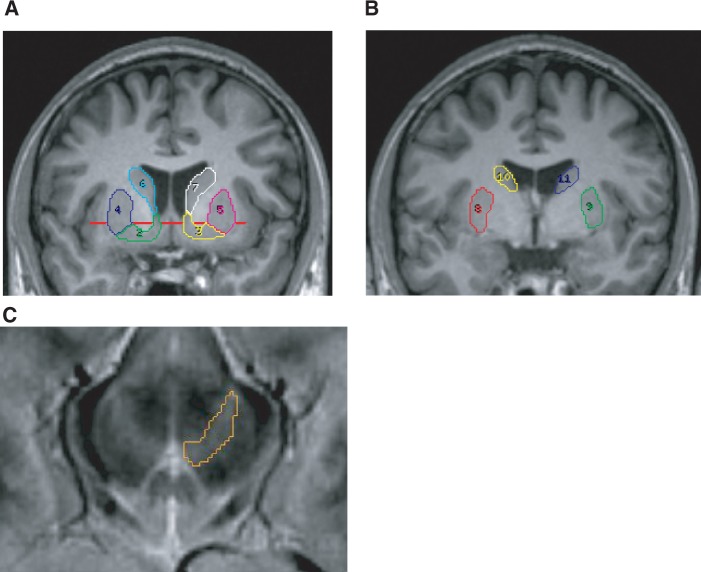
For each subject, regions of interest were manually drawn on coronal planes of the MRAC-PC with Analyze 7.0 (AnalyzeDirect) as previously described (Mawlawi et al., 2001; Martinez et al., 2003; Del Campo et al., 2011b). The following striatal subregions were defined bilaterally: ventral striatum, pre-commissural dorsal putamen, pre-commissural dorsal caudate, post-commissural putamen and post-commissural caudate (Fig. 2).
Figure 2.
(A and B) Coronal planes through an AC–PC aligned MPRAGE image showing striatal regions of interest. (A) Ventral striatum (2, 3), pre-commissural dorsal putamen (4, 5), pre-commissural dorsal caudate (6, 7); (B) post-commissural putamen (8, 9) and post-commissural caudate (10, 11). The red line helped to divide the pre-commissural caudate and putamen into ventral and dorsal. (C) Midbrain region of interest defined on a T2 scan co-registered to the MRAC-PC.
These subregions allowed classification of the striatum into the following functional modules: ventral striatum; associative striatum, comprising pre-commissural dorsal caudate, post-commissural caudate and pre-commissural dorsal putamen and sensorimotor striatum, consisting of the post-commissural putamen (Haber et al., 2000; Joel and Weiner, 2000; Haber, 2003). Evidence suggests that through their involvement in different cortico-striatal networks, these modules are functionally distinct, with the ventral striatum being implicated in emotion, motivation and reward-guided behaviours, the associative striatum in cognition, and the sensorimotor striatum in motor function (Martinez et al., 2003). The aggregate of bilateral ventral striatum, associative striatum and sensorimotor striatum is henceforth referred to as total striatum.
Bilateral midbrain regions of interest including the substantia nigra and the ventral tegmental area were delineated on transverse planes of the MRAC-PC-coregistered T2-weighted image as described elsewhere (Foster et al., 2008). To allow BPND values to be calculated using reference tissue modelling, a bilateral cerebellar region was drawn on the MRAC-PC.
Regional quantification of binding potential
Regional BPND was estimated by fitting the simplified reference tissue model (Gunn et al., 1997) to region of interest time activity curves. The cerebellar time activity curve between 75–120 min post-injection was estimated by fitting an exponential function from 50 min onwards. To calculate mean BPND for associative striatum, bilateral regions and total striatum, BPND values for the constituent regions of interest were weighted in proportion to region of interest volume. Changes in receptor availability following methylphenidate relative to placebo were estimated by BPND % change:
| (1) |
Statistical analysis
Group effects on ADHD self-rating scale scores and both group and methylphenidate effects on task performance and BPND were tested using univariate and repeated measures analysis of variance (ANOVAs) with group and medication status as between-subject factors. Where assumptions of sphericity were violated, degrees of freedom were corrected by applying Greenhouse-Geisser corrections. Significant differences were further examined using post hoc t-tests or, where appropriate, the equivalent versions for non-parametric data (Wilcoxon signed-rank test and Mann-Whitney U test).
Pearson correlations and where appropriate Spearman’s rank correlations were used to describe relationships between non-imaging measures and both BPND and BPND % change. Where relevant, methylphenidate plasma concentrations were partialled out. Relationships were investigated for left and right hemispheres separately, in-line with previous observations of lateralization differences in dopaminergic parameters in the healthy population (Larisch et al., 1998; Vernaleken et al., 2007) as well as in patients with ADHD (Volkow et al., 2007a, b, 2009). To allow assessment of group differences, correlation coefficients were transformed into z-scores. To control for the overall type 1 error rate, stepwise linear regression models were also used to establish the relative contributions of 18F-fallypride BPND to attentional performance variability, both at baseline and following methylphenidate. BPND measures of all regions of interest were entered into the models. An alpha level of 0.05 was chosen as the statistical threshold.
Results
Sample characteristics and cognitive performance
Demographic characteristics of the sample and mean scores on psychological measures are shown in Table 1. Both groups were matched for age and IQ. Patients with ADHD had significantly higher ADHD self-rating scale scores (P ≤ 0.01) and performed significantly worse on the RVP A’ signal detection parameter [main effect of group: F(1,29) = 8.31, P = 0.007; placebo data only: F(1,29) = 9.89, P = 0.004]. There were no between session practice effects on task performance (P > 0.1).
Table 1.
Subject characteristics
| ADHD patients (n = 16) | Controls (n = 16) | F-value | P-value | |
|---|---|---|---|---|
| Age, years | 30.3 (7.4) | 28.9 (6.0) | 0.3 | 0.57 |
| NART | 112.0 (9.2) | 115.7 (4.4) | 2.1 | 0.16 |
| Adult Self Report Scale | ||||
| Total | 54.1 (7.9) | 25.9 (6.7) | 68.4 | <0.001 |
| Inattention | 28.1 (4.8) | 14.5 (3.6) | 48.3 | <0.001 |
| Hyperactivity | 26.1 (4.8) | 11.4 (4.8) | 43.1 | <0.001 |
| RVP performance on placebo | ||||
| A’ | 0.92 (0.05) | 0.97 (0.03) | 9.9 | 0.004 |
| Mean latency, ms | 424 (59) | 373 (40) | 3.0 | 0.09 |
Values are mean (SD).
NART = National Adult Reading Test.
Group comparisons were carried out using ANOVAs, significant P-values (<0.05) are in bold.
Grey matter volume
No group differences were found in total intracranial volume and total grey matter volume (P > 0.1). However, patients with ADHD showed significantly reduced grey matter volume in left prefrontal cortical areas (middle frontal cortex, inferior portions of the orbitofrontal cortex and gyrus rectus), and in bilateral putamen, amygdala, hippocampus, fusiform, insula and cerebellum (Table 2 and Fig. 3).
Table 2.
Regions of decreased grey matter density in ADHD patients compared with controls
| Cluster | Region name (AAL) | Location of peak (x, y, z) [mm] | Voxels | F | ||
|---|---|---|---|---|---|---|
| GM 1 503 vox | Cerebellum crus II R | 40 | −66 | −48 | 35 | 3.86 |
| Cerebellum VIIB R | 38 | −66 | −48 | 70 | 3.79 | |
| Cerebellum VIII R | 22 | −74 | −48 | 392 | 3.66 | |
| GM 2 2089 vox | Superior frontal gyrus (orbital portion) L | −16 | 16 | −20 | 55 | 3.29 |
| Inferior frontal gyrus (pars triangularis) L | −40 | 26 | 2 | 71 | 4.11 | |
| Inferior frontal orbital gyrus L | −26 | 32 | −12 | 64 | 3.51 | |
| Olfactory cortex L | −12 | 8 | −18 | 49 | 3.76 | |
| Gyrus rectus L | −6 | 38 | −20 | 65 | 4.16 | |
| Insula L | −38 | 0 | −8 | 127 | 3.80 | |
| Hippocampus L | −22 | −12 | −12 | 297 | 4.59 | |
| Parahippocampal gyrus L | −28 | −24 | −24 | 133 | 3.74 | |
| Amygdala L | −26 | −4 | −14 | 81 | 3.78 | |
| Fusiform gyrus L | −28 | −30 | −20 | 173 | 3.86 | |
| Putamen L | −28 | 2 | −6 | 92 | 3.51 | |
| Middle temporal gyrus L | −46 | −2 | −16 | 57 | 4.39 | |
| Inferior temporal gyrus L | −38 | −20 | −28 | 23 | 2.54 | |
| Cerebellum IV, V L | −14 | −54 | −24 | 184 | 3.11 | |
| Cerebellum VI L | −14 | −58 | −24 | 213 | 3.52 | |
| Vermis IV, V | −2 | −60 | −14 | 23 | 2.55 | |
| GM 3 692 vox | Hippocampus R | 30 | −30 | −10 | 107 | 3.36 |
| Parahippocampal gyrus R | 26 | −34 | −12 | 106 | 3.31 | |
| Lingual gyrus R | 28 | −48 | −6 | 28 | 3.42 | |
| Fusiform gyrus R | 38 | −22 | −26 | 106 | 4.05 | |
| Cerebellum IV, V R | 26 | −38 | −24 | 166 | 4.25 | |
| Cerebellum VI R | 36 | −46 | −32 | 144 | 3.57 | |
| GM 4 749 vox | Insula R | 40 | −8 | 2 | 114 | 3.43 |
| Hippocampus R | 34 | −6 | −18 | 56 | 5.51 | |
| Amygdala R | 30 | −4 | −18 | 79 | 4.04 | |
| Putamen R | 34 | −2 | −6 | 202 | 3.89 | |
| GM 5 197 vox | Middle frontal gyrus | −28 | 40 | 20 | 231 | 6.53 |
Regional labels are clusters of the Automated Anatomical Labelling (AAL). Locations are in the stereotactic space of the MNI. Cluster peaks are highlighted in bold.
GM = grey matter.
Figure 3.
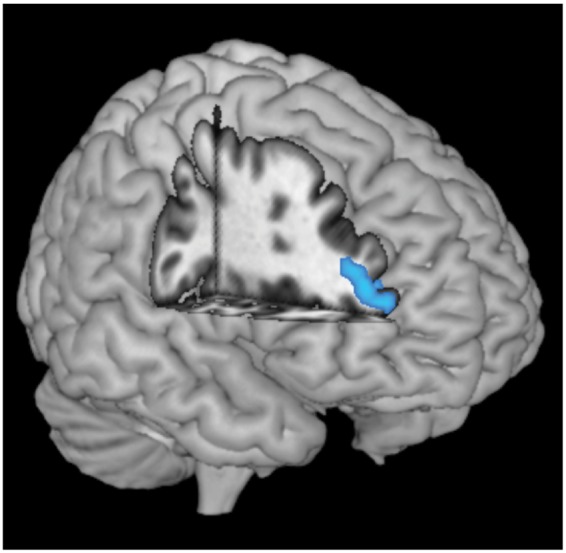
Cluster of greatest grey matter volume reduction in patients with ADHD compared with control subjects located in the left middle frontal gyrus, overlaid on a rendered standardized brain template.
For clarity, regional PET and behavioural results (and the relationship between both) are subsequently presented split by experimental session (placebo then methylphenidate). Supporting whole-brain voxel-wise PET results are presented and discussed in the Supplementary material.
Placebo results
Regional analysis of BPND on placebo
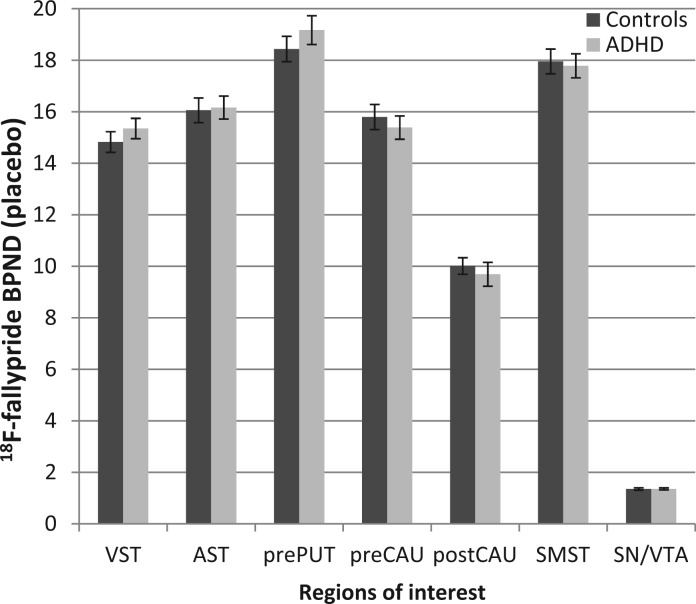
The mean BPND values were not significantly different between patients with ADHD and control subjects across all regions (either unilaterally or bilaterally), with bilateral values for the latter shown in Fig. 4. Regional BPND values pooled across all subjects are shown in Table 3. BPND values on placebo were in the same range as those previously reported in a study examining test–retest variability of baseline 18F-fallypride BPND across the same brain regions (Cropley et al., 2008) (Supplementary material).
Figure 4.
Regional variation in 18F-fallypride binding potential (BPND) on placebo in healthy control subjects and ADHD patients. Error bars denote standard error of the mean. VST = ventral striatum; AST = associative striatum; prePUT = pre-commissural putamen; preCAU = pre-commissural caudate; postCAU = post-commissural caudate; SMST = sensorimotor striatum; SN/VTA = substantia nigra/ventral tegmental area.
Table 3.
18F-fallypride BPND at baseline and after methylphenidate, pooled across all subjects
| BPND (Placebo) |
BPND (MPH) |
BPND % change (MPH relative to placebo) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Left | Right | Bilateral | Left | Right | Bilateral | Left | Right | Bilateral | |
| Striatum | 16.3 (1.6) | 16.4 (1.6) | 16.4 (1.6) | 15.3 (1.4) | 15.4 (1.5) | 15.4 (1.5) | −6.2 (5.8) | −5.1 (5.9) | −5.9 (5.5) | |
| VST | 15.3 (1.5) | 14.9 (1.7) | 15.1 (1.5) | 14.4 (1.6) | 14.5 (1.8) | 14.5 (1.6) | −5.6 (7.8) | −2.2 (10.1) | −4.0 (7.4) | |
| AST | 16.1 (1.8) | 16.2 (1.8) | 16.1 (1.8) | 15.1 (1.6) | 15.2 (1.6) | 15.2 (1.6) | −5.4 (7) | −5.5 (5.5) | −5.4 (5.9) | |
| Pre-PUT | 18.6 (2.1) | 18.9 (2.1) | 18.8 (2.0) | 17.5 (1.8) | 17.7 (1.9) | 17.6 (1.8) | −5.8 (7.5) | −6.5 (6.1) | −6.2 (5.9) | |
| Pre-CAU | 15.7 (2.0) | 15.5 (1.8) | 15.6 (1.8) | 14.8 (1.7) | 14.8 (1.7) | 14.8 (1.7) | −4.8 (7.5) | −4.6 (6.3) | −4.8 (6.2) | |
| Post-CAU | 9.9 (1.6) | 9.9 (1.7) | 9.9 (1.5) | 9.2 (1.5) | 9.3 (1.6) | 9.2 (1.5) | −6.4 (10) | −5.6 (10.3) | −6.1 (9.1) | |
| SMST | Post-PUT | 17.8 (2.0) | 17.9 (1.8) | 17.9 (1.8) | 16.4 (1.7) | 16.5 (1.7) | 16.5 (1.7) | −7.5 (7.2) | −7.7 (6.4) | −7.6 (6.1) |
| SN/VTA | 1.4 (0.2) | 1.3 (0.2) | 1.4 (0.2) | 1.3 (0.2) | 1.2 (0.2) | 1.2 (0.2) | −7.2 (10.5) | −8.0 (11.0)a | −7.8 (8.5) | |
Data are mean (SD).
AST = associative striatum; CAU = caudate; post = post-commissural; pre = pre-commissural; PUT = putamen; SMST = sensorimotor striatum; SN/VTA = substantia nigra/ventral tegmental area; VST = ventral striatum.
Levels of significance of BPND % change were assessed with paired-samples t-tests and, where appropriate, with a Wilcoxon Signed ranks test. BPND changes significant below Bonferroni corrected level of P < 0.003 for left and right regions and P < 0.005 for bilateral regions are in bold.
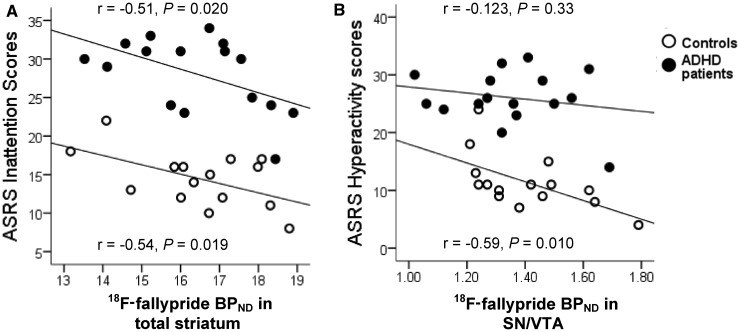
Relationship between ADHD self-rating scale and regional BPND on placebo
ADHD self-rating scale total and inattention sub-scores correlated negatively with total striatum BPND both in patients (r = −0.48, P = 0.032; r = −0.51, P = 0.020) and control subjects (r = −0.47, P = 0.04; r = −0.54, P = 0.019) (Fig. 5A) but did not correlate with BPND in substantia nigra/ventral tegmental area for either subject group. Conversely, self-rated hyperactivity was not associated with BPND in total striatum but was negatively correlated with BPND in substantia nigra/ventral tegmental area in control subjects (r = −0.59, P = 0.010) although not in patients (r = −0.123, P = 0.33) (Fig. 5B).
Figure 5.
Linear regressions and corresponding Pearson correlations between (A) adult ADHD self-rating scale (ASRS) inattention scores and BPND in total striatum and (B) ADHD self-rating scale hyperactivity scores and BPND in the substantia nigra/ventral tegmental area (SN/VTA) in patients with ADHD and control subjects.
Correlation coefficients for left and right striatal subregions and substantia nigra/ventral tegmental area were similar in patients and control subjects (P > 0.1). For patients and control subjects combined, inattention scores were negatively correlated with BPND in the portions of the associative striatum corresponding to pre-commissural dorsal caudate and post-commissural caudate (r = −0.393, P = 0.014; r = −0.375, P = 0.019) and in sensorimotor striatum (r = −0.324, P = 0.038). Hyperactivity scores for all subjects on the other hand were associated with BPND in left substantia nigra/ventral tegmental area (r = −0.34, P = 0.032) but not in any other region.
Relationship between sustained attention (A’) and regional BPND on placebo
In patients, low A’ scores were associated with low BPND in left pre-commissural dorsal caudate (r = 0.48, P = 0.031) and right post-commissural caudate (r = 0.43, P = 0.048). Although the correlation coefficients in control subjects were not significant, they were in the same direction as those observed in patients for all regions and were not significantly different from them (P > 0.1). Across the entire subject group, low A’ scores were associated with low BPND in left pre-commissural dorsal caudate (r = 0.314, P = 0.04) as well as in left and right post-commissural caudate (r = 0.308, P = 0.043 and r = 0.323, P = 0.036, respectively; mean: r = 0.33, P = 0.032).
Stepwise linear regression of all regional BPND values on attentional performance (in all subjects) revealed a significant best-fit model with the left pre-commissural dorsal caudate as independent variable [β = 0.386, P = 0.029; F(1,30) = 5.26, adjusted R2 = 0.21].
Methylphenidate results
Plasma level analysis and cardiovascular effects of methylphenidate
Plasma level analysis of methylphenidate confirmed randomization and compliance with discontinuation of therapeutic medication. Methylphenidate produced significant increases in systolic blood pressure [mean paired difference 5 mmHg; t(31) = 4.5; P < 0.001] and heart rate [mean paired difference 7 beats per min; t(31) = 4.4; P < 0.001] (Supplementary material). Mean methylphenidate plasma concentrations were similar in patients with ADHD and control subjects (P > 0.05).
Effects of methylphenidate on sustained attention
There was no significant main effect of methylphenidate on A’, nor was there an interaction of methylphenidate with group. Covarying for medication status within the patient groups did not change these results (interactions between treatment and group versus medication status all P > 0.05) (Fig. 6A).
Figure 6.
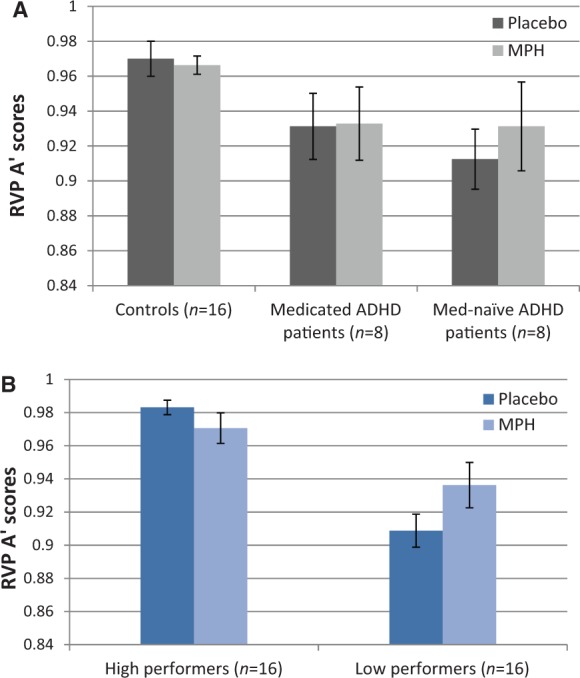
RVP A’ scores following placebo and methylphenidate (MPH) as a function of (A) ADHD diagnosis, with the ADHD group split by medication status and (B) baseline performance, with the combined ADHD and control groups split by the median of A’.
Based on accumulating evidence that effects of methylphenidate and related drugs on cognition are baseline performance-dependent (Kimberg et al., 1997; Mattay et al., 2000; Mehta et al., 2000; Gibbs and D’Esposito, 2005; Tipper et al., 2005; Finke et al., 2010), changes in sustained attention following methylphenidate were assessed as a function of baseline A’ scores, independent of group. We thus split participants into high and low performers based on the median for A’, as used previously (Kimberg et al., 1997). There was an overlap of performance between patients with ADHD and control subjects in about a third of all subjects. High and low performers were matched for age (mean 28 ± 6 years versus 31 ± 7 years; P = 0.17), IQ (mean 113 ± 8 versus 115 ± 7) and methylphenidate plasma concentrations (mean 10 ± 3.16 μg/l versus 9.87 ± 3.78 μg/l) (furthermore, four of the five patients with ADHD assigned to the high performing group were on prescribed ADHD medication).
This analysis showed that methylphenidate affected cognition differentially in high and low performing subjects [drug by group interaction: F(1,28) = 10.94, P = 0.003], improving A’ scores in low [t(15) = −2.610, P = 0.02] but not in high performers [t(15) = 1.656, P = 0.119] (Fig. 6B).
Effects of methylphenidate on regional BPND
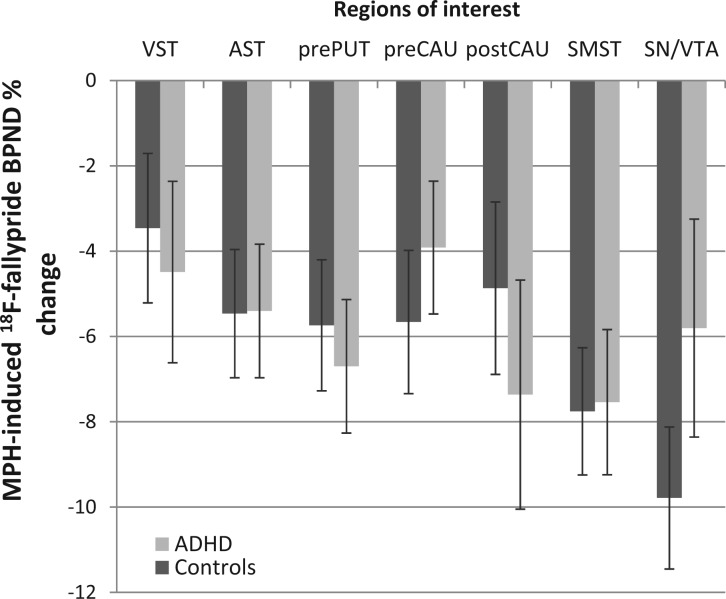
Methylphenidate significantly reduced BPND [main effect of treatment: F(1,29) = 30.51, P < 0.001], ranging from −4.0 to −7.8% depending on anatomical region {region of interest by treatment interaction [F(3,87) = 10.31, P < 0.001]}, being greatest in substantia nigra/ventral tegmental area and least in ventral striatum (Table 3 and Fig. 7). Methylphenidate also reduced BPND differentially across ventral striatum, associative striatum and sensorimotor striatum [region of interest by treatment interaction: F(2,42) = 8.34, P = 0.002], revealing a preferential effect in sensorimotor striatum. The magnitude of regional BPND % change was similar in patients with ADHD and control subjects (main effect of group and group by drug interaction: P > 0.05).
Figure 7.
Methylphenidate (MPH)-induced BPND % change in healthy control subjects and ADHD patients across regions of interest. Reductions in BPND were significant in all regions and similar in both groups. There was a trend for decreased BPND % change in substantia nigra/ventral tegmental area in patients with ADHD compared to control subjects [t(30) = −1.65, P = 0.055]. Error bars denote standard error of the mean.
VST = ventral striatum; AST = associative striatum; prePUT = pre-commissural putamen; preCAU = pre-commissural caudate; postCAU = post-commissural caudate; SMST = sensorimotor striatum; SN/VTA = substantia nigra/ventral tegmental area.
Baseline performance-dependent relationship between methylphenidate effects on sustained attention (A’ % change) and BPND % change
Methylphenidate-induced % change in A’ was negatively correlated with BPND % change in ventral striatum (r = −0.29, P = 0.05) and positively with substantia nigra/ventral tegmental area (r = 0.5, P = 0.002), with the correlation coefficients being highly significantly different (z = 3.25, P < 0.001).
After controlling for between-subject differences in methylphenidate plasma levels, improvements in sustained attention were associated with greater BPND % change in right ventral striatum (r = −0.318, P = 0.043) and lower BPND % change in substantia nigra/ventral tegmental area [r = 0.32, P = 0.043; r = 0.309, 8 P = 0.048; r = 0.374, P = 0.021; in left, right and bilateral substantia nigra/ventral tegmental area, respectively (Fig. 8)]. Correlation coefficients for ventral striatum and substantia nigra/ventral tegmental area were significantly different from each other (P < 0.01), but similar in patients with ADHD and control subjects.
Figure 8.
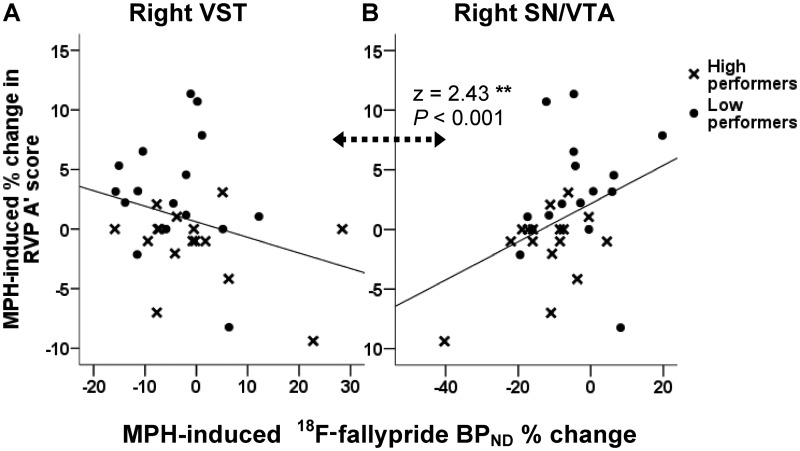
Regression lines representing the relationship between methylphenidate (MPH) effects on RVP A’ scores and 18F-fallypride BPND % change in (A) right ventral striatum (VST) and (B) right substantia nigra/ventral tegmental area (SN/VTA). Correlation coefficients in these regions were significantly different (z = 2.43, P < 0.001). Note the x-axis scales are different.
The above analyses were also calculated after excluding ceiling performers on the attentional task (five control subjects and one patient), yielding similar results (Supplementary material).
We hypothesized that the relationship between A’ % change and BPND % change for both ventral striatum and substantia nigra/ventral tegmental area would be dependent on A’ baseline scores, based on the aforementioned differential effect of methylphenidate in high and low performers. Controlling for between-subject differences in A’ scores (in addition to methylphenidate plasma levels) had no impact on the correlation between methylphenidate-induced changes in A’ scores and BPND % change in ventral striatum (r = −0.348, P = 0.032 for bilateral ventral striatum; r = −0.247, P = 0.098 and r = −0.349, P = 0.033 for left and right ventral striatum, respectively). Therefore, these effects were not baseline-dependent.
On the contrary, changes in sustained attention associated with BPND % change in substantia nigra/ventral tegmental area were dependent on baseline A’ scores, the correlations for this region no longer being significant (r = 0.223, P = 0.142 for bilateral substantia nigra/ventral tegmental area; r = 0.144, P = 0.228 and r = 0.218, P = 0.129 for left and right substantia nigra/ventral tegmental area, respectively). Corroborating this finding, repeated measures ANOVA revealed lower BPND % change in low compared with high performers in substantia nigra/ventral tegmental area (Fig. 9) [F(1,28) = 9.351, P = 0.005; F(1,28) = 5.46, P = 0.027 and F(1,28) = 5.2, P = 0.030 for bilateral, left and right substantia nigra/ventral tegmental area, respectively], but not in any other region.
Figure 9.
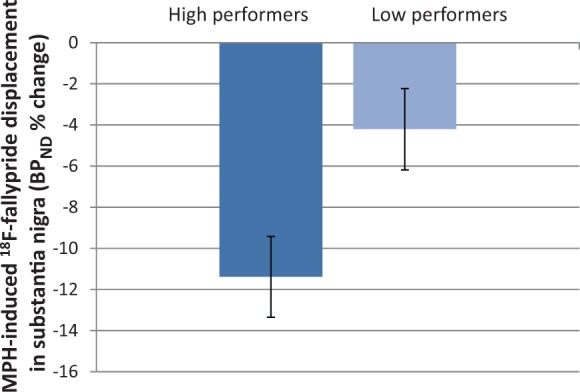
BPND % change in substantia nigra/ventral tegmental area for high and low performers. Low performers had decreased BPND % change following methylphenidate (MPH) (P = 0.007), suggesting that drug-induced increase in endogenous dopamine in this region was smaller than for high performers.
Stepwise linear regression modelling to determine the relative impact of methylphenidate-induced BPND changes across regions and methylphenidate plasma levels on drug-induced A’ changes confirmed the role of the right midbrain in predicting changes in attentional performance [β = 0.39, P = 0.30; F(1,30) = 5.21, adjusted R2 = 0.12]. A further regression model revealed that baseline performance levels on A’ predicted BPND changes specifically in the left midbrain [β = −0.471, P < 0.01; F(1,30) = 8.57, adjusted R2 = 0.20], confirming the above correlational results.
Baseline performance-dependent regional BPND
On placebo, low performers had reduced BPND in left pre-commissural dorsal caudate (Fig. 10A) [F(1,28) = 4.63, P = 0.04]. As shown in Fig. 10B, on methylphenidate this group difference was no longer observed [F(1,28) = 2.97, P = 0.096].
Figure 10.
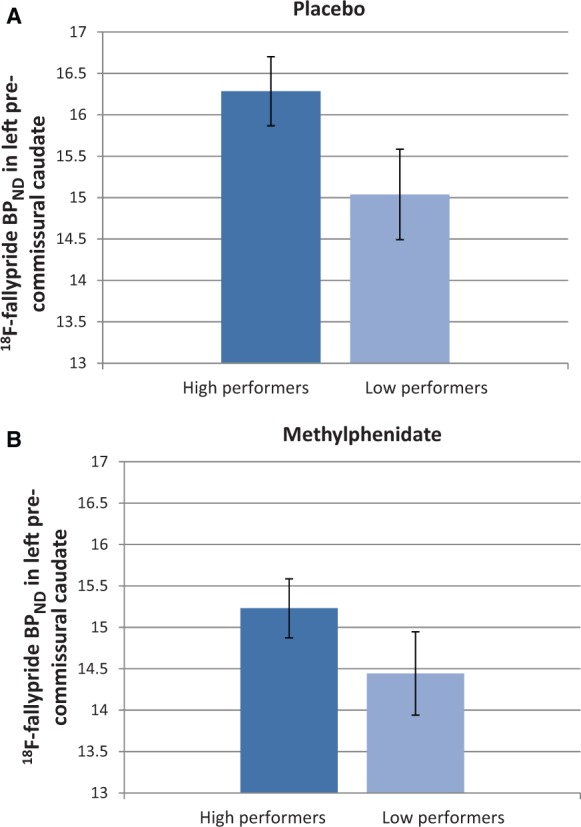
BPND in left pre-commissural caudate in high and low performers following (A) placebo and (B) methylphenidate (MPH). Low performers had reduced BPND in left pre-commissural caudate on placebo (P = 0.035), which was normalized by methylphenidate.
Discussion
This study examined the neural basis of the attentional deficits associated with ADHD and the mechanisms underpinning a single therapeutic dose of methylphenidate through the application of multi-modal state-of-the-art neuroimaging techniques. Adult patients with ADHD as a group had marked deficits in sustained attention and showed reduced grey matter volume in distributed frontostriatal and limbic circuits relative to control subjects. No significant group differences in D2/D3 receptor availability were found; however, poor performance on the computerized sustained attention task across both patient and control groups was associated with low BPND values in the left caudate. Methylphenidate increased dopamine levels across all striatal regions as well as the midbrain similarly in patients with ADHD and control subjects, thereby normalizing D2/D3 receptor availability in low performers. The drug improved sustained attention in a baseline-dependent manner independently from diagnosis. Subjects performing poorly at baseline also had significantly smaller drug-induced dopamine release in the midbrain. Collectively these novel results suggest that deficits in sustained attention are associated with both reduced D2/D3 receptor availability in the left caudate on placebo and reduced dopamine release in the midbrain in response to methylphenidate, and that these relationships hold irrespective of ADHD diagnosis.
Neural correlates of ADHD
The absence of overall group differences in 18F-fallypride binding in this study may seem to be at variance with previous reports of reduced 11C-raclopride binding in selected brain regions of the left hemisphere in adult patients with ADHD compared with control subjects (Volkow et al., 2007b, 2009). However, it is possible that the apparent discrepancy can be resolved by the following consideration: low performing subjects drawn from both ADHD and control groups on the computerized test of sustained attention in fact had significantly lower BPND (attributed to lower D2/D3 receptor availability) in the left pre-commissural caudate than high-performing subjects, though independent of diagnosis. This result is consistent with previous evidence implicating the left caudate in inattentiveness, not only in patients with ADHD, but also in healthy subjects (Schrimsher et al., 2002; Volkow et al., 2007a, b, 2009) and is also congruent with reports that lesions in the left caudate produce attentional deficits (Benke et al., 2003).
Our findings define a role for D2/D3 receptor availability in the manifestation of attentional deficits that can be explained in the light of the previously hypothesized continuum model of cognitive deficits and underlying dopaminergic dysregulation associated with ADHD (Sahakian and Robbins, 1977). Consistent with the model, the attentional deficits observed in ADHD fall along a single normal continuum extending into the healthy population, with patients with ADHD predominantly representing the lower end of the distribution. However, the lack of overall group differences in 18F-fallypride binding argue against a primary nigro-striatal-dependent dopamine dysfunction in adult ADHD. Thus, although our data suggest that dopamine plays a key role in attention deficits, they also imply that dopaminergic deficits alone do not account for an ADHD diagnosis. What is then different in ADHD in neural terms?
Of course it is possible that such differences in dopamine function may exist within other regions not addressed here such as the frontal cortex (e.g. Ernst et al., 1998, but see also Del Campo et al., 2011a for discussion). However, the sensitivity of 18F-fallypride to the methylphenidate dose administered in the current design was arguably suboptimal for detecting robust changes in dopamine levels in regions with low D2/D3 receptor density such as the prefrontal cortex. A further possibility is that the central noradrenergic system is selectively affected in ADHD. In support of this hypothesis, atomoxetine, a selective noradrenergic reuptake inhibitor, has been reported to be effective in the treatment of ADHD and some of its associated cognitive deficits (Chamberlain et al., 2007). Finally, it may also be argued that ADHD as described in the DSM-IV encompasses an array of phenotypes and underlying mechanisms too broad and heterogeneous to be associated with a primary neurobiological dysfunction common to all patients. The Research Domain Criteria project (RDoC) recently launched by the National Institute of Mental Health, which encourages the development of new ways of classifying psychopathology based on dimensions of observable behaviour and neurobiological measures, represents an important step forward for future research which may help encapsulate the heterogeneity in ADHD. However, it is striking that although no group differences were found in terms of dopamine activity in our study, the same group of adult patients with ADHD had significant brain tissue abnormalities in frontostriatal and limbic structures compared with control subjects.
Methylphenidate-induced dopamine increases: regional dependence and therapeutic implications
Some of the present findings on effects of methylphenidate are relevant to the more general understanding of stimulant drug action in healthy humans, other than simply in patients with ADHD. Thus, here we report, for the first time, that therapeutic doses of methylphenidate administered orally reduce 18F-fallypride binding, which can be attributed to methylphenidate-induced increases in synaptic dopamine levels. Effect sizes and rank order of 18F-fallypride BPND % change across subregions of the striatum and the midbrain were comparable with those documented following oral administration of amphetamine (Riccardi et al., 2006a; Cropley et al., 2008) (Supplementary material), the alternative first-line treatment for ADHD which also increases catecholamine levels, albeit via different cellular mechanisms (Faraone and Glatt, 2010). Consistent with previous reports, the substantia nigra/ventral tegmental area showed high levels of BPND % change, which were comparable to those observed in sensorimotor striatum.
These findings replicate the preferential effect of methylphenidate in the sensorimotor striatum when administered orally using 11C-raclopride binding (Clatworthy et al., 2009). The preferential effect of oral methylphenidate and amphetamine on dorsal compared to ventral regions of the striatum, however, is in contrast with the well-replicated finding that intravenous amphetamine leads to greater dopamine changes in ventral compared to dorsal striatum (Drevets et al., 1999, 2001; Martinez et al., 2003). Animal evidence suggests that the ability to increase dopamine in ventral striatum, involved with reward circuitry, is a common pharmacological effect underlying the reinforcing properties of virtually all drugs of abuse (Di Chiara and Imperato, 1988; Bradberry et al., 2000). Consistent with these data, in healthy humans, amphetamine-induced dopamine changes in ventral but not dorsal striatum were found to predict hedonic responses (Drevets et al., 2001; Leyton et al., 2002; Martinez et al., 2003).
The pattern of dopamine changes observed along the ventral–dorsal gradient of the striatum following oral versus intravenous administration of methylphenidate and amphetamine may be explained in light of the different pharmacokinetics associated with each route of drug administration. Whereas oral methylphenidate penetrates the brain only slowly, peaking 60–90 min after administration, intravenous methylphenidate rapidly enters the brain, peaking in <15 min. The large and abrupt dopamine increase after intravenous administration is perceived as reinforcing, an effect that appears to be reduced when the increase in dopamine is slow and progressive (Volkow, 2006).
Overall, the differences in regional patterns of methylphenidate-induced dopamine increase following oral versus intravenous methylphenidate administration suggest that the mechanisms underlying the effects of the drug to improve attention differ from those mediating its reinforcing actions.
Baseline performance-dependent effects of methylphenidate
Methylphenidate significantly improved sustained attention in a baseline-dependent manner in low performers from both groups. Although the lack of methylphenidate effects on attention in high performers was possibly due to ceiling effects, this does not undermine the key finding that the drug is capable of improving sustained attention in low-performing individuals, regardless of ADHD diagnosis. This result adds to previous evidence demonstrating equivalence of behavioural stimulant effects in individuals with and without ADHD (Rapoport et al., 1978, 1980), supporting a dimensional model of attentional deficits and underlying catecholaminergic mechanisms of ADHD which extends into the healthy population. It is also consistent with a growing literature documenting baseline performance-dependent effects of stimulants and related drugs, which have been explained by a hypothesized inverted U-shaped function, whereby optimal catecholamine levels determine optimal performance, and levels along the curve at either side of the optimum are associated with impaired performance (Robbins and Sahakian, 1979; Naylor et al., 1985; Williams and Goldman-Rakic, 1995; Kimberg et al., 1997; Arnsten and Goldman-Rakic, 1998; Rogers et al., 1999; Mattay et al., 2000, 2003; Tipper et al., 2005; Finke et al., 2010). Inverted U-shaped functions as invoked here accurately describe the dose-response relationships often observed in psychopharmacological studies of cognition (Dews, 1958; Sahakian and Robbins, 1977; Cools and Robbins, 2004; Robbins, 2010). Our data do not allow drawing conclusions on the declining portion of the hypothesized function. Further research is needed to test the full dose-response curve of methylphenidate, which would require higher doses than the one used here.
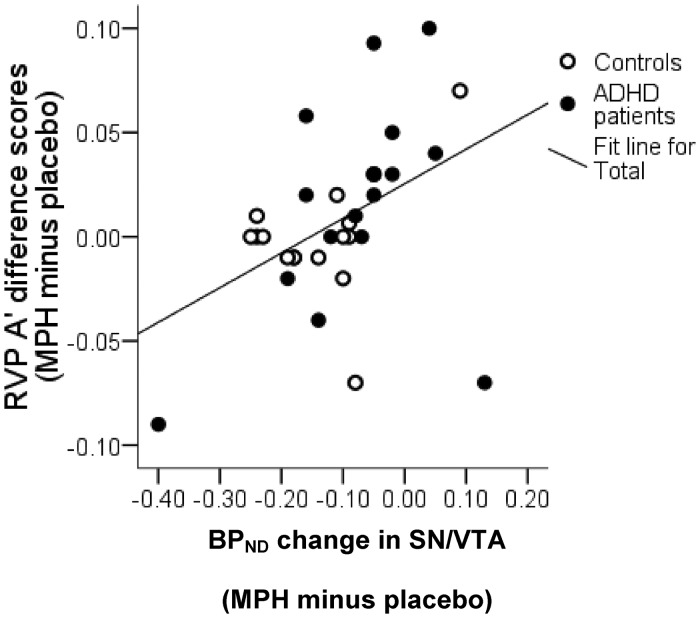
A central finding of this study was that the drug-induced changes in performance correlated with changes in midbrain dopamine levels (Fig. 11), a result that remained unchanged after excluding ceiling performers. Changes in midbrain dopamine levels mirrored the behavioural baseline-dependent effect: low performers had reduced methylphenidate-induced BPND reductions in the midbrain compared with high performers, with smaller dopamine changes being associated with greater improvements on the sustained attention task. These robust neurobiological correlates argue against the behavioural baseline-dependent effects resulting simply from a statistical regression towards the mean.
Figure 11.
Regression line representing the relationship between methylphenidate (MPH) effects on sustained attention and on 18F-fallypride BPND in the midbrain (r = 0.502; P = 0.002, two-tailed). SN/VTA = substantia nigra/ventral tegmental area.
These findings suggest that the effects of methylphenidate on midbrain dopamine levels play a hitherto unknown role in the modulation of attention. A further likely mechanism by which methylphenidate improved sustained attention in low performers is the observed normalization of dopamine levels in the left caudate.
Midbrain mechanisms underlying therapeutic effects of methylphenidate
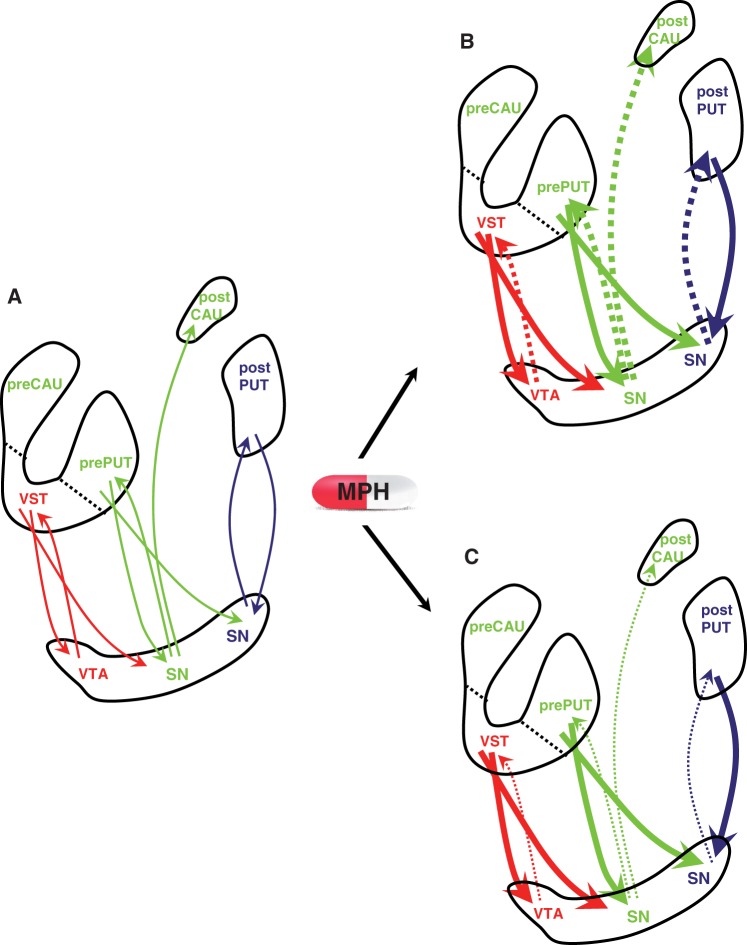
Methylphenidate-induced dopamine changes in substantia nigra/ventral tegmental area were negatively related to dopamine changes in ventral striatum but not associative striatum or sensorimotor striatum. These results may reflect regional differences in the response of midbrain dopamine neurons following methylphenidate administration. Dopamine neurons located in the midbrain receive γ-aminobutyric acid (GABA)-ergic projections from the same striatal subregion they project to through reciprocal connections, giving rise to topographically organized striato-nigro-striatal circuits (Haber et al., 2000). Additionally, this system provides non-reciprocal connections, facilitating dopamine transmission along a feed–forward spiral starting in the ventral striatum and finishing in the sensorimotor striatum, whose projections to the midbrain are confined to the regions from which it receives its dopamine input (Haber et al., 2000) (Fig. 12A). GABAergic neurons descending from the striatum to the midbrain can inhibit or stimulate dopamine cell firing depending on whether they contact dopamine neurons or GABAergic interneurons. After administration of psychostimulants (Bunney and Achajanian, 1976), including methylphenidate (Shi et al., 2004), the inhibitory effect prevails, leading to decreased responsiveness in dopaminergic neurons (Fig. 12B).
Figure 12.
Schematic model of dopamine activity along striato-nigro-striatal circuits at baseline (A) and after oral methylphenidate (MPH) in high (B) and low performers (C). Ascending arrows represent projections from dopamine (DA) neurons located in the substantia nigra (SN)/ventral tegmental area (VTA) to the striatum and descending arrows represent GABA-ergic projections from the striatum to the substantia nigra/ventral tegmental area. Different colours represent different striatal functional circuits (red = limbic, green = associative, blue = sensorimotor). Methylphenidate administration (B) increased postsynaptic catecholamine stimulation (solid lines), leading to inhibition of dopamine neuron firing (dashed lines) by activation of synthesis- and release-regulating dopamine autoreceptors. It is suggested that the level of responsivity of dopamine neurons and subsequent striato-nigro-striatal neuromodulation following methylphenidate administration was dependent on baseline dopamine activity in the striatum. Adapted from Haber et al. (2000) and Martinez et al. (2003). CAU = caudate PUT = putamen; VST = ventral striatum.
We hypothesize that the inverse relationship in dopamine release between substantia nigra/ventral tegmental area and ventral striatum but not associative striatum or sensorimotor striatum following methylphenidate reflects the asymmetrical nigro-striatal projection pattern. Ventral striatum is the only striatal region whose dopamine input is unaffected by non-reciprocal connections, its dopaminergic input thus being regulated to a greater extent by synthesis- and release-regulating midbrain dopamine autoreceptors. The observed inverse correlation may thus reflect the inhibitory influences exerted by midbrain dopamine neurons to regulate dopamine levels in ventral striatum. This inverse relationship did not hold for associative striatum and sensorimotor striatum, which can be explained by the fact that, via the aforementioned feed–forward spiralling connections, associative striatum receives additional inhibitory influences from ventral striatum and sensorimotor striatum from both ventral striatum and associative striatum. These findings possibly constitute the first direct evidence in humans supporting the notion that the doses used clinically to treat ADHD exert their therapeutic actions through presynaptic mechanisms. A key finding of this study is that midbrain dopamine autoreceptor regulation was reduced in low performers, as evidenced by the significantly smaller methylphenidate-induced increases in midbrain dopamine levels observed in low compared to high performers (Fig. 12C).
How are the changes in midbrain dopamine receptor availability related to the attention-enhancing effects of methylphenidate, whether in patients with ADHD or healthy control subjects? A plausible explanation is provided by the classical model of catecholamine neurotransmission regulation and psychostimulant action postulated by Grace (1991, 2001). According to this model, the amplitude of the phasic dopamine response is dynamically regulated by the influence of corticostriatal activity through the modulation of tonic dopamine levels. Low tonic dopamine activity is associated with abnormally high phasic dopamine responses, resulting in distractibility and impaired attention. Stimulants enhance tonic catecholamine levels, thereby increasing postsynaptic catecholamine receptor stimulation but also triggering negative feedback mechanisms through stimulation of dopamine autoreceptors. As a result, dopamine synthesis is reduced, dopamine neuron firing inhibited and subsequent phasic (spike-dependent) transmitter release reduced.
The idea that the clinical effects of stimulants are mediated by the ability of small increases in extracellular dopamine to produce a net decrease in phasic catecholamine release by the selective activation of dopamine autoreceptors (following an increase in tonic dopamine levels) (Solanto, 1984, 1998; Seeman and Madras, 1998) is supported by studies showing reduction in behavioural activity levels in children with ADHD following low doses of dopamine agonists in the range presumed to stimulate autoreceptors (Solanto, 1986) and from neurocomputational models (Oades et al., 1985; Sawaguchi et al., 1988; Daniel et al., 1991; Servan-Schreiber et al., 1998; Li et al., 2001; MacDonald et al., 2009). It can thus be concluded that stimulant-induced increases in endogenous midbrain dopamine in the presence of reduced tonic striatal dopamine levels observed in low performers may improve the signal-to-noise ratio, thereby normalizing the system to approximate that characterizing high performing subjects.
Strengths and limitations
In an attempt to identify putative biomarkers of ADHD, we used, for the first time, an interdisciplinary approach encompassing both PET and MRI in combination with neuropsychopharmacological assessment which allowed characterization of the disorder at clinical, cognitive, neurochemical and neuroanatomical levels. The current paper focused on the neuropsychopharmacological findings; a companion paper will address in more detail grey and white matter tissue in relation to the behavioural changes.
An important innovation of this study relative to previous ADHD PET studies was the use of high resolution co-registered MRI images to help localize the PET signal. This was crucial to allow segmentation of the striatum into its functional subregions, known to have different tracer uptake due to heterogeneous D2/D3 receptor density (Del Campo et al., 2011b).
A further strength was the experimental design, (double-blind, randomized, cross-over) which differs from the fixed design (placebo first, followed by drug) used in previous studies investigating the effects of methylphenidate in ADHD (Rosa-Neto et al., 2005; Volkow et al., 2007b). It might be argued that placebo is not equivalent to a null condition, and thus the comparison between studies using placebo as a control condition with those in which baseline measurements are acquired might be suboptimal. Indeed, PET evidence has shown that sensitization to amphetamine in humans leads to carry-over effects in subsequent sessions when placebo is administered (Boileau et al., 2007). These findings imply that placebo administered in the session subsequent to stimulant treatment can potentially reduce the power to detect true stimulant effects and identify neurobiological markers in psychiatric populations. However, fixed designs entail order effects, thereby introducing the confounding effect of drug anticipation. PET evidence suggests that anticipation of stimulants such as caffeine can induce dopaminergic responses in humans (Kaasinen et al., 2004). To address this issue, placebo 18F-fallypride BPND values were compared with 18F-fallypride BPND estimates documented by other groups in the same regions of interest at baseline in fixed designs (Riccardi et al., 2006a; Cropley et al., 2008; Slifstein et al., 2010) (Supplementary material). Values were largely comparable in terms of both magnitude and rank order, suggesting that the regional BPND values reported here following placebo administration were within the expected baseline ranges. One way future research might help to disentangle undesired effects of drug-order and stimulant carry-over effects is through implementation of a double-blind randomized design with parallel groups, as used by Aalto et al. (2009) where each subject underwent two scans; the first at baseline and the second after administration of either a psychostimulant or placebo. However, there is no evidence to suggest that our design produced anomalous results.
A caveat of dopamine receptor imaging technique as the one reported here is that receptor availability and dopamine release are inferred from estimates of BPND, which is a combined measure of receptor availability and radioligand affinity. Consequently, we were not able to assess whether dynamics such as receptor internalization (Laruelle, 2000) or changes in the affinity of D2/D3 receptors for dopamine and/or fallypride were implicated (Laruelle, 2000; Gjedde et al., 2005). In the absence of information regarding levels of endogenous dopamine, it was not possible to determine to what extent D2/D3 receptor availability reflected receptor density or basal occupancy. Moreover, this technique did not allow a distinction of the proportion of different D2/D3 receptor types (synaptic versus extra-synaptic, post versus presynaptic) or their state of affinity for dopamine. It is important to note also that the slow kinetics of 18F-fallypride necessitated a long acquisition period (3.15 h) and thus did not allow us to differentiate tonic from phasic dopamine activity in the midbrain, which can only be inferred.
A further methodological aspect that merits discussion concerns partial volume effects, defined as the loss in spatial resolution resulting in spill-over of image signal to and from adjacent structures. Specifically, partial volume effects are to be considered as a potential confounding factor of radiotracer uptake when the structures under study are small and/or when adjacent structures have high signal contrast. 18F-fallypride PET imaging of the striatum satisfies both these criteria and hence partial volume effects are theoretically an issue of concern in this study. Moreover, our finding of reduced grey matter volume in the putamen in patients with ADHD compared with control subjects implies that partial volume effects may have had a differential effect on sub-striatal BPND estimation in patients and control subjects, potentially masking group differences in regional D2/D3 receptor availability. In order to rule this out, an additional set of analyses was carried out to estimate partial volume error using an in-house implementation of a well-validated method (Rousset et al., 1998). A detailed account of the methodology used and the results can be found in the Supplementary material. Importantly, signal recovery due to partial volume effect was similar in patients with ADHD and control subjects, independently from brain region and experimental condition, and thus it can be concluded that the grey matter changes observed in patients did not confound the PET results here reported.
Finally, the medication history of the patients also needs discussing, as half of the patients in the current study had been previously exposed to ADHD medication. Although this study was not designed to resolve the question of whether patients with ADHD with a history of pharmacological treatment have different D2/D3 receptor availability compared to medication-naïve patients, we did not find any differences in PET measurements between patients with previous exposure to ADHD medication and medication-naïve patients (all P > 0.05). Importantly, to ensure complete washout at the time of scanning among patients under current treatment, compliance with treatment discontinuation 3 days before each PET scan was verified through urine screening. Moreover, medication status was controlled for in all repeated measures ANOVAs. There is a lack of clarity regarding chronic effects of ADHD medication, with different studies reporting contradicting results with regard to its impact on dopaminergic markers (Gill et al., 2012; Volkow et al., 2012). Further research and suitable study designs are needed to fully characterize the consequences of long-term pharmacological ADHD treatment both at the behavioural and at the neurobiological levels.
Conclusion
The present findings query the precise role of dopamine in the pathophysiology in adult ADHD. Although our findings are consistent with the modulation of attention by nigro-striatal dopamine and with poor attention being a key deficit in the clinical profile of ADHD, our data also suggest that dopamine dysregulation per se is unlikely to be the primary cause underlying ADHD pathology in adults. This conclusion is reinforced by evidence of structural brain changes in the same set of patients with adult ADHD.
We also conclude that a more precise explanation of the behavioural effects of psychostimulant treatment in both patients with ADHD and healthy control subjects can be derived from the combination of (i) dose and time course of methylphenidate actions; (ii) level of responsivity of the dopamine system determined by tonic dopamine levels; and (iii) baseline level of performance, possibly reflecting basal dopamine function in the striatum. The findings of reduced methylphenidate-induced midbrain dopamine increases and improved sustained attention in low performers (mostly patients with ADHD) implicate a hitherto neglected role of midbrain dopamine in the mediation of therapeutic effects of methylphenidate.
We have shown the considerable potential of combining the complementary strengths of structural MRI and neuroreceptor PET imaging in identifying and characterizing cognitive endophenotypes of ADHD, which may help our understanding of the diagnosis, prognosis and treatment of ADHD.
Supplementary Material
Acknowledgements
We thank all participants as well as the radiochemistry and scanning staff of the Wolfson Brain Imaging Centre for the successful completion of the study; Jasper van der Aart for technical assistance; Tim Croudace for statistical advice and Kevin Craig for providing medical cover.
Glossary
Abbreviations
- AC–PC
anterior commissure–posterior commissure
- ADHD
attention deficit/hyperactivity disorder
- BPND
18F-fallypride binding potential
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- MRAC-PC
scans manually aligned to the anterior commissure-posterior commissure line
- RVP
Rapid Visual Information Processing
Funding
This work was funded by a Pathfinder Award of the UK Medical Research Council [MRC, G0401099] and the Behavioural and Clinical Neuroscience Institute [BCNI], jointly funded by MRC and Wellcome Trust [MRC Ref G001354 and WT Ref 076244/Z/04/Z]. N.D.C. was funded by the Gates Cambridge Trust when the work here presented was completed and is now employed by Cambridge Cognition. S.R.C. consults for Cambridge Cognition, P1Vital, and Shire Pharmaceuticals. T.W.R. is a consultant for Cambridge Cognition, Pfizer, Eli Lilly, GlaxoSmithKline, Allon Therapeutics, and Lundbeck, and receives royalties from Cambridge Cognition and Springer-Verlag. B.J.S. consults for Cambridge Cognition and holds shares in CeNeS. She has consulted for Novartis, Shire, GlaxoSmithKline, Eli Lilly and Boehringer-Ingelheim. She also receives an honorarium from the Journal of Psychological Medicine. U.M. has received research and educational grant support from Janssen-Cilag and honoraria or travel expenses from Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, Lundbeck, Pharmacia-Upjohn, and UCB Pharma.
Supplementary material
Supplementary material is available at Brain online.
References
- Aalto S, Hirvonen J, Kaasinen V, Hagelberg N, Kajander J, Nagren K, et al. The effects of d-amphetamine on extrastriatal dopamine D2/D3 receptors: a randomized, double-blind, placebo-controlled PET study with [11C]FLB 457 in healthy subjects. Eur J Nucl Med Mol Imaging. 2009;36:475–83. doi: 10.1007/s00259-008-0969-9. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998;55:362–8. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Benke T, Delazer M, Bartha L, Auer A. Basal ganglia lesions and the theory of fronto-subcortical loops: neuropsychological findings in two patients with left caudate lesions. Neurocase. 2003;9:70–85. doi: 10.1076/neur.9.1.70.14374. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157:816–8. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M, et al. Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci. 2007;27:3998–4003. doi: 10.1523/JNEUROSCI.4370-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J Neurosci. 2000;20:3874–83. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Achajanian GK. d-Amphetamine-induced inhibition of central dopaminergic neurons: mediation by a striato-nigral feedback pathway. Science. 1976;192:391–3. doi: 10.1126/science.1257777. [DOI] [PubMed] [Google Scholar]
- Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biol Psychiatry. 2005;57:1273–84. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Castells X, Ramos-Quiroga JA, Rigau D, Bosch R, Nogueira M, Vidal X, et al. Efficacy of methylphenidate for adults with attention-deficit hyperactivity disorder: a meta-regression analysis. CNS Drugs. 2011;25:157–69. doi: 10.2165/11539440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Challman TD, Lipsky JJ. Methylphenidate: its pharmacology and uses. Mayo Clin Proc. 2000;75:711–21. doi: 10.4065/75.7.711. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Del Campo N, Dowson J, Muller U, Clark L, Robbins TW, et al. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry. 2007;62:977–84. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Clark L, Blackwell AD, Aron AR, Turner DC, Dowson J, Robbins TW, et al. Association between response inhibition and working memory in adult ADHD: a link to right frontal cortex pathology? Biol Psychiatry. 2007;61:1395–401. doi: 10.1016/j.biopsych.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Clatworthy PL, Lewis SJ, Brichard L, Hong YT, Izquierdo D, Clark L, et al. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci. 2009;29:4690–6. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Robbins TW. Chemistry of the adaptive mind. Philos Trans A Math Phys Eng Sci. 2004;362:2871–88. doi: 10.1098/rsta.2004.1468. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Frackowiak RS, Grasby PM. A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia. 1996;34:1085–95. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Hauser MD, Robbins TW. Serotonin selectively influences moral judgment and behavior through effects on harm aversion. Proc Natl Acad Sci USA. 2010;107:17433–8. doi: 10.1073/pnas.1009396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropley VL, Innis RB, Nathan PJ, Brown AK, Sangare JL, Lerner A, et al. Small effect of dopamine release and no effect of dopamine depletion on [18F]fallypride binding in healthy humans. Synapse. 2008;62:399–408. doi: 10.1002/syn.20506. [DOI] [PubMed] [Google Scholar]
- Daniel DG, Weinberger DR, Jones DW, Zigun JR, Coppola R, Handel S, et al. The effect of amphetamine on regional cerebral blood flow during cognitive activation in schizophrenia. J Neurosci. 1991;11:1907–17. doi: 10.1523/JNEUROSCI.11-07-01907.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011a;69:e145–57. doi: 10.1016/j.biopsych.2011.02.036. [DOI] [PubMed] [Google Scholar]
- Del Campo N, Muller U, Sahakian BJ. Neural and behavioral endophenotypes in ADHD. Curr Top Behav Neurosci. 2012;11:65–91. doi: 10.1007/7854_2012_200. [DOI] [PubMed] [Google Scholar]
- Del Campo N, Tait RJ, Acosta-Cabronero J, Hong YT, Izquierdo-Garcia D, Smith R, et al. Quantification of receptor-ligand binding potential in sub-striatal domains using probabilistic and template regions of interest. Neuroimage. 2011b;55:101–12. doi: 10.1016/j.neuroimage.2010.11.071. [DOI] [PubMed] [Google Scholar]
- Dews PB. Studies on behavior. IV. Stimulant actions of methamphetamine. J Pharmacol Exp Ther. 1958;122:137–47. [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JC, Kupfer DJ, Kinahan PE, Lopresti B, Holt D, et al. PET measures of amphetamine-induced dopamine release in ventral versus dorsal striatum. Neuropsychopharmacology. 1999;21:694–709. doi: 10.1016/S0893-133X(99)00079-2. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Matthews K, Bannerjea A, Rimmer J, Robbins TW. Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology (Berl) 1997;131:196–206. doi: 10.1007/s002130050284. [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci. 1998;18:5901–7. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Cohen RM. High midbrain [18F]DOPA accumulation in children with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1209–15. doi: 10.1176/ajp.156.8.1209. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71:754–63. doi: 10.4088/JCP.08m04902pur. [DOI] [PubMed] [Google Scholar]
- Finke K, Dodds CM, Bublak P, Regenthal R, Baumann F, Manly T, et al. Effects of modafinil and methylphenidate on visual attention capacity: a TVA-based study. Psychopharmacology (Berl) 2010;210:317–29. doi: 10.1007/s00213-010-1823-x. [DOI] [PubMed] [Google Scholar]
- Foster ER, Black KJ, Antenor-Dorsey JA, Perlmutter JS, Hershey T. Motor asymmetry and substantia nigra volume are related to spatial delayed response performance in Parkinson disease. Brain Cogn. 2008;67:1–10. doi: 10.1016/j.bandc.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gau SS, Huang WL. Rapid visual information processing as a cognitive endophenotype of attention deficit hyperactivity disorder. Psychol Med. 2013:1–12. doi: 10.1017/S0033291713000640. [DOI] [PubMed] [Google Scholar]
- Gibbs SE, D'Esposito M. Individual capacity differences predict working memory performance and prefrontal activity following dopamine receptor stimulation. Cogn Affect Behav Neurosci. 2005;5:212–21. doi: 10.3758/cabn.5.2.212. [DOI] [PubMed] [Google Scholar]
- Gill KE, Pierre PJ, Daunais J, Bennett AJ, Martelle S, Gage HD, et al. Chronic treatment with extended release methylphenidate does not alter dopamine systems or increase vulnerability for cocaine self-administration: a study in nonhuman primates. Neuropsychopharmacology. 2012;37:2555–65. doi: 10.1038/npp.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjedde A, Wong DF, Rosa-Neto P, Cumming P. Mapping neuroreceptors at work: on the definition and interpretation of binding potentials after 20 years of progress. Int Rev Neurobiol. 2005;63:1–20. doi: 10.1016/S0074-7742(05)63001-2. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA. Psychostimulant actions on dopamine and limbic system function: relevance to the pathophysiology and treatment of ADHD. New York: Oxford University Press; 2001. [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–87. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–30. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–82. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–74. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- Jucaite A, Fernell E, Halldin C, Forssberg H, Farde L. Reduced midbrain dopamine transporter binding in male adolescents with attention-deficit/hyperactivity disorder: association between striatal dopamine markers and motor hyperactivity. Biol Psychiatry. 2005;57:229–38. doi: 10.1016/j.biopsych.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Aalto S, Nagren K, Rinne JO. Expectation of caffeine induces dopaminergic responses in humans. Eur J Neurosci. 2004;19:2352–6. doi: 10.1111/j.1460-9568.2004.03310.x. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–9. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005;35:245–56. doi: 10.1017/s0033291704002892. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Mrzljak L, Gutierrez A, de la Calle A, Goldman-Rakic PS. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci USA. 1998;95:7731–6. doi: 10.1073/pnas.95.13.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg DY, D'Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 1997;8:3581–5. doi: 10.1097/00001756-199711100-00032. [DOI] [PubMed] [Google Scholar]
- Kinahan PE, Rogers JG. Analytic 3D image reconstruction using all detected events. IEEE Trans Nucl Sci. 1989;36:964–8. [Google Scholar]
- Koelega HS. Stimulant drugs and vigilance performance: a review. Psychopharmacology (Berl) 1993;111:1–16. doi: 10.1007/BF02257400. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, McLoughlin G, Asherson P. Attention deficit hyperactivity disorder. Neuromolecular Med. 2006;8:461–84. doi: 10.1385/NMM:8:4:461. [DOI] [PubMed] [Google Scholar]
- Larisch R, Meyer W, Klimke A, Kehren F, Vosberg H, Muller-Gartner HW. Left-right asymmetry of striatal dopamine D2 receptors. Nucl Med Commun. 1998;19:781–7. doi: 10.1097/00006231-199808000-00009. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–51. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15:1028–38. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Leo D, Sorrentino E, Volpicelli F, Eyman M, Greco D, Viggiano D, et al. Altered midbrain dopaminergic neurotransmission during development in an animal model of ADHD. Neurosci Biobehav Rev. 2003;27:661–9. doi: 10.1016/j.neubiorev.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2002;27:1027–35. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Sikstrom S. Aging cognition: from neuromodulation to representation. Trends Cogn Sci. 2001;5:479–86. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, Cervenka S, Farde L, Nyberg L, Backman L. Extrastriatal dopamine D2 receptor binding modulates intraindividual variability in episodic recognition and executive functioning. Neuropsychologia. 2009;47:2299–304. doi: 10.1016/j.neuropsychologia.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Callicott JH, Bertolino A, Heaton I, Frank JA, Coppola R, et al. Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage. 2000;12:268–75. doi: 10.1006/nimg.2000.0610. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–91. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–57. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- McNicol D. A primer of signal detection theory. London: Allen & Unwin; 1972. [Google Scholar]
- Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set-shifting in AD/HD: relationships to baseline memory capacity. J Child Psychol Psychiatry. 2004;45:293–305. doi: 10.1111/j.1469-7610.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri NB, Calabresi P, Bernardi G. The electrophysiological actions of dopamine and dopaminergic drugs on neurons of the substantia nigra pars compacta and ventral tegmental area. Life Sci. 1992;51:711–8. doi: 10.1016/0024-3205(92)90479-9. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Saiardi A, Bonci A, Picetti R, Calabresi P, Bernardi G, et al. Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience. 1997;79:323–7. doi: 10.1016/s0306-4522(97)00135-8. [DOI] [PubMed] [Google Scholar]
- Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry. 2011;168:1154–63. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- Naylor H, Halliday R, Callaway E. The effect of methylphenidate on information processing. Psychopharmacology (Berl) 1985;86:90–5. doi: 10.1007/BF00431690. [DOI] [PubMed] [Google Scholar]
- Nelson H. National Adult Reading Test (NART): test manual. Windsor, UK: NFER-Nelson; 1982. [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull. 2000;126:220–46. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biol Psychiatry. 2005;57:1424–35. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Oades R, Taghzouti K, Simon H, Le Moal M. Dopamine-sensitive alternation and collateral behaviour in a Y-maze: effects of d-amphetamine and haloperidol. Psychopharmacology (Berl) 1985;85:123–8. doi: 10.1007/BF00427335. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Mehta MA, Kuntsi J, Asherson P. Functional MRI in ADHD: a systematic literature review. Expert Rev Neurother. 2007;7:1337–56. doi: 10.1586/14737175.7.10.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Buchsbaum MS, Zahn TP, Weingartner H, Ludlow C, Mikkelsen EJ. Dextroamphetamine: cognitive and behavioral effects in normal prepubertal boys. Science. 1978;199:560–3. doi: 10.1126/science.341313. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Buchsbaum MS, Weingartner H, Zahn TP, Ludlow C, Mikkelsen EJ. Dextroamphetamine. Its cognitive and behavioral effects in normal and hyperactive boys and normal men. Arch Gen Psychiatry. 1980;37:933–43. doi: 10.1001/archpsyc.1980.01780210091010. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Li R, Ansari MS, Zald D, Park S, Dawant B, et al. Amphetamine-induced displacement of [18F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacology. 2006a;31:1016–26. doi: 10.1038/sj.npp.1300916. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Zald D, Li R, Park S, Ansari MS, Dawant B, et al. Sex differences in amphetamine-induced displacement of [(18)F]fallypride in striatal and extrastriatal regions: a PET study. Am J Psychiatry. 2006b;163:1639–41. doi: 10.1176/ajp.2006.163.9.1639. [DOI] [PubMed] [Google Scholar]
- Robbins TW. From behavior to cognition: functions of mesostriatal, mesolimbic and mesocortical dopamine systems. In: Iversen LL, Iversen SD, Dunnett SB, Bjorklund A, editors. Dopamine handbook. New York: Oxford University Press; 2010. pp. 203–14. [Google Scholar]
- Robbins TW, Sahakian BJ. “Paradoxical” effects of psychomotor stimulant drugs in hyperactive children from the standpoint of behavioural pharmacology. Neuropharmacology. 1979;18:931–50. doi: 10.1016/0028-3908(79)90157-6. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Blackshaw AJ, Middleton HC, Matthews K, Hawtin K, Crowley C, et al. Tryptophan depletion impairs stimulus-reward learning while methylphenidate disrupts attentional control in healthy young adults: implications for the monoaminergic basis of impulsive behaviour. Psychopharmacology (Berl) 1999;146:482–91. doi: 10.1007/pl00005494. [DOI] [PubMed] [Google Scholar]
- Romanos M, Weise D, Schliesser M, Schecklmann M, Loffler J, Warnke A, et al. Structural abnormality of the substantia nigra in children with attention-deficit hyperactivity disorder. J Psychiatry Neurosci. 2010;35:55–8. doi: 10.1503/jpn.090044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa-Neto P, Lou HC, Cumming P, Pryds O, Karrebaek H, Lunding J, et al. Methylphenidate-evoked changes in striatal dopamine correlate with inattention and impulsivity in adolescents with attention deficit hyperactivity disorder. Neuroimage. 2005;25:868–76. doi: 10.1016/j.neuroimage.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med. 1998;39:904–11. [PubMed] [Google Scholar]
- Sahakian B, Morein-Zamir S. Professor's little helper. Nature. 2007;450:1157–9. doi: 10.1038/4501157a. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Robbins TW. Are the effects of psychomotor stimulant drugs on hyperactive children really paradoxical? Med Hypotheses. 1977;3:154–8. doi: 10.1016/0306-9877(77)90065-2. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Matsumura M, Kubota K. Dopamine enhances the neuronal activity of spatial short-term memory task in the primate prefrontal cortex. Neurosci Res. 1988;5:465–73. doi: 10.1016/0168-0102(88)90030-2. [DOI] [PubMed] [Google Scholar]
- Schrimsher GW, Billingsley RL, Jackson EF, Moore BD., 3rd Caudate nucleus volume asymmetry predicts attention-deficit hyperactivity disorder (ADHD) symptomatology in children. J Child Neurol. 2002;17:877–84. doi: 10.1177/08830738020170122001. [DOI] [PubMed] [Google Scholar]
- Seeman P, Madras BK. Anti-hyperactivity medication: methylphenidate and amphetamine. Mol Psychiatry. 1998;3:386–96. doi: 10.1038/sj.mp.4000421. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1263–72. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Bruno RM, Carter CS, Cohen JD. Dopamine and the mechanisms of cognition: Part I. A neural network model predicting dopamine effects on selective attention. Biol Psychiatry. 1998;43:713–22. doi: 10.1016/s0006-3223(97)00448-4. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, et al. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID) J Clin Psychiatry. 2010;71:313–26. doi: 10.4088/JCP.09m05305whi. [DOI] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhou Y. Psychostimulants induce low-frequency oscillations in the firing activity of dopamine neurons. Neuropsychopharmacology. 2004;29:2160–7. doi: 10.1038/sj.npp.1300534. [DOI] [PubMed] [Google Scholar]
- Simon V, Czobor P, Balint S, Meszaros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194:204–11. doi: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- Slifstein M, Kegeles LS, Xu X, Thompson JL, Urban N, Castrillon J, et al. Striatal and extrastriatal dopamine release measured with PET and [(18)F] fallypride. Synapse. 2010;64:350–62. doi: 10.1002/syn.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV. Neuropharmacological basis of stimulant drug action in attention deficit disorder with hyperactivity: a review and synthesis. Psychol Bull. 1984;95:387–409. [PubMed] [Google Scholar]
- Solanto MV. Behavioral effects of low-dose methylphenidate in childhood attention deficit disorder: implications for a mechanism of stimulant drug action. J Am Acad Child Psychiatry. 1986;25:96–101. doi: 10.1016/s0002-7138(09)60604-x. [DOI] [PubMed] [Google Scholar]
- Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998;94:127–52. doi: 10.1016/s0166-4328(97)00175-7. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Adler LA, McGough JJ, Muniz R, Jiang H, Pestreich L. Efficacy and safety of dexmethylphenidate extended-release capsules in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1380–7. doi: 10.1016/j.biopsych.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Strauss J, Lewis JL, Klorman R, Peloquin LJ, Perlmutter RA, Salzman LF. Effects of methylphenidate on young adults' performance and event-related potentials in a vigilance and a paired-associates learning test. Psychophysiology. 1984;21:609–21. doi: 10.1111/j.1469-8986.1984.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Tipper CM, Cairo TA, Woodward TS, Phillips AG, Liddle PF, Ngan ET. Processing efficiency of a verbal working memory system is modulated by amphetamine: an fMRI investigation. Psychopharmacology (Berl) 2005;180:634–43. doi: 10.1007/s00213-005-0025-4. [DOI] [PubMed] [Google Scholar]
- Turner DC, Blackwell AD, Dowson JH, McLean A, Sahakian BJ. Neurocognitive effects of methylphenidate in adult attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 2005;178:286–95. doi: 10.1007/s00213-004-1993-5. [DOI] [PubMed] [Google Scholar]
- Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Relative lack of cognitive effects of methylphenidate in elderly male volunteers. Psychopharmacology (Berl) 2003;168:455–64. doi: 10.1007/s00213-003-1457-3. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vernaleken I, Weibrich C, Siessmeier T, Buchholz HG, Rosch F, Heinz A, et al. Asymmetry in dopamine D(2/3) receptors of caudate nucleus is lost with age. Neuroimage. 2007;34:870–8. doi: 10.1016/j.neuroimage.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Volkow ND. Stimulant medications: how to minimize their reinforcing effects? Am J Psychiatry. 2006;163:359–61. doi: 10.1176/appi.ajp.163.3.359. [DOI] [PubMed] [Google Scholar]
- Volkow ND. Long-term safety of stimulant use for ADHD: findings from nonhuman primates. Neuropsychopharmacology. 2012;37:2551–2. doi: 10.1038/npp.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302:1084–91. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn J, Fowler JS, Telang F, Solanto MV, et al. Brain dopamine transporter levels in treatment and drug naive adults with ADHD. Neuroimage. 2007a;34:1182–90. doi: 10.1016/j.neuroimage.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007b;64:932–40. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–5. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.