Abstract
Inflammatory bowel diseases, ulcerative colitis, and Crohn’s disease, are chronic intestinal disorders of unknown etiology in which in genetically susceptible individuals, the mucosal immune system shows an aberrant response towards commensal bacteria. The gastrointestinal tract has developed ingenious mechanisms to coexist with its autologous microflora, but rapidly responds to invading pathogens and then returns to homeostasis with its commensal bacteria after the pathogenic infection is cleared. In case of disruption of this tightly-regulated homeostasis, chronic intestinal inflammation may be induced. Previous studies showed that some commensal bacteria are detrimental while others have either no influence or have a protective action. In addition, each host has a genetically determined response to detrimental and protective bacterial species. These suggest that therapeutic manipulation of imbalance of microflora can influence health and disease. This review focuses on new insights into the role of commensal bacteria in gut health and disease, and presents recent findings in innate and adaptive immune interactions. Therapeutic approaches to modulate balance of intestinal microflora and their potential mechanisms of action are also discussed.
Keywords: Commensal bacteria, Prebiotics, Probiotics, Innate immunity
INTRODUCTION
The continuous contact between commensal bacterial flora and the single epithelial cell layer of the mucosal tissue is a characteristic feature of the gastrointestinal system. In particular, interaction between commensal bacteria and mucosal immune system plays an important role in keeping health and disease development. Inflammatory bowel diseases (IBD), ulcerative colitis (UC), and Crohn’s disease (CD), are chronic intestinal disorders of unknown etiology in which in genetically susceptible individuals, the mucosal immune system shows an aberrant response towards luminal antigens such as dietary factors and/or commensal bacteria[1]. The relation between a dysregulated bacterial ecosystem and mucosal inflammation in IBD has been demonstrated in a variety of clinical and basic literatures[2-4]. For example, intestinal lesions of IBD predominate in the distal parts of the gastrointestinal tract where the commensal bacteria are most abundant. The presence of intestinal bacteria is essential for development of experimental colitis in several animal models, such as interleukin (IL)-10 gene knockout (KO) mice[5], T cell receptor α-deficient mice[6] and HLA-B27 transgenic rats[7]. In CD, fecal stream diversion reduces gut inflammation and induces mucosal healing in the excluded intestinal segment, whereas infusion of intestinal contents rapidly induced flare-up of disease[8].
The gastrointestinal tract has developed elaborate mechanisms to coexist with its autologous microflora, but rapidly respond to invading pathogens and then return to homeostasis with its commensal bacteria after the pathogenic infection is cleared. If these tightly regulated homeostatic mechanisms are disturbed, chronic intestinal inflammation may be induced[9]. Previous studies demonstrated that some commensal bacteria are detrimental, and others have either no effect or have a protective action. In addition, each host has a genetically determined response to detrimental and protective bacterial species. Environmental and genetic factors modulate the relative balance of beneficial and detrimental bacterial species, suggesting that therapeutic manipulation of this balance can influence health and disease. This review focuses on new insights into the role of commensal bacteria in gut health and disease, and presents recent advances in therapeutic approaches to modulate imbalance of intestinal microflora in IBD patients.
THE INTESTINAL MICROFLORA IN HEALTH AND DISEASE
The gastrointestinal tract host a complex and dynamic microorganisms enviroment. Most members are from the domain bacteria, but there are also representatives from archaea and eukarya, as well as virus[10]. The intestinal habitat of an adult human individual contain more than 500 different species of bacteria, with 30-40 species comprising up to 99% of the total population[10]. There is a progressive increase in the number of bacteria along the small bowel, from approximately 104 in the jejunum to 107 colony-forming units (CFU) per gram of luminal content at the ileal end, with predominance of gram-negative aerobes and some obligate anaerobes[10]. Anaerobes are predominant in the colon, and bacterial counts reach around 1012 CFU per gram of luminal content. Bacteria contribute to 60% of the fecal mass. Individuals exhibit variation in the types and numbers of species within their microflora. Conventional culturing techniques could detect only -30% of total bacteria in the gut[11], but the use of molecular biologic techniques enhanced detection capability of numbers and diversity of microflora[4,11].
Intestinal bacteria include native species that permanently colonize the tract and a variable set of microorganisms that transit temporarily through the gastrointestinal tract. Native bacteria are primarily acquired at birth and during the first year, but transient bacteria are being ingested continuously from the external environment. The fetal gut is sterile, and bacterial colonization, which is driven by contact between the infant and its environment, is influenced by the mode of delivery, hygiene levels and medications[4]. At a few days after birth infant feces are rich in enterobacteria species, such as E coli and Bifidobacterium, and these are soon influenced by feeding habits. Early colonization may also depend on genetic influences[4]. The pattern appear to be determined in part by the host genotype, because similarity in fecal bacterial species is much higher within twins than in genetically unrelated couples who share environment and dietary habits[10]. Intestinal microflora plays an essential role in the development of the gut immune system. Animals bred in a germ-free environment possess architectural abnormalities with crypt hyperplasia and lack of lymphoid follicle development. Immediately after exposure to microbes, the number of mucosal lymphocytes expands in the lamina propria and increases the number of IgA-secreting cells[12,13].
Several studies using different methods have repeatedly demonstrated that the fecal microflora as well as metabolic activity differs between subjects with IBD and healthy controls[14,15]. Recent molecular biology technique revealed that in CD patients the proportion of enterobacteria is increased[16,17], and this finding is compatible with previous reports based in culture techniques[14]. A large part of the dominant microflora (30%) was characterized in undefined phylogenetic groups, indicating a presence of major differences between CD and healthy individuals. Other studies confirmed that Bacteroides vulgatus was the only species shared by all CD patients in spite of unusual dominant species. From the results of analyses of mucosa-associated flora, Swidsinski et al found high concentrations of mucosal bacteria in patients with bowel inflammation, but not in controls[18]. The concentrations of mucosal bacteria increased progressively with the severity of disease, both in inflamed and non-inflamed colon[18]. They hypothesize that the healthy mucosa is capable of holding back fecal bacteria and that this function is profoundly disturbed in patients with IBD. These observations are compatible with the report by Kleessen et al[19]. They demonstrated that more bacteria were detected on the mucosal surface of IBD patients than on those of non-IBD controls[19]. Bacterial invasion of the mucosa was evident in colonic specimens from the UC patients, in the ileal and the colonic specimens from the CD patients, but no bacteria were detected in the tissues of the controls. Colonic UC specimens were colonized by a variety of organisms, such as bacteria belonging to the gamma subdivision of Proteobacteria, the Enterobacteriaceae, the Bacteroides/Prevotella cluster, the Clostridium histolyticum/Clostridium lituseburense group, the Clostridium coccoides/Eubacterium rectale group, high G + C Gram-positive bacteria, or sulphate-reducing bacteria, while CD samples harbored mainly bacteria belonging to the former three groups. Previously, we also reported that the bacterial counts for both aerobes and anaerobes increased in UC patients[20]. In particular, we detected the highest bacterial counts of Bacteroides vulgatus (Table 1). A high agglutination titer against B. vulgatus, B. fragilis, and C. ramosum was detected in most UC patients, and the percentage of positive immunoreactivity was much higher in UC patients than in healthy controls. The serum immunoreactivity (IgG) against 26-kDa protein derived from B. vulgatus outer-membrane was much higher in UC patients (53.8%) than in the control sera (9.1%) (Figure 1). These results suggest that B. vulgatus and a specific antibody response directed against it may play an important role in the pathogenesis of UC[20].
Table 1.
Comparison of rectal mucosal flora in UC patients
| Bacterial counts (mean ± SD) | Positive rate (%) | ||||
| UC | Control | UC | Control | ||
| Total | 6.63 ± 0.96 | 5.47 ± 0.96 | |||
| Aerobes | 6.23 ± 0.50 | 5.28 ± 2.47 | |||
| Anaerobes | 6.42 ± 1.05 | 5.01 ± 0.76 | |||
| Clostridium | 4.64 ± 2.07 | 3.74 ± 2.02 | 61.1 | 42.9 | |
| Bacteroides | 5.33 ± 1.71 | 3.96 ± 1.66 | 94.4 | 85.7 | |
| Bifidobacterium | 5.32 ± 2.64 | 4.25 ± 2.24 | 44.4 | 28.6 | |
| Eubacterium | 4.73 ± 2.13 | 2.73 ± 1.53 | 33.3 | 57.1 | |
| Fusobacterium | 0 | 4.14 ± 1.95 | 0 | 28.6 | |
| Actinomyces | 2.28 ± 0.83 | 0 | 5.5 | 0 | |
| Veillonella | 4.82 ± 1.79 | 0 | 11.1 | 0 | |
| Peptostreptococcus | 4.53 ± 2.17 | 1.80 ± 1.14 | 38.8 | 28.6 | |
| Streptococcus | 4.93 ± 2.33 | 3.03 ± 1.47 | 27.7 | 14.3 | |
| Peptococcu | 3.35 ± 1.34 | 0 | 11.1 | 0 | |
log10 numbers of organism/g tissue. Reproduction from reference 20.
Figure 1.
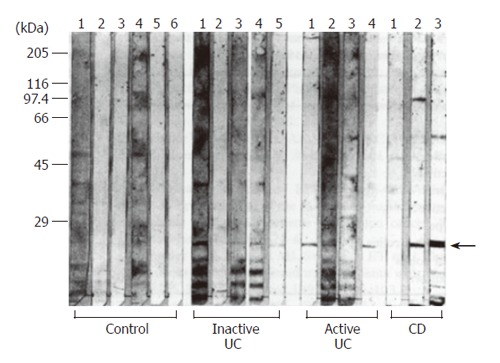
Western blot analysis of serum antibodies (IgG) agaist outer-membrane antigens of Bacteroides vulgatus isolated from an IBD patient. The arrow indicates specific band. Reproduced from reference 20 with permission.
MUCOSAL RESPONSE TO LUMINAL BACTERIA
General background
The search for specific pathogens that trigger intestinal inflammation failed to produce conclusive results[21]. Instead, it has been found that reconstitution of germ-free mice with commensal bacteria can be enough to induce IBD in several gene-deficient as well as T cell transfer models of IBD in mice[22-25]. Therefore, instead of a specific pathogen, a broad spectrum of bacteria may contribute to the induction of intestinal inflammation. Although metabolically active microbial cells and cell wall components, such as lipopolysaccharide (LPS) and peptidoglycan, present and contact to the host intestinal mucosa, pro-inflammatory responses are absent in the mucosa exposed to the resident luminal microflora. In contrast, the capability to respond to luminal pathogenic bacteria through recruitment of inflammatory cells from systemic circulation is remaining. The probable mechanisms underlying these responses are explained by the standpoints of innate and adaptive immune response[26]. In the intestine, components of innate immunity are preexisting or rapidly activated, resulting in induction or regulation of the highly specific adaptive immune responses.
Recently, novel functions of mucosal dendritic cells (DC) have been reported. Dendritic cells are critical to innate and adaptive immunity as specialized antigen-presenting cells. Hendrik et al showed that lamina propria DCs form transepithelial dendrites which enable the cells to directly sample antigens, such as commensal bacterial components[27]. It is likely that DCs take up directly intestinal antigens through transepithelial dendrites and activate an innate immune pathway that protects the mucosa from pathogenic bacteria.
Toll-like receptors and their signaling
Highly conserved structures of pathogenic and commensal bacteria, designated MAMPs (microbe-associated molecular patterns), are recognized by pattern-recognition receptors, such as TLR (Toll-like receptor)[28-30]. TLRs comprise a family of pattern-recognition receptors that detect conserved molecular products of microorganisms, such as LPS and lipoteichoic acid (LTA), recognized by TLR4 and TLR2, respectively. TLRs are expressed both in epithelial cells and in phagocytic cells, thus functioning as sensors of microbial infection. They are critical to initiation of inflammatory and immune defense responses. The bacterial ligands recognized by TLRs are not unique to pathogens, but are rather shared by entire classes of bacteria, and are produced by commensal bacteria as well. However, it remains unclear how the host distinguishes between pathogenic and commensal bacteria. Two major pathways are activated by TLRs[28,30-32]. The first culminate in activating the transcription factor NF-κB, which acts as a master switch for inflammation. It regulates the transcription of many genes that encode proteins involved in immunity and inflammation. The second leads to activation of MAP kinases p38 and Jun amino-terminal kinase (JNK), which also participate in increased transcription and regulate the stability of mRNAs that contain AU repeats. With the exception of TLR3, all TLRs activate NF-κB and MAP kinases via a pathway that involves MyD88, IRAK (IL-1 receptor associated kinase)-4 and IRAK-1[30]. There are specific differences in the ultimate gene expression profile that results from the activation of individual TLRs, although the precise mechanisms are unclear. A set of adaptor proteins that are differentially recruited to TLRs may be involved in the molecular basis of this specific gene induction[33].
MyD88 is an adaptor protein for TLRs, and similar to TLRs, it has a Toll-IL-1 receptor (TIR) domain[30-32,34]. Signaling may be initiated by recruitment of MyD88 to TLRs through TIR-TIR interactions. Interaction between MyD88 and TLRs leads to the recruitment of IRAK-4. IRAK-4 becomes activated and is phosphorylated to IRAK-1, resulting in activation of TRAF6[34]. TRAF6 activation results in activation of NF-κB and MAP kinases. Other proteins, such as Tollip, ECSIT, Pellinos, MEKK1 and MEKK3 have also been implicated in this pathway.
Recently, two different groups reported that MyD88-deficient mice reveal an increased susceptibility to dextran sodium sulfate (DSS) colitis[35,36]. MyD88-deficient mice showed an increased mortality and morbidity, as well as aggravation of colitis, following DSS administration. In MyD88-deficient mice, mucosal proliferative zone was expanded and number of proliferating cells increased, indicating dysregulated proliferation and differentiation of intestinal epithelium. Interestingly, similar responses were also observed in TLR2- and TLR4-deficient mice. Increased susceptibility to intestinal injury in MyD88-deficient mice was accompanied with defective production of cytoprotective and reparative factors, such as IL-6, TNF, KC-1 and heat-shock proteins[36]. These findings indicate that TLR signaling via the MyD88-dependent pathway conferred protection from the mortality, morbidity and colonic damage caused by the administration of the injurious agent DSS. Since DSS is capable to induce severe morbidity and mortality in wild mice where commensal microflora had been depleted by antibiotics, , Authors have concluded that recognition of commensal microflora by TLRs is required for keeping intestinal homeostasis.
Several mechanisms have been proposed to explain how the epithelium discriminates pathogens from commensals in order to trigger TLR signaling. Although they express similar MAMP, pathogens differ from commensals mainly in their ability to colonize host mucosal surfaces and invade the host. Differences attributed to the differential expression of adhesion molecules. Recently-proposed speculation is that in the gut, the commensal-specific TLR/MyD88 pathway permits the symbiotic relationship between the microflora and the host, while pathogen-specific virulence factors are required to trigger pro-inflammatory responses via the usage of additional TLR co-receptor and/or adaptor molecules for disease-causing organisms[29,37].
NOD2/CARD15
The putative intracellular peptidoglycan receptor NOD2 (CARD15) is a member of the Apaf-1/CARD superfamily and is composed of an N-terminal caspase recruitment domain (CARD), a centrally located nucleotide-binding oligomerization domain (NOD) and 10C-terminal-located leucine-rich repeats (LRRs)[38,39]. NOD2 was found to be expressed in antigen-presenting cells such as monocytes/macrophages, but more recent studies revealed abundant presence of NOD2 in epithelial Paneth cells of the small intestine as well as in other epithelial cells[39,40]. NOD2 has been shown to recognize intracellular peptidoglycan fragments (e.g. muramyl dipeptide, MDP) through its LRR region leading to pro-inflammatory responses through activation of NF-κB. NOD2 serves as an intracellular pattern recognition receptor to enhance host defense by inducing the production of antimicrobial peptides such as human beta-defensin-2[39].
Several studies have shown that mutations in the LRR region of NOD2 are associated with susceptibility to Crohn’s disease[41]. The molecular mechanisms by which mutations in the NOD2 gene cause Crohn’s disease are still emerging. However, it is supposed that decreased production of antimicrobial peptides, such as defensins, may promote bacterial-mediated inflammation in Crohn’s disease[39]. Recent study demonstrated that NOD2 mutation in CD potenciates NF-κB activity and IL-1β processing, suggesting initiation and/or promotion of mucosal inflammation[42].
THERAPEUTIC STRATEGIES TARGETING MICROFLORA IN IBD
IBD continues to be an enigmatic disorder with obvious potential to improve therapeutic target and outcomes[43]. Established therapies for IBD include the aminosalicylates, corticosteroids, and immunosuppressive drugs. An increasing number of novel and alternative therapeutic approaches are in progress[43]. New biologic therapies include the targeting of proinflammatory cytokines, enhancement or infusion of anti-inflammatory cytokines, blocking intravascular adhesion molecules, and modifying T-cell functions. Recently, therapeutic approaches to modifying intestinal microflora have been attempted by using prebiotics and probiotics. In addition, antibiotic therapies continue to be used[2,44-47].
Prebiotics
Prebiotics are nondigestible food constituents that benefit the host by selectively stimulating the growth or activity of one or a limited number of bacterial species already resident in the colon[2]. Some examples of prebiotics are dietary fiber and some types of oligosaccharides. Intake of prebiotics can significantly alter the colonic microflora by increasing the populations of certain bacteria and thereby quantitatively changing the composition of the microflora[48,49]. These alterations may act beneficially, in part, by causing a luminal reduction of short-chain fatty acids (SCFAs), which are both important nutrients for the intestine and inducers of an acidic environment[47,49-52]. Among the SCFAs, butyrate most effectively protects intestinal mucosa against injury and promotes mucosal healing[49,53].
Lactosucrose: Lactosucrose, an indigestible oligosaccharide, is a water-soluble dietary fiber with the potency of modulating microflora. Ohkusa et al reported that a daily 6-gram intake of lactosucrose significantly increased the percentage of Bifidobacterium sp and the total number of bacteria in healthy subjects[54,55]. The treatment also reduced fecal ammonia levels but had no effect on fecal SCFA, pH, and water content. It has been reported that an elemental diet or low-fat, low-residual diet decreases anaerobic bacteria and changes the composition of microflora in IBD patients. Teramoto et al showed that the continuous administration of lactosucrose for 2 wk led to an increase of Bifidobacterium and a decrease of Bacteroidaceae in patients with IBD[55].
Oligofructose and inulin: Inulin and oligofructose are comparable to dietary fiber in that they are composed of multiple saccharide units, which are indigestible by the enzymes in mammalian small intestine. The saccharide chain in inulin is longer than in oligofructose. Inulin and oligofructose show similar physiological functions in the intestine. It is generally recognized that inulin stimulates the generation of butyrate and the growth of lactic acid bacteria (LAB) in the colons of healthy subjects[50,56]. Videla et al examined the efficacy of inulin in a dextran sodium sulfate (DSS)-induced colitis model, demonstrating that it significantly attenuates inflammation as assessed by mucosal damage and both colonic eicosanoid and myeloperoxidase concentrations[50]. The treatment also led to an increase of Lactobacillus and a decrease of luminal pH and fecal water content. To our knowledge, no clinical trials to confirm the benefit from either inulin or oligofructose have been performed.
Bran: The laxative effect of wheat bran has long been recognized. Although not potent, bran is used widely because of its harmlessness. A Swedish group examined the effect of wheat bran supplementation on the composition of fecal bile acid and microflora in juvenile patients with UC. Although the clinical activity was not described, wheat bran significantly decreased the number of Bacteroides and the concentration of total and unconjugated bile acid[57,58].
Psyllium: Psyllium, also called Ispaghula husk or Plantago ovata, is a water-soluble dietary fiber. Buhman et al suggested that psyllium has a hypocholesterolemic effect, based on its hydrocolloid, a gel-forming potency[59,60]. Feeding of psyllium significantly decreases the cholesterol content as well as the cholesterol 7-alpha hydroxylase activity in the rat liver. Hallert et al published the first report describing the clinical efficacy of psyllium in patients with UC[61]. Psyllium significantly attenuates clinical symptoms compared with placebo treatment. After this first report was published, a large-scale clinical trial was organized in UC patients by Fernandez-Banares et al[62]. In this trial, they found no significant differences in the remission periods of patients given psyllium treatment and patients given sulfasalazine treatment, although there was an increase in fecal butyrate in the psyllium patients. The authors therefore concluded that psyllium may be as effective as sulfasalazine in maintaining remission in patients with CD.
Germinated barley foodstuff (GBF): GBF, which is derived from the aleuronic layer and scutellum fractions of germinated barley, consists mainly of dietary fiber and glutamine-rich protein[47,48,51,52]. The fiber fraction of GBF consists mainly of low-lignified hemicellulose, which has a large water-holding capacity[63]. GBF contains glutamine, which is another important nutrient for epithelial cells[47,48,52,63]. In germination process, GBF obtains these two unique characteristics of being a glutamine-rich protein and having a conspicuous water holding capacity[64]. In the intestinal lumen, the dietary fiber fraction of GBF is utilized efficiently by Bifidobacterium or Lactobacillus and converted to lactate and acetate. Coexistence of Bifidobacterium and Eubacterium limosum increases butyrate production from GBF[65,66]. The endogenous bacterial butyrate produced from GBF is immediately absorbed by the intestinal epithelial cells and utilized by them as an efficient fuel. The water holding capacity of GBF is much higher than other representative water-insoluble dietary fibers, for example, wheat bran, cellulose powder, and sugar beet fiber.
Treatment of rat DSS colitis with GBF in a preventive mode led to a significant improvement of the clinical and pathological signs of colitis and a decrease in serum IL-8 and alpha 1-acid-glycoprotein[64]. The improvements were associated with an induction of luminal butyrate and beneficial organisms, such as Bifidobacterium and Eubacterium. In a therapeutic mode, GBF was comparably effective against mucosal inflammation and more effective against diarrhea when compared with sulfasalazine[64]. The anti-inflammatory action of GBF was markedly reduced by the concomitant administration of SCFA β-oxidation inhibitor (ibuprofen) and GBF, indicating a butyrate-dependent anti-inflammatory mechanism[67]. In addition to its role as a preferential nutrient for colonocytes, the butyrate also acts as an anti-inflammatory agent by functionally inactivating nuclear factorκB[68]. Of the GBF constituents, the fiber fraction, but not its protein fraction, drastically mitigated mucosal damage with an increase of luminal butyrate. GBF significantly increased the number of Eubacterium and Bifidobacterium, with a concomitant decrease in luminal pH. In the HLA-B27 transgenic rat, a representative model of spontaneous colitis, GBF improves the clinical and pathological signs of colitis with an increase in luminal butyrate levels[69].
The first trial enrolled 10 patients with mild to moderately active UC who had been unresponsive to or intolerant of standard treatment[70]. The patients consumed 30 g of GBF 3 times daily for 4 wk in a nonrandomized, open-label fashion. At 4 wk, treatment with GBF resulted in clinical and endoscopic improvement with an increase in fecal butyrate. Despite continued treatment with standard drugs, the patients had an exacerbation of the disease within 4 wk after discontinuing GBF treatment. A subsequent multicenter trial with 28 patients conducted in the same fashion showed a similar benefit from GBF. Eighteen patients with mild to moderately active UC were divided into two groups using a random allocation protocol. The control group (n = 7) were given a baseline anti-inflammatory therapy, and the GBF-treated group (n = 11) received 20 to 30 g of GBF daily together with the baseline treatment. After 4 wk of observation, the GBF group showed a significant decrease in clinical activity index scores compared with the control group. No side effects related to GBF were observed[48]. GBF increased fecal concentrations of Bifidobacterium and Eubacterium limosum. Twenty-one patients with moderately active UC patients were treated with 20-30 g/d of GBF for 24 wk. GBF significantly reduced clinical activity as compared to the control group[52]. Furthermore, GBF prolonged remission in UC patients[51].
Probiotics
In the intestinal lumen of IBD patients, balance between commensal and detrimental bacteria has been broken down with secondary harm on immune system activities. However, the changes in microbiotic composition may be transient; and implantation of exogenous bacteria will have a limited applicability[2,44,46]. Probiotics are live microorganisms administered to alter the intestinal microflora and confer a beneficial effect on health[2]. Potential mechanisms of probiotic action include competitive interactions, production of antimicrobial metabolites, influences on the epithelium, and immune modulation[2,44,46]. However, such changes may be transient, and therefore the implantation of exogenous bacteria has a limited usefulness at present. Restoring the microbial balance using probiotics may be the most physiologic and non-toxic way to prevent and treat IBD.
VSL#3: IL-10 knockout mice develop colitis when they are raised under conventional facilities but not under germ-free conditions. Prior treatment of IL-10 knockout mice with antibiotics prevented the subsequent onset of colitis, suggesting that the exposure to intestinal bacteria during the neonatal period influences later disease progression. The use of VSL#3, a probiotic preparation containing three strains of Bifidobacterium, four strains of Lactobacilli, and one strain of Streptococcus salivarius ssp. thermophilus completely normalized the physiological transport function and barrier integrity and also inhibited mucosal TNFα and IFNγ production[71]. In vitro studies showed that epithelial barrier function and resistance to Salmonella invasion could be enhanced by exposure to a proteinaceous soluble factor secreted by the bacteria found in the VSL#3 compound[72]. There are increasing number of reports describing anti-inflammatory effects of VSL#3[46].
In a clinical trial, daily administration of VSL#3 prevented relapse of chronic pouchitis after induction of remission by antibiotics. Moreover, every patients relapsed within 3 mo of stopping VSL#3[73]. These were replicated[74,75], and prospective study was performed[76]. In this study, 2 of 20 patients (2%) receiving VSL#3 for one year developed pouchitis versus 40% of placebo-treated patients. Uncontrolled pilot studies have indicated that VSL#3 maintained remission of UC in 75% of patients over 12 mo[73,77]. The results showed that this probiotic preparation could colonize the intestine, and might be useful in maintaining remission in UC. As another study, 32 ambulatory patients with active UC received open label VSL#3, 3600 billion bacteria daily in two divided doses for 6 wk. Treatment of patients with mild to moderate UC, not responding to conventional therapy, with VSL#3 resulted in a combined induction of remission/response rate of 77% with no adverse events. At least some of the bacterial species incorporated in the probiotic product reached the target site in amounts that could be detected[78].
Nissle1917: Previous trials have examined the efficacy of a non-pathogenic strain of Escherichia coli originally called Nissle1917. In the first pilot study, capsules containing this strain of E coli were compared with a placebo for the maintenance of prednisolone-induced remission of colonic CD[79]. After 12 wk of treatment, there was no significant difference between the Nissle and control groups. Rembacken et al described a single-center trial in which 116 patients with UC were randomized to receive either mesalazine or this non-pathogenic E coli strain[80]. Initial responses to treatment were similar, with remission being noted in 75% and 68% of those receiving mesalazine and E coli, respectively. Even more impressive were the maintenance benefits; respectively, 73% and 67% of patients remained in remission for 12 mo[80].
Clostridium butyricum: Araki et al reported that the anti-colitis effect of Japanese microbial preparation (MIYAIRI-588) was examined in a dextran sulfate sodium (DSS)-induced rodent colitis model[81]. This preparation itself did not display any therapeutic effect. Another probiotic mixture (Lactobacillus, Clostridium butyricum and Streptococcus faecalis; Biothree: Towa Kasei Co., Ltd, Tokyo, Japan) was also evaluated in a DSS colitis model, by Fukuda et al[82]. Although the benefits on colon histology were not significant, diarrhea was significantly decreased by treatment in comparison to the control group 66) Further trials in patients with IBD would be desirable.
Bifidobacterium-fermented milk: The preventive effect of lactic acid bacteria (LAB - Bifidobacterium breve, Bifidobacterium bifidum and Lactobacillus acidophilus)-fermented milk was determined in SAMP1/Yit mice[83]. Administration of LAB-fermented milk to mice reduced histological injury scores, compared with those in saline-treated or unfermented milk-treated mice. Treatment with LAB-fermented milk also reduced ileal tissue weight and myeloperoxidase activity. Moreover, the tissue contents of immunoglobulin such as IgG1 and IgG2a were lower in the inflammatory regions in the LAB-fermented milk-treated group than in the control group. A decreased release of Info and TNFα with an increase of IL-10 from mesenteric lymph node cells were observed in the LAB-fermented milk treated group.
Lactococci-secreting IL-10: Steidler et al described the use of transgenic Lactococcus lactis capable of secreting bioactive IL-10 in both the DSS and IL-10 knockout models of colitis[84,85]. The authors observed an inhibition of spontaneous colitis development in IL-10 knockout mice that was mediated by relatively low concentrations of the Lactococcus-borne cytokine. These experiments provide the basis for the use of genetically modified organisms designed for delivery of biologically relevant therapeutic molecules.
Synbiotics
A synbiotic is a combination of one or more probiotics and prebiotics[86-88]. Prebiotics may enhance the survival of probiotic strains, as well as stimulating activity of the host’s endogenous bacteria. Bengmark suggests that clinical effects vary from modest effects to significant effects as one goes from single-strain of probiotics < multistrain probiotics < or - < single-strain/single fiber synbiotics < multistrain/multifiber synbiotics[87]. Kanamori et al reported that combination therapy with Bifidobacterium breve, Lactobacillus casei, and galactooligosaccharides dramatically improved the intestinal function in a girl with short bowel syndrome[89]. Roller et al demonstrated that combination of oligofructose-enriched inulin combined with Lactobacillus and Bifidobacterium suppressed colon carcinogenesis by modulating functions of gut-associated lymphoid tissue[90]. They also reported that same synbiotic formula stimulated secretion of secretory-IgA and IL-10 production in the cecum[91]. A symbiotic preparation of Bifidobacterium combined with galacto-oligosaccharides protects against Salmonella infection in mice[92]. Thus, synbiotics modulate mucosal immune responses and exert anti-inflammatory effects. We are not aware of any trial design to evaluate potential benefit of synbiotics in IBD patients.
Antibiotics
A few trials of antibacterial agents have been conducted in UC with controversial results. Oral vancomycin and intravenous metronidazole were not beneficial in active UC both as single or adjunctive given therapy[93,94]. Tobramycin, a nonabsorbable, gram-negative specificantibiotic , induced a significant improvement in the short term compared to placebo in UC. However, the improvement was lost at long term follow-up. In addition, the association of tobramycin and metronidazole did not implemented the outcome in patients with severe UC treated by conventional therapy (steroids)[95]. In a a, double-blind placebo-controlled trial run on a small sample, the use of rifaximin (a nonabsorbable, wide-spectrum antibiotic) led to a significant improvement in both clinical and endoscopic activity[96].
A more definite role for antibiotics in UC is in the treatment of pouchitis, where conditions are favourable to bacterial overgrowth[2]. It has been suggested that anaerobes induce pouch inflammation, but some investigators have found a relative increase of aerobic bacteria in pouchitis. In pouchitis, Bacteroides species are present in low numbers, whereas E coli numbers are increased, but not correlated with the degree of inflammation. Luminal pH is increased, enhancing proteolytic enzyme activity and mucin degradation. Current data suggest that dysbiosis of luminal organisms contributes to pouch inflammation in a susceptible host. Treatment with metronidazole, ciproxin and/or rifaximin leads to a significant decrease of the total number of the following anaerobes and aerobes in fecal samples: Enterococci, Lactobacilli, Bifidovacteria, and Bacteroides[96].
Antibiotics are largely used in clinical practice for treating active CD and provide more satisfactory results. Controlled trials have supported this treatment[97,98]. In a placebo-controlled trial, both low-dose (10 mg/kg per day) and high-dose (20 mg/kg per day) metronidazole were more effective than a placebo in colonic CD. In a controlled trial to study the combination of metronidazole and ciprofloxacin versus methylprednisolone for active CD, after 12 wk there was no statistically significant difference in remission rates of the two groups. More recently, ciprofloxaxin was shown to be effective as mesalazine in inducing remission in patients with active CD. Antibiotics do have a role in at least a subset of cases; but we must keep in mind that treatment with antibiotics may have some disadvantages, such as non-specific effect on the enteric flora, the possibility of inducing an antibiotic resistance, and the risk of Clostridium difficile superinfection.
In conclusion, the pathogenesis of IBD may be associated with imbalance in the intestinal microflora with a relative predominance of "aggressive" bacteria and paucity of "protective" organisms. Manipulation of the intestinal flora may represent a highly physiologic, nontoxic way to prevent and treat IBD. Although these strategies appear promising and may be actually useful in specific settings, more studies are are needed to establish the relevance of these therapies.
Footnotes
S- Editor Pan BR L- Editor Chiarioni G E- Editor Liu WF
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 3.Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut. 2004;53:1–4. doi: 10.1136/gut.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahida YR, Rolfe VE. Host-bacterial interactions in inflammatory bowel disease. Clin Sci (Lond) 2004;107:331–341. doi: 10.1042/CS20040136. [DOI] [PubMed] [Google Scholar]
- 5.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kishi D, Takahashi I, Kai Y, Tamagawa H, Iijima H, Obunai S, Nezu R, Ito T, Matsuda H, Kiyono H. Alteration of V beta usage and cytokine production of CD4+ TCR beta beta homodimer T cells by elimination of Bacteroides vulgatus prevents colitis in TCR alpha-chain-deficient mice. J Immunol. 2000;165:5891–5899. doi: 10.4049/jimmunol.165.10.5891. [DOI] [PubMed] [Google Scholar]
- 7.Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernandez-Sueiro JL, Balish E, Hammer RE. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harper PH, Lee EC, Kettlewell MG, Bennett MK, Jewell DP. Role of the faecal stream in the maintenance of Crohn‘s colitis. Gut. 1985;26:279–284. doi: 10.1136/gut.26.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz M, Scholmerich J, Rath HC. Rationale for probiotic and antibiotic treatment strategies in inflammatory bowel diseases. Dig Dis. 2003;21:105–128. doi: 10.1159/000073243. [DOI] [PubMed] [Google Scholar]
- 10.Guarner F. The intestinal flora in inflammatory bowel disease: normal or abnormal. Curr Opin Gastroenterol. 2005;21:414–418. [PubMed] [Google Scholar]
- 11.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 12.Yamanaka T, Helgeland L, Farstad IN, Fukushima H, Midtvedt T, Brandtzaeg P. Microbial colonization drives lymphocyte accumulation and differentiation in the follicle-associated epithelium of Peyer's patches. J Immunol. 2003;170:816–822. doi: 10.4049/jimmunol.170.2.816. [DOI] [PubMed] [Google Scholar]
- 13.Helgeland L, Dissen E, Dai KZ, Midtvedt T, Brandtzaeg P, Vaage JT. Microbial colonization induces oligoclonal expansions of intraepithelial CD8 T cells in the gut. Eur J Immunol. 2004;34:3389–3400. doi: 10.1002/eji.200425122. [DOI] [PubMed] [Google Scholar]
- 14.Marteau P, Lepage P, Mangin I, Suau A, Doré J, Pochart P, Seksik P. Review article: gut flora and inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20 Suppl 4:18–23. doi: 10.1111/j.1365-2036.2004.02062.x. [DOI] [PubMed] [Google Scholar]
- 15.van Nuenen MH, Venema K, van der Woude JC, Kuipers EJ. The metabolic activity of fecal microbiota from healthy individuals and patients with inflammatory bowel disease. Dig Dis Sci. 2004;49:485–491. doi: 10.1023/b:ddas.0000020508.64440.73. [DOI] [PubMed] [Google Scholar]
- 16.Sokol H, Seksik P, Rigottier-Gois L, Lay C, Lepage P, Podglajen I, Marteau P, Doré J. Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:106–111. doi: 10.1097/01.MIB.0000200323.38139.c6. [DOI] [PubMed] [Google Scholar]
- 17.Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, Jian R, Dore J. Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut. 2003;52:237–242. doi: 10.1136/gut.52.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, Weber J, Hoffmann U, Schreiber S, Dietel M, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122:44–54. doi: 10.1053/gast.2002.30294. [DOI] [PubMed] [Google Scholar]
- 19.Kleessen B, Kroesen AJ, Buhr HJ, Blaut M. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand J Gastroenterol. 2002;37:1034–1041. doi: 10.1080/003655202320378220. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda H, Fujiyama Y, Andoh A, Ushijima T, Kajinami T, Bamba T. Characterization of antibody responses against rectal mucosa-associated bacterial flora in patients with ulcerative colitis. J Gastroenterol Hepatol. 2000;15:61–68. doi: 10.1046/j.1440-1746.2000.02045.x. [DOI] [PubMed] [Google Scholar]
- 21.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 22.Kullberg MC, Ward JM, Gorelick PL, Caspar P, Hieny S, Cheever A, Jankovic D, Sher A. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foltz CJ, Fox JG, Cahill R, Murphy JC, Yan L, Shames B, Schauer DB. Spontaneous inflammatory bowel disease in multiple mutant mouse lines: association with colonization by Helicobacter hepaticus. Helicobacter. 1998;3:69–78. doi: 10.1046/j.1523-5378.1998.08006.x. [DOI] [PubMed] [Google Scholar]
- 24.Aranda R, Sydora BC, McAllister PL, Binder SW, Yang HY, Targan SR, Kronenberg M. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. J Immunol. 1997;158:3464–3473. [PubMed] [Google Scholar]
- 25.Andoh A, Endo Y, Kushima R, Hata K, Tsujikawa T, Sasaki M, Mekata E, Tani T, Fujiyama Y. A case of Crohn's disease involving the gallbladder. World J Gastroenterol. 2006;12:977–978. doi: 10.3748/wjg.v12.i6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi D, Das J, Das G. Inflammatory bowel disease requires the interplay between innate and adaptive immune signals. Cell Res. 2006;16:70–74. doi: 10.1038/sj.cr.7310009. [DOI] [PubMed] [Google Scholar]
- 27.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 28.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 29.Sirard JC, Bayardo M, Didierlaurent A. Pathogen-specific TLR signaling in mucosa: mutual contribution of microbial TLR agonists and virulence factors. Eur J Immunol. 2006;36:260–263. doi: 10.1002/eji.200535777. [DOI] [PubMed] [Google Scholar]
- 30.O'Neill LA. How Toll-like receptors signal: what we know and what we don't know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 31.McGettrick AF, O'Neill LA. The expanding family of MyD88-like adaptors in Toll-like receptor signal transduction. Mol Immunol. 2004;41:577–582. doi: 10.1016/j.molimm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q, Dziarski R, Kirschning CJ, Muzio M, Gupta D. Micrococci and peptidoglycan activate TLR2-->MyD88-->IRAK-->TRAF-->NIK-->IKK-->NF-kappaB signal transduction pathway that induces transcription of interleukin-8. Infect Immun. 2001;69:2270–2276. doi: 10.1128/IAI.69.4.2270-2276.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber AN, Moncrieffe MC, Gangloff M, Imler JL, Gay NJ. Ligand-receptor and receptor-receptor interactions act in concert to activate signaling in the Drosophila toll pathway. J Biol Chem. 2005;280:22793–22799. doi: 10.1074/jbc.M502074200. [DOI] [PubMed] [Google Scholar]
- 34.Gohda J, Matsumura T, Inoue J. Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not toll/IL-1 receptor domain-containing adaptor-inducing IFN-beta (TRIF)-dependent pathway in TLR signaling. J Immunol. 2004;173:2913–2917. doi: 10.4049/jimmunol.173.5.2913. [DOI] [PubMed] [Google Scholar]
- 35.Araki A, Kanai T, Ishikura T, Makita S, Uraushihara K, Iiyama R, Totsuka T, Takeda K, Akira S, Watanabe M. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J Gastroenterol. 2005;40:16–23. doi: 10.1007/s00535-004-1492-9. [DOI] [PubMed] [Google Scholar]
- 36.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Fischer H, Yamamoto M, Akira S, Beutler B, Svanborg C. Mechanism of pathogen-specific TLR4 activation in the mucosa: fimbriae, recognition receptors and adaptor protein selection. Eur J Immunol. 2006;36:267–277. doi: 10.1002/eji.200535149. [DOI] [PubMed] [Google Scholar]
- 38.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 39.Voss E, Wehkamp J, Wehkamp K, Stange EF, Schröder JM, Harder J. NOD2/CARD15 mediates induction of the antimicrobial peptide human beta-defensin-2. J Biol Chem. 2006;281:2005–2011. doi: 10.1074/jbc.M511044200. [DOI] [PubMed] [Google Scholar]
- 40.Ogura Y, Lala S, Xin W, Smith E, Dowds TA, Chen FF, Zimmermann E, Tretiakova M, Cho JH, Hart J, et al. Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut. 2003;52:1591–1597. doi: 10.1136/gut.52.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 42.Maeda S, Hsu LC, Liu H, Bankston LA, Iimura M, Kagnoff MF, Eckmann L, Karin M. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–738. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 43.Hibi T, Ogata H. Novel pathophysiological concepts of inflammatory bowel disease. J Gastroenterol. 2006;41:10–16. doi: 10.1007/s00535-005-1744-3. [DOI] [PubMed] [Google Scholar]
- 44.Sartor RB. Probiotic therapy of intestinal inflammation and infections. Curr Opin Gastroenterol. 2005;21:44–50. [PubMed] [Google Scholar]
- 45.Gordon JN, Di Sabatino A, Macdonald TT. The pathophysiologic rationale for biological therapies in inflammatory bowel disease. Curr Opin Gastroenterol. 2005;21:431–437. [PubMed] [Google Scholar]
- 46.Dotan I, Rachmilewitz D. Probiotics in inflammatory bowel disease: possible mechanisms of action. Curr Opin Gastroenterol. 2005;21:426–430. [PubMed] [Google Scholar]
- 47.Kanauchi O, Serizawa I, Araki Y, Suzuki A, Andoh A, Fujiyama Y, Mitsuyama K, Takaki K, Toyonaga A, Sata M, et al. Germinated barley foodstuff, a prebiotic product, ameliorates inflammation of colitis through modulation of the enteric environment. J Gastroenterol. 2003;38:134–141. doi: 10.1007/s005350300022. [DOI] [PubMed] [Google Scholar]
- 48.Kanauchi O, Suga T, Tochihara M, Hibi T, Naganuma M, Homma T, Asakura H, Nakano H, Takahama K, Fujiyama Y, et al. Treatment of ulcerative colitis by feeding with germinated barley foodstuff: first report of a multicenter open control trial. J Gastroenterol. 2002;37 Suppl 14:67–72. doi: 10.1007/BF03326417. [DOI] [PubMed] [Google Scholar]
- 49.Andoh A, Tsujikawa T, Fujiyama Y. Role of dietary fiber and short-chain fatty acids in the colon. Curr Pharm Des. 2003;9:347–358. doi: 10.2174/1381612033391973. [DOI] [PubMed] [Google Scholar]
- 50.Videla S, Vilaseca J, Antolín M, García-Lafuente A, Guarner F, Crespo E, Casalots J, Salas A, Malagelada JR. Dietary inulin improves distal colitis induced by dextran sodium sulfate in the rat. Am J Gastroenterol. 2001;96:1486–1493. doi: 10.1111/j.1572-0241.2001.03802.x. [DOI] [PubMed] [Google Scholar]
- 51.Hanai H, Kanauchi O, Mitsuyama K, Andoh A, Takeuchi K, Takayuki I, Araki Y, Fujiyama Y, Toyonaga A, Sata M, et al. Germinated barley foodstuff prolongs remission in patients with ulcerative colitis. Int J Mol Med. 2004;13:643–647. [PubMed] [Google Scholar]
- 52.Kanauchi O, Mitsuyama K, Homma T, Takahama K, Fujiyama Y, Andoh A, Araki Y, Suga T, Hibi T, Naganuma M, et al. Treatment of ulcerative colitis patients by long-term administration of germinated barley foodstuff: multi-center open trial. Int J Mol Med. 2003;12:701–704. [PubMed] [Google Scholar]
- 53.Steinhart AH, Brzezinski A, Baker JP. Treatment of refractory ulcerative proctosigmoiditis with butyrate enemas. Am J Gastroenterol. 1994;89:179–183. [PubMed] [Google Scholar]
- 54.Ohkusa T, Ozaki Y, Sato C, Mikuni K, Ikeda H. Long-term ingestion of lactosucrose increases Bifidobacterium sp. in human fecal flora. Digestion. 1995;56:415–420. doi: 10.1159/000201269. [DOI] [PubMed] [Google Scholar]
- 55.Teramoto F, Rokutan K, Kawakami Y, Fujimura Y, Uchida J, Oku K, Oka M, Yoneyama M. Effect of 4G-beta-D-galactosylsucrose (lactosucrose) on fecal microflora in patients with chronic inflammatory bowel disease. J Gastroenterol. 1996;31:33–39. doi: 10.1007/BF01211184. [DOI] [PubMed] [Google Scholar]
- 56.Schneeman BO. Fiber, inulin and oligofructose: similarities and differences. J Nutr. 1999;129:1424S–1427S. doi: 10.1093/jn/129.7.1424S. [DOI] [PubMed] [Google Scholar]
- 57.Pattee PL, Thompson WG. Drug treatment of the irritable bowel syndrome. Drugs. 1992;44:200–206. doi: 10.2165/00003495-199244020-00004. [DOI] [PubMed] [Google Scholar]
- 58.Jacobs LR, Lupton JR. Effect of dietary fibers on rat large bowel mucosal growth and cell proliferation. Am J Physiol. 1984;246:G378–G385. doi: 10.1152/ajpgi.1984.246.4.G378. [DOI] [PubMed] [Google Scholar]
- 59.Buhman KK, Furumoto EJ, Donkin SS, Story JA. Dietary psyllium increases expression of ileal apical sodium-dependent bile acid transporter mRNA coordinately with dose-responsive changes in bile acid metabolism in rats. J Nutr. 2000;130:2137–2142. doi: 10.1093/jn/130.9.2137. [DOI] [PubMed] [Google Scholar]
- 60.Buhman KK, Furumoto EJ, Donkin SS, Story JA. Dietary psyllium increases fecal bile acid excretion, total steroid excretion and bile acid biosynthesis in rats. J Nutr. 1998;128:1199–1203. doi: 10.1093/jn/128.7.1199. [DOI] [PubMed] [Google Scholar]
- 61.Hallert C, Kaldma M, Petersson BG. Ispaghula husk may relieve gastrointestinal symptoms in ulcerative colitis in remission. Scand J Gastroenterol. 1991;26:747–750. doi: 10.3109/00365529108998594. [DOI] [PubMed] [Google Scholar]
- 62.Fernández-Bañares F, Hinojosa J, Sánchez-Lombraña JL, Navarro E, Martínez-Salmerón JF, García-Pugés A, González-Huix F, Riera J, González-Lara V, Domínguez-Abascal F, et al. Randomized clinical trial of Plantago ovata seeds (dietary fiber) as compared with mesalamine in maintaining remission in ulcerative colitis. Spanish Group for the Study of Crohn's Disease and Ulcerative Colitis (GETECCU) Am J Gastroenterol. 1999;94:427–433. doi: 10.1111/j.1572-0241.1999.872_a.x. [DOI] [PubMed] [Google Scholar]
- 63.Kanauchi O, Iwanaga T, Andoh A, Araki Y, Nakamura T, Mitsuyama K, Suzuki A, Hibi T, Bamba T. Dietary fiber fraction of germinated barley foodstuff attenuated mucosal damage and diarrhea, and accelerated the repair of the colonic mucosa in an experimental colitis. J Gastroenterol Hepatol. 2001;16:160–168. doi: 10.1046/j.1440-1746.2001.02427.x. [DOI] [PubMed] [Google Scholar]
- 64.Kanauchi O, Nakamura T, Agata K, Mitsuyama K, Iwanaga T. Effects of germinated barley foodstuff on dextran sulfate sodium-induced colitis in rats. J Gastroenterol. 1998;33:179–188. doi: 10.1007/s005350050067. [DOI] [PubMed] [Google Scholar]
- 65.Kanauchi O, Matsumoto Y, Matsumura M, Fukuoka M, Bamba T. The beneficial effects of microflora, especially obligate anaerobes, and their products on the colonic environment in inflammatory bowel disease. Curr Pharm Des. 2005;11:1047–1053. doi: 10.2174/1381612053381675. [DOI] [PubMed] [Google Scholar]
- 66.Kanauchi O, Fukuda M, Matsumoto Y, Ishii S, Ozawa T, Shimizu M, Mitsuyama K, Andoh A. Eubacterium limosum ameliorates experimental colitis and metabolite of microbe attenuates colonic inflammatory action with increase of mucosal integrity. World J Gastroenterol. 2006;12:1071–1077. doi: 10.3748/wjg.v12.i7.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kanauchi O, Iwanaga T, Mitsuyama K, Saiki T, Tsuruta O, Noguchi K, Toyonaga A. Butyrate from bacterial fermentation of germinated barley foodstuff preserves intestinal barrier function in experimental colitis in the rat model. J Gastroenterol Hepatol. 1999;14:880–888. doi: 10.1046/j.1440-1746.1999.01971.x. [DOI] [PubMed] [Google Scholar]
- 68.Andoh A, Fujiyama Y, Hata K, Araki Y, Takaya H, Shimada M, Bamba T. Counter-regulatory effect of sodium butyrate on tumour necrosis factor-alpha (TNF-alpha)-induced complement C3 and factor B biosynthesis in human intestinal epithelial cells. Clin Exp Immunol. 1999;118:23–29. doi: 10.1046/j.1365-2249.1999.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanauchi O, Andoh A, Iwanaga T, Fujiyama Y, Mitsuyama K, Toyonaga A, Bamba T. Germinated barley foodstuffs attenuate colonic mucosal damage and mucosal nuclear factor kappa B activity in a spontaneous colitis model. J Gastroenterol Hepatol. 1999;14:1173–1179. doi: 10.1046/j.1440-1746.1999.02025.x. [DOI] [PubMed] [Google Scholar]
- 70.Mitsuyama K, Saiki T, Kanauchi O, Iwanaga T, Tomiyasu N, Nishiyama T, Tateishi H, Shirachi A, Ide M, Suzuki A, et al. Treatment of ulcerative colitis with germinated barley foodstuff feeding: a pilot study. Aliment Pharmacol Ther. 1998;12:1225–1230. doi: 10.1046/j.1365-2036.1998.00432.x. [DOI] [PubMed] [Google Scholar]
- 71.Madsen KL. Inflammatory bowel disease: lessons from the IL-10 gene-deficient mouse. Clin Invest Med. 2001;24:250–257. [PubMed] [Google Scholar]
- 72.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 73.Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–309. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 74.Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108–114. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kühbacher T, Ott SJ, Helwig U, Mimura T, Rizzello F, Kleessen B, Gionchetti P, Blaut M, Campieri M, Fölsch UR, et al. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut. 2006;55:833–841. doi: 10.1136/gut.2005.078303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–1209. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 77.Venturi A, Gionchetti P, Rizzello F, Johansson R, Zucconi E, Brigidi P, Matteuzzi D, Campieri M. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1103–1108. doi: 10.1046/j.1365-2036.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 78.Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539–1546. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- 79.Hockertz S. Augmentation of host defence against bacterial and fungal infections of mice pretreated with the non-pathogenic Escherichia coli strain Nissle 1917. Arzneimittelforschung. 1997;47:793–796. [PubMed] [Google Scholar]
- 80.Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354:635–639. doi: 10.1016/s0140-6736(98)06343-0. [DOI] [PubMed] [Google Scholar]
- 81.Araki Y, Fujiyama Y, Andoh A, Koyama S, Kanauchi O, Bamba T. The dietary combination of germinated barley foodstuff plus Clostridium butyricum suppresses the dextran sulfate sodium-induced experimental colitis in rats. Scand J Gastroenterol. 2000;35:1060–1067. doi: 10.1080/003655200451180. [DOI] [PubMed] [Google Scholar]
- 82.Fukuda M, Kanauchi O, Araki Y, Andoh A, Mitsuyama K, Takagi K, Toyonaga A, Sata M, Fujiyama Y, Fukuoka M, et al. Prebiotic treatment of experimental colitis with germinated barley foodstuff: a comparison with probiotic or antibiotic treatment. Int J Mol Med. 2002;9:65–70. [PubMed] [Google Scholar]
- 83.Matsumoto S, Watanabe N, Imaoka A, Okabe Y. Preventive effects of Bifidobacterium- and Lactobacillus-fermented milk on the development of inflammatory bowel disease in senescence-accelerated mouse P1/Yit strain mice. Digestion. 2001;64:92–99. doi: 10.1159/000048846. [DOI] [PubMed] [Google Scholar]
- 84.Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–1355. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 85.Schotte L, Steidler L, Vandekerckhove J, Remaut E. Secretion of biologically active murine interleukin-10 by Lactococcus lactis. Enzyme Microb Technol. 2000;27:761–765. doi: 10.1016/s0141-0229(00)00297-0. [DOI] [PubMed] [Google Scholar]
- 86.Bengmark S, Martindale R. Prebiotics and synbiotics in clinical medicine. Nutr Clin Pract. 2005;20:244–261. doi: 10.1177/0115426505020002244. [DOI] [PubMed] [Google Scholar]
- 87.Bengmark S. Synbiotics and the mucosal barrier in critically ill patients. Curr Opin Gastroenterol. 2005;21:712–716. doi: 10.1097/01.mog.0000182858.65927.81. [DOI] [PubMed] [Google Scholar]
- 88.Rastall RA, Maitin V. Prebiotics and synbiotics: towards the next generation. Curr Opin Biotechnol. 2002;13:490–496. doi: 10.1016/s0958-1669(02)00365-8. [DOI] [PubMed] [Google Scholar]
- 89.Kanamori Y, Hashizume K, Sugiyama M, Morotomi M, Yuki N. Combination therapy with Bifidobacterium breve, Lactobacillus casei, and galactooligosaccharides dramatically improved the intestinal function in a girl with short bowel syndrome: a novel synbiotics therapy for intestinal failure. Dig Dis Sci. 2001;46:2010–2016. doi: 10.1023/a:1010611920750. [DOI] [PubMed] [Google Scholar]
- 90.Roller M, Pietro Femia A, Caderni G, Rechkemmer G, Watzl B. Intestinal immunity of rats with colon cancer is modulated by oligofructose-enriched inulin combined with Lactobacillus rhamnosus and Bifidobacterium lactis. Br J Nutr. 2004;92:931–938. doi: 10.1079/bjn20041289. [DOI] [PubMed] [Google Scholar]
- 91.Roller M, Rechkemmer G, Watzl B. Prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis modulates intestinal immune functions in rats. J Nutr. 2004;134:153–156. doi: 10.1093/jn/134.1.153. [DOI] [PubMed] [Google Scholar]
- 92.Asahara T, Nomoto K, Shimizu K, Watanuki M, Tanaka R. Increased resistance of mice to Salmonella enterica serovar Typhimurium infection by synbiotic administration of Bifidobacteria and transgalactosylated oligosaccharides. J Appl Microbiol. 2001;91:985–996. doi: 10.1046/j.1365-2672.2001.01461.x. [DOI] [PubMed] [Google Scholar]
- 93.Dickinson RJ, O’Connor HJ, Pinder I, Hamilton I, Johnston D, Axon AT. Double blind controlled trial of oral vancomycin as adjunctive treatment in acute exacerbations of idiopathic colitis. Gut. 1985;26:1380–1384. doi: 10.1136/gut.26.12.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gilat T, Leichtman G, Delpre G, Eshchar J, Bar Meir S, Fireman Z. A comparison of metronidazole and sulfasalazine in the maintenance of remission in patients with ulcerative colitis. J Clin Gastroenterol. 1989;11:392–395. doi: 10.1097/00004836-198908000-00008. [DOI] [PubMed] [Google Scholar]
- 95.Burke DA, Axon AT, Clayden SA, Dixon MF, Johnston D, Lacey RW. The efficacy of tobramycin in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 1990;4:123–129. doi: 10.1111/j.1365-2036.1990.tb00456.x. [DOI] [PubMed] [Google Scholar]
- 96.Gionchetti P, Rizzello F, Ferrieri A, Venturi A, Brignola C, Ferretti M, Peruzzo S, Miglioli M, Campieri M. Rifaximin in patients with moderate or severe ulcerative colitis refractory to steroid-treatment: a double-blind, placebo-controlled trial. Dig Dis Sci. 1999;44:1220–1221. doi: 10.1023/a:1026648812439. [DOI] [PubMed] [Google Scholar]
- 97.Ursing B, Alm T, Bárány F, Bergelin I, Ganrot-Norlin K, Hoevels J, Huitfeldt B, Järnerot G, Krause U, Krook A, et al. A comparative study of metronidazole and sulfasalazine for active Crohn's disease: the cooperative Crohn's disease study in Sweden. II. Result. Gastroenterology. 1982;83:550–562. [PubMed] [Google Scholar]
- 98.Sutherland L, Singleton J, Sessions J, Hanauer S, Krawitt E, Rankin G, Summers R, Mekhjian H, Greenberger N, Kelly M. Double blind, placebo controlled trial of metronidazole in Crohn's disease. Gut. 1991;32:1071–1075. doi: 10.1136/gut.32.9.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]


