Abstract
AIM: To investigate the effect of hot water-extracted Lycium barbarum (LBE) and Rehmannia glutinosa (RGE) on cell proliferation and apoptosis in rat and/or human hepatocellular carcinoma (HCC) cells.
METHODS: Rat (H-4-II-E) and human HCC (HA22T/VGH) cell lines were incubated with various concentrations (0-10 g/L) of hot water-extracted LBE and RGE. After 6-24 h incubation, cell proliferation (n = 6) was measured by a colorimetric method. The apoptotic cells (n = 6) were detected by flow cytometry. The expression of p53 protein (n = 3) was determined by SDS-PAGE and Western blotting.
RESULTS: Crude LBE (2-5 g/L) and RGE (2-10 g/L) dose-dependently inhibited proliferation of H-4-II-E cells by 11% (P < 0.05) to 85% (P < 0.01) after 6-24 h treatment. Crude LBE at a dose of 5 g/L suppressed cell proliferation of H-4-II-E cells more effectively than crude RGE after 6-24 h incubation (P < 0.01). Crude LBE (2-10 g/L) and RGE (2-5 g/L) also dose-dependently inhibited proliferation of HA22T/VGH cells by 14%-43% (P < 0.01) after 24 h. Crude LBE at a dose of 10 g/L inhibited the proliferation of HA22T/VGH cells more effectively than crude RGE (56.8% ± 1.6% vs 70.3% ± 3.1% of control, P = 0.0003 < 0.01). The apoptotic cells significantly increased in H-4-II-E cells after 24 h treatment with higher doses of crude LBE (2-5 g/L) and RGE (5-10 g/L) (P < 0.01). The expression of p53 protein in H-4-II-E cells was 119% and 143% of the control group compared with the LBE-treated (2, 5 g/L) groups, and 110% and 132% of the control group compared with the RGE -treated (5, 10 g/L) groups after 24 h.
CONCLUSION: Hot water-extracted crude LBE (2-5 g/L) and RGE (5-10 g/L) inhibit proliferation and stimulate p53-mediated apoptosis in HCC cells.
Keywords: Lycium barbarum extract, Rehmannia glutinosa extract, Proliferation, Apoptosis, Hepatocellular carcinoma
INTRODUCTION
According to the official report by the Department of Health, Taiwan, malignant tumor is the first leading cause of death in 2004. Among cancers, hepatocellular carcinoma (HCC) is the second leading cause of death. The mortality rate for HCC is 31.17 per 100 000, accounting for 17.9% of cancer deaths in 2004. The rising incidence of HCC in at-high-risk patients with chronic hepatitis B or C is an important issue in Taiwan. Although early diagnosis and treatment improve survival, HCC is rarely cured and recurs frequently after regional therapy or transplantation[1]. Recently, preventing HCC formation and HCC therapy are major research focuses.
Both Lycium barbarum (LBE) L Rehmannia glutinosa (RGE) have been commonly used as traditional Chinese medicine and herbal foods for health promotion in China. The active components of the fruit of LBE L the dried root of RGE primarily contain water-soluble polysaccharides. LBE L RGE can be extracted with hot water followed by precipitation with ethanol to obtain high quantity of polysaccharides[2-4]. Polysaccharide-containing active components purified from these herbs have been recently studied for their physiological and pharmaceutical activities. LBE polysaccharides as glycopeptides isolated from the fruit of LBE[5,6] are water soluble and potent in immunomodulation, anti-lipid peroxidation[6-9], and antitumor[3]. RGE polysaccharides isolated from the dried root of RGE have also shown the properties of immunomodulation[10-14] and antitumor[15]. To few studies have investigated the effect of LBE and RGE extracts on HCC. The purpose of this study was to investigate the effect of crude hot water-extracted LBE and RGE on cell proliferation and apoptosis in HCC cells.
MATERIALS AND METHODS
Preparation of crude herbal extracts
LBE L Radix RGE (processed and dried RGE) were purchased from Chien Yuan Hang (Taipei, Taiwan). To maintain the quality and consistency of the ingredients, the crude extracts were prepared in a single batch with adequate quantity for this study. Dried LBE L Radix RGE (100 g) were incubated with 900 mL deionized water at 100°C for 2 h. The herbal juice was then centrifuged at 9000 r/min for 20 min to remove the precipitate. The remaining herbal juice was filtered with gauze. The filtered supernatant containing polysaccharides was precipitated with three volumes of 950 mL/L ethanol, concentrated (rotavapor R200 with glass assembly V; BÜCHI Labortechnik AG, Flawil, Switzerland) and lyophilized (freeze dry system Lyph-Lock 6; Labconco Corp., Kansas City, MO, USA)[16].
Composition analysis of herbal extracts
The total carbohydrate content in hot water-extracted LBE and RGE was determined by phenol-sulfuric acid assay using glucose as a standard[17]. The contents of crude protein, crude fat, moisture, and ash were measured using the Association of Official Analytical Chemists (AOAC) methods (981.10, 991.36, 925.10, 923.03)[18].
Cell cultures and treatments
Rat (H-4-II-E, BCRC no. 60209) and human HCC (HA22T/VGH, BCRC no. 60168) cell lines were purchased from the Bioresources Collection and Research Center (BCRC) of the Food Industry Research and Development Institute (Hsinchu, Taiwan). Rat and human HCC cells (1 × 105 cells/mL) were grown in 850 mL/L minimum essential medium (MEM; GIBCO™, Invitrogen Corp., Carlsbad, CA, USA) with 150 mL/L fetal bovine serum (FBS; GIBCO™) or 900 mL/L Dulbecco’s modified Eagle’s medium (DMEM; GIBCO™) with 100 mL/L FBS, respectively, at 37°C in a humidified atmosphere of 950 mL/L air and 50 mL/L CO2. Prior to addition of the treatment, the cells were grown to 80%-90% confluency and synchronized by incubating in serum-free basal medium (MEM or DMEM) for 24 h. The cells were then treated with various concentrations of crude LBE (0-5 g/L) or RGE (0-10 g/L) in the absence of serum for 0-24 h. The cells and medium were collected. Protein contents in the cells and medium were determined by the modified method of Lowry et al[19] using a Bio-Rad DC protein kit (Bio-Rad Laboratories, Hercules, CA, USA).
Cell proliferation assay
Cell proliferation was colorimetrically measured at 490 nm using a commercial proliferation assay kit (CellTiter 96® AQueous; Promega Corp., Madison, WI, USA). After treatment with various concentrations of crude LBE (0-5 g/L) or RGE (0-10 g/L) for 0-24 h, rat and human HCC cells (n = 6) in the 96-well plate were incubated with 20 μL MTS (3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) solution containing 1.90 g/L MTS and 300 μmol/L phenazine methosulfate in Dulbecco’s PBS (pH 6.0) for 2 h at 37°C in a humidified 50 mL/L CO2 atmosphere[20]. The absorbance of soluble formazan produced by cellular reduction of MTS was determined at 490 nm using an ELISA reader (Multiskan RC; Labsystems, Helsinki, Finland).
Flow cytometric analysis of cellular DNA content
The percentage of cells undergoing apoptosis and distributing in different phases of cell cycle were determined by propidium iodide (PI)-staining method using flow cytometry. After treatment for 24 h, the conditioned medium of rat H-4-II-E cells (n = 6) was centrifuged at 800 r/min for 5 min at 4°C to remove the supernatant. The trypsinized cells and cell pellet of the conditioned medium were washed with PBS, fixed in 2 mL of 700 mL/L cold ethanol, and stored at 4°C overnight. After washed twice with PBS, the ethanol-fixed cells were incubated with 3 μL RNase (10 g/L) at 37°C for 30 min, and stained with 1 mL PI (40 mg/L) in the dark. The cell suspension was then filtered through a 35 μm mesh, and analyzed by a flow cytometer (FACSCalibur; Becton Dickinson Biosciences, San Jose, CA, USA) within 2 h. Cellular DNA content was calculated using CellQuest software (Becton Dickinson Biosciences).
Analysis of p53 protein
After incubation with various concentrations of LBE (0-5 g/L) or RGE (0-10 g/L) for 24 h, the conditioned medium of rat H-4-II-E cells was centrifuged at 800 r/min for 5 min at 4°C to remove the supernatant. Rat H-4-II-E cells and cell pellet in the conditioned medium were re-suspended in lysis buffer (2 mol/L Tris, 5 mol/L NaCl, 50 g/L NP-40, 100 g/L sodium dodecylsulfate (SDS), and 5 g/L phenylmethylsulfonyl fluoride), centrifuged at 300 r/min for 5 min at 4°C. The supernatant (40 μg total protein) pooled from 3 independent experiments (n = 3) was mixed with 4 × sample buffer (0.25 mol/L Tris-HCl, pH 6.8, 80 g/L SDS, 200 ml/L glycerol, 100 g/L β-mercaptoethanol, and 1 g bromophenol blue)[21] denatured at 100°C for 3 min, and applied to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (Bio-Rad Mini-PROTEAN 3 Cell; Bio-Rad Laboratories). Proteins were separated by 125 mL/L resolving gel (acrylamide:bisacrylamide = 37.5:1) with 40 mL/L stacking gel in the running buffer (25 mmol/L Tris, pH 8.3, 192 mmol/L glycine, and 1 g/L SDS) at 3 EV/cm per hour. Then the proteins were transferred onto nitrocellulose membrane (0.45 μm) using a semi-dry transfer unit (Hoefer Semiphor TE 70, Amersham Biosciences Corp., San Francisco, CA, USA) in Towbin buffer (25 mmol/L Tris, 192 mmol/L glycine, 1 g/L SDS, and 100 mL/L methanol) at 6 mA/cm per hour[22]. The membrane was washed with PBS, and incubated with a blocking buffer (50 g/L skim milk and 2 mL/L Tween-20 in PBS) for 2 h. Then the membrane was incubated with an anti-mouse monoclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) against p53 (Pab-246; 2 mg/L) or α-tubulin (TU-02; 1 mg/L) at room temperature for 2 h. Alpha-tubulin was used as an internal control. The membrane was washed three times with the wash buffer (2 mL/L Tween-20 in PBS), and incubated with 4 mg/L goat anti-mouse IgG-horseradish peroxide conjugate (Santa Cruz Biotechnology, Inc.) for 2 h. The blot was washed three times again with the wash buffer, incubated with luminol reagent (PerkinElmer Life and Analytical Sciences, Inc., Boston, MA, USA) for 2 min, and exposed to an X-ray film (Eastman Kodak Co., Rochester, NY, USA) for 3-5 min. The bands were quantitated by an image analysis system (0.2-megapixel charge-coupled device camera, gel analysis system; EverGene Biotechnology, Taipei, Taiwan) and Phoretix 1D Lite software (version 4.0; Phoretix International Ltd., Newcastle upon Tyne, UK).
Statistical analysis
Data are expressed as mean ± SD. Data were analyzed by one- and two-way analysis of variance (ANOVA) to determine the treatment effect using the Statistical Analysis System (SAS version 8.2; SAS Institute Inc., Cary, NC, USA). Fisher’s least significant difference test was used to make post hoc comparisons if the treatment effect was demonstrated. P < 0.05 was considered statistically significant.
RESULTS
Composition analysis of herbal extracts
The extraction rate for crude LBE and RGE was 704 mg/g and 724 mg/g, respectively. Carbohydrate content, the major component, was 762.8 mg/g and 700.4 mg/g in crude LBE and RGE, respectively (Table 1). Moisture and protein contents in crude LBE and RGE were 221.6 mg/g and 134.7 mg/g as well as 92.4 mg/g and 47.2 mg/g, respectively. Fat and ash contents in these crude extracts were less than 5 mg/g.
Table 1.
Composition of hot water-extracted Lycium barbarum and Rehmannia glutinosa1
| Ingredient (mg/g) | Lycium barbarum extract | Rehmannia glutinosa extract |
| Carbohydrate | 762.8 | 700.4 |
| Protein | 134.7 | 47.2 |
| Fat | 3.9 | 2.9 |
| Moisture | 221.6 | 92.4 |
| Ash | 0.5 | 0.4 |
1The samples were at least triplicated.
Cell proliferation assay
Crude LBE and RGE at a dose of 1 g/L did not inhibit cell proliferation up to 24 h incubation in rat H-4-II-E cells (Table 2). However, crude RGE at a dose of 1 g/L slightly increased cell proliferation of H-4-II-E cells at 18 h incubation compared with the control group (111.4% ± 11.7% vs 100.0% ± 5.9% of control, P = 0.03 < 0.05). Crude LBE (2-5 g/L) and RGE (2-10 g/L) dose-dependently inhibited cell proliferation by 11% (P < 0.05) to 85% (P < 0.01) compared with the control group after 6-24 h incubation. The inhibitory effect of crude LBE and RGE at lower doses (1-2 g/L) on cell proliferation was not significantly different. A higher dose (5 g/L) of crude LBE suppressed cell proliferation more efficiently (P < 0.01) than that of crude RGE. From the curves of four time points, the mean values of IC50 for crude LBE and RGE were 3.8 g/L and 6.9 g/L, respectively. Similar results in human HA22T/VGH cells, crude LBE (2-10 g/L) and RGE (2-5 g/L) dose-dependently inhibited cell proliferation by 14%-43% (P < 0.01) compared with the control group after 24 h incubation (Table 3). Cell proliferation was inhibited equivalently by crude LBE and RGE at lower doses (2-5 g/L). However, crude LBE at a higher dose (10 g/L) showed better suppression (56.8% ± 1.6% vs 70.3% ± 3.1% of control, P = 0.0003 < 0.01) on cell proliferation compared with the same dosage of crude RGE. The IC50 value for both crude LBE and RGE was above 10 g/L.
Table 2.
Effect of hot water-extracted Lycium barbarum (LBE) and Rehmannia glutinosa (RGE) on cell proliferation in rat hepatocellular carcinoma (H-4-II-E) cells (n = 6, mean ± SD)
| Cell proliferation (% of control) | ||||
| 6 h | 12 h | 18 h | 24 h | |
| Control | 100.0 ± 0.7ehj | 100.0 ± 8.6ehj | 100.0 ± 5.9fhj | 100.0 ± 5.9fhj |
| 1 g/L LBE | 100.3 ± 9.8fh | 100.7 ± 8.2eh | 100.1 ± 5.7f,h | 97.8 ± 6.3fh |
| 2 g/L LBE | 84.4 ± 6.6adh | 89.3 ± 7.5ach | 83.8 ± 7.5bdh | 86.3 ± 2.9bdh |
| 5 g/L LBE | 37.6 ± 6.9bdf | 23.8 ± 4.6bdf | 18.0 ± 5.3bdf | 15.1 ± 3.1bdf |
| 1 g/L RGE | 99.5 ± 7.1fhj | 97.0 ± 13.4hj | 111.4 ± 11.7afhj | 96.1 ± 5.3fhj |
| 2 g/L RGE | 80.9 ± 18.4bdhj | 88.3 ± 9.4ahj | 84.6 ± 14.4bdhj | 84.4 ± 6.4bdhj |
| 5 g/L RGE | 58.3 ± 11.4bdfj | 74.6 ± 7.0bdfj | 57.1 ± 10.1bdfj | 71.2 ± 6.7bdfj |
| 10 g/L RGE | 29.2 ± 6.9bdfh | 29.5 ± 6.3bdfh | 25.0 ± 5.7bdfh | 24.2 ± 7.1bdfh |
P < 0.05,
P < 0.01 vs control,
P < 0.05,
P < 0.01 vs 1 g/L corresponding treatment,
P < 0.05,
P < 0.01 vs 2 g/L corresponding treatment; gP < 0.05,
P < 0.01 vs 5 g/L corresponding treatment,
P < 0.01 vs 10 g/L corresponding treatment within the same column.
Table 3.
Effect of hot water-extracted LBE and RGE on cell proliferation in human hepatocellular carcinoma (HA22T/VGH) cells after 24 h incubation (n = 6, mean ± SD)
| Cell proliferation (% of control) | |
| Control | 100.0 ± 4.2dfh |
| 2 g/L LBE | 86.0 ± 5.9bch |
| 5 g/L LBE | 77.0 ± 5.1abh |
| 10 g/L LBE | 56.8 ± 3.9bdf |
| 2 g/L RGE | 86.3 ± 5.9bfh |
| 5 g/L RGE | 74.3 ± 7.5bd |
| 10 g/L RGE | 70.3 ± 7.7bd |
P < 0.01 vs control;
P < 0.05,
P < 0.01 vs 2 g/L corresponding treatment;
P < 0.05,
P < 0.01 vs 5 g/L corresponding treatment;
P < 0.01 vs 10 g/L corresponding treatment.
Flow cytometric analysis of cellular DNA content
Cellular DNA content in cell cycle distribution was determined, and apoptosis was quantitated by the percentage of cells with sub-G0 DNA content by flow cytometry (Figure 1A). Crude LBE and RGE at the doses above 2 g/L and 5 g/L, respectively, significantly increased the percentage of cells in sub-G0 phase (27.5% ± 0.5% and 91.2% ± 0.3% for 2 g/L and 5 g/L LBE, and 2.4% ± 0.2% and 48.1% ± 2.0% for 5 g/L and 10 g/L RGE, P < 0.01) compared with the control group (1.0% ± 0.2%) (Figure 1B). The percentage of cells in G0/G1 phase was dose-dependently decreased by crude LBE and RGE. Crude LBE at lower doses (1-2 g/L) significantly increased the percentage of cells in G2/M phase (24.1% ± 0.4% and 25.8% ± 0.3%, P < 0.01) compared with the control group (15.8% ± 0.4%), but decreased the percentage of cells to 1.5% ± 0.1% (P < 0.01) at a higher dose (5 g/L). Likewise, crude RGE at lower doses (2 g/L and 5 g/L) significantly increased the percentage of cells in G2/M phase (18.3% ± 0.4% and 39.3% ± 2.1%, P < 0.01), but decreased the percentage of cells to 8.3% ± 0.7% (P < 0.01) at a higher dose (10 g/L).
Figure 1.
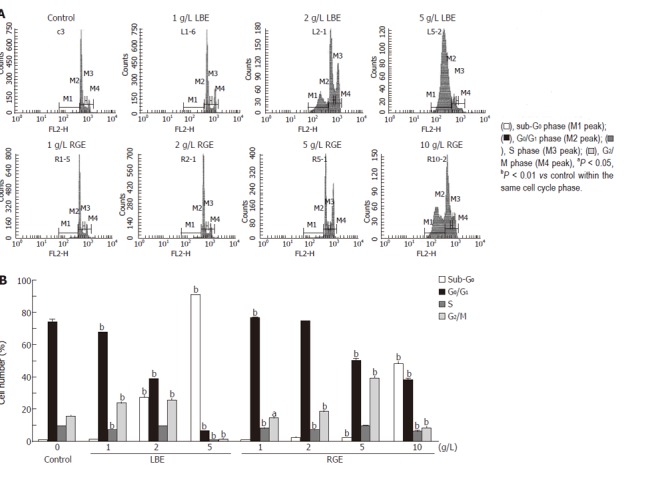
Representative DNA histograms (A) and percentage of cells in different cell cycle phases (B) after incubated with various concentrations of hot water-extracted LBE and RGE for 24 h in rat H-4-II-E cells determined by flow cytometry. Values are mean ± SD (n = 6).
Analysis of p53 protein
After 24 h incubation with crude LBE and RGE, the expression of p53 protein in rat H-4-II-E cells was analyzed by SDS-PAGE and Western blotting (Figure 2A). After calibrated by an internal control (α-tubulin), the expression of p53 protein increased with the dosage of crude LBE and RGE (Figure 2B). Crude LBE at higher doses (2-5 g/L) increased p53 expression to 119% and 143% of the control group, respectively. Similarly, crude RGE at higher doses (5-10 g/L) increased p53 expression to 110% and 136% of the control group, respectively. However, lower doses of crude LBE (1 g/L) and RGE (1-2 g/L) suppressed p53 expression to 63%-70% of the control group.
Figure 2.
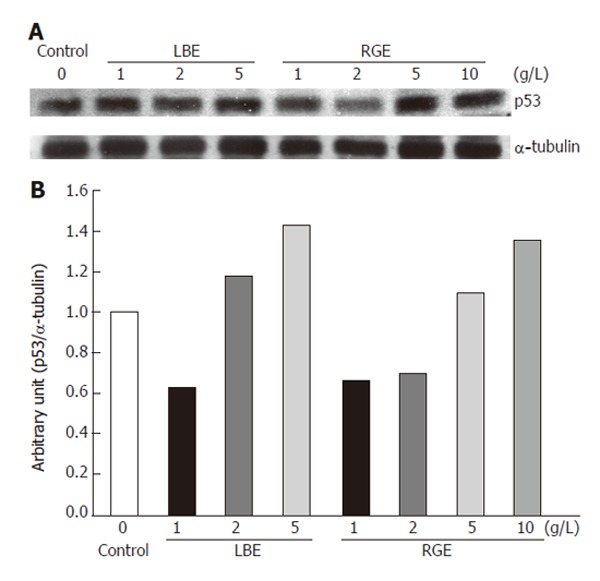
Expression of p53 protein with the molecular weight of 53 ku visualized by Western blotting (A) and quantitated by an image analysis system (B) after incubation of rat H-4-II-E cells with various concentrations of hot water-extracted LBE and RGE for 24 h. Samples were pooled from 3 independent experiments (n = 3). Alpha-tubulin (55 ku) was used as an internal control.
DISCUSSION
Polysaccharides, a water-soluble bioactive component from LBE L Radix RGE, showed antitumorgenic activity[3,15]. The methods for the isolation of polysaccharides from these herbs are various in different studies. Different extraction methods and fractions could affect not only the ingredients but also the physiological activity of the extract. Our crude herbal extracts were prepared by hot water (100°C) extraction followed by ethanol (950 mL/L) precipitation. Gan et al[3] extracted LBE with distilled water at 80°C after removal of pigment by acetone/petroleum (1:1) and oligosaccharides by 800 mL/L ethanol. The crude polysaccharide-protein complex was precipitated by ethanol, and the acidic fraction was further purified by diethylaminoethyl-cellulose anion exchange and Sephadex G200 column chromatography. Finally, a purified fraction (LBP3p) contains 63.6% neutral sugars, 24.8% acidic sugars, and 7.6% proteins. The purified fraction showed antiproliferating and immunomodulating activity in S180-bearing mice. Compared with the purified LBP3p fraction, our crude LBE contains lower sugars (88.4% vs 76.3%) and higher proteins (7.6% vs 13.5%). Zhang et al[4] prepared purified fraction of RGE oligosaccharides by extraction with hot water and isolation with cation/anion exchange and charcoal column chromatography. The eluted fraction containing monosaccharides, disaccharides, trisaccharides, and other oligosaccharides but not polysaccharides exert a significant hypoglycemic effect in normal and alloxan-induced diabetic rats. Our crude extract did not contain certain water-insoluble active components in LBE and RGE. Scopoletin (7-hydroxy-6-methoxycoumarin), slightly soluble in water, could be excluded as extracted by hot water, and has been reported as an active component of the fruit of LBE for inhibiting cell proliferation of human prostate cancer PC3 cells[23]. Furan derivatives, isolated from chloroform extract of the dried roots of RGE, have the immunomodulating and anti-coagulating
activity[24].
Our study found that both crude LBE and RGE suppressed cell proliferation in rat and human HCC cells in a dose-dependent manner. Consistent with our findings, Zhang et al[25] showed that polysaccharide containing LBE extract inhibits cell proliferation of human hepatoma QGY7703 cells. Additionally, LBE polysaccharides (20-1000 mg/L) dose-dependently suppress cell growth of human leukemia HL-60 cells[26]. The previous in vivo studies also showed that LBE exhibits the antitumorgenic activity in tumor-bearing mice[3,27]. A polysaccharide-protein complex from LBE (LBP3p) at 10 μg/g significantly reduces tumor weight and enhances immune functions in S180-bearing mice[3]. RGE polysaccharide b with an average molecular weight of 160 ku has antitumorgenic and immunomodulating activity in S180-bearing mice[13]. A clinical trial found 40.9% response rate in the advanced cancer patients with lymphokine-activated killer (LAK) cells and interleukin-2 (IL-2) treatment combined with LBE polysaccharides compared with 16.1% response rate (P < 0.05) in those treated with LAK/IL-2 alone. These data indicate that LBE polysaccharides can be used as an adjuvant for the treatment of cancer[28]. Chinese medicinal herbs containing RGE have been demonstrated to alleviate the adverse effects of high-dose methotrexate plus vincristine in postoperative osteogenic sarcoma patients with chemotherapy, suggesting that Chinese medicinal herbs containing RGE are less toxic compared with a traditional chemotherapy[29]. The extract of LBE at the dose of 25 g/L and 5 g/L inhibited cell proliferation by 14% and 85%, and stimulated p53 protein expression by 19% and 43% in rat H-4-II-E cells after 24 h incubation. The extract of RGE at the dose of 5 g/L and 10 g/L inhibited cell proliferation by 23% and 76%, and stimulated p53 protein expression by only 10% and 36% in rat H-4-II-E cells after 24 h incubation. The inhibition of cell proliferation by LBE at the dose of 2 g/L was almost parallel to the stimulation of p53. However, the relationship between cell proliferation inhibition and p53 expression was not obvious in rat H-4-II-E cells treated with higher doses of LBE and RGE.
The percentage of cells in sub-G0 phase significantly increased in H-4-II-E cells after 24 h treatment with higher doses of crude LBE (2-5 g/L) and RGE (5-10 g/L). A dramatic increased cell percentage in sub-G0 phase by crude LBE and RGE at higher doses was probably due to late apoptotic/necrotic cells in sub-G0 phase if undergoing autolysis, which could overestimate the apoptotic cells. The proliferation-inhibiting and apoptosis-inducing activity of crude LBE at higher doses (≥ 5 g/L) in HCC cells was more effective than that of crude RGE according to the mean values of IC50 and the proportion of apoptotic cells. As initiating a significant increase in the percentage of cells in sub-G0 phase by crude LBE (2 g/L) and RGE (5 g/L), the percentage of cells in G2/M phase significantly increased. Additionally, the expression of p53 protein was correspondently stimulated as well after 24 h incubation with 2 g/L crude LBE and 5 g/L crude RGE. However, lower doses of crude LBE and RGE decreased the expression of p53 protein compared with the control group, which could be due to the overexposure to α-tubulin. The expression of p53 protein tended to be dose-dependently increased by crude LBE and RGB. The results suggest that higher doses of crude LBE and RGE arrest cells in G2/M phase and p53 may be involved in mediating apoptosis. The mechanism of promoting G2/M arrest by LBE and RGE has not been understood. It is proposed that LBE and RGE may inhibit nuclear factor-κB to alter the expression of regulatory cell cycle proteins such as cyclin B and/or p21WAF1/Cip1. A previous study reported that LBE polysaccharides arrest cells in S phase and induce apoptosis with increased intracellular calcium in human hepatoma QGY7703 cells[25]. Additionally, LBE (20-1000 mg/L) results in DNA fragmentation and positive TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) signals in human leukemia HL-60 cells, indicating that LBE induces apoptosis[26]. Although the effect of LBE and RGE on apoptosis in normal hepatocytes has not been studied yet, the previous studies[30,31] found that LBE protects normal cells from apoptosis rather than stimulating apoptosis in tumor cells. LBE dose-dependently inhibits apoptosis induced by hydrocortisone in rat spleen in vitro[30]. LBE extract shows cytoprotective effect against β-amyloid peptide-induced apoptosis in primary rat cortical neurons and dithiothreitol-induced caspase-3 activation in normal neural endoplasmic reticulum[31]. The effect of LBE on apoptosis could be various due to different extraction methods, extraction fractions, dosages, tissues, and cell types (normal vs malignant). The low-molecular-weight RGE polysaccharides at the doses of 20 μg/g and 40 μg/g markedly increase the level of p53 mRNA to 3.3- and 3.2-fold, respectively, in Lewis lung cancer tissue of C57BL/6 mice[15]. Activation of p53 tumor suppressor protein can lead to cell cycle arrest and apoptosis in response to DNA damage[32]. Cell death induced through p53-mediating pathway is subsequently initiated by the activation of caspases followed by the characteristic apoptotic phenotype.
The mechanisms underlying regulation of apoptosis by LBE and RGE have not been clearly understood. Besides the induction of p53-mediated apoptosis, immunomodulation may contribute to antitumorgenesis. A polysaccharide-protein complex from LBE (LBP3p) dose-dependently increases the expression of IL-2 and tumor necrosis factor (TNF)-α at the levels of mRNA and protein in human peripheral blood mononuclear cells[33]. An in vivo study showed that LBP3p administered orally at 10 μg/g for 10 d inhibits cell growth of transplantable sarcoma S180 cells as well as increases macrophage phagocytosis, cell proliferation of spleen lymphocytes, the activity of cytotoxic T lymphocytes (CTL), and the expression of IL-2 mRNA in S180-bearing mice[3]. RGE polysaccaride b at the intraperitoneal injection dose of 10 μg/g or 20 μg/g attenuates the decrease in CTL activity caused by excessive tumor growth through increasing the production of CD8+ (Lyt-2+) CTL and lowering the ratio of CD4+ to CD8+ (L3T4+) T lymphocyte subset in S180-bearing mice[13]. An aqueous extract of RGE-steamed root dose-dependently suppresses the secretion of TNF-α by mouse astrocytes stimulated with substance P and lipopolysaccharide through the inhibition of IL-1 secretion, suggesting that RGE has the anti-inflammatory activity[14]. Although crude LBE and RGE show antitumorgenic activity in vitro, it still remains to determine the antitumorgenic components of crude LBE and RGE, and molecular mechanisms for regulating the proliferation of HCC cells.
In conclusion, hot water-extracted LBE (2-5 g/L) and RGE (5-10 g/L) inhibit proliferation and stimulate apoptosis probably involved in p53 mediation in HCC cells. It is not known that whether hot water-extracted LBE and RGE have antiproliferative and apoptosis-stimulating effects on HCC in vivo and further studies are still required to verify their in vivo effects on both normal and malignant hepatocytes.
ACKNOWLEDGMENTS
The authors gratefully thank Dr. Ting-Jang Lu in the Institute of Food Science and Technology, National Taiwan University for providing suggestions in the preparation of crude polysaccharide extracts.
Footnotes
S- Editor Wang J L- Editor Wang XL E- Editor Bi L
References
- 1.Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145–151. doi: 10.1097/00000658-199308000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhi F, Zheng W, Chen P, He M. [Study on the extraction process of polysaccharide from Lycium barbarum] Zhongyaocai. 2004;27:948–950. [PubMed] [Google Scholar]
- 3.Gan L, Hua Zhang S, Liang Yang X, Bi Xu H. Immunomodulation and antitumor activity by a polysaccharide-protein complex from Lycium barbarum. Int Immunopharmacol. 2004;4:563–569. doi: 10.1016/j.intimp.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Zhang R, Zhou J, Jia Z, Zhang Y, Gu G. Hypoglycemic effect of Rehmannia glutinosa oligosaccharide in hyperglycemic and alloxan-induced diabetic rats and its mechanism. J Ethnopharmacol. 2004;90:39–43. doi: 10.1016/j.jep.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Peng X, Tian G. Structural characterization of the glycan part of glycoconjugate LbGp2 from Lycium barbarum L. Carbohydr Res. 2001;331:95–99. doi: 10.1016/s0008-6215(00)00321-9. [DOI] [PubMed] [Google Scholar]
- 6.Huang LJ, Tian GY, Ji GZ. Structure elucidation of glycan of glycoconjugate LbGp3 isolated from the fruit of Lycium barbarum L. J Asian Nat Prod Res. 1999;1:259–267. doi: 10.1080/10286029908039874. [DOI] [PubMed] [Google Scholar]
- 7.Zhang JP, Qian DH. Antitumor activity and tumor necrosis factor production of Phytolacca acinosa polysaccharides I in mice. Zhongguo Yaoli XueBao. 1993;14:542–545. [PubMed] [Google Scholar]
- 8.Zhang B, Zhang X, Li W. [The injury of Xenopus laevis oocytes membrane and its acetylcholine receptor by free radical and the protection of lycium barbarum polysaccharide] Zhongguo Yingyong Shenglixue Zazhi. 1997;13:322–325. [PubMed] [Google Scholar]
- 9.Kim SY, Lee EJ, Kim HP, Kim YC, Moon A, Kim YC. A novel cerebroside from lycii fructus preserves the hepatic glutathione redox system in primary cultures of rat hepatocytes. Biol Pharm Bull. 1999;22:873–875. doi: 10.1248/bpb.22.873. [DOI] [PubMed] [Google Scholar]
- 10.Luo ZH. [The use of Chinese traditional medicines to improve impaired immune functions in scald mice] Zhonghua Zhengxing Shaoshang Waike Zazhi. 1993;9:56–58, 80. [PubMed] [Google Scholar]
- 11.Tomoda M, Miyamoto H, Shimizu N. Structural features and anti-complementary activity of rehmannan SA, a polysaccharide from the root of Rehmannia glutinosa. Chem Pharm Bull (Tokyo) 1994;42:1666–1668. doi: 10.1248/cpb.42.1666. [DOI] [PubMed] [Google Scholar]
- 12.Tomoda M, Miyamoto H, Shimizu N, Gonda R, Ohara N. Characterization of two polysaccharides having activity on the reticuloendothelial system from the root of Rehmannia glutinosa. Chem Pharm Bull (Tokyo) 1994;42:625–629. doi: 10.1248/cpb.42.625. [DOI] [PubMed] [Google Scholar]
- 13.Chen LZ, Feng XW, Zhou JH. Effects of Rehmannia glutinosa polysaccharide b on T-lymphocytes in mice bearing sarcoma 180. Zhongguo Yaolixue Bao. 1995;16:337–340. [PubMed] [Google Scholar]
- 14.Kim HM, An CS, Jung KY, Choo YK, Park JK, Nam SY. Rehmannia glutinosa inhibits tumour necrosis factor-alpha and interleukin-1 secretion from mouse astrocytes. Pharmacol Res. 1999;40:171–176. doi: 10.1006/phrs.1999.0504. [DOI] [PubMed] [Google Scholar]
- 15.Wei XL, Ru XB. [Effects of low-molecular-weight Rehmannia glutinosa polysaccharides on p53 gene expression] Zhongguo Yaoli XueBao. 1997;18:471–474. [PubMed] [Google Scholar]
- 16.Ramesh HP, Tharanathan RN. Water-extracted polysaccha-rides of selected cereals and influence of temperature on the extractability of polysaccharides in sorghum. Food Chem. 1999;64:345–350. [Google Scholar]
- 17.Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F. A colorimetric method for the determination of sugars. Nature. 1951;168:167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 18.Association of Official Analytical Chemists. Official methods of analysis of AOAC International. 16th ed. Maryland: AOAC International; 1995. [Google Scholar]
- 19.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Dunigan DD, Waters SB, Owen TC. Aqueous soluble tetrazolium/formazan MTS as an indicator of NADH- and NADPH-dependent dehydrogenase activity. Biotechniques. 1995;19:640–649. [PubMed] [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu XL, Sun JY, Li HY, Zhang L, Qian BC. [Extraction and isolation of active component for inhibiting PC3 cell proliferation in vitro from the fruit of Lycium barbarum L] Zhongguo Zhongyao Zazhi. 2000;25:481–483. [PubMed] [Google Scholar]
- 24.Li YS, Chen ZJ, Zhu DY. A novel bis-furan derivative, two new natural furan derivatives from Rehmannia glutinosa and their bioactivity. Nat Prod Res. 2005;19:165–170. doi: 10.1080/14786410410001704787. [DOI] [PubMed] [Google Scholar]
- 25.Zhang M, Chen H, Huang J, Li Z, Zhu C, Zhang S. Effect of lycium barbarum polysaccharide on human hepatoma QGY7703 cells: inhibition of proliferation and induction of apoptosis. Life Sci. 2005;76:2115–2124. doi: 10.1016/j.lfs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Gan L, Wang J, Zhang S. [Inhibition the growth of human leukemia cells by Lycium barbarum polysaccharide] Weisheng Yanjiu. 2001;30:333–335. [PubMed] [Google Scholar]
- 27.Lu CX, Cheng BQ. Radiosensitizing effects of Lycium barbarum polysaccharide for Lewis lung cancer. Zhongxiyi Jiehe Zazhi. 1991;11:611–612, 582. [PubMed] [Google Scholar]
- 28.Cao GW, Yang WG, Du P. [Observation of the effects of LAK/IL-2 therapy combining with Lycium barbarum polysaccharides in the treatment of 75 cancer patients] Zhonghua Zhongliu Zazhi. 1994;16:428–431. [PubMed] [Google Scholar]
- 29.Liu JQ, Wu DW. 32 cases of postoperative osteogenic sarcoma treated by chemotherapy combined with Chinese medicinal herbs. Zhongguo Zhongxiyi Jiehe Zazhi. 1993;13:150–152, 132. [PubMed] [Google Scholar]
- 30.Lu X, Xian X, Lu W, Wu X, Gu H. [The regulation of Lycium barbarum on apoptosis of rat spleen in vitro] Zhongyaocai. 1999;22:250–251. [PubMed] [Google Scholar]
- 31.Yu MS, Ho YS, So KF, Yuen WH, Chang RC. Cytoprotective effects of Lycium barbarum against reducing stress on endoplasmic reticulum. Int J Mol Med. 2006;17:1157–1161. [PubMed] [Google Scholar]
- 32.Schuler M, Green DR. Mechanisms of p53-dependent apoptosis. Biochem Soc Trans. 2001;29:684–688. doi: 10.1042/0300-5127:0290684. [DOI] [PubMed] [Google Scholar]
- 33.Gan L, Zhang SH, Liu Q, Xu HB. A polysaccharide-protein complex from Lycium barbarum upregulates cytokine expression in human peripheral blood mononuclear cells. Eur J Pharmacol. 2003;471:217–222. doi: 10.1016/s0014-2999(03)01827-2. [DOI] [PubMed] [Google Scholar]


