Abstract
AIM: To study the effects of chitosan gel and blending chiston/gelatin film on preventing peritoneal adhesion in rats.
METHODS: SD rats were randomly divided into 2 groups, group A treated with chitosan gel and group B with blending chiston/gelatin film. In group A, rats were randomly subdivided into 3 subgroups as groups A1, A2 and A3, and different methods were used to induce peritoneal adhesions at the dead end of vermiform process in each group as follows: Group A1 with trauma, A2 with talc powder and A3 with ligation of blood vessel. In each subgroup, rats were redivided into control group and experimental group whose treated vermiform processes were respectively coated with chitosan gel and normal saline immediately after the adhesion-induced treatments. In group B, all the rats received traumatic adhesion-induced treatments and then were randomly divided into 4 groups (groups B1, B2, B3, B4). Group B1 served as control group and were coated with normal saline in the vermiform processes immediately after the treatments, and groups B2, B3 and B4 with 100% chitosan film, chitosan film containing 10% gelatin and chiston film containing 50% gelatin, respectively. At 2 and 4 wk after the above treatments, half of the rats in each terminal group were belly opened, and the peritoneal adhesive situation was graded and histopathological changes were examined.
RESULTS: (1) In group A, regarding peritoneal adhesion situation: At both 2 and 4 wk after the treatments, for groups A1 and A3, the adhesive grades of experimental groups were significantly lower than those of the control group (2 wk: H = 4.305, P < 0.05 for A1, H = 6.743, P < 0.01 for A3; 4 wk: H = 4.459, P < 0.05 for A1, H = 4.493, P < 0.05 for A3). However, of group A2, there was no significant difference between the experimental and control groups (2 wk: H = 0.147, P > 0.05; 4 wk: H = 1.240, P > 0.05). Regarding pathological changes: In groups A1 and A3, the main pathological change was fibroplasia. In group A2, the main changes were massive foreign-body giant cell reaction and granuloma formation with fibroplasia of different degrees. (2) In group B, regarding degradation of film: With increase of the blended gelatin concentration, degrading speed of the film accelerated significantly. Regarding peritoneal adhesion situation: At both 2 and 4 wk after the treatments, the adhesive grades of B1 were the lowest among the four subgroups of B (2 wk: H = 29.679, P < 0.05; 4 wk: H = 18.791, P < 0.05). At 2 wk after the treatments, the grades of group B2 were significantly lower than that of groups B3 and B4 (H = 4.025, P < 0.05 for B2 vs B3; H = 4.361, P < 0.05 for B2 vs B4). At 4 wk, there were no significant differences of the grades between groups B2, B3 and B4. Regarding pathological changes: Inflammatory cell infiltration and fibroplastic proliferation were observed in the local treated serous membranes, which was the mildest in group B1. Slight foreign-body giant cell reactions were also found in groups B2, B3, and B4.
CONCLUSION: (1) Chitosan gel has preventive effect on traumatic or ischemic peritoneal adhesion, but no obvious effect on foreign body-induced peritoneal adhesion. (2) Chitosan film may exacerbate the peritoneal adhesion. Blending with gelatin to chitosan film can accelerate the degradation of the film, but can simultaneously facilitate the formation of peritoneal adhesion.
Keywords: Chitosan, Gelatin, Peritoneal adhesion, Rat
INTRODUCTION
Chitosan is the derivant of the chitin after deacetylation, and chitin is a main ingredient of the arthropod shells (such as shrimps, crabs and insects, etc), which is a kind of renewable natural resources and profuse in amount. Since chitosan is innocuous, biodegradable and with ideal biocompatibility, it has been applied to develop biomaterials[1,2]. In medical field, chitosan has antiseptic function and can facilitate the healing of wound. It has been widely studied on its potential use to be medical biomaterials. Because of its inhibitory effect on fibroblast growth and the function of mechanical isolation[3], chitosan has been regarded highly in the prevention of peritoneal adhesion. To evaluate the effects of different types of chitosan on preventing traumatic peritoneal adhesion, a control study in rats was done in this paper.
MATERIALS AND METHODS
Experimental animals and grouping
Two hundred and forty SD rats, 120 females and 120 males, weighing 200-250 g, were provided by the Laboratory Animal Center of Zhejiang Province. They were randomly divided into group A (144 rats) and group B (96 rats). Group A was treated with chitosan gel and group B with blending chiston/gelatin film. In Group A, 144 rats were randomly divided into 3 subgroups (groups A1, A2, A3) with 48 rats in each. Different methods were used to induce peritoneal adhesion in the dead ends of the vermiform processes as follows: group A1 with trauma, group A2 with talc powder and group A3 with ligation of blood vessel. For each subgroup, 48 rats were randomly redivided into control group and experimental group with 24 rats in each. In group B, all the 96 rats received traumatic adhesion-induced treatments as group A1 and then were randomly divided into 4 groups (groups B1, B2, B3, B4) with 24 rats for each. Group B1 served as control group and groups B2, B3, and B4 served as experimental groups treated with 100% chitosan film, chitosan film containing 10% gelatin and chiston film containing 50% gelatin, respectively.
All the rats were fed under the same condition: 24°C-26°C of environmental temperature, about 40% of humidity, alternating 12 h light/dark cycle, free access to food and water.
Surgical methods: Under general anaesthesia with intraperitoneal injection of 3% amobarbital (60 mg/kg), the rats were immobilised in dorsal position, routinely degermed, abdominally incised through a median incision of 2-3 cm long, and the vermiform processes were searched and pulled out of the incision, then the terminal vermiform processes within a distance of 3 cm were treated as follows: In group A1, the anterior surface of serous membrane was scraped slightly with surgical blade till obvious congestion and small bleeding drops appeared. In group A2, talc powders were evenly smeared over the anterior surface of serous membrane. In group A3, the vermiform artery stem was ligated with No 0 surgical thread at the point of 3 cm from the dead end in the following way: loosely knotting the first loop, thrilling a thread with equivalent diameter to the vermiform artery stem through the first loop, tightening the first loop, knotting and tightening the second loop of the ligation knot,and pulling out the thrilled thread. The ligation resulted in a stricture of vermiform artery which induced ischemia of the distal vermiform tissue from the ligation point. This method had been proved successful in inducing peritoneal adhesion in our former experiments. After the above treatments, for the experimental groups, the treated serous membranes within 3 cm distance from the dead ends were coated with chitosan gel in a dosage of 0.5 mL for each, and the vermiform processes were put back into the abdominal cavities, which were then closed. For the control groups, all the treatments were the same as those of the experimental groups except that the chitosan gel was replaced by normal saline. The duration from opening to closing the abdominal cavity was 5 min, so that the duration of exposure of intestines to air was the same for each rat. In group B, of all the rats, the anterior surfaces of vermiform processes were scraped slightly with surgical blade just as group A1, and the scraped surfaces of B2, B3, B4 were coated with films containing different percentages of gelatin at 0%, 10%, and 50% respectively. Group B1 was treated with normal saline as control. The duration from opening to closing the abdominal cavity was controlled just for 5 min.
At 2 and 4 wk after the surgery, 12 rats (6 females and 6 males) in each subgroup were randomly selected respectively for further study. The abdominal cavity was reopened under anaesthesia, and the grades of peritoneal adhesion were evaluated which existed between the treated vermiform processes and intestines, mesenteria and abdominal walls. After that, the vermiform processes with adhesions were resected, fixed with formalin and histopathologically examined.
Grading standard for the peritoneal adhesion
Referring to Phillips’[4] grading method of 5 levels and considering the characteristics of peritoneal adhesion in rats, we offered the following grading standard: Grade 0: no adhesions; Grade I: the ratio of adhesive area/the total treated area in the vermiform processes < 20%; Grade II: the ratio is about 40% Grade III: the ratio is about 60%;Grade IV: the ratio is ≥ 60%. Each rat was graded by three referees blindly and the average grade of the three was accepted as the adhesive grade of the rat.
Statistical analysis
The H-test of non-parametric statistics for ranked grouped data was used to analyze the differences of the peritoneal adhesive grades between the experimental and control groups. P < 0.05 was taken as significant.
RESULTS
Group A
Observation with naked eyes: The skin incisions of all rats healed in first grade. No obvious infection appeared in the abdominal cavity in all rats. In the experimental groups, no obvious residual of chitosan gel could be found at 2 wk after surgery.
Comparison of peritoneal adhesion level: As it shows in Table 1, in groups A1 (trauma-induced adhesion) and A3 (ischemia-induced adhesion), peritoneal adhesion grades of the experimental groups were significantly lower than those of the control groups (P < 0.05 or P < 0.01) both at 2 and 4 wk after the surgical treatments. While in group A2 (talc powder-induced adhesion), there was no significant difference in the peritoneal adhesion between the experimental and control groups (P > 0.05) both at 2 and 4 wk. The above results indicated that chitosan gel has perfect effect on preventing peritoneal adhesion induced by trauma and ischemia, but no obvious effect on adhesion induced by talc powder.
Table 1.
Comparison of peritoneal adhesion between experimental and control groups in groups A1, A2, and A3
| Group |
Experimental group (total
n
= 12) |
Control group (total
n
= 12) |
H | P | |||||||||
| 0 | I | II | III | IV | 0 | I | II | III | IV | ||||
| A1 | 2 wk | 1 | 7 | 4 | 0 | 0 | 5 | 6 | 1 | 0 | 0 | 4.305 | < 0.05 |
| 4 wk | 2 | 7 | 3 | 0 | 0 | 6 | 6 | 0 | 0 | 0 | 4.459 | < 0.05 | |
| A2 | 2 wk | 0 | 1 | 1 | 4 | 6 | 0 | 0 | 2 | 3 | 7 | 0.147 | > 0.05 |
| 4 wk | 0 | 0 | 1 | 2 | 9 | 0 | 0 | 0 | 1 | 11 | 1.240 | > 0.05 | |
| A3 | 2 wk | 0 | 5 | 5 | 2 | 0 | 2 | 8 | 2 | 0 | 0 | 6.743 | < 0.01 |
| 4 wk | 2 | 5 | 4 | 1 | 0 | 6 | 5 | 1 | 0 | 0 | 4.493 | < 0.05 | |
Comparison of pathological changes: In group A1, at 2 wk after the surgical treatments, there existed obvious fibroplasia and diffused lymphocytes infiltration in the treated serous membrane of the vermiform processes. While at 4 wk after the treatments, the main pathological change was fibroplasia. The above pathological changes in the experimental group were obviously milder than those in the control group. In group A2, at 2 wk, massive foreign-body giant cell reaction (FBGCR) and granuloma formation appeared in the treated vermiform processes with diffuse inflammatory cell infiltration. At 4 wk after the surgical treatment, foreign body granuloma and fibroplasia reaction aggravated with crystal appearing in the foreign-body giant cell. There was no significant difference in the adhesive severity between the experimental and the control groups. In group A3, 2 wk after the surgical treatments, full-thickness fibroplasias and lymphocyte infiltration appeared in the vermiform processes which was more severe in the serous layer. While at 4 wk after the treatment, the lymphocyte infiltration became lighter, but the fibroplasias aggravated obviously. The above pathological changes in the experimental group were obviously milder than that in the control group 2 wk after the treatment. However, at 4 wk, there was no significant difference between the experimental and control groups.
Group B
Observation with naked eyes: The skin incisions of all rats healed in first grade. No obvious infection appeared in the abdominal cavities at 2 wk after the treatments in all rats. Chitosan film degradation: In group B2, no obvious changes occurred in the chitosan film at 2 wk, and even no obvious rupture could be found in the film at 4 wk after the surgical treatments. The films were only swollen and thinned at 4 wk. In group B3, the films with 10% gelatin were swollen at 2 wk, and degradated into fragments at 4 wk after the treatments. In group B4, the films with 50% gelatin were broken into pieces at 2 wk, and disappeared at 4 wk after the surgical treatments.
Comparison of peritoneal adhesion degree: As lis-ted in Table 2, both at 2 wk and 4 wk after the surgical treatments, there existed significant differences among the four groups (P < 0.05) in the peritoneal adhesive degree. At 2 wk, all the differences were significant between every 2 groups (P < 0.05) except between groups B3 and B4. At 4 wk, there were significant differences between groups B1 and B2, B1 and B3, B1 and B4 (P < 0.05), but there was no significant difference between groups B2 and B3, B2 and B4, B3 and B4 (P > 0.05). All the above results indicated that both pure chitosan film and blending chiston/gelatin films could exacerbate peritoneal adhesion, as well as the blended gelatin.
Table 2.
Comparison of peritoneal adhesion in group B rats
| Group | n |
2 wk (n) |
4 wk (n) |
||||||||
| 0 | I | II | III | IV | 0 | I | II | III | IV | ||
| B1 | 24 | 1 | 7 | 4 | 0 | 0 | 2 | 7 | 3 | 0 | 0 |
| B2 | 24 | 1 | 3 | 3 | 4 | 1 | 0 | 3 | 3 | 5 | 1 |
| B3 | 24 | 0 | 0 | 2 | 4 | 6 | 0 | 2 | 2 | 3 | 5 |
| B4 | 24 | 0 | 0 | 0 | 2 | 10 | 0 | 2 | 1 | 5 | 4 |
| H | 29.679 | 18.791 | |||||||||
| P | < 0.05 | < 0.05 | |||||||||
Comparison of pathological changes: (1) At 2 wk after the surgical treatments, the adhesive vermiform processes of all rats were mainly with various degrees of edema, dilation and congestion of capillaries, infiltration of inflammatory cell and fibroplasias. The reaction became severer in the serous membrane than in other layers. The reactions in groups B2, B3, and B4 were more severe than those in group B1. There were slight foreign-body giant cell reactiond in groups B2, B3 and B4 (Figure 1). (2)At 4 wk after the treatments, the acute inflammatory reaction decreased obviously in all groups except group B1. In all the other groups (B2, B3 and B4), the fibroplasia was aggravated obviously which caused formation of complete fibrous capsules around the implantation, with a thickest capsule wall in group B4 and a thinnest one in group B2. Furthermore, groups B2, B3, and B4 had obvious foreign-body giant cell reaction (Figure 2).
Figure 1.
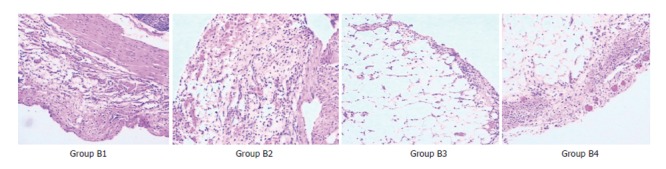
Histopathologic changes of the four B-groups at 2 wk after the surgical treatment.
Figure 2.
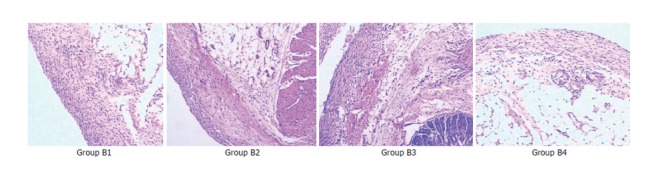
Histopathologic changes of the four B-groups at 4 wk after the surgical treatment.
DISCUSSION
Peritoneal adhesion is an inevitable phenomenon of the natural repairing processes after peritoneal injury. In recent years, many researches have been done on the process and mechanism of peritoneal adhesion. To understand the mechanism of peritoneal adhesion is very important for the prevention of its formation.
Animal studies indicated that the serous membrane injury caused by mechanical injury, ischemia of tissue, stimulation of foreign body and peritonitis was the main reason of peritoneal adhesion. However, whether it results in permanent fibrinous adhesions or not depends on the integrity of the fibrinolytic system[5,6]. Generally speaking, the formation of peritoneal adhesion needs the following steps: step 1 is the formation of fibrinous gelatinous matrix. This happens within 3 h after the injury. The gelatinous matrix locates among the injured peritoneal membranes, which is the initiation of peritoneal adhesion. Step 2 occurs between 1-5 d after the injury. The fibrinous matrix is gradually replaced by the vascular granular tissues which contain fibroblasts, macrophagocytes and giant cells. Most of the fibrins disappear and are replaced by a large number of fibroblasts and collagen fibers, and then the fibrin network is formed. Step 3 takes place during 5-10 d after the injury. The fibroblasts acquire a regular alignment gradually and the collagen deposition is enriched. Step 4 is at 2 wk after the injury. The component cells, especially the fibroblasts, decrease obviously and are covered by mesothelial cells. Eventually, the fibrinous adhesions are formed. However, after peritoneal injury, the fibrin can be decomposed by fibrinolysin into fibrin degradation products (FDPs) in the course of fibrinous gelatinous matrix formation, which is unrelated with peritoneal adhesion. It generally occurs at 72-76 h after the injury. In brief, when peritoneal injury occurs, whether the wounds heal through adhesive fusion or through epithelization, mainly depends on the degree of local fibrinolysis and whether epilesional juxtaposition exists[7].
Based on how and why the peritoneal adhesion happens, more than 10 preventive methods have been proposed. Among them, mechanical isolation seems to have the brightest prospect. At present, close attention has been paid to the following 4 materials which are used as mechanical isolation: EPTEE, oxidized regenerated cellulose (ORC), HA-CMC and chitosan[8,9]. Chitosan is highly regarded as a biomaterial for prevention of peritoneal adhesion, because it has the functions of anti-infection, hemostasis, inhibiting growth of fibroblasts, mechanical isolation and moreover, it is biodegradable.
Chitosan is the derivant of deacetylized chitin. Chitin was discovered in 1811. Its molecular structure was determined by chemical method and X-ray diffraction in 1887. Chitin can be converted into chitosan after deacetylation. Chitosan is soluble, easily to be chemically modified because it contains many amid and hydroxyl. Meanwhile, this kind of natural polysaccharose is alkaline and has good biocompatibility and is biologically degradable. Its degradation products are acetylglucosamine and aminoglucose, which are atoxic for human bodies. The low molecular weight of chitosan and its oligosaccharide produced during degradation render it to have no immuno-genicity and not to accumulate in vivo. The biological activity of chitosan mainly includes: (1) inhibiting the growth of bacteria and mold; (2) antineoplasmic activity: It can selectively agglutinate the L1210 cells in leukemia and Ehrlich’s cells in carcinomatous ascites, but does not affect the normal erythroid bone marrow cells; (3) immunological enhancement: It can efficaciously enhance the function of macrophages and the activity of hydrolase, stimulate the macrophage to produce lymphokine and initiate the immune response. But it cannot promote the production of antibody; (4) anticoagulant activity: The chitosan after thioesterification has a similar chemical structure with heparin so that it has a good anticoagulant activity; (5) promoting tissue repair and hemostasis: Since the degradating product of chitosan is charged, it can induce platelet aggregation and activate the coagulation system. Chitosan can inhibit fibrous hyperplasia during wound healing and efficaciously enhance wound healing. Animal experiments in recent years have proven that chitosan gel has good effect in preventing peritoneal adhesion[3,4].
All the 3 different animal models of peritoneal adhesion used in our study were monofactor-induced peritoneal adhesion, which made the study of effects of chitosan on preventing peritoneal adhesion due to different causes technologically possible. Observation at 2 and 4 wk after the surgical treatments in all the control groups showed that the incidence of peritoneal adhesion was 90.9% and 83.3% respectively, and most of the adhesions were graded as I and II. This proved that the animal model was reliable and stable.
In our study, different chitosan materials were used for the prevention of peritoneal adhesion, which turned out to have completely different results. Because the peritoneal adhesions in each group were caused by different methods, different preventing effect was observed. In group A (treated with chitosan gel), the chitosan gel film had satisfactory effects on the prevention of peritoneal adhesion due to injury or ischemia. At 2 wk after the treatments, the chitosan gel was completely biologically degraded. Moreover, the peritoneal adhesion level and pathological change in both groups A1 and A3 were milder than those in the control groups. This indicated that the chitosan gel had an evident preventing effect against peritoneal adhesion due to injury or ischemia. In group A2 (adhesions induced by talc powder), both histopathologic examination and adhesion grades indicated that the chitosan gel had no evident preventing effects on peritoneal adhesion induced by talc powder. The probable cause may be related with the pathological change in adhesions induced by talc powder, which mainly was foreign body granuloma reaction with massive fibroplasias. As long as the foreign body existed, the foreign body granuloma and fibroplasia reaction would remain persistent. Since the chitosan gel could be fastly degradated in vivo (within 2 wk after surgery), the acting time of the chitosan gel was too short to prevent against persistent foreign body granuloma reaction.
Chitosan gel is still imperfect because it is highly flowable. When chitosan gel is smeared at the surface of wounded peritoneum, it cannot come to a high concentration in the focus, resulting in weakened effect since its acting time is shortened. In order to improve the concentration of chitosan in the intraabdominal focus and make a more thoroughly mechanical isolation, some researchers used pure chitosan film instead to get a better effect on peritoneal adhesion prevention. However, our study indicated that such effort was disappointing.
In Group B2 (treated with pure chitosan film), at 4 wk after the treatments, the chitosan film was still undegraded. Such slow degradating speed was detrimental for an anti-peritoneal adhesion material because if the postsurgical initial membranous adhesions cannot be degraded in time, it will form irreversible fibrinous adhesions, which cannot be inhibited by chitosan. On the contrary, the intraabdominal residual of undegraded chitosan film can evoke the foreign body reaction and result in fibrous capsule formation, which facilitates the formation of peritoneal adhesion. These were proved in our study: the adhesion grade in group B2 was higher than that in control group, and histopathologic examination indicated obvious foreign-body giant cell reaction at 2 and 4 wk after the surgical treatments.
Because the slow degradation of chitosan film weakened its anti-adhesive function, blending chiston/gelatin film was suggested to improve the in vivo degra-dation speed of chiston film. And experiments proved that the more gelatin added in the film, the more fastly the blending film in vivo degradated. However, there have been no experimental data about the effect of the blending chiston/gelatin film on the prevention of peritoneal adhesion.
Gelatin is a polypeptide mixture which is soluble in hot water. It is widely used in medical field as haemostat and dermagraft and dressing materials of wounds because it can be degradated quickly in vivo. In our study, the blending chiston/gelatin films with different concentration of gelatin were used in group B2 (10% gelatin) and B4 (50% gelatin), and the results indicated that the higher concentration of gelatin in the film, the more fastly it was degradated in the abdominal cavity of rats. However, our study indicated that the anti-peritoneal adhesion effect of the blending film was not ideal. The peritoneal adhesion grade was not only lower than that of the control group, but also lower than that of group B2 (with pure chiston film). The probable reasons are: although the blending chiston/gelatin film had a faster degradation speed, the film still could not be completely degradated even at 4 wk after the treatments in group B3 and at 2 wk in group B2. The residual blending film may cause foreign body reaction. In addition, gelatin is a polypeptide mixture which is probably antigenic and can cause immunological rejection. This in turn promotes and facilitates the formation of local peritoneal adhesions.
We conclude that: (1) Chitosan gel has perfect effect on preventing peritoneal adhesion due to injury or ischemia, but no evident effect on peritoneal adhesion induced by talc powder; (2) Pure chitosan film could not prevent peritoneal adhesion because of low in vivo degradation speed; (3) Chitosan film blended with gelatin could exacerbate peritoneal adhesion.
Footnotes
S- Editor Wang J L- Editor Zhu LH E- Editor Liu WF
References
- 1.Hirano S. Chitin biotechnology applications. Biotechnol Annu Rev. 1996;2:237–258. doi: 10.1016/s1387-2656(08)70012-7. [DOI] [PubMed] [Google Scholar]
- 2.Shigemasa Y, Minami S. Applications of chitin and chitosan for biomaterials. Biotechnol Genet Eng Rev. 1996;13:383–420. doi: 10.1080/02648725.1996.10647935. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy R, Costain DJ, McAlister VC, Lee TD. Prevention of experimental postoperative peritoneal adhesions by N,O-carboxymethyl chitosan. Surgery. 1996;120:866–870. doi: 10.1016/s0039-6060(96)80096-1. [DOI] [PubMed] [Google Scholar]
- 4.Phillips RK, Dudley HA. The effect of tetracycline lavage and trauma on visceral and parietal peritoneal ultrastructure and adhesion formation. Br J Surg. 1984;71:537–539. doi: 10.1002/bjs.1800710722. [DOI] [PubMed] [Google Scholar]
- 5.Holmdahl L, Eriksson E, al-Jabreen M, Risberg B. Fibrinolysis in human peritoneum during operation. Surgery. 1996;119:701–705. doi: 10.1016/s0039-6060(96)80196-6. [DOI] [PubMed] [Google Scholar]
- 6.Almdahl SM, Burhol PG. Peritoneal adhesions: causes and prevention. Dig Dis. 1990;8:37–44. doi: 10.1159/000171238. [DOI] [PubMed] [Google Scholar]
- 7.Luijendijk RW, de Lange DC, Wauters CC, Hop WC, Duron JJ, Pailler JL, Camprodon BR, Holmdahl L, van Geldorp HJ, Jeekel J. Foreign material in postoperative adhesions. Ann Surg. 1996;223:242–248. doi: 10.1097/00000658-199603000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.diZerega GS. Contemporary adhesion prevention. Fertil Steril. 1994;61:219–235. doi: 10.1016/s0015-0282(16)56507-8. [DOI] [PubMed] [Google Scholar]
- 9.Burns JW, Skinner K, Colt J, Sheidlin A, Bronson R, Yaacobi Y, Goldberg EP. Prevention of tissue injury and postsurgical adhesions by precoating tissues with hyaluronic acid solutions. J Surg Res. 1995;59:644–652. doi: 10.1006/jsre.1995.1218. [DOI] [PubMed] [Google Scholar]


