Abstract
AIM: To investigate the expression of vascular endothelial growth factor-c (VEGF-C) mRNA and microvessel density (MVD) in human esophageal squamous cell carcinoma (ESCC) and its relationship with clinical significance.
METHODS: Specimens obtained from 43 patients undergoing surgical resection for ESCC were used in this study. The expression of VEGF-C mRNA was examined by in situ hybridization. Tumor MVD was determined immunohistochemically with anti-CD31 antibody and estimated by image analysis. Ten sections of adjacent normal mucosa were also examined.
RESULTS: VEGF-C mRNA expression was detected in cytoplasm of carcinoma cells. Of the 43 ESCC patients studied, 18 cases (41.9%) were positive for VEGF-C mRNA. No VEGF-C mRNA expression was observed in normal esophageal mucosa. VEGF-C mRNA expression correlated significantly with lymph node metastasis, TNM stage and depth of invasion (P < 0.05). Furthermore, histological grade (differentiation) tended to correlate with VEGF-C mRNA expression, but was not statistically significant (P > 0.05). In tumor lesions, the MVD was significantly greater than that in normal esophageal mucosa. MVD correlated significantly with lymph node metastasis, TNM stage and depth of invasion (P < 0.05), but not with histological grade (differentiation) (P > 0.05). Lesions with VEGF-C mRNA expression had a significantly higher MVD than that of those without VEGF-C mRNA expression (P < 0.05).
CONCLUSION: VEGF-C plays a role in lymphatic metastasis via lymphangiogenesis and angiogenesis in ESCC. VEGF-C is one of the important predictors of the biological behavior in ESCC.
Keywords: Vascular endothelial growth factor-c, Esophageal carcinoma, Angiogenesis, Microvessel density, Lymph node metastasis, In situ hybridization
INTRODUCTION
Lymph node metastasis is an important prognostic factor for human esophageal squamous cell carcinoma (ESCC)[1]. Angiogenesis induced by a variety of growth factors such as vascular endothelial growth factor (VEGF), has been recently considered necessary for the growth, invasion and metastasis of solid tumors[2]. Thus, measurement of vascular growth may be clinically important in esophageal cancer specimens. To assess angiogenesis, several markers of blood vessel endothelium have been developed for microscopic estimation of microvessel density(MVD) by immunohistochemistry, including CD31/PECAM-1, CD34, and factor VIII-related antigen(von Willebrand factor or vWF)[3]. VEGF-C, a member of the VEGF family, stimulates the proliferation of both vascular and lymphatic endothelial cells via VEGF receptor (VEGFR)-2 and VEGFR-3 in many physiological and pathological processes[2,4]. Previous reports have shown that VEGF-C expression in cancer tissues has a positive correlation with the risk of lymphatic metastasis in a variety of cancers[5] including prostatic[6], gastric[7], oral[8], lung[9], colorectal[10,11] and bladder carcinoma[12]. A similar tendency has been reported for esophageal cancers, although a significant correlation between VEGF-C expression and the frequency of nodal metastasis is not always found[13-15]. Hence, the precise role of VEGF-C in ESCC has not been clearly understood[1]. Therefore, in the present study, VEGF-C mRNA expression in biopsy specimens of ESCC was examined by in situ hybridization (ISH) and the association between VEGF-C mRNA expression and clinicopathological factors, including the intratumoroal microvessel density (MVD) as a measure of tumor angiogenesis in ESCC was invested.
MATERIALS AND METHODS
Patients and tumor specimens
Tumor specimens from 43 ESCC patients (32 males and 11 females, mean age 62.5 years, range 47-76 years) undergoing surgical treatment in Jinhua People’s Hospital from 1997 to 2002 were included in the study. Specimens were classified according to the TNM classification system by UICC and 10 normal esophageal tissues were collected as control. None of the patients received any radiotherapy or chemotherapy prior to the study. Histological grades, depth of invasion and lymph node metastasis are shown in Table 1.
Table 1.
Correlation between VEGF-C mRNA expression in ESCC tissue and clinicopathological factors
| Clinicopathological factors | n | VEGF-C mRNA n (%) | MVD/mm2 (mean ± SD) |
| TNM stage | |||
| I,II | 33 | 11 (33.3) | 60.14 ± 20.2 |
| III, IV | 10 | 7 (70.0)a | 83.68 ± 33.6a |
| Histological grade | |||
| G1 | 12 | 4 (33.3) | 60.5 ± 31.7 |
| G2 | 20 | 8 (45.0) | 66.30 ± 21.6 |
| G3 | 11 | 6 (54.6) | 71.66 ± 30.8 |
| Depth of invasion | |||
| T1, T2 | 23 | 5 (21.7) | 64.24 ± 19.3 |
| T3, T4 | 20 | 13 (65.0)b | 68.86 ± 31.7 |
| Lymph node metastasis | |||
| Negative | 31 | 10 (32.3) | 58.66 ± 18.8 |
| Positive | 12 | 8 (66.7)a | 85.30 ± 32.7a |
P < 0.05 vs negative lymph node metastasis and TNM stages III and IV;
P < 0.01 vs depth of tumor invasion of T3 and T4.
Methods
In situ mRNA hybridization (ISH) analysis was performed as described previously with minor modifications[16]. VEGF-C specific oligonucleotide probes were labeled with digoxin at the 5’-end and synthesized by Beijing Dinguo Co. Ltd (Dingguo, Beijing, China). The DNA oligonucleotide sequences used are 5'-TGTACAAGTGTCAGCTAAGG-3' and 5'-CCACATCTATACACACCTCC-3'. Tissue sections (4 μm-thick) were incubated with 50 mg/L proteinase K, and then hybridization was performed in a moist chamber for 16 h at 45°C, using a prove concentration of 100 ng/L. The sections were then incubated with a staining solution containing NBT/BCIP in a dark box for 1-2 h. Negative controls ware performed in all cases by omitting the probes.
A mouse CD31 mAb (JC/70A, DAKO Corporation, Denmark) was used as a vascular endothelial marker[3,17]. Immunohistochemical examination for CD31 was performed with EnVision kits to access MVD. In brief, after deparaffinization and rehydration, the sections were denatured for 3 min in a microwave oven in citrate buffer (0.01 mol/L, pH 6.0) for antigen retrieval. Endogenous peroxide activity was quenched with hydrogen peroxide, and the sections were incubated with goat serum for 20 min at room temperature to block nonspecific binding of anti-CD31 mAb (JC/70A, 1:50 dilution) and then for 16 h at 4°C at 1:50 dilution. Bound antibodies were detected by the EnVision system (DAKO Corporation, Denmark) and using 3, 3’-diamindoenzidine as the chromogen while nuclei were counterstained with hematoxylin. Negative controls were obtained by omission of the primary antibodies.
The results of ISH and MVD were examined by two of the authors with no prior knowledge of the identification of each section, using Leica Qwin image system (Leica Corporation, Germany). The expression of VEGF-C mRNA in the tissue was defined as positive if distinct staining was observed in at least 10% of tumor cells[18]. For determination of MVD, the number of microvessels with positive reaction to JC/70A was counted in five randomly selected microcopy fields on the vascular areas within a section (× 200, 0.7386 mm2/field) and the average was then calculated.
Statistical analysis
The data were evaluated by t test, Fisher’s exact probability test or chi square test. P < 0.05 was considered statistically significant for all tests. All statistical analyses were performed using the SPSS 10.0 statistical package (SPSS, Inc, Chicago, IL).
RESULTS
Expression of VEGF-C mRNA in ESCC tissues
Of the 43 patients studied, 18 cases (41.9%) were positive for VEGF-C mRNA expression in cytoplasm. The specimens were divided into two categories by the staining pattern of VEGF-C mRNA, diffuse or focal staining of carcinoma cells (Figures 1A and 1B). In contrast, no VEGF-C mRNA in specimens of adjacent normal esophageal mucosa was stained.
Figure 1.
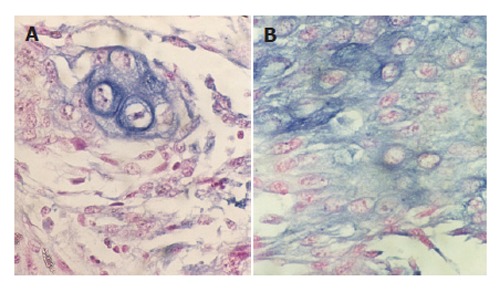
Focal (A) and diffuse (B) expression of VEGF-C mRNA in ESCC tissue as blue granules in cytoplasm of tumor cells (in situ hybridization × 400).
CD 31 expression and MVD in ESCC tissues
Immunohistochemical expression of CD31 was detected in cytoplasm of vascular endothelial cells. Brown capillaries or small clusters standing out sharply from other tissues were found to be microvessels (Figures 2A and 2B). At the deepest invasive site of tumors, the MVD ranging 43-144/mm2 (76.4 ± 20.3/mm2) was significantly higher than that in normal esophageal mucosa (P < 0.01).
Figure 2.
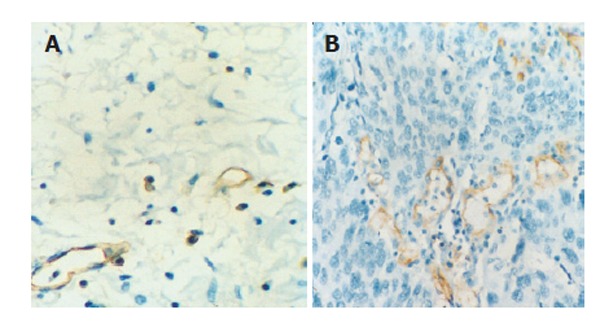
Immunohistochemical staining of CD 31 in normal esophageal (A) and ESCC (B) tissue (EnVision × 400).
Correlation between VEGF-C mRNA expression and MVD and clinicopatholgical factors
VEGF-C mRNA expression in ESCC tissues was associated with the depth of tumor invasion, TNM stage and lymph node metastasis (P < 0.05), but not with histological grade (P > 0.05). Furthermore, MVD also correlated with TNM stage and lymph node metastasis in ESCC tissue (P < 0.05). No association was found between MVD and depth of tumor invasion and histological grade (P > 0.05). The patients were divided into VEGF-C mRNA positive and negative groups. The VEGF-C mRNA positive cases showed a higher MVD (79.5 ± 30.9/mm2) than the VEGF-C mRNA negative cases (57.5 ± 18.4/mm2) (P < 0.05) (Table 1).
DISCUSSION
Recent studies have shown a significant relation between MVD, VEGF expression and prognosis in a variety of tumors including esophageal carcinoma[19]. Among the VEGF family members, VEGF-C appears to affect the lymphatic system as a lignant for VEGFK-3, which is specifically expressed on lymphatic vessels, and VEGF-C is suspected to play an important role in lymphangioge-nesis[2,4,5]. But little is known about the mechanisms of lymphangiogenesis and lymphatic metastasis, while the effects of VEGF-C in ESCC remain controversial[1]. In this study, we investigated the clinicopathalogical significance of VEGF-C mRNA expression in relation to the angiogenesis in ESCC. The results showed that 41.9% of patients with ESCC had expression of VEGF-C mRNA, which correlated significantly with lymph mode metastasis, depth of tumor invasion and TNM stage, suggesting that VEGF-C plays a role in ESCC. It was reported that VEGF-C can be detected using immunohistochemical staining and RT-PCR, and VEGF-C expression in ESCC may play a key role in tumor progression and lymphatic metastasis[13,14]. It was also reported that only histological grade, but not parameters involved in lymphatic spread, correlates with VEGF-C expression in ESCC[15]. These contradictory findings can be explained by difference in analytic method. Even in immunohistochemial analysis, the result may differ depending on the selected site for assessment.
More interestingly, we found that VEGF-C mRNA expression was significantly correlated with MVD, indicating the grade of angiogenesis in ESCC. Tsurusaki et al[6] and Akagi et al[18] have failed to find any remarkable differences in blood vessel density between VEGF-C positive and negative prostatic and colorectal carcinoma specimens. Also, VEGF-C expression is not correlated with MVD in human gallbladder cancer[20]. Whereas other studies reported that VEGF-C is associated with angiogenesis in breast cancer[21], colorectal carcinoma[22,23] and bladder transitional cell carcinoma[12], and plays an important role in angiogenesis and lymphangiogenesis in lung cancer[9] and malignant mesothelioma[24]. It has been reported that VEGF-C can bind to both VEGFR-2 and VEGFK-3[25]. The specificity of VEGF-C for its 2 receptor is known to be regulated by proteolytic processing. Accordingly, only fully processed VEGF-C activates both VEGFR-2 and VEGFK-3, whereas the partially processed precursors act only through VEGFK-3[26]. Activation of VEGFR-2 results in mitogenesis of vascular endothelial cells, whereas VEGFR-3 activation by VEGF-C is considered to induce proliferation of lymphatic endothelial cells. Furthermore, recent studies have revealed that VEGFR-3 is expressed in angiogenic blood vessels in certain pathological conditions. For example, VEGFR-3 expression can detected in intratumor blood vessels of human breast cancer[21], cutaneous melanoma[27], head and neck squamous cell carcinoma[28], gliomas as well as in vascular endothelial cells activated during formation of granulation tissue in skin[29]. Thus, angiogenesis and lymphangiogenesis responses to VEGF-C have been found to depend on the expression of its receptors in blood and lymphatic endothelial cells of the target tissue.
In our present study, patients with VEGF-C mRNA expression had a significantly higher MVD that those without VEGF-C mRNA expression, suggesting that VEGF-C secreted from tumor cells may stimulate not only lymphangiogenesis but also angiogenesis, which are significantly correlated with lymph node metastasis in ESCC. As a rule, once cancer cells enter blood microvessels, they can reach regional lymph nodes through lymphatic vessels from blood vessels, via blood vessel-lymph vessel junctions. In fact, the lymphatic and vascular systems have numerous connections allowing disseminating tumor cells to pass rapidly from one system to the other. Furthermore, metastatic tumor cells expressing VEGF-C could grow in regional lymph nodes that they reached.
In conclusion, VEGF-C mRNA is expressed heterogeneously in tumor cells and plays an important role in angiogenesis and lymphangiogenesis, as well as growth and metastasis of ESCC. The combined examination of VEGF-C expression and MVD in biopsy specimens from ESCC patients can predict lymph node metastasis and select appropriate treatments.
Footnotes
S- Editor Pan BR L- Editor Wang XL E- Editor Bi L
References
- 1.Duff SE, Li C, Jeziorska M, Kumar S, Saunders MP, Sherlock D, O'Dwyer ST, Jayson GC. Vascular endothelial growth factors C and D and lymphangiogenesis in gastrointestinal tract malignancy. Br J Cancer. 2003;89:426–430. doi: 10.1038/sj.bjc.6601145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathanson SD. Insights into the mechanisms of lymph node metastasis. Cancer. 2003;98:413–423. doi: 10.1002/cncr.11464. [DOI] [PubMed] [Google Scholar]
- 3.Uzzan B, Nicolas P, Cucherat M, Perret GY. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res. 2004;64:2941–2955. doi: 10.1158/0008-5472.can-03-1957. [DOI] [PubMed] [Google Scholar]
- 4.Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev. 2002;82:673–700. doi: 10.1152/physrev.00005.2002. [DOI] [PubMed] [Google Scholar]
- 5.Stacker SA, Baldwin ME, Achen MG. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002;16:922–934. doi: 10.1096/fj.01-0945rev. [DOI] [PubMed] [Google Scholar]
- 6.Tsurusaki T, Kanda S, Sakai H, Kanetake H, Saito Y, Alitalo K, Koji T. Vascular endothelial growth factor-C expression in human prostatic carcinoma and its relationship to lymph node metastasis. Br J Cancer. 1999;80:309–313. doi: 10.1038/sj.bjc.6690356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amioka T, Kitadai Y, Tanaka S, Haruma K, Yoshihara M, Yasui W, Chayama K. Vascular endothelial growth factor-C expression predicts lymph node metastasis of human gastric carcinomas invading the submucosa. Eur J Cancer. 2002;38:1413–1419. doi: 10.1016/s0959-8049(02)00106-5. [DOI] [PubMed] [Google Scholar]
- 8.Kishimoto K, Sasaki A, Yoshihama Y, Mese H, Tsukamoto G, Matsumura T. Expression of vascular endothelial growth factor-C predicts regional lymph node metastasis in early oral squamous cell carcinoma. Oral Oncol. 2003;39:391–396. doi: 10.1016/s1368-8375(02)00143-4. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Dong X, Gu W, Qiu X, Wang E. Clinical significance of co-expression of VEGF-C and VEGFR-3 in non-small cell lung cancer. Chin Med J (Engl) 2003;116:727–730. [PubMed] [Google Scholar]
- 10.Onogawa S, Kitadai Y, Tanaka S, Kuwai T, Kimura S, Chayama K. Expression of VEGF-C and VEGF-D at the invasive edge correlates with lymph node metastasis and prognosis of patients with colorectal carcinoma. Cancer Sci. 2004;95:32–39. doi: 10.1111/j.1349-7006.2004.tb03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia YT, Li ZX, He YT, Liang W, Yang HC, Ma HJ. Expression of vascular endothelial growth factor-C and the relationship between lymphangiogenesis and lymphatic metastasis in colorectal cancer. World J Gastroenterol. 2004;10:3261–3263. doi: 10.3748/wjg.v10.i22.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki K, Morita T, Tokue A. Vascular endothelial growth factor-C (VEGF-C) expression predicts lymph node metastasis of transitional cell carcinoma of the bladder. Int J Urol. 2005;12:152–158. doi: 10.1111/j.1442-2042.2005.01010.x. [DOI] [PubMed] [Google Scholar]
- 13.Kitadai Y, Amioka T, Haruma K, Tanaka S, Yoshihara M, Sumii K, Matsutani N, Yasui W, Chayama K. Clinicopathological significance of vascular endothelial growth factor (VEGF)-C in human esophageal squamous cell carcinomas. Int J Cancer. 2001;93:662–666. doi: 10.1002/ijc.1379. [DOI] [PubMed] [Google Scholar]
- 14.Kimura Y, Watanabe M, Ohga T, Saeki H, Kakeji Y, Baba H, Maehara Y. Vascular endothelial growth factor C expression correlates with lymphatic involvement and poor prognosis in patients with esophageal squamous cell carcinoma. Oncol Rep. 2003;10:1747–1751. doi: 10.3892/or.10.6.1747. [DOI] [PubMed] [Google Scholar]
- 15.Noguchi T, Takeno S, Shibata T, Uchida Y, Yokoyama S, Müller W. VEGF-C expression correlates with histological differentiation and metastasis in squamous cell carcinoma of the esophagus. Oncol Rep. 2002;9:995–999. [PubMed] [Google Scholar]
- 16.Wauke K, Nagashima M, Ishiwata T, Asano G, Yoshino S. Expression and localization of vascular endothelial growth factor-C in rheumatoid arthritis synovial tissue. J Rheumatol. 2002;29:34–38. [PubMed] [Google Scholar]
- 17.Hironaka S, Hasebe T, Kamijo T, Ohtsu A, Boku N, Yoshida S, Saitoh H, Ochiai A. Biopsy specimen microvessel density is a useful prognostic marker in patients with T(2-4)M(0) esophageal cancer treated with chemoradiotherapy. Clin Cancer Res. 2002;8:124–130. [PubMed] [Google Scholar]
- 18.Akagi K, Ikeda Y, Miyazaki M, Abe T, Kinoshita J, Maehara Y, Sugimachi K. Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br J Cancer. 2000;83:887–891. doi: 10.1054/bjoc.2000.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du JR, Jiang Y, Zhang YM, Fu H. Vascular endothelial growth factor and microvascular density in esophageal and gastric carcinomas. World J Gastroenterol. 2003;9:1604–1606. doi: 10.3748/wjg.v9.i7.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakashima T, Kondoh S, Kitoh H, Ozawa H, Okita S, Harada T, Shiraishi K, Ryozawa S, Okita K. Vascular endothelial growth factor-C expression in human gallbladder cancer and its relationship to lymph node metastasis. Int J Mol Med. 2003;11:33–39. [PubMed] [Google Scholar]
- 21.Valtola R, Salven P, Heikkila P, Taipale J, Joensuu H, Rehn M, Pihlajaniemi T, Weich H, deWaal R, Alitalo K. VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am J Pathol. 1999;154:1381–1390. doi: 10.1016/S0002-9440(10)65392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furudoi A, Tanaka S, Haruma K, Kitadai Y, Yoshihara M, Chayama K, Shimamoto F. Clinical significance of vascular endothelial growth factor C expression and angiogenesis at the deepest invasive site of advanced colorectal carcinoma. Oncology. 2002;62:157–166. doi: 10.1159/000048262. [DOI] [PubMed] [Google Scholar]
- 23.Kaio E, Tanaka S, Kitadai Y, Sumii M, Yoshihara M, Haruma K, Chayama K. Clinical significance of angiogenic factor expression at the deepest invasive site of advanced colorectal carcinoma. Oncology. 2003;64:61–73. doi: 10.1159/000066511. [DOI] [PubMed] [Google Scholar]
- 24.Ohta Y, Shridhar V, Bright RK, Kalemkerian GP, Du W, Carbone M, Watanabe Y, Pass HI. VEGF and VEGF type C play an important role in angiogenesis and lymphangiogenesis in human malignant mesothelioma tumours. Br J Cancer. 1999;81:54–61. doi: 10.1038/sj.bjc.6690650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 26.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarijs R, Schalkwijk L, Hofmann UB, Ruiter DJ, de Waal RM. Induction of vascular endothelial growth factor receptor-3 expression on tumor microvasculature as a new progression marker in human cutaneous melanoma. Cancer Res. 2002;62:7059–7065. [PubMed] [Google Scholar]
- 28.Neuchrist C, Erovic BM, Handisurya A, Fischer MB, Steiner GE, Hollemann D, Gedlicka C, Saaristo A, Burian M. Vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 expression in squamous cell carcinomas of the head and neck. Head Neck. 2003;25:464–474. doi: 10.1002/hed.10235. [DOI] [PubMed] [Google Scholar]
- 29.Witmer AN, van Blijswijk BC, Dai J, Hofman P, Partanen TA, Vrensen GF, Schlingemann RO. VEGFR-3 in adult angiogenesis. J Pathol. 2001;195:490–497. doi: 10.1002/path.969. [DOI] [PubMed] [Google Scholar]


