Abstract
Ophthalmological complications with interferon therapy are usually mild and reversible, not requiring the withdrawal of the treatment. We report a case of a patient who had visual loss probably associated with interferon therapy. Chronic hepatitis C virus infection (genotype 1a) was diagnosed in a 33-year old asymptomatic man. His past medical history was unremarkable and previous routine ophthalmologic check-up was normal. Pegylated interferon alpha and ribavirin were started. Three weeks later he reported painless reduction of vision. Ophthalmologic examination showed extensive intraretinal hemorrhages and cotton-wool spots, associated with inferior branch retinal vein thrombosis. Antiviral therapy was immediately discontinued, but one year later he persists with severely decreased visual acuity. This case illustrates the possibility of unpredictable and severe complications during pegylated interferon therapy.
Keywords: Hepatitis C, Interferon, Visual loss
INTRODUCTION
Pegylated interferon has been shown to be associated with high rates of sustained virological responses and has become the standard therapy of chronic hepatitis C. However, interferon therapy may be associated with severe adverse effects. We report the case of a patient who developed retinal vein thrombosis and visual loss probably related to the antiviral therapy.
CASE REPORT
A 33-year old asymptomatic Caucasian man was referred for investigation of abnormal liver enzymes. Chronic hepatitis C virus infection, genotype 1a, with viral load 220 400 IU/mL, was diagnosed, and a liver biopsy showed chronic hepatitis, with inflammatory activity A2 and fibrosis F2 according to the METAVIR system. Past medical history was unremarkable and negative for diabetes mellitus, arterial hypertension, dislipidemia, obesity, regular use of medications, smoking and illicit drug or alcohol abuse. Previous routine ophthalmologic check-up was normal, with a visual acuity 20/20. Pegylated interferon α 2b was started at the dose of 1.5 μg/kg once weekly in combination with ribavirin 1000 mg/d. Three weeks later he reported painless blurring of vision. The ophthalmologic examination confirmed the reduction of the visual acuity and showed extensive intraretinal hemorrhages and cotton-wool spots, associated with inferior branch retinal vein thrombosis. Fluorescein angiography confirmed the venous occlusion (Figure 1). The antiviral therapy was immediately discontinued. Hematocrit, platelet count, glucose, prothrombin activity, creatinine, cholesterol and triglycerides levels were normal. Activated protein C resistance phenotype was normal. The levels of protein S, antithrombin III, homocysteine and fibrinogen were within the normal ranges. Plasma protein C was 50% (normal 58%-125%), compatible with heterozygous state. Cryoglobulins and antiphospholipids antibodies were negative. Factor V Q 506 and prothrombin gene 3’-UTR G20210A mutant alleles were absent. Doppler ultrasound of the heart and carotid arteries was normal. The patient developed macular edema which required panretinal photocoagulation. One year later, he persists with severely decreased visual acuity.
Figure 1.
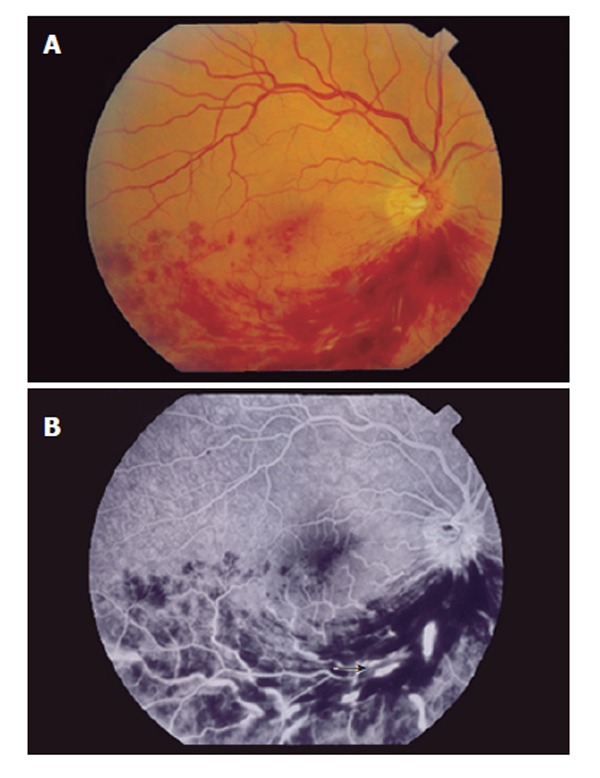
A: Fundus photograph of the right eye with extensive intraretinal hemorrhages and cotton-wool spots in the inferonasal and inferotemporal regions; B: Fluorescein angiography with segmental hypoperfusion, dilation and tortuosity of the retinal veins (arrow), compatible with inferior branch retinal vein thrombosis.
DISCUSSION
Ophthalmologic complications with interferon alpha therapy, such as retinopathy with cotton-wool spots, hemorrhages and macular edema, optic neuropathy and thrombotic microangiopathy, occur in less than 1% of treated patients. Individuals with diabetes, hypertension, dislipidemia and hypercoagulable states are more prone to develop those changes. In most cases they are subclinical, mild and reversible, not requiring the withdrawal of the treatment.
The physiopathology of retinal vein thrombosis in patients with viral hepatitis is poorly understood, but may be related to both background predisposition and side effects of therapy. Protein C deficiency occurs in 1 of 250 controls and leads to impaired inhibition of clot formation, which is associated with an increased risk for venous thrombosis (relative risk, 7.3; annual incidence, 1%)[1]. However, the frequency of venous thrombosis in protein C deficiency state is highly variable. Only a minority of affected individuals develop thrombosis, suggesting that the presence of another simultaneous risk factor is needed.
Both chronic hepatitis C and interferon therapy are associated with the formation of procoagulant antibodies, in particular antiphospholipids antibodies, which may predispose to thrombosis. In addition, interferon therapy induces the increase of plasma-activated complement C5a, a potent intravascular aggregator of granulocytes, favoring the development of microthrombi in small vessels[2,3].
The patient developed a severe, early and sight losing complication of antiviral therapy. Most reported cases occurred in predisposed individuals 2 to 11 months after the beginning of standard alpha interferon therapy. However, one could speculate that interferon pegylation could modify both the pharmacokinetics of the drug and the timing of retinal vein thrombosis.
Since there are few reported cases of retinal vein thrombosis[3-5], this case may represent a coincidence, not associated with interferon therapy. Nevertheless, it is noteworthy that the patient was asymptomatic and had a normal vision before antiviral therapy. Although current guidelines of therapy of chronic viral hepatitis do not support routine ophthalmologic screening and work-up for hypercoagulable states in all patients treated with interferon, this case illustrates the possibility of unpredictable and severe complications during pegylated interferon therapy.
Footnotes
S- Editor Wang J L-Editor Zhu LH E- Editor Ma N
References
- 1.Thomas RH. Hypercoagulability syndromes. Arch Intern Med. 2001;161:2433–2439. doi: 10.1001/archinte.161.20.2433. [DOI] [PubMed] [Google Scholar]
- 2.Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:S237–S244. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- 3.Nadir A, Amin A, Chalisa N, van Thiel DH. Retinal vein thrombosis associated with chronic hepatitis C: a case series and review of the literature. J Viral Hepat. 2000;7:466–470. doi: 10.1046/j.1365-2893.2000.00245.x. [DOI] [PubMed] [Google Scholar]
- 4.Sugano S, Suzuki T, Watanabe M, Ohe K, Ishii K, Okajima T. Retinal complications and plasma C5a levels during interferon alpha therapy for chronic hepatitis C. Am J Gastroenterol. 1998;93:2441–2444. doi: 10.1111/j.1572-0241.1998.00701.x. [DOI] [PubMed] [Google Scholar]
- 5.Rubio JE Jr, Charles S. Interferon-associated combined branch retinal artery and central retinal vein obstruction. Retina. 2003;23:546–548. doi: 10.1097/00006982-200308000-00019. [DOI] [PubMed] [Google Scholar]


