Abstract
Animal models have allowed detailed study of hemodynamic alterations typical of portal hypertension and the molecular mechanisms involved in abnormalities in splanchnic and systemic circulation associated with this syndrome. Models of prehepatic portal hypertension can be used to study alterations in the splanchnic circulation and the pathophysiology of the hyperdynamic circulation. Models of cirrhosis allow study of the alterations in intrahepatic microcirculation that lead to increased resistance to portal flow. This review summarizes the currently available literature on animal models of portal hypertension and analyzes their relative utility. The criteria for choosing a particular model, depending on the specific objectives of the study, are also discussed.
Keywords: Cirrhosis, Nitric oxide, Portal vein
INTRODUCTION
As for all pathologic conditions, the use of animal models is of enormous importance for the study of pathophysiological disturbances of portal hypertension, since they allow comprehensive study of questions that cannot be addressed in human studies. The historical evolution of the knowledge of portal hypertension clearly illustrates this. The concept that portal hypertension is not only the consequence of an increased resistance to portal blood flow, but also of an increase in portal inflow could not be definitely demonstrated until the development of an adequate methodology to conduct detailed hemodynamic studies in experimental animals[1,2]. Later, the isolation and ex vivo study of the two vascular beds implicated in the syndrome, the mesenteric bed and the liver vasculature, allowed the physiological characterization of the vasoactive mediators involved in mesenteric vasodilation and in the increased vascular tone of the cirrhotic liver. The next step was the introduction of introduction of molecular biology tools to molecular biology to identify the alterations in the signaling pathways responsible for the dysregulation of these mediators[3]. This allowed the development and validation of therapeutic targets that have been ultimately tested in patients with cirrhosis and portal hypertension.
This review will cover the most commonly used animal models of portal hypertension and their relative utility for the study of different aspects of this syndrome. In the selection of an animal model for the study of portal hypertension some general concepts, which apply to every animal model, must be considered[4] (Table 1). The final choice will largely depend on the specific alteration of the pathophysiology of portal hypertension to be studied, because not all models express all disturbances characteristic of the portal hypertension syndrome. The first step is species choice. Rat and rabbit are the species used most often. More recently, the methodology for hemodynamic studies in rat and rabbit have been implemented to mice[5-7]. This advance has enormously widened the research possibilities due to the availability of knock out and transgenic mice. The use of animals like dogs or pigs[8] offers an advantage for the instrumentation and dissection of vascular structures due to their size, but this practice has been abandoned due to high cost. This review will focus on commonly used models at present.
Table 1.
General considerations in choosing animal models (modified from Mullen & McCullough[4])
| Reproducibility: % of animals reaching the desired state. Consistent time frame to attain desired state. |
| Specificity: The model should have the desired abnormality without other complicating problems. |
| Costs: Consider not only the direct costs, but also indirect costs such as animal housing (and, therefore, the time to achieve the desired state). An expensive but reliable model could be cheaper than a cheap but inconsistent model. |
| Safety: Animal and induction method should not be a risk for the personal. |
| Size: Blood volume sample requirements or need for vascular access may determine the size of the animal. The size also determines drug spending. |
| Ethics: Different ethics committees can have different opinions about the acceptability of one model. |
| Feasibility: Whether the laboratory has the expertise, manpower facilities, etc, to generate or handle the model. |
MODELS OF PORTAL HYPERTENSION
The portal pressure gradient is the result of the interaction between portal blood flow and the vascular resistance that opposes that flow. This relationship is defined by Ohm’s law in the equation: ∆P = Q × R where ∆P is the portal pressure gradient (the difference between portal pressure and inferior vena cava pressure), Q is blood flow within the entire portal venous system (which in portal hypertension includes also the portal-systemic collaterals), and R is the vascular resistance of the entire portal venous system. It follows that portal pressure may increase because of an increase in portal blood flow, an increase in vascular resistance, or by a combination of both[3]. It is well established that the primary factor leading to portal hypertension is an increased resistance to portal blood flow[9-11]. This increased resistance can be prehepatic (portal vein thrombosis), intrahepatic (liver cirrhosis) or posthepatic (Budd-Chiari syndrome). Independently of the cause, portal hypertension is associated with severe disturbances in the systemic and splanchnic circulation, characterized by vasodilation, hypotension, activation of vasoactive systems, plasma volume expansion and increased cardiac output[12]. This is known as the hyperdynamic circulatory syndrome, and it leads to an increase in portal blood inflow that contributes to maintain or worsen portal hypertension despite the development of portal-systemic collaterals (Figure 1). This means that for the study of hyperdynamic circulation both models of pre-hepatic and intra-hepatic portal hypertension are useful. For the study of the intrahepatic circulation specific models of disease are needed.
Figure 1.
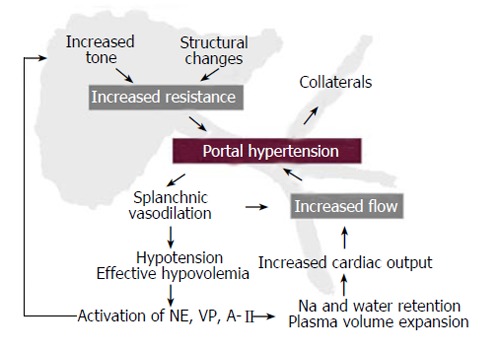
Summary of the pathophysiology of portal hypertension. The increase in hepatic resistance leads to an increase in portal pressure. This leads to a cascade of disturbances in the splanchnic and systemic circulation characterized by vasodilation, sodium and water retention and plasma volume expansion, that are major players in the pathogenesis of ascites and hepato-renal syndrome. Additionally, these alterations lead to an increase in portal blood inflow that contributes to maintain and aggravates portal hypertension. Another characteristic feature is the development of porto-systemic collaterals that are resposible for complications such as variceal bleeding and hepatic encephalopathy.
Models of pre-hepatic portal hypertension
Partial portal vein ligation: Partial portal vein ligation model (PVL) has been widely used in the study of the pathophysiology of portal hypertension. This model has been developed in rats[1,13,14], mice[6,7] and rabbits[15]. The portal vein is freed from surrounding tissue after a midline abdominal incision. A ligature (silk 3-0) is placed around a blunt-tipped needle lying along the portal vein. Subsequent removal of the needle yields a calibrated stenosis of the portal vein that has the diameter of the needle. In the conventional rat PVL model a 20G needle is used (0.889 mm diameter)[1,13,14]. By using needles of greater caliber, less severe stenosis and thus less severe degrees of portal hypertension are induced[16,17]. The diameters of the needles and resulting levels of stenosis are as follows: 16G: 1.651mm, 18G: 1.270 mm, 20G: 0.889 mm. The conventional needles for mice and rabbits are 27G[6,7] and 18G[15], respectively. Surgery must be conducted in aseptic conditions. Numerous websites provide information on surgical techniques and pre- and post-operative care of the animals, including information on anesthesia, analgesia and antibiotic prophylaxis (http://info.med.yale.edu/yarc/vcs/). For portal vein ligation no antibiotic prophylaxis is needed.
PVL model has been extensively used because the procedures are easy to perform, inexpensive, reproducible and portal hypertension develops very fast. One week after portal vein ligation rats develop the complete portal hypertensive syndrome, with hyperdynamic circulation and portal-systemic shunting. Portal-systemic shunting is already detectable at two days. The percentage of portal-systemic shunting; i.e. the amount of portal blood inflow diverted to collaterals, approaches 100% after the 7th day[10]. Mesenteric vasodilation and increased cardiac output are detectable at 4 d[10,13]. The main drawback of the model is that portal hypertension developes acutely. Thus, contrary to the majority of situations found in clinical practice, the degree of portal hypertension is maximal at 24 h, and decreases afterwards due to the development of portal-systemic collaterals[10].
Models of intrahepatic portal hypertension
Intrahepatic portal hypertension can be classified as presinusoidal, sinusoidal and postsinusoidal. Models of cirrhosis, the most common cause of portal hypertension in western countries, have a double component, which are the pre- and post-sinusoidal components, but for practical purposes they will be discussed with the models of sinusoidal portal hypertension.
Presinusoidal intrahepatic portal hypertension: (1)Schistosomiasis Experimental infection with Schistosoma mansoni has been characterized in mice and hamsters. This model is achieved by injecting cercariae of the parasite in the abdominal wall. Portal hypertension develops 5 to 7 wk after inoculation[18,19]. An important feature of this model is that portal hypertension develops progressively. In the hamster Schistosoma infection does not induce the development of portal-systemic shunting despite the presence of portal hypertension[20]. On the contrary, mice infected with Schistosoma mansoni develop portal hypertension with portal-systemic shunting. Shunting is detectable from wk 9 and reaches 15% at wk 11[18,19]. Currently this model is seldom used, and no studies have been published in the last decade.
Sinusoidal portal hypertension: A number of models of cirrhosis have been described. We will limit our discussion to those that have been used for the study of portal hypertension.
(1) Common bile duct ligation (CBDL) CBDL is a model of secondary biliary cirrhosis. It has been mainly developed in rats[21], which are especially appropriate due to the lack of a gallbladder, but it has also been developed in rabbits[22] and mice[23]. Mice, however, develop a marked dilation of the gallbladder after bile duct ligation, which may lead to perforation and choleperitoneum. The intervention consists of the isolation of the common bile duct followed by a double ligature. The first ligature is made below the junction of the hepatic ducts. The second is made above the entrance of the pancreatic ducts. The portion of the bile duct between the two ligatures is resected to avoid repermeabilization. Mortality is high after the 5th wk (20%). The use of prophylactic antibiotics (Ampicilin 100 mg/Kg s.c. or similars) before surgery and weekly administration of vit K (50 mcg s.c.)[24] notably improve the survival of CBDL rats. One of the drawbacks of this model is the potential formation of a biliary cyst, which may compress the portal vein at the hilum. This problem can be solved by gently injecting 10% formalin (120 μL/100 g) through a P10 catheter in the bile duct before ligation[14,25]. Other authors have prevented cyst formation by injecting Ethibloc®[26], a substance developed for vessel embolization, or by ligating the biliary duct of each lobule[27,28].
This model develops biliary fibrosis-cirrhosis in 4-6 wk. Histology shows marked cholangiolar proliferation and expansive portal fibrosis (Figure 2A), but the architectural disturbances typical of cirrhosis are seldom found[29]. At 2 wk rats develop mild portal hypertension[30] and at 4 wk severe portal hypertension, hyperdynamic circulation and portal-systemic shunting of 30%-60%[21,31,32]. Approximately, 60% of the rats develop ascites. Portal hypertension in this model has a presinusoidal component[30]. A major drawback of this model is that it is not adequate for pharmacological studies with drugs that are eliminated through the biliary route.
Figure 2.
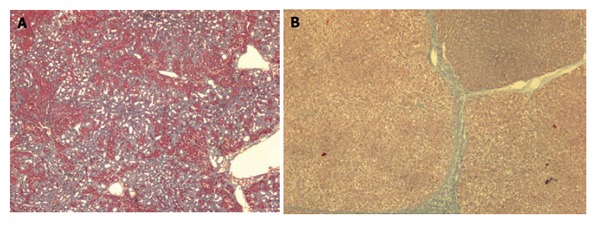
A comparison of microscopic aspect of a liver 6 wk after CBDL (A) and 12 wk after TAA (B) administration.
(2) Carbon tetrachloride induced cirrhosis (CCl4) Acute administration of carbon tetrachloride induces acute hepatitis of primary perivenular localization. Continuous administration induces chronic liver injury that leads to cirrhosis. This methodology to induce cirrhosis has been used in rats[33,34], mice[35] and rabbits[22]. Route of administration varies among laboratories, but the most effective are oral[36,37], intraperitoneal[38,39] or inhalatory[34,40,41]. Subcutaneous route is not recommended due to its low yield of cirrhosis. The use of different administration schedules, even using the same route of administration, could explain the variability in the yield and time to cirrhosis in different laboratories. In our unit, Phenobarbital (0.3 g/L) is added to drinking water to increase the yield of cirrhosis, starting one week before first CCl4 administration. Hemodynamic studies are performed 5-7 d after stopping CCl4 and Phenobarbital.
Twelve to 15 wk after CCl4 administration the rats develop micronodular cirrhosis (Figure 3A), portal hypertension, portal-systemic shunting (30%-60%) and hyperdynamic circulation[2]. If maintained for 12 to 20 wk, most rats develop ascites. A major complexity of this model is the different sensitivity of the rats to CCl4, which makes it difficult to obtain a homogeneous group of cirrhotic rats. Proctor et al[36] proposed a solution that consists of the individualization of the dose according to weight gain/loss of the animal in response to the previous dose.
Figure 3.
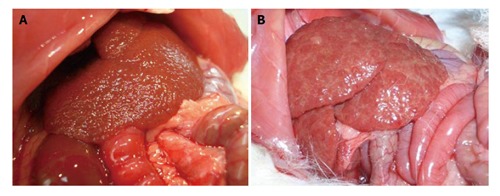
Macroscopical images of livers from CCl4 (A) and TAA (B) models.
(3) Cirrhosis induced by thioacetamide (TAA) This is another widely used model of toxic cirrhosis. The toxin affects both perivenular and periportal areas. It has been used in rats[42] and mice[43]. TAA can be administered in drinking water[42] or by i.p. injection[44,45]. I.p. injection offers much more consistent results[45].
This model develops macronodular cirrhosis with portal hypertension in 12 wk (Figures 2B and 3B)[42,45,46]. Longer periods of induction might be required for the instauration of overt hyperdynamic circulation[46]. Approximately 40% develop ascites[42]. One particular feature of this model is that, contrary to what occurs with the CCl4 model, fibrosis remains stable for weeks after TAA withdrawal[45]. After 18 wk of TAA administration the rats might develop cholangiocarcinoma[47].
(4) Dimethylnitrosamine induced cirrhosis (DMNA) DMNA is another hepatotoxin that induces hepatocellular necrosis. After continuous administration (generally i.p.) the rats develop fibrosis with portal hypertension, already present at 5 wk, but at this time the animals do not have cirrhosis nor features of hyperdynamic circulation[48]. Overt cirrhosis with ascites develops in 13 wk[49,50]. This model has been seldom used for the study of the pathophysiology of portal hypertension, probably due to restrictions in the use of DMNA due to its high carcinogenetic potential.
(5) Diet induced cirrhosis A diet deficient in choline and methionin, or a diet with low protein and choline and enriched with fat, induces liver steatosis associated with marked oxidative stress that induces inflammation and fibrosis[49,51]. Cirrhosis is developed after 12-24 wk. These models have not been well-characterized from the hemodynamic point of view and have not been used for the study of portal hypertension.
Postsinusoidal portal hypertension: Recently, a model that reproduces the pathological and clinical characteristics of veno-occlusive disease has been developed[52]. This is achieved by the administration of monocrotalin by oral gavage. Rats develop hyperbilirrubinemia, hepatomegaly and ascites at 4-5 d. This model is useful for the study of the pathophysiology of veno-occlusive disease, but has not been characterized from the hemodynamic point of view.
Posthepatic portal hypertension
The aim of these models is to reproduce the features of the Budd-Chiari syndrome; i.e. liver injury derived from hepatic venous outflow obstruction. This has been achieved by placing an ameroid, which is a stainless steel device that allows slow expansion inside upon contact with the wet tissue, in the hepatic veins inducing a progressive occlusion of hepatic venous outflow. This model has been developed in dogs[53]. However, in the rat it is almost impossible to dissect the hepatic veins, so hepatic venous outflow occlusion has been induced by occluding the inferior vena cava cranially to the hepatic veins[54]. This is not a pure model of Budd-Chiari. These models have been very seldom used, and therefore it is uncertain whether they have any utility in the study of the hemodynamics of Budd-Chiari syndrome.
SELECTION OF A MODEL FOR THE STUDY OF THE PATHOPHYSIOLOGY OF PORTAL HYPERTENSION AND ITS COMPLICATIONS (TABLE 2)
Abnormalities in the intrahepatic circulation in cirrhosis
The most frequent cause of portal hypertension in western countries is liver cirrhosis. The primary factor leading to portal hypertension is an increased resistance to portal blood flow. This is not only the result of the disruption of the liver architecture, but is also due to an increased hepatic vascular tone. This concept has been demonstrated in isolated liver perfusion[55], which allows evaluation of the hepatic vascular tone and its response to vasoconstrictors and vasodilators. This is problematic in in vivo studies because it is very difficult to discern the effects of a particular vasoactive substance that depend on changes in systemic, splanchnic and collateral circulation from those derived from changes on hepatic resistance. Another way of evaluating intrahepatic microcirculation is by intravital microscopy[56].
The model most frequently used for isolated perfusion has been CCl4 induced cirrhosis. In these livers, it has been demonstrated that there is an increased hepatic vascular tone, hyperresponse to vasoconstrictors and hyporresponse to vasodilators. The main mechanism mediating these abnormal vascular responses is endothelial dysfunction with insufficient NO production and an increased production of vasoconstrictive eicosanoids[57,58]. Vascular responses have also been studied in other models of cirrhosis, such as CBDL[59,60] and TAA[46,61], but data are still scarce and less consistent than that obtained with the CCl4 model. Moreover, it must be stressed that the CBDL model has an important presinusoidal component in the increase in hepatic resistance[30].
Abnormalities in the systemic, mesenteric and collateral circulation in portal hypertension
These studies include in vivo hemodynamic studies and ex vivo perfusion of the mesenteric vascular bed and the portal-systemic collaterals.
The in vivo studies are very useful for the study of the pathophysiology of the hyperdynamic circulation associated with portal hypertension. The hyperdynamic circulation has been described in the PVL model[1,13] and in the models of cirrhosis induced by CCl4[2], CBDL[21] and TAA[46,62]. The in vivo studies, on the other hand, globally evaluate the effects of a drug on portal pressure, which is the result of the integrated effects of the drug on portal blood inflow, collateral resistance and hepatic resistance.
Any model of portal hypertension is valid, in theory, for the study of mesenteric circulation. This has been evaluated by the isolated perfusion of the mesenteric vascular bed (McGregor’s preparation[63]), by the study of vascular responses in isolated mesenteric vessels[64] or by the perfusion of intestinal microvasculature[65]. The characteristic hyporesponse to vasoconstrictors of the mesenteric circulation has only been demonstrated so far in the PVL and the CCl4 models[40,64-66].
Collateral circulation has been studied with different methodologies. The most common has been the evaluation of portal-systemic shunting by the injection of radioactive, colored or fluorescent microspheres[67,68]. If different isotopes or colored or fluorescent markers are used, changes in portal-systemic shunting after different interventions can be evaluated in the same animal[9]. Another way of studying the collateral circulation is the measurement, with transit-time flow probes, of the blood flow of the spontaneous splenorenal shunt, a major collateral developed after portal hypertension[48]. Mosca et al developed a system for ex vivo perfusion of collaterals[69], in which the vascular responses of the collateral vascular bed to different vasoactive substances can be tested. The interpretation of the results obtained with this methodology might differ among models, because in the PVL model shunting is about 100%, while in the cirrhosis models shunting does not go beyond 60%.
The availability of the PVL model has shown great advantage for the study of the abnormalities of systemic, mesenteric and collateral circulation, because it is a very rapid model and much less expensive than models of cirrhosis. In the last 20 years, most investigators have chosen to generate and test hypothesis first in the PVL model, and subsequently confirm those hypothesis in the more laborious and expensive models of cirrhosis.
Ascites and renal dysfunction
The vast majority of studies in this field have been performed using the CCl4 cirrhosis model[70-72]. CCl4 administration is maintained until the animal develops ascites at physical examination, which occurs between 12-20 wk. The PVL model, akin to what happens in patients with prehepatic portal hypertension, does not develop ascites. Even though, since this model is ideal for sequential studies, it has been instrumental in the description of hyperdynamic circulation[13] and in the validation of the peripheral arterial vasodilation hypothesis as the trigger for sodium and water retention in cirrhosis[13,73-75]. CBDL and CCl4 models have been also used for longitudinal studies aimed at demonstrating the sequence vasodilation-sodium retention-ascites[76-78], but the temporal evolution of these models (especially for CCl4) is less consistent.
Portal hypertensive gastropathy
Several studies have demonstrated that PVL and cirrhotic rats show abnormalities of the gastric microvasculature comparable to those observed in portal hypertensive gastropathy in humans. Studies in these models have been useful to test therapeutic interventions that subsequently showed some efficacy in patients bleeding from portal hypertensive gastropathy[79,80].
Hepatopulmonary syndrome
CBDL rats develop alterations analogous to those of hepatopulmonary syndrome seen in humans, such as intrapulmonary vascular dilatations and an increased alveolar to arterial oxygen gradient[81]. These alterations are obvious from the 2nd wk after bile duct ligation[44]. Other models of portal hypertension, such as the PVL and the TAA, do not develop hepatopulmonary syndrome[44,81].
Models of portal hypertension-related bleeding
Our laboratory has recently described a portal hyper-tension-related bleeding model that has been useful in evaluating therapeutic interventions in acute variceal bleeding, such as for determining the best policy for volume replacement and the effects of vasoactive drugs on the outcome of bleeding[14,25,82]. This model consists of the isolation and section of a branch of the ileocolic vein (Figure 4). The severity of hemorrhage depends on the degree of portal hypertension and the size of the sectioned branch[14]. This model has been characterized in PVL and CBDL rats[14,25,82]. A section of the first order branch of the ileocolic vein results in 50% mortality in CBDL rats, whereas mortality is 0% in PVL rats. Subsequently, a modification of the model was developed, in which 2 successive sections of a first order branch of ileocolic vein are performed in PVL rats. In this way the second bleeding is induced when the rat is already hypovolemic. This modification increases mortality to 50%, and allows testing of the vasoactive drugs in hypovolemic conditions, a situation that better reproduces the clinical context in which these drugs are applied[82].
Figure 4.
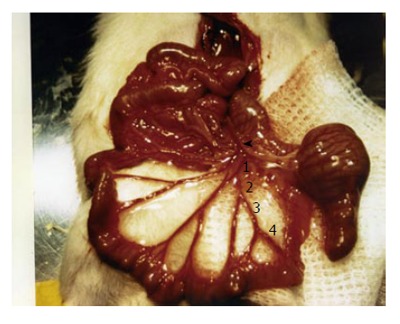
Model of portal hypertension-related bleeding. In these model a section of a first (1), second (2), third (3) or fourth order branch of the ileocolic vein is isolated and sectioned[14,25,82].
Footnotes
Supported by Fondo de Investigación Sanitaria (CM04/00031 and PI050519), Ministerio de Educación y Ciencia, No.SAF 04/04783; Instituto de Salud Carlos III, No. C03/02
S- Editor Liu Y L- Editor Luzte M E- Editor Bi L
References
- 1.Vorobioff J, Bredfeldt JE, Groszmann RJ. Hyperdynamic circulation in portal-hypertensive rat model: a primary factor for maintenance of chronic portal hypertension. Am J Physiol. 1983;244:G52–G57. doi: 10.1152/ajpgi.1983.244.1.G52. [DOI] [PubMed] [Google Scholar]
- 2.Vorobioff J, Bredfeldt JE, Groszmann RJ. Increased blood flow through the portal system in cirrhotic rats. Gastroenterology. 1984;87:1120–1126. [PubMed] [Google Scholar]
- 3.Groszmann RJ, Abraldes JG. Portal hypertension: from bedside to bench. J Clin Gastroenterol. 2005;39:S125–S130. doi: 10.1097/01.mcg.0000155552.14396.3d. [DOI] [PubMed] [Google Scholar]
- 4.Mullen KD, McCullough AJ. Problems with animal models of chronic liver disease: suggestions for improvement in standardization. Hepatology. 1989;9:500–503. doi: 10.1002/hep.1840090326. [DOI] [PubMed] [Google Scholar]
- 5.Sarin SK, Sabba C, Groszmann RJ. Splanchnic and systemic hemodynamics in mice using a radioactive microsphere technique. Am J Physiol. 1990;258:G365–G369. doi: 10.1152/ajpgi.1990.258.3.G365. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez M, Vizzutti F, Garcia-Pagan JC, Rodes J, Bosch J. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology. 2004;126:886–894. doi: 10.1053/j.gastro.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Iwakiri Y, Cadelina G, Sessa WC, Groszmann RJ. Mice with targeted deletion of eNOS develop hyperdynamic circulation associated with portal hypertension. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1074–G1081. doi: 10.1152/ajpgi.00145.2002. [DOI] [PubMed] [Google Scholar]
- 8.Bosch J, Enriquez R, Groszmann RJ, Storer EH. Chronic bile duct ligation in the dog: hemodynamic characterization of a portal hypertensive model. Hepatology. 1983;3:1002–1007. doi: 10.1002/hep.1840030618. [DOI] [PubMed] [Google Scholar]
- 9.Kroeger RJ, Groszmann RJ. Increased portal venous resistance hinders portal pressure reduction during the administration of beta-adrenergic blocking agents in a portal hypertensive model. Hepatology. 1985;5:97–101. doi: 10.1002/hep.1840050120. [DOI] [PubMed] [Google Scholar]
- 10.Sikuler E, Kravetz D, Groszmann RJ. Evolution of portal hypertension and mechanisms involved in its maintenance in a rat model. Am J Physiol. 1985;248:G618–G625. doi: 10.1152/ajpgi.1985.248.6.G618. [DOI] [PubMed] [Google Scholar]
- 11.Witte CL, Tobin GR, Clark DS, Witte MH. Relationship of splanchnic blood flow and portal venous resistance to elevated portal pressure in the dog. Gut. 1976;17:122–126. doi: 10.1136/gut.17.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groszmann RJ. Hyperdynamic circulation of liver disease 40 years later: pathophysiology and clinical consequences. Hepatology. 1994;20:1359–1363. [PubMed] [Google Scholar]
- 13.Colombato LA, Albillos A, Groszmann RJ. Temporal relationship of peripheral vasodilatation, plasma volume expansion and the hyperdynamic circulatory state in portal-hypertensive rats. Hepatology. 1992;15:323–328. doi: 10.1002/hep.1840150224. [DOI] [PubMed] [Google Scholar]
- 14.Castañeda B, Debernardi-Venon W, Bandi JC, Andreu V, Pérez-del-Pulgar S, Moitinho E, Pizcueta P, Bosch J. The role of portal pressure in the severity of bleeding in portal hypertensive rats. Hepatology. 2000;31:581–586. doi: 10.1002/hep.510310306. [DOI] [PubMed] [Google Scholar]
- 15.Cahill PA, Foster C, Redmond EM, Gingalewski C, Wu Y, Sitzmann JV. Enhanced nitric oxide synthase activity in portal hypertensive rabbits. Hepatology. 1995;22:598–606. [PubMed] [Google Scholar]
- 16.Lozeva V, Montgomery JA, Tuomisto L, Rocheleau B, Pannunzio M, Huet PM, Butterworth RF. Increased brain serotonin turnover correlates with the degree of shunting and hyperammonemia in rats following variable portal vein stenosis. J Hepatol. 2004;40:742–748. doi: 10.1016/j.jhep.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Abraldes JG, Iwakiri Y, Loureiro-Silva M, Haq O, Sessa WC, Groszmann RJ. Mild increases in portal pressure upregulate vascular endothelial growth factor and endothelial nitric oxide synthase in the intestinal microcirculatory bed, leading to a hyperdynamic state. Am J Physiol Gastrointest Liver Physiol. 2006;290:G980–G987. doi: 10.1152/ajpgi.00336.2005. [DOI] [PubMed] [Google Scholar]
- 18.Sarin SK, Groszmann RJ, Mosca PG, Rojkind M, Stadecker MJ, Bhatnagar R, Reuben A, Dayal Y. Propranolol ameliorates the development of portal-systemic shunting in a chronic murine schistosomiasis model of portal hypertension. J Clin Invest. 1991;87:1032–1036. doi: 10.1172/JCI115062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarin SK, Mosca P, Sabba C, Groszmann RJ. Hyperdynamic circulation in a chronic murine schistosomiasis model of portal hypertension. Hepatology. 1991;13:581–584. [PubMed] [Google Scholar]
- 20.Morgan JS, Groszmann RJ, Rojkind M, Enriquez R. Hemodynamic mechanisms of emerging portal hypertension caused by schistosomiasis in the hamster. Hepatology. 1990;11:98–104. doi: 10.1002/hep.1840110117. [DOI] [PubMed] [Google Scholar]
- 21.Lee SS, Girod C, Braillon A, Hadengue A, Lebrec D. Hemodynamic characterization of chronic bile duct-ligated rats: effect of pentobarbital sodium. Am J Physiol. 1986;251:G176–G180. doi: 10.1152/ajpgi.1986.251.2.G176. [DOI] [PubMed] [Google Scholar]
- 22.Burns RC, Wu Y, Sitzmann JV. Role of cirrhosis in the hemodynamic response to hemorrhage in portal hypertension. Surgery. 1995;117:488–493. doi: 10.1016/s0039-6060(05)80246-6. [DOI] [PubMed] [Google Scholar]
- 23.Biecker E, Neef M, Sägesser H, Shaw S, Koshy A, Reichen J. Nitric oxide synthase 1 is partly compensating for nitric oxide synthase 3 deficiency in nitric oxide synthase 3 knock-out mice and is elevated in murine and human cirrhosis. Liver Int. 2004;24:345–353. doi: 10.1111/j.1478-3231.2004.0933.x. [DOI] [PubMed] [Google Scholar]
- 24.Beck PL, Lee SS. Vitamin K1 improves survival in bile-duct-ligated rats with cirrhosis. J Hepatol. 1995;23:235. doi: 10.1016/0168-8278(95)80345-9. [DOI] [PubMed] [Google Scholar]
- 25.Castañeda B, Morales J, Lionetti R, Moitinho E, Andreu V, Pérez-Del-Pulgar S, Pizcueta P, Rodés J, Bosch J. Effects of blood volume restitution following a portal hypertensive-related bleeding in anesthetized cirrhotic rats. Hepatology. 2001;33:821–825. doi: 10.1053/jhep.2001.23437. [DOI] [PubMed] [Google Scholar]
- 26.Cho JJ, Hocher B, Herbst H, Jia JD, Ruehl M, Hahn EG, Riecken EO, Schuppan D. An oral endothelin-A receptor antagonist blocks collagen synthesis and deposition in advanced rat liver fibrosis. Gastroenterology. 2000;118:1169–1178. doi: 10.1016/s0016-5085(00)70370-2. [DOI] [PubMed] [Google Scholar]
- 27.Aller MA, Lorente L, Alonso S, Arias J. A model of cholestasis in the rat, using a microsurgical technique. Scand J Gastroenterol. 1993;28:10–14. doi: 10.3109/00365529309096038. [DOI] [PubMed] [Google Scholar]
- 28.Aller MA, Duran M, Ortega L, Arias JL, Nava MP, Prieto I, Arias J. Comparative study of macro- and microsurgical extrahepatic cholestasis in the rat. Microsurgery. 2004;24:442–447. doi: 10.1002/micr.10153. [DOI] [PubMed] [Google Scholar]
- 29.Kountouras J, Billing BH, Scheuer PJ. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br J Exp Pathol. 1984;65:305–311. [PMC free article] [PubMed] [Google Scholar]
- 30.Franco D, Gigou M, Szekely AM, Bismuth H. Portal hypertension after bile duct obstruction: effect of bile diversion on portal pressure in the rat. Arch Surg. 1979;114:1064–1067. doi: 10.1001/archsurg.1979.01370330086016. [DOI] [PubMed] [Google Scholar]
- 31.Heller J, Shiozawa T, Trebicka J, Hennenberg M, Schepke M, Neef M, Sauerbruch T. Acute haemodynamic effects of losartan in anaesthetized cirrhotic rats. Eur J Clin Invest. 2003;33:1006–1012. doi: 10.1046/j.1365-2362.2003.01251.x. [DOI] [PubMed] [Google Scholar]
- 32.Sikuler E, Buchs AE, Yaari A, Keynan A. Hemodynamic characterization of conscious and ketamine-anesthetized bile duct-ligated rats. Am J Physiol. 1991;260:G161–G166. doi: 10.1152/ajpgi.1991.260.1.G161. [DOI] [PubMed] [Google Scholar]
- 33.Jimenez W, Claria J, Arroyo V, Rodes J. Carbon tetrachloride induced cirrhosis in rats: a useful tool for investigating the pathogenesis of ascites in chronic liver disease. J Gastroenterol Hepatol. 1992;7:9097. doi: 10.1111/j.1440-1746.1992.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 34.Graupera M, Garcia-Pagan JC, Titos E, Claria J, Massaguer A, Bosch J, Rodes J. 5-lipoxygenase inhibition reduces intrahepatic vascular resistance of cirrhotic rat livers: a possible role of cysteinyl-leukotrienes. Gastroenterology. 2002;122:387–393. doi: 10.1053/gast.2002.31040. [DOI] [PubMed] [Google Scholar]
- 35.Kamada Y, Tamura S, Kiso S, Matsumoto H, Saji Y, Yoshida Y, Fukui K, Maeda N, Nishizawa H, Nagaretani H, et al. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology. 2003;125:1796–1807. doi: 10.1053/j.gastro.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 36.Proctor E, Chatamra K. High yield micronodular cirrhosis in the rat. Gastroenterology. 1982;83:1183–1190. [PubMed] [Google Scholar]
- 37.Kobayashi N, Ito M, Nakamura J, Cai J, Gao C, Hammel JM, Fox IJ. Hepatocyte transplantation in rats with decompensated cirrhosis. Hepatology. 2000;31:851–857. doi: 10.1053/he.2000.5636. [DOI] [PubMed] [Google Scholar]
- 38.Hernández-Muñoz R, Díaz-Muñoz M, Suárez-Cuenca JA, Trejo-Solís C, López V, Sánchez-Sevilla L, Yáñez L, De Sánchez VC. Adenosine reverses a preestablished CCl4-induced micronodular cirrhosis through enhancing collagenolytic activity and stimulating hepatocyte cell proliferation in rats. Hepatology. 2001;34:677–687. doi: 10.1053/jhep.2001.27949. [DOI] [PubMed] [Google Scholar]
- 39.Constandinou C, Henderson N, Iredale JP. Modeling liver fibrosis in rodents. Methods Mol Med. 2005;117:237–250. doi: 10.1385/1-59259-940-0:237. [DOI] [PubMed] [Google Scholar]
- 40.Sieber CC, Lopez-Talavera JC, Groszmann RJ. Role of nitric oxide in the in vitro splanchnic vascular hyporeactivity in ascitic cirrhotic rats. Gastroenterology. 1993;104:1750–1754. doi: 10.1016/0016-5085(93)90655-v. [DOI] [PubMed] [Google Scholar]
- 41.Loureiro-Silva MR, Cadelina GW, Groszmann RJ. Deficit in nitric oxide production in cirrhotic rat livers is located in the sinusoidal and postsinusoidal areas. Am J Physiol Gastrointest Liver Physiol. 2003;284:G567–G574. doi: 10.1152/ajpgi.00452.2002. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Benjamin IS, Alexander B. Reproducible production of thioacetamide-induced macronodular cirrhosis in the rat with no mortality. J Hepatol. 2002;36:488–493. doi: 10.1016/s0168-8278(02)00011-9. [DOI] [PubMed] [Google Scholar]
- 43.Okuyama H, Nakamura H, Shimahara Y, Uyama N, Kwon YW, Kawada N, Yamaoka Y, Yodoi J. Overexpression of thioredoxin prevents thioacetamide-induced hepatic fibrosis in mice. J Hepatol. 2005;42:117–123. doi: 10.1016/j.jhep.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 44.Luo B, Liu L, Tang L, Zhang J, Ling Y, Fallon MB. ET-1 and TNF-alpha in HPS: analysis in prehepatic portal hypertension and biliary and nonbiliary cirrhosis in rats. Am J Physiol Gastrointest Liver Physiol. 2004;286:G294–G303. doi: 10.1152/ajpgi.00298.2003. [DOI] [PubMed] [Google Scholar]
- 45.Popov Y, Patsenker E, Bauer M, Niedobitek E, Schulze-Krebs A, Schuppan D. Halofuginone induces matrix metalloproteinases in rat hepatic stellate cells via activation of p38 and NFkappaB. J Biol Chem. 2006;281:15090–15098. doi: 10.1074/jbc.M600030200. [DOI] [PubMed] [Google Scholar]
- 46.Laleman W, Vander Elst I, Zeegers M, Servaes R, Libbrecht L, Roskams T, Fevery J, Nevens F. A stable model of cirrhotic portal hypertension in the rat: thioacetamide revisited. Eur J Clin Invest. 2006;36:242–249. doi: 10.1111/j.1365-2362.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 47.Yeh CN, Maitra A, Lee KF, Jan YY, Chen MF. Thioacetamide-induced intestinal-type cholangiocarcinoma in rat: an animal model recapitulating the multi-stage progression of human cholangiocarcinoma. Carcinogenesis. 2004;25:631–636. doi: 10.1093/carcin/bgh037. [DOI] [PubMed] [Google Scholar]
- 48.Veal N, Oberti F, Moal F, Vuillemin E, Fort J, Kaassis M, Pilette C, Cales P. Spleno-renal shunt blood flow is an accurate index of collateral circulation in different models of portal hypertension and after pharmacological changes in rats. J Hepatol. 2000;32:434–440. doi: 10.1016/s0168-8278(00)80394-3. [DOI] [PubMed] [Google Scholar]
- 49.Tsukamoto H, Matsuoka M, French SW. Experimental models of hepatic fibrosis: a review. Semin Liver Dis. 1990;10:56–65. doi: 10.1055/s-2008-1040457. [DOI] [PubMed] [Google Scholar]
- 50.Jenkins SA, Grandison A, Baxter JN, Day DW, Taylor I, Shields R. A dimethylnitrosamine-induced model of cirrhosis and portal hypertension in the rat. J Hepatol. 1985;1:489–499. doi: 10.1016/s0168-8278(85)80747-9. [DOI] [PubMed] [Google Scholar]
- 51.Nanji AA. Animal models of nonalcoholic fatty liver disease and steatohepatitis. Clin Liver Dis. 2004;8:559–74, ix. doi: 10.1016/j.cld.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 52.DeLeve LD, McCuskey RS, Wang X, Hu L, McCuskey MK, Epstein RB, Kanel GC. Characterization of a reproducible rat model of hepatic veno-occlusive disease. Hepatology. 1999;29:1779–1791. doi: 10.1002/hep.510290615. [DOI] [PubMed] [Google Scholar]
- 53.Sweat ER, Musicant ME, Annetts DL, Goodhead B, Orloff MJ. Production of hepatic outflow block and ascites with an ameroid constrictor. Surg Forum. 1966;17:376–378. [PubMed] [Google Scholar]
- 54.Orloff MJ, Daily PO, Girard B. Treatment of Budd-Chiari syndrome due to inferior vena cava occlusion by combined portal and vena caval decompression. Am J Surg. 1992;163:137–142; discussion 142-143. doi: 10.1016/0002-9610(92)90266-t. [DOI] [PubMed] [Google Scholar]
- 55.Bhathal PS, Grossman HJ. Reduction of the increased portal vascular resistance of the isolated perfused cirrhotic rat liver by vasodilators. J Hepatol. 1985;1:325–337. doi: 10.1016/s0168-8278(85)80770-4. [DOI] [PubMed] [Google Scholar]
- 56.Zhang JX, Pegoli W, Clemens MG. Endothelin-1 induces direct constriction of hepatic sinusoids. Am J Physiol. 1994;266:G624–G632. doi: 10.1152/ajpgi.1994.266.4.G624. [DOI] [PubMed] [Google Scholar]
- 57.Graupera M, García-Pagán JC, Abraldes JG, Peralta C, Bragulat M, Corominola H, Bosch J, Rodés J. Cyclooxygenase-derived products modulate the increased intrahepatic resistance of cirrhotic rat livers. Hepatology. 2003;37:172–181. doi: 10.1053/jhep.2003.50004. [DOI] [PubMed] [Google Scholar]
- 58.Gupta TK, Toruner M, Chung MK, Groszmann RJ. Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats. Hepatology. 1998;28:926–931. doi: 10.1002/hep.510280405. [DOI] [PubMed] [Google Scholar]
- 59.Yokoyama Y, Xu H, Kresge N, Keller S, Sarmadi AH, Baveja R, Clemens MG, Zhang JX. Role of thromboxane A2 in early BDL-induced portal hypertension. Am J Physiol Gastrointest Liver Physiol. 2003;284:G453–G460. doi: 10.1152/ajpgi.00315.2002. [DOI] [PubMed] [Google Scholar]
- 60.Kamath PS, Tyce GM, Miller VM, Edwards BS, Rorie DK. Endothelin-1 modulates intrahepatic resistance in a rat model of noncirrhotic portal hypertension. Hepatology. 1999;30:401–407. doi: 10.1002/hep.510300235. [DOI] [PubMed] [Google Scholar]
- 61.Noda S, Masumi S, Moriyama M, Kannan Y, Ohta M, Sugano T, Yamate J. Population of hepatic macrophages and response of perfused liver to platelet-activating factor during production of thioacetamide-induced cirrhosis in rats. Hepatology. 1996;24:412–418. doi: 10.1053/jhep.1996.v24.pm0008690413. [DOI] [PubMed] [Google Scholar]
- 62.Hori N, Okanoue T, Sawa Y, Mori T, Kashima K. Hemo-dynamic characterization in experimental liver cirrhosis induced by thioacetamide administration. Dig Dis Sci. 1993;38:2195–2202. doi: 10.1007/BF01299895. [DOI] [PubMed] [Google Scholar]
- 63.McGregor DD, Smirk FH. Vascular responses in mesenteric arteries from genetic and renal hypertensive rats. Am J Physiol. 1968;214:1429–1433. doi: 10.1152/ajplegacy.1968.214.6.1429. [DOI] [PubMed] [Google Scholar]
- 64.Sogni P, Sabry S, Moreau R, Gadano A, Lebrec D, Dinh-Xuan AT. Hyporeactivity of mesenteric resistance arteries in portal hypertensive rats. J Hepatol. 1996;24:487–490. doi: 10.1016/s0168-8278(96)80170-x. [DOI] [PubMed] [Google Scholar]
- 65.Joh T, Granger DN, Benoit JN. Intestinal microvascular responsiveness to norepinephrine in chronic portal hypertension. Am J Physiol. 1991;260:H1135–H1143. doi: 10.1152/ajpheart.1991.260.4.H1135. [DOI] [PubMed] [Google Scholar]
- 66.Sieber CC, Groszmann RJ. In vitro hyporeactivity to methoxamine in portal hypertensive rats: reversal by nitric oxide blockade. Am J Physiol. 1992;262:G996–1001. doi: 10.1152/ajpgi.1992.262.6.G996. [DOI] [PubMed] [Google Scholar]
- 67.Chojkier M, Groszmann RJ. Measurement of portal-systemic shunting in the rat by using gamma-labeled microspheres. Am J Physiol. 1981;240:G371–G375. doi: 10.1152/ajpgi.1981.240.5.G371. [DOI] [PubMed] [Google Scholar]
- 68.Theodorakis NG, Wang YN, Skill NJ, Metz MA, Cahill PA, Redmond EM, Sitzmann JV. The role of nitric oxide synthase isoforms in extrahepatic portal hypertension: studies in gene-knockout mice. Gastroenterology. 2003;124:1500–1508. doi: 10.1016/s0016-5085(03)00280-4. [DOI] [PubMed] [Google Scholar]
- 69.Mosca P, Lee FY, Kaumann AJ, Groszmann RJ. Pharmacology of portal-systemic collaterals in portal hypertensive rats: role of endothelium. Am J Physiol. 1992;263:G544–G550. doi: 10.1152/ajpgi.1992.263.4.G544. [DOI] [PubMed] [Google Scholar]
- 70.Claria J, Jimenez W, Ros J, Rigol M, Angeli P, Arroyo V, Rivera F, Rodes J. Increased nitric oxide-dependent vasorelaxation in aortic rings of cirrhotic rats with ascites [see comments] Hepatology. 1994;20:1615–1621. doi: 10.1002/hep.1840200635. [DOI] [PubMed] [Google Scholar]
- 71.Claria J, Jimenez W, Ros J, Asbert M, Castro A, Arroyo V, Rivera F, Rodes J. Pathogenesis of arterial hypotension in cirrhotic rats with ascites: role of endogenous nitric oxide. Hepatology. 1992;15:343–349. doi: 10.1002/hep.1840150227. [DOI] [PubMed] [Google Scholar]
- 72.Ros J, Clària J, Jiménez W, Bosch-Marcé M, Angeli P, Arroyo V, Rivera F, Rodés J. Role of nitric oxide and prostacyclin in the control of renal perfusion in experimental cirrhosis. Hepatology. 1995;22:915–920. [PubMed] [Google Scholar]
- 73.Albillos A, Colombato LA, Groszmann RJ. Vasodilatation and sodium retention in prehepatic portal hypertension. Gastroenterology. 1992;102:931–935. doi: 10.1016/0016-5085(92)90179-3. [DOI] [PubMed] [Google Scholar]
- 74.Colombato LA, Albillos A, Groszmann RJ. The role of central blood volume in the development of sodium retention in portal hypertensive rats. Gastroenterology. 1996;110:193–198. doi: 10.1053/gast.1996.v110.pm8536856. [DOI] [PubMed] [Google Scholar]
- 75.Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodes J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151–1157. doi: 10.1002/hep.1840080532. [DOI] [PubMed] [Google Scholar]
- 76.Martinez-Prieto C, Ortiz MC, Fortepiani LA, Ruiz-Macia J, Atucha NM, Garcia-Estan J. Haemodynamic and renal evolution of the bile duct-ligated rat. Clin Sci (Lond) 2000;98:611–617. [PubMed] [Google Scholar]
- 77.Jimenez W, Martinez-Pardo A, Arroyo V, Bruix J, Rimola A, Gaya J, Rivera F, Rodes J. Temporal relationship between hyperaldosteronism, sodium retention and ascites formation in rats with experimental cirrhosis. Hepatology. 1985;5:245–250. doi: 10.1002/hep.1840050215. [DOI] [PubMed] [Google Scholar]
- 78.López C, Jiménez W, Arroyo V, Clària J, La Villa G, Asbert M, Gaya J, Rivera F, Rodés J. Temporal relationship between the decrease in arterial pressure and sodium retention in conscious spontaneously hypertensive rats with carbon tetrachloride-induced cirrhosis. Hepatology. 1991;13:585–589. [PubMed] [Google Scholar]
- 79.Panés J, Casadevall M, Piqué JM, Bosch J, Whittle BJ, Terés J. Effects of acute normovolemic anemia on gastric mucosal blood flow in rats: role of nitric oxide. Gastroenterology. 1992;103:407–413. doi: 10.1016/0016-5085(92)90828-m. [DOI] [PubMed] [Google Scholar]
- 80.Panés J, Casadevall M, Fernández M, Piqué JM, Bosch J, Casamitjana R, Cirera I, Bombí JA, Terés J, Rodés J. Gastric microcirculatory changes of portal-hypertensive rats can be attenuated by long-term estrogen-progestagen treatment. Hepatology. 1994;20:1261–1270. doi: 10.1002/hep.1840200525. [DOI] [PubMed] [Google Scholar]
- 81.Fallon MB, Abrams GA, McGrath JW, Hou Z, Luo B. Common bile duct ligation in the rat: a model of intrapulmonary vasodilatation and hepatopulmonary syndrome. Am J Physiol. 1997;272:G779–G784. doi: 10.1152/ajpgi.1997.272.4.G779. [DOI] [PubMed] [Google Scholar]
- 82.Morales J, Moitinho E, Abraldes JG, Fernandez M, Bosch J. Effects of the V1a vasopressin agonist F-180 on portal hypertension-related bleeding in portal hypertensive rats. Hepatology. 2003;38:1378–1383. doi: 10.1016/j.hep.2003.09.023. [DOI] [PubMed] [Google Scholar]


