Abstract
Surgical resection with lymphadenectomy is the mainstay of treatment for all resectable esophagogastric junction tumors, prior to systemic generalization of the disease. This makes accurate pre-treatment staging and classification of the tumors most demanding. A well-established and internationally accepted classification for adenocarcinomas of the esophagogastric junction (AEG) helps to choose the appropriate surgical approach and to make results from different institutions comparable. Distal esophageal adenocarcinomas (AEGI) are distinguished from true cardia carcinomas (AEG II) and subcardiac gastric cancers (AEG III). Substantial advancements in this surgical field during the preceding decades have clearly revealed that individualization of the surgical strategy is the key to successfully approaching these entities. In this review we discuss the surgical management of esophagogastric junction tumors with a tailored surgical strategy.
Keywords: Adenocarcinoma of the esophagogastric junction, Esophageal cancer, Gastric cancer, Surgical resection
INTRODUCTION
Different tumor entities arise in the vicinity of the esophagogastric junction. The appropriate classification of these entities is essential for choosing the appropriate surgical approach and making results from different instutions comparable. Surgical resection is the mainstay of treatment for esophagogastric junction tumors of all resectable tumor stages prior to systemic generalization of the disease. Therefore a meticulous pretreatment staging is mandatory for planning the therapeutic approach. The major goal of surgical resection is complete removal of primary tumor together with its lymphatic drainage, because R0-resection (microscopic complete removal of the tumor) as well as nodal status and lymph node ratio (number of infiltrated nodes per node removed) are major prognostic factors. In locally advanced tumors, with low chances for R0 resection by means of primary surgery, multimodality treatment is attempted, aiming at downsizing the primary tumor and possibly downstaging the disease.
CLASSIFICATION
The appropriate and uniform classification of carcinomas arising within the vicinity of the esophagogastric junction is essential both for planning therapeutic/ surgical approaches and for making results from different institutions comparable. Well-established and meanwhile increasingly used world-wide is the classification of “adenocarcinomas of the esophagogastric junction” (AEG)[1,2]. Adenocarcinomas of the distal esophagus (AEG typeI) are distinguished from carcinomas arising at the level of the anatomical cardia (AEG type II) and subcardiac gastric cancers (AEG III). The classification has been introduced 17 years before from a surgical viewpoint[1,2]. But meanwhile it becomes more and more evident, that this classification also reflects the pathophysiology of different entities very well[3].
The center of the main tumor mass in relation to the anatomical cardia comprises the basis for the AEG-classification. For clinical usage, a definition of the anatomical cardia from the endoscopist’s viewpoint is required. The cardia is localized, where the gastric folds end. The Z-line (correlative to the squamocolumnar junction) is shifted proximally in Barrett’s esophagus but not at the level of the cardia, like it is under physiologic conditions[4,5]. The AEG classification is recommended by the consensus conference of the International Society for Diseases of the Esophagus[2] and increasingly accepted and used worldwide[6-11].
PATHOPYSIOLOGY AND EPIDEMIOLOGY
AEG typeItumors are found in the majority of patients with Barrett’s cancers, arising within the precancerous Barrett’s esophagus[12]. In patients with no evidence of Barrett’s metaplasia on initial work-up, this can be due to an advanced primary tumor “overgrowing” the intestinal metaplasia. It has been shown that Barrett’s metaplasia can get “unmasked” by preoperative chemotherapy in a substantial number of cases[13]. In the series by Theisen et al[13] over 97% of the patients with AEGItumors are associated with Barrett’s esophagus.
Carcinogenesis within the specialized intestinal metaplasia follows a metaplasia-intraepithelial neoplasia-carcinoma sequence. Initiation and progression of the disease process are promoted by the chronically damaging effect of gastroesophageal reflux[4,5]. Gastroesophageal reflux disease and its complication Barrett’s esophagus are associated with a compromised lower esophageal sphincter. Patients have a high prevalence of hiatal hernia, which has been evaluated as a risk factor for esophageal adenocarcinoma[14,15]. Furthermore, patients are often obese (pathologically elevated body mass index), and only occasional alcohol drinkers in sharp contrast to patients with squamous cell cancers of the esophagus. It has been clearly demonstrated that the histological tumor types (esophageal adenocarcinomas and squamous cell cancers) comprise two entirely different entities[16] occurring in completely different types of patients.
The association of hiatal hernias and obesity with cancer development decreases in patients with tumors localized more distally. In patients with AEG II/III tumors long-lasting GERD and correlated morbidity (hiatal hernias, obesity) have diminished importance as risk factors[14,17]. AEG II and III tumors also have only a weak or no association with specialized intestinal metaplasia of the esophagus in the majority of patients[3]. Regarding the pathophysiology, these tumors seem to have more similarities with gastric cancers. A strong association with H pylori and intestinal metaplasia at or below the gastric cardia has been demonstrated[18].
Nevertheless, there are numerous hints supporting the concept of distinguishing these entities from gastric cancers, and regarding them as own entities. Especially striking is the fact that their incidence is increasing, compared to the decreasing incidence of gastric cancers.
PRE-TREATMENT STAGING
Accurate pre-treatment staging is most demanding, because therapy for esophagogastric junction tumors must be performed by adjusting stage of the disease. Tailored surgical strategies are based on accurate localization of the primary tumor and its classifcation.
For all upper gastrointestinal tumors, endoscopy is the basic staging modality, allowing direct visualization of the primary tumor, exact localization and establishment of the diagnosis by means of biopsy. An up-to-date practice guideline by the American Society for Gastrointestinal Endoscopy deals with the use of endoscopy for esophageal cancer, and addresses the broad spectrum of endoscopy. New developments in the field of endoluminal diagnostics make recognition of early lesions more and more precise, especially by introduction and evaluation of new technologies, i.e. high resolution devices[19].
The depth of tumor invasion defines the T-category according to the TNM-classification system of the UICC, which is commonly used for staging[20]. For defining depth of invasion of a primary tumor in the clinical setting, endoscopic ultrasound (EUS) is the best staging modality. It has a major impact on choosing the appropriate treatment strategy (retrospective, but blinded evaluation)[21]. Furthermore, definition of T- and N-categories by EUS has been demonstrated to be of value for predicting long-term survival (prospective evaluation of 150 patients)[22].
CT scan remains the preferred staging method for exclusion of systemic tumor spread[23], providing anatomical information. The more functional positron emission tomography with fluorodeoxyglucose (FDG-PET), visualizing the regional glucose metabolism, is increasingly used for staging[23,24] and response evaluation during multimodality treatment[24,25]. Many studies have assessed the value of PET as a staging method and the former is meanwhile decreasing to a more realistic view. By critically reviewing their data, Kneist et al have demonstrated that the use of PET does neither lead to a different therapeutic approach nor provide new information on the indication for surgery.
In our experience, the use of FDG-PET as a staging method should be limited to early tumor stages, namely early cancers (T1) with very low prevalence of lymphatic or systemic tumor spread, and systemically disseminated disease. This is the clue to make usage of FDG-PET cost-effective.
MULTITREATMENT MODALITY
In the Western world, neoadjuvant treatment concepts for administering systemic antineoplastic therapy (i.e. chemotherapy or chemoradiation prior to subsequent surgical resection) are preferred over adjuvant chemotherapy. Studies on treating adenocarcinomas of the esophagogastric junction are scarce, due to non-uniform classification. These tumors are either included in esophageal or gastric cancer trials. Furthermore some studies on esophageal cancer still have not distinguished adenocarcinoma from squamous cell cancers, although this is essential, because these histological tumor types comprise two entirely different entities[16].
Only two studies on neoadjuvant treatment of esophageal cancer have been able to show a survival benefit with this concept[26,27]. Meta-analyses including 9 and 11 randomized trials[28,29] have demonstrated decent survival benefits. But these studies deal with esophageal cancer, and not exclusively adenocarcinoma. Nevertheless, the major message of these trials is probably true for carcinomas of the esophagogastric junction. Only a subgroup of patients undergoing neoadjuvant treatment experiences a survival benefit. These are the ‘responders’. ‘Non-responders’ by contrast do progress or deteriorate during preoperative treatment. In our experience the response frequency to the preoperative antineoplastic regimens accounts for 30%-60%[26,27].
An amazing concept for assessing the early response during the course of the antineoplastic regimen is response evaluation with FDG-PET. This has been demonstrated in esophageal squamous cell cancers[24,30] and also in AEG tumors and gastric cancers[25,31]. It has been recognized that tumors responding to chemotherapy show an early decrease of glucose uptake. This tool has been used and intensively studied concerning the selective usage of neoadjuvant protocols.
SURGICAL STRATEGIES
Complete removal of the primary tumor (R0 resection) is one of the major prognostic factors in adenocarcinomas of the esophagogastric junction[12]. Its predictive value is strong as demonstrated by univariate analysis[32] (Figure 1). Multivariate analysis demonstrates that R0 resection is independent of other strong predictors of survival, like T, N and M[12]. Thus, the primary goal of surgical resection of esophagogastric junction tumors is complete removal of the primary tumor, together with its lymphatic drainage. Which surgical approach best suits this purpose is still controversial. A vast variety of approaches for surgical resection of tumors of the esophagogastric junction has been proposed, including abdominothoracic en bloc esophagogastrectomy, subtotal esophagectomy with resection of the proximal stomach, total gastrectomy with transhiatal resection of the distal esophagus, limited resection of the esophagogastric junction.
Figure 1.
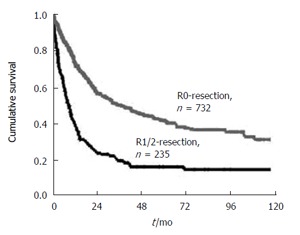
Overall 10-year survival rate of patients with resected adenocarcinoma of the esophagogastric junction. Patients with complete macroscopic and microscopic tumor resection (R0 resection) versus patients with resection (R1/R2 resection) (Date of the Chirurgische Klinik und Poliklinik, Klinikum rechts der lsar der TU Munchen 1982-1999).
In the past 20 years we have operated on more than 1500 patients with AEG tumors. A variety of approaches have been assessed[13]. Based on this surgical experience tailored surgical strategies have been developed, with respect to distinct requirements of the different AEG tumor types and different stages of the disease. Although still practiced[33], complete esophagogastrectomy has been abandoned as a procedure for carcinomas within the esophagogastric junction.
AEGItumors (distal esophageal adenocarcinomas)
It is beyond dispute that AEGItumors require an esophagectomy. Resection must include complete removel of the precancerous Barrett’s esophagus[1]. For this purpose, preoperative clipping of the oral margin (level of the squamocolumnar junction) by the endoscopist is recommended. These tumors, which are mostly Barrett’s cancers, have been shown to metastasize predominantly to the mediastinal lymph nodes[34]. But lymphatic spread occurs later than in esophageal squamous cell cancers and the prevalence of lymphatic metastases is lower in distal esophageal adenocarcinomas[12].
The transthoracic or transhiatal resection of Barrett’s cancers is the best approach which is a topic of intensive research. Transthoracic esophagectomy with en bloc removal of esophagus and adjacent lymph nodes is the optimal approach in respect to radical resection of tumors. These lymph nodes are left behind with transhiatal (transmediastinal) esophagectomy, because a formal lymphadenectomy is not performed with this technique. The transhiatal approach can result in a reduced postoperative morbidity and mortality, because thoracotomy is avoided. This view is not supported by a recent multicenter trial from the USA[35]. In this large scale investigation the reported differences between transhiatal and transthoracic approaches in respect to morbidity and mortality are not statistically significant.
Another prospective trial, a recent single center study from Amsterdam/The Netherlands, comparing the two approaches in a series of patients with distal esophageal adenocarcinomas[36] showed that patients may benefit from transthoracic resection, thus having a longer survival. A clear superiority of either procedure has not been demonstrated, requiring individualized strategy. For all patients who are likely to benefit from the complete nodal clearance, transthoracic en bloc esophagectomy appears to be the procedure of first choice. In terms of radical resection, transhiatal esophagectomy is appropriate for earlier distal esophageal adenocarcinomas (with low propability of lymphatic involvement) and patients with substantial co-morbidity (who may benefit from avoiding the thoracotomy)[3,34].
AEG II/ III tumors (cardia carcinomas and subcardiac gastric cancers)
In our experience, total gastrectomy with transhiatal resection of the distal esophagus (transhiatally extended gastrectomy) is the best approach for AEG II tumors[37]. Short-term postoperative results, i.e. morbidity and mortlity, are better with this approach, compared to transhiatal esophagectomy (e.g. 5.6% vs 1.9% mortality in a consecutive patient series of 46 transmediastinal esophagectomies and 103 extended total gastrectomies). Multivariate analysis has shown that R0 resection is the single most important prognostic factor. Regression analysis of the subgroup of R0-resected patients has demonstrated that absence of lymph node metastases and extended gastrectomy are two independet predictors of long-term survival[37] (Figure 2). This approach (extended gastrectomy superior to transhiatal esophagectomy for AEG II) is also supported by data from other institutions[38,39].
Figure 2.
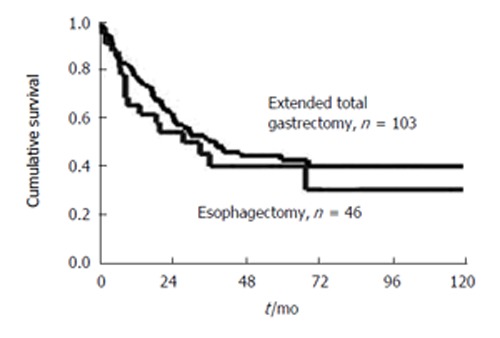
Ten-year survival rate of patients with R0-resected true carcinoma of the gastric cardia (AEG Type-II). Radical transmediastinal esophagectomy versus extend total gastrectomy (Date of the Chirurgische Klinik und Poliklinik, Klinikum rechts der lsar der TU Munchen 1982-1999).
EXTENT OF LYMPHADENECTOMY AND SPLENECTOMY
The required extent of lymphadenectomy for AEG tumors has never been studied systematically. Similar to the findings of Japanese institutions, we have demonstrated that patients with tumors limited to the mucosa (pT1a) have virtually no lymph node involvement. Furthermore lymph node metastases are uncommon in carcinomas invading only the submucosa (pT1b). This holds also true, when more sensitive methods (immunohistochemistry/PCR techniques) are used for detection of micrometastases. Patients with more advanced AEG II carcinomas harbor metastases in lymph nodes of paracardial region, lesser and greater curvatures, left gastric artery towards celiac axis, splenic artery, superior border of the pancreas towards the splenic hilum, lower posterior mediastinum, left adrenal gland and left renal vein[34,27,40,41].
This comprises the basis for the current concept of standard lymphadenectomy for AEG II and III tumors. Lymphadenectomy (in addition to lymph nodes adjacent to the gastrectomy specimen) starts with removal of the lymph nodes along the splenic artery towards the splenic hilum. Lymph nodes around the left renal vein are included. Formerly a retroperitoneal lymphadenectomy with left-sided pancreatic resection plus splenectomy is frequently performed in addition. Although the number of resected lymph nodes is increased with this procedure, the negative side-effects are predominant. A substantial number of septic complications, pancreatic fistulae and abscess formation have been observed[42-44].
LIMITED RESECTION
Extended gastrectomies, especially esophagectomies, are associated with a considerable morbidity and mortality. Although these indicators for short-term postoperative outcome have been markedly improved during recent years[2,45,46], the remaining risk is nevertheless substantial. Furthermore, the quality of life after esophagectomy and gastrectomy is compromised. This fact has led to limited resections of adenocarcinomas of the esophagogastric junction. Resection of the distal esophagus and esophagogastric junction, with regional lymphadenectomy and jejunal interposition for reconstruction, has been described as a suitable surgical alternative[5,6]. Reconstruction is done by jejunal interposition and the short- and long-term results are excellent and the quality of life is improved as expected.
THERAPEUTIC STRATEGIES
Esophagectomy is the appropriate approach for surgical resection of AEGItumors, whereas transhiatally extended gastrectomy is recommended for AEG II and III tumors. In our experience it is not necessary to perform more extended procedures in most cases, like esophagogastrectomy. Limited resection of the esophagogastric junction and reconstruction with interposition of a jejunal loop can be successfully applied to early cancers arising in the vicinity of the EGJ[6]. The value of neoadjuvant treatment concepts is not entirely clear as yet. Although a subset of patients benefits from chemotherapy preoperatively, the effective tool for response prediction is positron emission tomography (PET).
FUTURE PERSPECTIVES
Further individualization of surgical strategies can be expected. The most striking problem with surgery of esophagogastric junction tumors is that the subset of patients who benefit from neoadjuvant protocols is unknown. Therefore the patients who do not respond to this aggressive regimen would suffer from the side-effects of this therapy. Response prediction for defining subsets of patients benefiting most from neoadjuvant treatment regimens would become a matter of molecular characterization. It might become possible to distinguish responders from non-responders before initiating the treatment according to their genetic profiles. The molecular characterization by genomic profiling perhaps can predict lymph node status and survival, as in gastric cancer[47] and Barrett’s cancer[48] and other entities. Regarding the surgical technique, progress can be achieved with the technique of sentinel lymph node biopsy[49]. This technique helps us to individualize the extent of required lymphadenectomy, which is important because it is a major factor contributing to postoperative morbidity and mortality.
In the near future, new technical devices like a new FDG-PET hand device, help to identify metastatic lymph node intraoperatively. Further development in the field of nuclear medicine with new tracers and more sensitive detection systems is perhaps helpful regarding pre-treatment staging and intra-operative identification of metastatic disease. The combination of functional (PET/scintigraphy) and antomical (CT) information helps to summarize information of the major staging methods for esophagogastric junction tumors. Although at its very beginning of clinical application[50], PET/CT is an amazing tool.
KEY ISSUES
Surgical resection is the mainstay of treatment for esophagogastric junction tumor prior to its systemic generalization.
A meticulous preoperative staging based on appropriate classification is required for choosing the appropriate therapeutic approach and surgical strategy.
Endoscopy, endoscopic ultrasound, pharyngoeso-phagography and CT scan are the basic staging modalities. The functional FDG-PET scan, visualizing areas of increased glucose uptake, is increasingly used for staging and response evaluation during multi treatment modality.
Uniform classification of tumors within the vicinity of the esophagogastric junction is important for choosing the appropriate surgical approach and making results from different institutions comparable. The classification of adenocarcinomas of the esophagogastric junction (AEG), can distinguish distal esophageal adenocarcinomas (AEG I) from true cardia carcinomas (AEG II) and subcardiac gastric cancers (AEG III).
AEGItumors are usually Barrett’s cancers arising in the precancerous Barrett’s esophagus under the chronically damaging effect of acid. AEG II and III tumors share more properties with gastric cancers, but comprise nevertheless distinct entities
Esophagectomy is the appropriate surgical procedure for AEGItumors, whereas transhiatally extended gastrectomy (with resection of an esophageal sleeve) is the best procedure for AEG II and III tumors. Esophagogastrectomy has been abandoned in most institutions, because it is associated with a substantial morbidity and mortality as well as a bad quality of life of the patients. Although its value has not been fully defined yet, a substantial number of patients with locally advanced tumors seem to benefit from neoadjuvant treatment concepts, aiming at down staging and down sizing of the primary tumor.
Footnotes
S- Editor Wang GP L- Editor Wang XL E- Editor Ma WH
References
- 1.Siewert JR, Hölscher AH, Becker K, Gössner W. Kardia-karzinom: Versuch einer therapeutisch relevanten Klassifikation. Chirurg. 1987;58:25–34. [PubMed] [Google Scholar]
- 2.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457–1459. doi: 10.1046/j.1365-2168.1998.00940.x. [DOI] [PubMed] [Google Scholar]
- 3.Stein HJ, Feith M, von Rahden BH, Siewert JR. Approach to early Barrett's cancer. World J Surg. 2003;27:1040–1046. doi: 10.1007/s00268-003-7059-8. [DOI] [PubMed] [Google Scholar]
- 4.Spechler SJ. Clinical practice. Barrett's Esophagus. N Engl J Med. 2002;346:836–842. doi: 10.1056/NEJMcp012118. [DOI] [PubMed] [Google Scholar]
- 5.von Rahden BH, Stein HJ, Siewert JR. Barrett's esophagus and Barrett's carcinoma. Curr Oncol Rep. 2003;5:203–209. doi: 10.1007/s11912-003-0111-x. [DOI] [PubMed] [Google Scholar]
- 6.Hardwick RH, Williams GT. Staging of oesophageal adenocarcinoma. Br J Surg. 2002;89:1076–1077. doi: 10.1046/j.1365-2168.2002.02175.x. [DOI] [PubMed] [Google Scholar]
- 7.Ichikura T, Ogawa T, Kawabata T, Chochi K, Sugasawa H, Mochizuki H. Is adenocarcinoma of the gastric cardia a distinct entity independent of subcardial carcinoma? World J Surg. 2003;27:334–338. doi: 10.1007/s00268-002-6776-8. [DOI] [PubMed] [Google Scholar]
- 8.de Manzoni G, Pedrazzani C, Pasini F, Di Leo A, Durante E, Castaldini G, Cordiano C. Results of surgical treatment of adenocarcinoma of the gastric cardia. Ann Thorac Surg. 2002;73:1035–1040. doi: 10.1016/s0003-4975(01)03571-8. [DOI] [PubMed] [Google Scholar]
- 9.Swisher SG, Pisters PW, Komaki R, Lahoti S, Ajani JA. Gastroesophageal junction adenocarcinoma. Curr Treat Options Oncol. 2000;1:387–398. doi: 10.1007/s11864-000-0066-1. [DOI] [PubMed] [Google Scholar]
- 10.Mariette C, Balon JM, Maunoury V, Taillier G, Van Seuningen I, Triboulet JP. Value of endoscopic ultrasonography as a predictor of long-term survival in oesophageal carcinoma. Br J Surg. 2003;90:1367–1372. doi: 10.1002/bjs.4307. [DOI] [PubMed] [Google Scholar]
- 11.Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T. Adenocarcinoma of the gastroesophageal junction in Japan: relevance of Siewert's classification applied to 177 cases resected at a single institution. J Am Coll Surg. 1999;189:594–601. doi: 10.1016/s1072-7515(99)00201-x. [DOI] [PubMed] [Google Scholar]
- 12.Siewert JR, Feith M, Werner M, Stein HJ. Adenocarcinoma of the esophagogastric junction. Results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232:353–361. doi: 10.1097/00000658-200009000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theisen J, Stein HJ, Dittler HJ, Feith M, Moebius C, Kauer WK, Werner M, Siewert JR. Preoperative chemotherapy unmasks underlying Barrett's mucosa in patients with adenocarcinoma of the distal esophagus. Surg Endosc. 2002;16:671–673. doi: 10.1007/s00464-001-8307-3. [DOI] [PubMed] [Google Scholar]
- 14.Wu AH, Tseng CC, Bernstein L. Hiatal hernia, reflux symptoms, body size, and risk of esophageal and gastric adenocarcinoma. Cancer. 2003;98:940–948. doi: 10.1002/cncr.11568. [DOI] [PubMed] [Google Scholar]
- 15.Avidan B, Sonnenberg A, Schnell TG, Chejfec G, Metz A, Sontag SJ. Hiatal hernia size, Barrett's length, and severity of acid reflux are all risk factors for esophageal adenocarcinoma. Am J Gastroenterol. 2002;97:1930–1936. doi: 10.1111/j.1572-0241.2002.05902.x. [DOI] [PubMed] [Google Scholar]
- 16.Siewert JR, Stein HJ, Feith M, Brucher BL, Bartels H, Fink U. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1000 consecutive resections at a single center in the Western world. Ann Surg. 2001;234:360–369. doi: 10.1097/00000658-200109000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 18.Chalasani N, Wo JM, Hunter JG, Waring JP. Significance of intestinal metaplasia in different areas of esophagus including esophagogastric junction. Dig Dis Sci. 1997;42:603–607. doi: 10.1023/a:1018863529777. [DOI] [PubMed] [Google Scholar]
- 19.May A, Gunter E, Roth F, Gossner L, Stolte M, Vieth M, Ell C. Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: a comparative, prospective, and blinded trial. Gut. 2004;53:634–640. doi: 10.1136/gut.2003.029421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobin LH, Wittekind Ch, editors . UICC. TNM Classification of Malignant Tumors, 6th ed. New York: Wiley-Liss; 2002. [Google Scholar]
- 21.Preston SR, Clark GW, Martin IG, Ling HM, Harris KM. Effect of endoscopic ultrasonography on the management of 100 consecutive patients with oesophageal and junctional carcinoma. Br J Surg. 2003;90:1220–1224. doi: 10.1002/bjs.4268. [DOI] [PubMed] [Google Scholar]
- 22.Mariette C, Castel B, Balon JM, Van Seuningen I, Triboulet JP. Extent of oesophageal resection for adenocarcinoma of the oesophagogastric junction. Eur J Surg Oncol. 2003;29:588–593. doi: 10.1016/s0748-7983(03)00109-4. [DOI] [PubMed] [Google Scholar]
- 23.Rasanen JV, Sihvo EI, Knuuti MJ, Minn HR, Luostarinen ME, Laippala P, Viljanen T, Salo JA. Prospective analysis of accuracy of positron emission tomography, computed tomography, and endoscopic ultrasonography in staging of adenocarcinoma of the esophagus and the esophagogastric junction. Ann Surg Oncol. 2003;10:954–960. doi: 10.1245/aso.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Downey RJ, Akhurst T, Ilson D, Ginsberg R, Bains MS, Gonen M, Koong H, Gollub M, Minsky BD, Zakowski M, et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol. 2003;21:428–432. doi: 10.1200/JCO.2003.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Ott K, Weber WA, Fink U, Helmberger H, Becker K, Stein HJ, Müller J, Schwaiger M, Siewert JR. Fluorodeoxyglucose-positron emission tomography in adenocarcinomas of the distal esophagus and cardia. World J Surg. 2003;27:1035–1039. doi: 10.1007/s00268-003-7058-9. [DOI] [PubMed] [Google Scholar]
- 26.Lordick F, Stein HJ, Peschel C, Siewert JR. Neoadjuvant therapy for oesophagogastric cancer. Br J Surg. 2004;91:540–551. doi: 10.1002/bjs.4575. [DOI] [PubMed] [Google Scholar]
- 27.Siewert JR, Stein HJ, von Rahden BH. Multimodal treatment of gastrointestinal tract tumors: consequences for surgery. World J Surg. 2005;29:940–948. doi: 10.1007/s00268-005-0010-4. [DOI] [PubMed] [Google Scholar]
- 28.Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185:538–543. doi: 10.1016/s0002-9610(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 29.Kaklamanos IG, Walker GR, Ferry K, Franceschi D, Livingstone AS. Neoadjuvant treatment for resectable cancer of the esophagus and the gastroesophageal junction: a meta-analysis of randomized clinical trials. Ann Surg Oncol. 2003;10:754–761. doi: 10.1245/aso.2003.03.078. [DOI] [PubMed] [Google Scholar]
- 30.Wieder HA, Brücher BL, Zimmermann F, Becker K, Lordick F, Beer A, Schwaiger M, Fink U, Siewert JR, Stein HJ, et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 2004;22:900–908. doi: 10.1200/JCO.2004.07.122. [DOI] [PubMed] [Google Scholar]
- 31.Ott K, Fink U, Becker K, Stahl A, Dittler HJ, Busch R, Stein H, Lordick F, Link T, Schwaiger M, et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol. 2003;21:4604–4610. doi: 10.1200/JCO.2003.06.574. [DOI] [PubMed] [Google Scholar]
- 32.Stein HJ, Feith M, Muller J, Werner M, Siewert JR. Limited resection for early adenocarcinoma in Barrett's esophagus. Ann Surg. 2000;232:733–742. doi: 10.1097/00000658-200012000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemeş R, Curcă T, Paraliov T, Munteanu M, Paşalega M, Meşină C, Dincă N, Martin L, Cheie M. The esophagogastric junction cancer: diagnosis and surgical treatment challenges. Rom J Gastroenterol. 2003;12:193–197. [PubMed] [Google Scholar]
- 34.Feith M, Stein HJ, Siewert JR. Pattern of lymphatic spread of Barrett's cancer. World J Surg. 2003;27:1052–1057. doi: 10.1007/s00268-003-7060-2. [DOI] [PubMed] [Google Scholar]
- 35.Rentz J, Bull D, Harpole D, Bailey S, Neumayer L, Pappas T, Krasnicka B, Henderson W, Daley J, Khuri S. Transthoracic versus transhiatal esophagectomy: a prospective study of 945 patients. J Thorac Cardiovasc Surg. 2003;125:1114–1120. doi: 10.1067/mtc.2003.315. [DOI] [PubMed] [Google Scholar]
- 36.Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, Stalmeier PF, ten Kate FJ, van Dekken H, Obertop H, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–1669. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- 37.Siewert JR, Stein HJ. Adenocarcinoma of the gastroesophageal junction: Classification, pathology and extent of resection. Dis Esophagus. 1996;9:173–182. [Google Scholar]
- 38.Graham AJ, Finley RJ, Clifton JC, Evans KG, Fradet G. Surgical management of adenocarcinoma of the cardia. Am J Surg. 1998;175:418–421. doi: 10.1016/S0002-9610(98)00040-3. [DOI] [PubMed] [Google Scholar]
- 39.Papachristou DN, Fortner JG. Adenocarcinoma of the gastric cardia. The choice of gastrectomy. Ann Surg. 1980;192:58–64. doi: 10.1097/00000658-198007000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Manzoni G, Morgagni P, Roviello F, Di Leo A, Saragoni L, Marrelli D, Guglielmi A, Carli A, Folli S, Cordiano C. Nodal abdominal spread in adenocarcinoma of the cardia. Results of a multicenter prospective study. Gastric Cancer. 1998;1:146–151. doi: 10.1007/s101200050009. [DOI] [PubMed] [Google Scholar]
- 41.Wang LS, Wu CW, Hsieh MJ, Fahn HJ, Huang MH, Chien KY. Lymph node metastasis in patients with adenocarcinoma of gastric cardia. Cancer. 1993;71:1948–1953. doi: 10.1002/1097-0142(19930315)71:6<1948::aid-cncr2820710604>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 42.Siewert JR, Bottcher K, Stein HJ, Roder JD, Busch R. Problem of proximal third gastric carcinoma. World J Surg. 1995;19:523–531. doi: 10.1007/BF00294713. [DOI] [PubMed] [Google Scholar]
- 43.Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T, Kito T. Lack of benefit of combined pancreaticosplenectomy in D2 resection for proximal-third gastric carcinoma. World J Surg. 1997;21:622–627; discussion 627-628. doi: 10.1007/s002689900283. [DOI] [PubMed] [Google Scholar]
- 44.Kitamura K, Nishida S, Ichikawa D, Taniguchi H, Hagiwara A, Yamaguchi T, Sawai K. No survival benefit from combined pancreaticosplenectomy and total gastrectomy for gastric cancer. Br J Surg. 1999;86:119–122. doi: 10.1046/j.1365-2168.1999.00967.x. [DOI] [PubMed] [Google Scholar]
- 45.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, Welch HG, Wennberg DE. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 46.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 47.Weiss MM, Kuipers EJ, Postma C, Snijders AM, Siccama I, Pinkel D, Westerga J, Meuwissen SG, Albertson DG, Meijer GA. Genomic profiling of gastric cancer predicts lymph node status and survival. Oncogene. 2003;22:1872–1879. doi: 10.1038/sj.onc.1206350. [DOI] [PubMed] [Google Scholar]
- 48.Brabender J, Marjoram P, Salonga D, Metzger R, Schneider PM, Park JM, Schneider S, Hölscher AH, Yin J, Meltzer SJ, et al. A multigene expression panel for the molecular diagnosis of Barrett's esophagus and Barrett's adenocarcinoma of the esophagus. Oncogene. 2004;23:4780–4788. doi: 10.1038/sj.onc.1207663. [DOI] [PubMed] [Google Scholar]
- 49.Burian M, Stein HJ, Sendler A, Piert M, Nahrig J, Feith M, Siewert JR. Sentinel node detection in Barrett's and cardia cancer. Ann Surg Oncol. 2004;11:255S–258S. doi: 10.1007/BF02523640. [DOI] [PubMed] [Google Scholar]
- 50.Cook GJ, Wegner EA, Fogelman I. Pitfalls and artifacts in 18FDG PET and PET/CT oncologic imaging. Semin Nucl Med. 2004;34:122–133. doi: 10.1053/j.semnuclmed.2003.12.003. [DOI] [PubMed] [Google Scholar]


