Abstract
AIM: To evaluate and compare the expression profiles of CXCL12 (SDF-1), CCL19 (MIP-3β), CCL20 (MIP-3α) and CCL21 (6Ckine, Exodus2) and their receptors on RNA and protein levels in hepatocellular carcinoma (HCC) versus colorectal liver metastases (CRLM) and to elucidate their impact on the carcinogenesis and progression of malignant liver diseases.
METHODS: Chemokine expression was analyzed by RT-PCR and ELISA in 11 cases of HCC specimens and in 23 cases of CRLM and corresponding adjacent non-tumorous liver tissues, respectively. Expressions of their receptors CXCR4, CCR6 and CCR7 were analyzed by RT-PCR and Western blot analysis in the same cases of HCC and CRLM.
RESULTS: Significant up-regulation for CCL20/CCR6 was detected in both cancer types. Moreover, CCL20 demonstrated significant overexpression in CRLM in relation to the HCC tissues. Being significantly up-regulated only in CRLM, CXCR4 displayed an aberrant expression pattern with respect to the HCC tissues.
CONCLUSION: Correlation of CXCR4 expression with CRLM suggests CXCR4 as a potential predictive factor for CRLM. High level expression of CCL20 and its receptor CCR6 in HCC and CRLM with marked up-regulation of CCL20 in CRLM in relation to HCC tissues indicates involvement of the CCL20/CCR6 ligand-receptor pair in the carcinogenesis and progression of hepatic malignancies.
Keywords: Chemokines, Chemokine receptors, Gene expression, Hepatocellular carcinoma, Colorectal liver metastases
INTRODUCTION
Chemokines represent a family of small chemotactic cytokines, initially identified as mediators of leucocyte trafficking and homing. In the last few years, chemokines have been shown to participate also in tumor growth and the lymphatic and even distant spread of malignant tumors[1,2]. Here we compare the expression profiles of several chemokine/chemokine receptor pairs, namely CXCL12/CXCR4, CCL20/CCR6 and CCL19/CCL21/CCR7 in hepatocellular carcinoma (HCC) versus colorectal liver metastases (CRLM). We chose to investigate this group of chemokines and their receptors because their roles in tumor growth and metastasis have recently gained increasing importance[3,4]. Like HCC, which is a highly malignant tumor with a poor prognosis due to its rapidly progressing and infiltrating growth, colorectal cancer (CRC) also still remains one of the leading causes of cancer-related death worldwide[5-8]. The mortality of CRC is principally attributable to the development of metastases, which primarily infest the liver, and CRLM are present in up to 95% of patients in the advanced disease stage.
The CC-chemokines CCL19 and CCL21 are highly expressed in lymph nodes and signal through a common receptor, the lymphocyte chemoattractant receptor CCR7[9-10]. While CCR7 was recently reported to predict lymph node metastasis in colorectal carcinoma and other cancer types[11,12], the cognate CXCL12 receptor CXCR4 has been suggested as a risk factor for the outgrowth of colon carcinoma micrometastases[13] and the invasion and spreading of several other cancers[15-17]. Also CCL20/CCR6 involvement in the neoplastic progression and cancer-specific metastasis of several tumor types is presently reported, with major focus on the amplification of local necroinflammatory response in the liver[18-19], suggesting CCR6 as an important factor in the recruitment of lymphocytes from peripheral blood to HCC[20-21] . However, data concerning the expression profiles and clinical impact of CCL20/CCR6 in HCC and CRLM are still limited and no expression data are currently available concerning the pathophysiological relationship of chemokines in HCC and CRLM. While our group and others previously suggested an association between the CCL20/CCR6 expression in CRC and the promotion of CRLM[22-23], we now demonstrate a correlation between the CCL20/CCR6 expression profile in HCC and CRLM with a marked overexpression of the CCL20 gene product in relation to HCC tissues. Consequently, we hypothesize that the CCL20/CCR6 chemokine receptor pair may be of general importance in the development of hepatic malignancies of different origins.
MATERIALS AND METHODS
Patients
HCC and CRLM specimens and corresponding non-tumorous liver tissues were collected from 11 HCC and 23 CRLM patients who underwent resection in our department between 2002 and 2006. All patients provided informed consent for tissue procurement, which was approved by the ethics commission of the Ärztekammer of the Saarland. The clinical variables presented in Table 1 were obtained from clinical and pathological records according to the UICC TNM classification[24] system.
Table 1.
Clinical characteristics of patients with HCC and CRLM
| Factor | HCC2n = 11 | CRLM3n = 23 |
| Localization of primary tumor | ||
| Liver | 11 | - |
| Colon | - | 14 |
| Rectum | - | 9 |
| Gender | ||
| Male | 5 | 17 |
| Female | 6 | 6 |
| Age, yr4 | 62.6 (29-82) | 65.0 (39-76) |
| Hepatitis (A, B or C) | ||
| Positive | 4 | 1 |
| Negative | 7 | 22 |
| Liver chirrhosis | ||
| Positive | 5 | 6 |
| Negative | 6 | 17 |
| Fibrosis | ||
| Positive | 1 | 3 |
| Negative | 10 | 20 |
| Largest tumor diameter (cm)4 | 4.3 (1.2-6.8) | 10.3 (1.3-19) |
| TNM1 stage of primary tumor | ||
| I | 2 | 2 |
| II | 4 | 2 |
| III | 5 | 15 |
| IV | 0 | 3 |
| Grading | ||
| I | 0 | 2 |
| II | 7 | 16 |
| III | 4 | 5 |
| IV | 0 | 0 |
| Lymphatic permeation | ||
| Positive | 1 | 16 |
| Negative | 10 | 7 |
| Vascular invasion | ||
| Positive | 4 | 1 |
| Negative | 7 | 22 |
| Chemotherapy before operation | 0 | 13 |
| Radiotherapy before operation | 0 | 3 |
Tumor-node-metastasis;
Hepatocellular carcinoma;
Colorectal liver metastases;
Median with range in parentheses.
Tissue preparation
Immediately after resection tissue samples were collected and processed under nucleic acid sterile conditions, snap frozen in liquid nitrogen and then stored at -80°C until RNA and protein were extracted. For corresponding normal tissue we used adjacent healthy tissue from the same resected liver specimen. All tissues obtained were reviewed by an experienced pathologist and examined for the presence of tumor cells. As minimum criteria for usefulness for our studies we only chose tumor tissues in which tumor cells occupied a major component (> 60%) of the tumor biopsy.
Single-strand cDNA synthesis
Total RNA was isolated using RNeasy columns from Qiagen (Hilden, Germany) following the manufacturer’s instructions and RNA integrity was confirmed spectrophotometrically and by electrophoresis on 1% agarose gels. For cDNA synthesis, 5 μg of each patient’s
total RNA sample were reverse-transcribed in a final reaction volume of 50 μL containing 1 × TaqMan RT buffer, 2.5 μmol/L random hexamers, 500 μmol/L each dNTP, 5.5 mmol/L MgCl2, 0.4 U/μL RNase inhibitor, and 1.25 U/μL Multiscribe RT. All RT-PCR reagents were purchased from Applied Biosystems (Applied Biosystems, Foster City, CA). The reaction conditions were 10 min at 25°C, 30 min at 48°C, and 5 min at 95°C.
Real-time PCR
All qRT assays containing the primers and probe mix were purchased from Applied Biosystems, (Applied Biosystems, Foster City, CA) and utilized according to the manufacturer’s instructions. PCR reactions were carried out using 10 μL 2 × Taqman PCR Universal Master Mix No AmpErase® UNG and 1 μL gene assay (Applied Biosystems, Foster City, CA), 8 μL Rnase-free water and 1 μL cDNA template (50 mg/L). The theoretical basis of the qRT assays is described in detail elsewhere[25]. All reactions were run in duplicates along with no template controls and an additional reaction in which reverse transcriptase was omitted to allow for assessment of genomic DNA contamination in each RNA sample. For the signal detection, ABI Prism 7900 sequence detector was programmed to an initial step of 10 min at 95°C, followed by 40 thermal cycles of 15 s at 95°C and 10 min at 60°C and the log-linear phase of amplification was monitored to obtain CT values for each RNA sample. Gene expression of all target genes was analyzed in relation to the levels of the slope matched housekeeping genes Cyclophilin C (CycC) and ß2-Microtubulin (B2M)[26]. Since reporting of data obtained from raw CT values falsely represent the variations, we converted the individual CT values to the linear form as follows:
Fold difference = 2-(mean CT pathological tissue-mean CT calibrator) = 2-delta CT
Hence, the liver of the tumor-neighboring tissue became the 1 × sample, and all other quantities were expressed as an n-fold difference relative to this tissue.
Isolation of total protein
Protein lysates from frozen tissues were precipitated with the RIPA buffer. Protein quantitation was performed using the Pierce BCA protein assay reagent kit (Pierce, Rockford, USA).
Western blot analysis
Chemokine receptors were detected with anti-CXCR4 (1:500, Serotec, AHP442 rabbit anti-human, Serotec, Oxford, UK; 1:5000, BioRad cat. 170-6515 goat anti-rabbit HRP, BioRad, Muenchen, Germany), anti CCR6 (1:500, Biomol, goat anti-human C2099-70B, Biomol, Hamburg, Germany; 1:5000 Santa Cruz, sc-2056 donkey anti-goat HRP) and anti-CCR7 (1:500, Santa Cruz, sc-9700 goat anti-human; 1:5000, Santa Cruz, sc-2056 donkey anti-goat HRP, Santa Cruz Biotechnology, Santa Cruz, CA USA), visualized by ECL Western blotting analysis system (Amersham Biosciences, Piscataway, NJ, USA), and quantified densitometrically. Human cell lysates HL-60 (sc-2209, Santa Cruz Biotechnology, Santa Cruz, CA, USA), Imgenex cell lysates A375, and HeLa (Imgenex, San Diego, USA) served as positive controls.
Enzyme-linked immunosorbant assay
Chemokine protein levels in the different tissue lysates were determined by sandwich-type ELISA according to the manufacturer’s protocol: R&D systems (R&D Systems Inc. Minneapolis, Minnesota, USA) for the quantification of CCL19, CCL20, CXCL12 or ProSci (ProSci, San Diego, USA) for CCL21. The absorbance was read at 450 nm.
Laser capture microdissection
Laser microbeam microdissection (LMM) was employed for obtaining pure tumor cell and pure normal cell samples for subsequent genetic analysis. LMM was performed on three samples for each tissue type and each chemokine, respectively. Histochemical staining was used on cryo sections before microdissection. Specimen preparation, microdissection and catapulting were performed following a laser pressure catapulting protocol according to the manufacturer’s instructions (P.A.L.M. Microlaser Technologies, Bernried, Germany). RNA was extracted using the P.A.L.M. RNA extraction kit and for reverse transcription the invitrogen reverse transcription kit (Invitrogen Life Technologies, Karlsruhe, Germany) was applied. Subsequently quantitative PCR analysis was performed as described earlier.
Statistical analysis
The statistical significance of differences in chemokine and chemokine receptor expression were summarized using mean and SEM (standard error of the mean). All statistical calculations were done with the MedCalc software package (MedCalc software, Mariakerke, Belgium)[27]. Where appropriate, either the Student’s t-test or the Wilcoxon’s rank sum test was applied to test for group differences of continuous variables. P < 0.05 was considered significant.
RESULTS
Chemokine/chemokine receptor expression
To assess potential differences in chemokine/chemokine receptor expression levels in HCC vs CRLM, we performed quantitative RT-PCR analysis in 11 HCC and 23 CRLM specimens and their corresponding tumor neighboring tissues, respectively. The adjacent, non tumor invaded liver tissues of the HCC and CRLM patients served as control groups. qRT analysis of the chemokine ligand CXCL12 displayed no significant difference in gene expression between the tumor and the tumor neighboring tissues in HCC or CRLM as shown in Figure 1A. In contrast to its ligand, the CXCL12 receptor CXCR4 demonstrated significant up-regulation in the CRLM compared with the tumor neighboring liver tissues (P < 0.05) thus demonstrating that CXCL12 and CXCR4 are inversely expressed in CRLM (Figure 1A). However, no significant difference in gene expression was detected for CXCR4 in the HCC tissues between the tumor and the tumor neighboring liver tissues thus indicating a clear difference in CXCR4 expression between HCC and CRLM. CC-chemokine CCL20 was found to be significantly up-regulated in the tumor tissue of patients with HCC and CRLM in comparison to their tumor neighboring tissues (P < 0.05), as shown in Figure 1B. Similarly, the CCL20 receptor CCR6 revealed a significantly higher mRNA expression in the tumor specimens of both HCC and CRLM patients in relation to the corresponding normal liver tissues, respectively (P < 0.05) (Figure 1B). In contrast, we observed no significant difference in CCL19 and CCL21 gene expression between the tumor and tumor neighboring tissues in either tissue type as demonstrated in Figure 1C. Likewise, no significant up- or down-regulation was detected for the corresponding receptor CCR7 in the HCC or CRLM tissues, respectively (Figure 1C). Analysing the differences between gene expressions from matched normal/cancer samples corresponded widely with the results presented in Figure 1 thus ensuring that averaging out the Ct values did not mask significant differences between individual paired samples. Sections of tumor and normal cells have been microdissected in three CRLM and HCC tissue specimens, respectively, followed by subsequent chemokine qRT gene expression analysis that corresponded well with the results presented in Figure 1.
Figure 1.
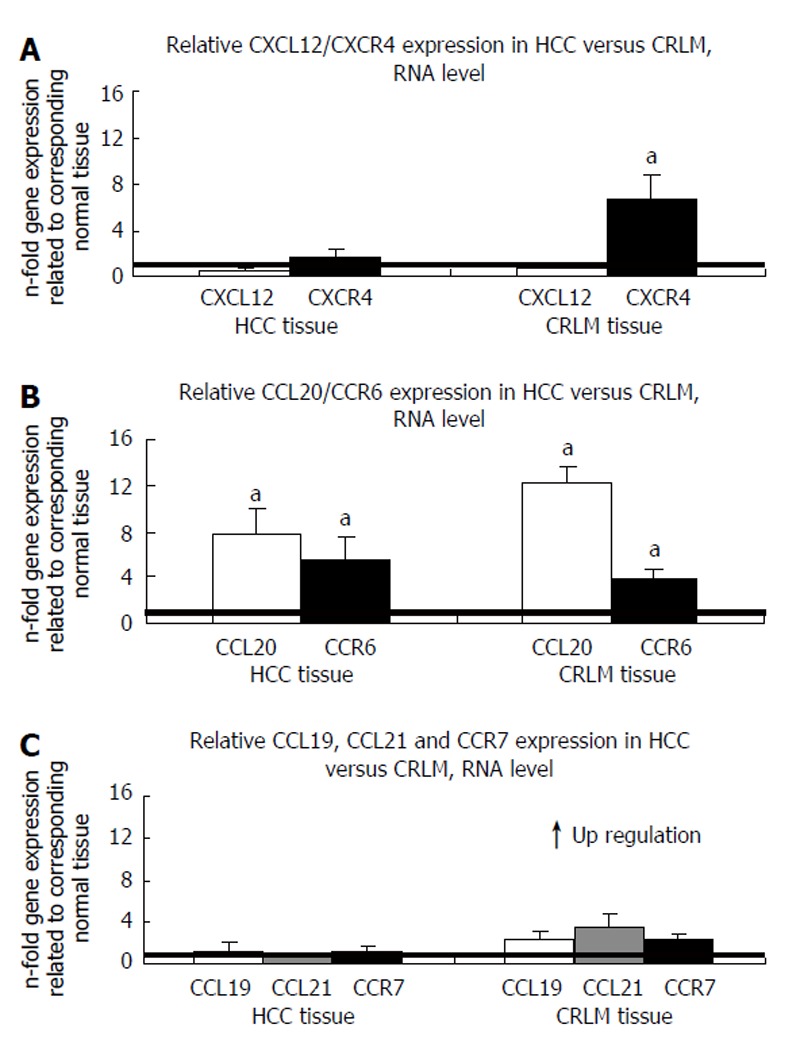
Expression of chemokine/chemokine receptor pairs in HCC and CRLM as determined by Q-RT-PCR. A: CXCL12/CXCR4 expression; B: CCL20/CCR6 expression; C: CCL19/CCL21/CCR7 expression (mean ± SE, aP < 0.05, n = 11 and 23, respectively).
Chemokine/chemokine receptor expression on the protein level
Consistent with our RNA data, gene expression data for CXCL12, as assessed by enzyme-linked immunosorbant assay (ELISA), showed no significant difference in expression between the tumor and tumor neighboring tissues in either tissue type (Figure 2). Similarly, absolute CXCL12 protein quantities were largely the same in HCC and CRLM tissues. In contrast, we found statistically relevant up-regulation of CCL20 protein expression in both CRLM and HCC tissues compared with the respective tumor neighboring tissues (P < 0.05). These findings are again well in line with the RNA expression profiles. Comparative analysis of the absolute protein quantities between CRLM and HCC tissues revealed a significantly higher CCL20 expression of almost 24 000 ng/L CCL20 in the CRLM tissues compared to approximately 6000 ng/L CCL20 in the HCC tissues (P < 0.05) as shown in Figure 2. In accordance with our qRT results we found no significant difference in protein expression for CCL19 and CCL21 between the tumor and tumor neighboring tissues in either tissue type (Figure 2). Despite a seemingly higher CCL21 protein expression level in the CRLM tissues compared with the HCC tissues, this difference was statistically not significant.
Figure 2.
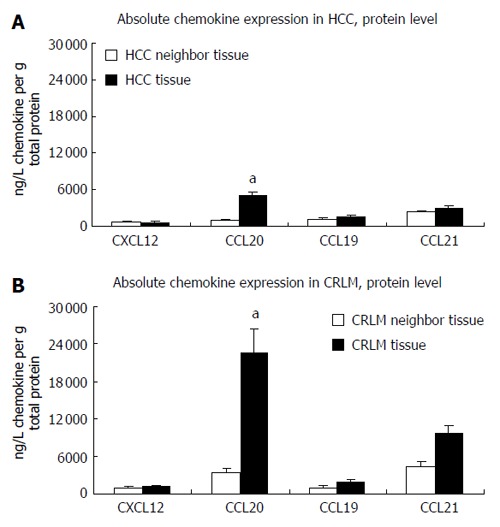
Expression of CXCL12, CCL20, CCL19 and CCL21 in HCC and CRLM as determined by the enzyme-linked immunosorbant assay (ELISA). A: Chemokine expression in HCC; B: Chemokine expression in CRLM. (ng/L chemokine ligand related to 1 g total protein for the HCC and CRLM tissues. mean ± SE, aP < 0.05; n = 11 and 23, respectively).
As assessed by western blot analysis and subsequent densitometric measurements, no significant difference in gene expression was observed for CXCR4 in the HCC tissues, whereas a statistically relevant 4-fold up-regulation of CXCR4 expression was detected in the CRLM tissues (P < 0.05), thus confirming the RNA transcript level analysis. Likewise, we found statistically relevant up-regulation of CCR6 expression in both tissue types (P < 0.05) as shown in Figure 3, but no significant difference in CCR7 expression between the HCC and CRLM tissues thus paralleling our qRT results.
Figure 3.
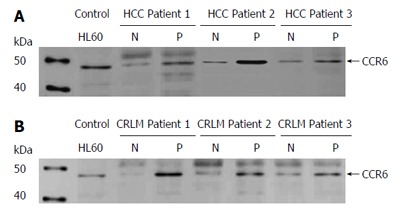
Expression of chemokine receptor CCR6 in HCC and CRLM as determined by Western blot analysis. A: Chemokine expression in HCC; B: Chemokine expression in CRLM. Total cell lysates of tumor (P) and corresponding normal tissues (N) of three patients with HCC and CRLM, respectively, were immunoblotted with antibodies specifically recognizing chemokine receptor CCR6. Cell line HL60 served as a positive control for the detection of CCR6. (n = 11 and 23, respectively).
DISCUSSION
To date, various studies implicate chemokines CXCL12, CCL20, CCL19 and CCL21 and their corresponding receptors not only in inflammatory cell recruitment but also in the tumorigenic process and metastatic homing of tumor cells. Recent data focus special emphasis on their roles in CRC, HCC and liver metastasis of different origins[21,28-31]. These findings prompted us to comparatively investigate their expression profiles in HCC and CRLM as the primary site of hematogenous metastases in CRC.
In recent years chemokine receptor CCR7 attracted considerable interest as a key receptor in determining lymph node metastasis in various malignant processes and tumor types such as leukemia, melanoma, gastric or non-small cell lung cancer[12,32-35], whereas also anti-tumorigenic effects have been demonstrated for the corresponding CCR7 ligand CCL21[36]. However, we observed no correlation between CCR7 expression and HCC or CRLM, respectively. Similarly, the corresponding ligands CCL19 and CCL21 showed no significant difference in their gene expression between the tumor and tumor neighboring tissues in either cancer type. Therefore, we believe that an association of these chemokine/receptor pairs with the progression of CRLM or HCC is rather unlikely.
While investigating CXCL12/CXCR4 expression, we made the interesting observation that CXCR4 was significantly up-regulated in the CRLM compared with the tumor neighboring liver tissues, yet no significant difference in gene expression was detected for CXCR4 in the HCC tissues thus indicating a distinct difference in the CXCR4 expression pattern between HCC and CRLM. These results are in line with recent findings that report CXCR4 gene expression in primary CRC demonstrated significant associations with recurrence and survival suggesting CXCR4 as a prognostic factor for poor disease outcome[29]. Unlike CXCR4, the chemokine ligand CXCL12 displayed no significant difference in gene expression between the tumor and the tumor neighboring tissues in HCC or CRLM thus indicating that CXCL12 and CXCR4 are inversely expressed in CRLM. This type of expression pattern was also demonstrated for CRC cell lines[37]. CXCL12 is presently discussed controversially with respect to its role in promoting tumor growth and metastasis. Various studies suggest CXCL12 involvement in metastasis, angiogenic activity and modulation of tumor immunity[3,14,15,38-40], while others describe efficient antitumor responses promoted by the CXCL12/CXCR4 interaction, suggesting that secretion of CXCL12 in tumors may mediate T-cell-dependent antitumor responses[41-43].
CCL20 is also presently controversially discussed with respect to its role in tumorigenesis. With regard to the chemoattractant properties of CCL20 for dendritic cells (DC), Fushimi et al reported on the tumor suppressive properties of this chemokine showing that CCL20 transgenes attract DC to established murine tumors and suppress tumor growth[44]. However, other studies correlate CCL20 transfection into a mouse tumor cell line with decreased immunogenicity and enhanced tumor growth[45] and recent data correlate increased serum levels of CCL20 in HCC with cancer-related factors[46]. Other reports allocating tumor growth promoting qualities with CCL20, associate CCR6 expression with hepatic metastasis in a rodent model and recent results, based on chemotactic and actin polymerization assays, correlate CCR6 expression with intrahepatic metastasis of HCC[30,47]. In our study, CCL20/CCR6 was the only pair among the chemokine ligand/receptor pairs under investigation that displayed a prominent expression pattern in HCC and CRLM, showing significant up-regulation in the tumor tissues of patients of both cancer types in comparison to the tumor neighboring tissues, respectively. We assume that these high expression levels in the malignant liver tissues of different origins - in one case primary tumor, in the other case liver metastases - indicate a possible pathogenetic role of CCL20 and its receptor in the development of hepatic malignancies. Moreover, we detected significantly higher CCL20 expression in the CRLM tissues compared with the HCC tissues. One possible explanation for this marked CCL20 overexpression in the hepatic metastases could be related to the ferocious malignity of metastatic cells. In other words, the malignant status of a cancer cell might be correlated with CCL20 expression. It is well known that the survival rate for patients is far worse when they have developed metastases at the time of surgery as compared to patients with the same primary tumor who have not developed metastases. It seems that as the chemokine metabolism of a cancer cell becomes increasingly unbalanced the further the malignity of the cell proceeds and the more aggressive a cancer tissue turns. This assumption is supported by previous clinicopathological findings of our group[19], which demonstrate a significant increase in CCL20 expression rates in HCC tissues from grade III tumors in comparison to HCC tissues from grade II tumors. Consequently, the marked CCL20 up-regulation in CRLM in comparison to the HCC tissues reported here could be interpretated as an indication of the extent of alteration in the tumor cells. In line with this theory, we would expect higher CCL20 expression rates in liver metastases in comparison to the moderately differentiated tumor cells of the HCC tissues, since metastatic cells virtually show the highest grading level of tumor differentiation, usually bearing no resemblance with normal liver cells.
In summary, the results presented in this study suggest an association of the CCL20/CCR6 pair and the development and progression of hepatic malignancies and we propose CCL20 as a predictive pathogenetic marker for tumor grading and the existence of liver metastases. Since we also presented evidence for a correlation between CXCR4 expression and CRLM, our results also support theories that suggest CXCR4 as a potential predictive factor for colorectal metastasis of the liver. Since the identification of chemokines as key targets in cancer and metastasis has emerged as a quickly progressing research topic, various chemokine receptor antagonist compounds are presently being developed. The identification of the CCL20/CCR6 and CXCL12/CXCR4 pairs as novel targets in CRLM and other metastatic processes may be of potential clinical value for the staging of primary tumors and the prevention of hepatic recurrences.
Footnotes
S- Editor Pan BR L- Editor Lutze M E- Editor Ma WH
References
- 1.Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 2.Wang JM, Deng X, Gong W, Su S. Chemokines and their role in tumor growth and metastasis. J Immunol Methods. 1998;220:1–17. doi: 10.1016/s0022-1759(98)00128-8. [DOI] [PubMed] [Google Scholar]
- 3.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 4.Kimsey TF, Campbell AS, Albo D, Wilson M, Wang TN. Co-localization of macrophage inflammatory protein-3alpha (Mip-3alpha) and its receptor, CCR6, promotes pancreatic cancer cell invasion. Cancer J. 2004;10:374–380. doi: 10.1097/00130404-200411000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Qian J, Feng GS, Vogl T. Combined interventional therapies of hepatocellular carcinoma. World J Gastroenterol. 2003;9:1885–1891. doi: 10.3748/wjg.v9.i9.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu AX. Hepatocellular carcinoma: are we making progress? Cancer Invest. 2003;21:418–428. doi: 10.1081/cnv-120018233. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 8.McLeod HL, McKay JA, Collie-Duguid ES, Cassidy J. Therapeutic opportunities from tumour biology in metastatic colon cancer. Eur J Cancer. 2000;36:1706–1712. doi: 10.1016/s0959-8049(00)00150-7. [DOI] [PubMed] [Google Scholar]
- 9.Campbell JJ, Bowman EP, Murphy K, Youngman KR, Siani MA, Thompson DA, Wu L, Zlotnik A, Butcher EC. 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3beta receptor CCR7. J Cell Biol. 1998;141:1053–1059. doi: 10.1083/jcb.141.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida R, Imai T, Hieshima K, Kusuda J, Baba M, Kitaura M, Nishimura M, Kakizaki M, Nomiyama H, Yoshie O. Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem. 1997;272:13803–13809. doi: 10.1074/jbc.272.21.13803. [DOI] [PubMed] [Google Scholar]
- 11.Günther K, Leier J, Henning G, Dimmler A, Weissbach R, Hohenberger W, Förster R. Prediction of lymph node metastasis in colorectal carcinoma by expressionof chemokine receptor CCR7. Int J Cancer. 2005;116:726–733. doi: 10.1002/ijc.21123. [DOI] [PubMed] [Google Scholar]
- 12.Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, Inoue H, Mori M. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;62:2937–2941. [PubMed] [Google Scholar]
- 13.Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63:3833–3839. [PubMed] [Google Scholar]
- 14.Geminder H, Sagi-Assif O, Goldberg L, Meshel T, Rechavi G, Witz IP, Ben-Baruch A. A possible role for CXCR4 and its ligand, the CXC chemokine stromal cell-derived factor-1, in the development of bone marrow metastases in neuroblastoma. J Immunol. 2001;167:4747–4757. doi: 10.4049/jimmunol.167.8.4747. [DOI] [PubMed] [Google Scholar]
- 15.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–1837. [PubMed] [Google Scholar]
- 16.Ishikawa T, Nakashiro K, Hara S, Klosek SK, Li C, Shintani S, Hamakawa H. CXCR4 expression is associated with lymph-node metastasis of oral squamous cell carcinoma. Int J Oncol. 2006;28:61–66. [PubMed] [Google Scholar]
- 17.Hu J, Deng X, Bian X, Li G, Tong Y, Li Y, Wang Q, Xin R, He X, Zhou G, et al. The expression of functional chemokine receptor CXCR4 is associated with the metastatic potential of human nasopharyngeal carcinoma. Clin Cancer Res. 2005;11:4658–4665. doi: 10.1158/1078-0432.CCR-04-1798. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu Y, Murata H, Kashii Y, Hirano K, Kunitani H, Higuchi K, Watanabe A. CC-chemokine receptor 6 and its ligand macrophage inflammatory protein 3alpha might be involved in the amplification of local necroinflammatory response in the liver. Hepatology. 2001;34:311–319. doi: 10.1053/jhep.2001.26631. [DOI] [PubMed] [Google Scholar]
- 19.Rubie C, Frick VO, Wagner M, Rau B, Weber C, Kruse B, Kempf K, Tilton B, König J, Schilling M. Enhanced expression and clinical significance of CC-chemokine MIP-3 alpha in hepatocellular carcinoma. Scand J Immunol. 2006;63:468–477. doi: 10.1111/j.1365-3083.2006.001766.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Poon RT, Feng X, Yu WC, Luk JM, Fan ST. Reduced expression of chemokine receptors on peripheral blood lymphocytes in patients with hepatocellular carcinoma. Am J Gastroenterol. 2004;99:1111–1121. doi: 10.1111/j.1572-0241.2004.30265.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Poon RT, Hughes J, Feng X, Yu WC, Fan ST. Chemokine receptors support infiltration of lymphocyte subpopulations in human hepatocellular carcinoma. Clin Immunol. 2005;114:174–182. doi: 10.1016/j.clim.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Rubie C, Oliveira V, Kempf K, Wagner M, Tilton B, Rau B, Kruse B, Konig J, Schilling M. Involvement of chemokine receptor CCR6 in colorectal cancer metastasis. Tumour Biol. 2006;27:166–174. doi: 10.1159/000092777. [DOI] [PubMed] [Google Scholar]
- 23.Ghadjar P, Coupland SE, Na IK, Noutsias M, Letsch A, Stroux A, Bauer S, Buhr HJ, Thiel E, Scheibenbogen C, et al. Chemokine receptor CCR6 expression level and liver metastases in colorectal cancer. J Clin Oncol. 2006;24:1910–1916. doi: 10.1200/JCO.2005.04.1822. [DOI] [PubMed] [Google Scholar]
- 24.Ghadjar P ICC. TNM classification of malignant tumors, 5th ed. New York: Wiley-Liss; 1997. [Google Scholar]
- 25.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 26.Rubie C, Kempf K, Hans J, Su T, Tilton B, Georg T, Brittner B, Ludwig B, Schilling M. Housekeeping gene variability in normal and cancerous colorectal, pancreatic, esophageal, gastric and hepatic tissues. Mol Cell Probes. 2005;19:101–109. doi: 10.1016/j.mcp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Schoonjans F, Zalata A, Depuydt CE, Comhaire FH. MedCalc: a new computer program for medical statistics. Comput Methods Programs Biomed. 1995;48:257–262. doi: 10.1016/0169-2607(95)01703-8. [DOI] [PubMed] [Google Scholar]
- 28.Schimanski CC, Schwald S, Simiantonaki N, Jayasinghe C, Gönner U, Wilsberg V, Junginger T, Berger MR, Galle PR, Moehler M. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11:1743–1750. doi: 10.1158/1078-0432.CCR-04-1195. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Takeuchi H, Lam ST, Turner RR, Wang HJ, Kuo C, Foshag L, Bilchik AJ, Hoon DS. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 30.Dellacasagrande J, Schreurs OJ, Hofgaard PO, Omholt H, Steinsvoll S, Schenck K, Bogen B, Dembic Z. Liver metastasis of cancer facilitated by chemokine receptor CCR6. Scand J Immunol. 2003;57:534–544. doi: 10.1046/j.1365-3083.2003.01263.x. [DOI] [PubMed] [Google Scholar]
- 31.Shibuta K, Mori M, Shimoda K, Inoue H, Mitra P, Barnard GF. Regional expression of CXCL12/CXCR4 in liver and hepatocellular carcinoma and cell-cycle variation during in vitro differentiation. Jpn J Cancer Res. 2002;93:789–797. doi: 10.1111/j.1349-7006.2002.tb01321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takanami I. Overexpression of CCR7 mRNA in nonsmall cell lung cancer: correlation with lymph node metastasis. Int J Cancer. 2003;105:186–189. doi: 10.1002/ijc.11063. [DOI] [PubMed] [Google Scholar]
- 33.Hasegawa H, Nomura T, Kohno M, Tateishi N, Suzuki Y, Maeda N, Fujisawa R, Yoshie O, Fujita S. Increased chemokine receptor CCR7/EBI1 expression enhances the infiltration of lymphoid organs by adult T-cell leukemia cells. Blood. 2000;95:30–38. [PubMed] [Google Scholar]
- 34.Mori T, Kim J, Yamano T, Takeuchi H, Huang S, Umetani N, Koyanagi K, Hoon DS. Epigenetic up-regulation of C-C chemokine receptor 7 and C-X-C chemokine receptor 4 expression in melanoma cells. Cancer Res. 2005;65:1800–1807. doi: 10.1158/0008-5472.CAN-04-3531. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi H, Fujimoto A, Tanaka M, Yamano T, Hsueh E, Hoon DS. CCL21 chemokine regulates chemokine receptor CCR7 bearing malignant melanoma cells. Clin Cancer Res. 2004;10:2351–2358. doi: 10.1158/1078-0432.ccr-03-0195. [DOI] [PubMed] [Google Scholar]
- 36.Yang SC, Batra RK, Hillinger S, Reckamp KL, Strieter RM, Dubinett SM, Sharma S. Intrapulmonary administration of CCL21 gene-modified dendritic cells reduces tumor burden in spontaneous murine bronchoalveolar cell carcinoma. Cancer Res. 2006;66:3205–3213. doi: 10.1158/0008-5472.CAN-05-3619. [DOI] [PubMed] [Google Scholar]
- 37.Brand S, Dambacher J, Beigel F, Olszak T, Diebold J, Otte JM, Göke B, Eichhorst ST. CXCR4 and CXCL12 are inversely expressed in colorectal cancer cells and modulate cancer cell migration, invasion and MMP-9 activation. Exp Cell Res. 2005;310:117–130. doi: 10.1016/j.yexcr.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Koshiba T, Hosotani R, Miyamoto Y, Ida J, Tsuji S, Nakajima S, Kawaguchi M, Kobayashi H, Doi R, Hori T, et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000;6:3530–3535. [PubMed] [Google Scholar]
- 39.Mirshahi F, Pourtau J, Li H, Muraine M, Trochon V, Legrand E, Vannier J, Soria J, Vasse M, Soria C. SDF-1 activity on microvascular endothelial cells: consequences on angiogenesis in in vitro and in vivo models. Thromb Res. 2000;99:587–594. doi: 10.1016/s0049-3848(00)00292-9. [DOI] [PubMed] [Google Scholar]
- 40.Vianello F, Papeta N, Chen T, Kraft P, White N, Hart WK, Kircher MF, Swart E, Rhee S, Palù G, et al. Murine B16 melanomas expressing high levels of the chemokine stromal-derived factor-1/CXCL12 induce tumor-specific T cell chemorepulsion and escape from immune control. J Immunol. 2006;176:2902–2914. doi: 10.4049/jimmunol.176.5.2902. [DOI] [PubMed] [Google Scholar]
- 41.Nomura T, Hasegawa H, Kohno M, Sasaki M, Fujita S. Enhancement of anti-tumor immunity by tumor cells transfected with the secondary lymphoid tissue chemokine EBI-1-ligand chemokine and stromal cell-derived factor-1alpha chemokine genes. Int J Cancer. 2001;91:597–606. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1107>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 42.Dunussi-Joannopoulos K, Zuberek K, Runyon K, Hawley RG, Wong A, Erickson J, Herrmann S, Leonard JP. Efficacious immunomodulatory activity of the chemokine stromal cell-derived factor 1 (SDF-1): local secretion of SDF-1 at the tumor site serves as T-cell chemoattractant and mediates T-cell-dependent antitumor responses. Blood. 2002;100:1551–1558. [PubMed] [Google Scholar]
- 43.Shi M, Hao S, Su L, Zhang X, Yuan J, Guo X, Zheng C, Xiang J. Vaccine of engineered tumor cells secreting stromal cell-derived factor-1 induces T-cell dependent antitumor responses. Cancer Biother Radiopharm. 2005;20:401–409. doi: 10.1089/cbr.2005.20.401. [DOI] [PubMed] [Google Scholar]
- 44.Fushimi T, Kojima A, Moore MA, Crystal RG. Macrophage inflammatory protein 3alpha transgene attracts dendritic cells to established murine tumors and suppresses tumor growth. J Clin Invest. 2000;105:1383–1393. doi: 10.1172/JCI7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonnotte B, Crittenden M, Larmonier N, Gough M, Vile RG. MIP-3alpha transfection into a rodent tumor cell line increases intratumoral dendritic cell infiltration but enhances (facilitates) tumor growth and decreases immunogenicity. J Immunol. 2004;173:4929–4935. doi: 10.4049/jimmunol.173.8.4929. [DOI] [PubMed] [Google Scholar]
- 46.Yamauchi K, Akbar SM, Horiike N, Michitaka K, Onji M. Increased serum levels of macrophage inflammatory protein-3alpha in hepatocellular carcinoma: relationship with clinical factors and prognostic importance during therapy. Int J Mol Med. 2003;11:601–605. [PubMed] [Google Scholar]
- 47.Uchida H, Iwashita Y, Sasaki A, Shibata K, Matsumoto T, Ohta M, Kitano S. Chemokine receptor CCR6 as a prognostic factor after hepatic resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 2006;21:161–168. doi: 10.1111/j.1440-1746.2005.04157.x. [DOI] [PubMed] [Google Scholar]


