Abstract
AIM: To review all studies in the literature that have assessed Hematopoietic cell transplantation (HCT) and Crohn’s disease (CD) with the ultimate aims of determining if this is a viable treatment option for those with CD. A secondary aim was to review the above literature and determine if the studies shed further light on the mechanisms involved in the pathogenesis of CD.
METHODS: An extensive Medline search was performed on all articles from 1970 to 2005 using the keywords; bone marrow transplant, stem cell, hematopoietic cell, Crohn’s disease and inflammatory bowel disease.
RESULTS: We identified one case in which a patient developed CD following an allogeneic HCT from a sibling suffering with CD. Evidence for transfer of the genetic predisposition to develop CD was also identified with report of a patient that developed severe CD following an allogeneic HCT. Following HCT it was found that the donor (that had no signs or symptoms of CD) and the recipient had several haplotype mismatches in HLA class III genes in the IBD3 locus including a polymorphism of NOD2/CARD15 that has been associated with CD. Thirty three published cases of patients with CD who underwent either autologous or allogeneic HCT were identified. At the time of publication 29 of these 33 patients were considered to be in remission. The median follow-up time was seven years, and twenty months for allogeneic and autologous HCT respectively. For patients who underwent HCT primarily for treatment of their CD there have been no mortalities related to transplant complications.
CONCLUSION: Overall these preliminary data suggest that both allogeneic and autologous HCT may be effective in inducing remission in refractory CD. This supports the hypothesis that the hemolymphatic cells play a key role in CD and that resetting of the immune system may be a critical approach in the management or cure of CD.
Keywords: Crohn’s disease, Inflammatory bowel disease, Bone marrow transplant, Stem cells, Hematopoietic cell transplantation
INTRODUCTION
Crohn’s disease (CD) is a chronic inflammatory disease of the gastrointestinal tract which commonly affects young adults. It follows a relapsing and remitting course and there is no known cure. However, approximately 10% to 15% have chronic unremitting active disease, with only 10% remaining in remission over many years[1]. A recent systematic review of population based cohorts estimated the prevalence of CD to be 26.0 to 198.5 cases per 100 000 persons and the incidence to be 3.1 to 14.6 cases per 100 000 person-years in North America[1]. In Europe the overall incidence per 100 000 person-years was 5.6[2]. Health related quality of life and general quality of life is lower in Crohn’s disease patients compared to the normal population[3] and quality of life is inversely related to active disease, hospitalization and surgery[4-7]. Achieving disease remission improves overall health related quality of life, employment rate, mental and physical functioning[8,9]. In three trials patients with CD have increased mortality compared to the normal population. Standardized mortality ratios were 1.4, 1.51 and 1.3 respectively[10-12]. Loftus et al[13] also showed that 20 year survival was 79% for those with CD versus 86% in the normal population. A recent study revealed that up to 32% of all deaths in patients with CD are directly related to complications of their disease[14].
Economic consequences for CD relates both to the loss of productivity, as CD most commonly affects adults in their working years, and the costs of hospitalization, medical and surgical treatment. Longobardi et al[15] estimated the cost of non participation in the labour force in 1998 to be 104.2 million dollars in Canada for patients with inflammatory bowel disease. A study of Crohn’s disease patients in the United States found that surgery accounts for the majority of hospitalization and 40% of the total cost of hospitalization[16]. Crohn’s disease patients who suffer an acute exacerbation requiring hospitalization in the United Kingdom have a 20 fold increase in healthcare costs compared to patients with stable disease[17].
Present therapy is aimed at relieving inflammation and treating signs and symptoms. Therapy consists of non-specific anti-inflammatory agents such as 5-ASA, glucocorticoids, immunomodulators, and anti-tumor necrosis factor therapy. The goals of therapy should include the induction and maintenance of remission and an attempt to heal mucosa with the ultimate goal being restitution of normal intestinal function. Despite the growing use of immunomodulators and new biologic agents the need for intestinal resection has remained stable[18]. Within five years of diagnosis of CD up to 43% of patients require surgical intervention[19]. The impact of infliximab, a monoclonal antibody to tumor necrosis factor has been substantial in treating patients with CD who were previously refractory to standard treatment. In our center response rates to infliximab are 75%-78%. However, the use of infliximab is often limited by its cost. In Canada the median cost of one year of infliximab maintenance is $27 000. For those patients who do not respond to infliximab currently there is no standard medical therapy. The goal of this review is to discuss one current area of research in hematopoietic cell transplantation and Crohn’s disease.
HEMATOPOIETIC CELL TRANSPLAN-TATION (HCT) IN OTHER AUTOIMMUNE DISEASES
HCT includes conditioning (high dose chemotherapy, total body irradiation and/or anti-lymphocyte antibodies) followed by the infusion of the hematopoietic cells. The hematopoietic cells are either directly harvested from the marrow or mobilized from marrow to blood (for e.g. by filgrastim) and harvested by apheresis. In case of autologous transplantation for autoimmune diseases, the graft is typically depleted of T cells (e.g. by immunomagnetic selection of CD34 cells) to reduce the likelihood of reinfusing presumed disease-causing T cells. In spite of this, a small number of T cells may be reinfused and some T cells may survive the conditioning. Allogeneic HCT should theoretically be associated with a higher likelihood of cure of an autoimmune disease than autologous HCT, as typically there is a graft-vs-host reaction that eliminates the presumed disease-causing T cells that survived conditioning. On the other hand, due to graft-vs.-host disease (GVHD), allogeneic transplantation is typically associated with higher transplant-related morbidity and mortality. For this reason, far more autologous than allogeneic transplants have been performed for various autoimmune diseases, in an attempt to “reset the immune system”.
HCT has been performed in more than 700 patients with autoimmune diseases[20]. The most frequent indications have been systemic sclerosis (SSc), multiple sclerosis (MS), rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE) (Figure 1). The results are reviewed below. For MS, the European Blood and Marrow Transplant (EBMT) registry reported data on autologous transplantation in 85 patients with severe progressive disease given cyclophosphamide and/or growth factors for stem cell mobilization, then combination chemotherapy ± total body irradiation (TBI) and anti-lymphocyte globulin. Transplant related mortality at three years was 6% with progression free survival of 74% and overall survival 90%[21]. Marked improvement in gadolinium-enhancing MRI lesions was seen. Unfortunately, filgrastim-induced neurological deterioration was seen in 22 patients; this was typically transient but six patients had persistent deficits. Recently updated analysis of 183 patients reported to the EBMT registry reveals 5.5% treatment-related mortality[20].
Figure 1.
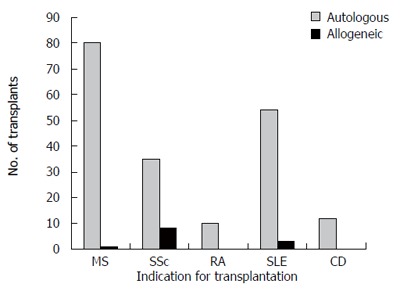
Number of hematopoietic cell transplants for multiple sclerosis (MS), scleroderma/systemic sclerosis (SSc), rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and Crohn’s disease (CD) registered to the Center for International Blood and Marrow Transplant Research (CIBMTR) by 30 teams in North America, by September, 2005.
Several single-center PhaseI/II studies with 15 or more patients have been completed in Europe and show a progression-free survival at one to three years ranging from 70% to 80% with transplant-related mortality ranging from 0%-3%[22]. A US multicenter PhaseI/II study of 26 MS patients who received autologous stem cell transplant after conditioning with cyclophosphamide, TBI and anti-thymocyte globulin (ATG) conditioning and granulocyte colony stimulating factor (G-CSF) mobilization demonstrated a three year projected survival of 91% and disease progression of 27% at 24 mo median follow-up[23]. One patient had a neurological flare during filgrastim therapy and one deteriorated during transplant; prophylactic steroid use may have prevented this in further patients. A Phase III randomized trial is underway in Europe (ASTIMS trial) comparing autologous HCT using high dose chemotherapy (carmustine, etoposide, araC and melphalan) and ATG vs low dose chemotherapy (mitoxantrone) alone.
Systemic sclerosis (SSc) is a debilitating multiorgan disease with a mortality rate of 40% to 50% at five years in high risk patients with high skin scores and involvement of the heart, lung or kidney[24-26]. Per the EBMT/EULAR (European League Against Rheumatism) registry, of the first 57 patients transplanted for SSc, 50 have been followed up for more than 6 mo (median 22.9 mo) and had an overall functional response rate of 92% (14 complete responses, 35 partial responses) and no response in 8%[27]. Skin score improvement > 25% was durable at two years in 79% of patients and lung function remained stable overall. Of responding patients, 35% showed evidence of relapse at nine months with projected 48% disease progression at five years and 72% survival at one year. Treatment-related mortality was 8.7%. In a US study of 30 SSc patients of autologous HCT using conditioning with cyclophosphamide, TBI and anti-thymocyte globulin (ATG) resulted in 75% of patients surviving at median follow-up of 36 mo with 12 of 12 assessable patients showing significant improvement in skin scores or the Health Assessment Questionnaire (HAQ) disability index at 12 mo[28]. Two of the first eight patients died of pulmonary toxicity, prevented in subsequent patients by lung shielding during TBI, two other patients died of treatment-related causes and two of progressive disease. In 19 assessable patients major organ function (lung, cardiac, renal) was stable. A French study showed response in 8 of 11 patients given autologous transplant with 36% of patients deceased at 18 mo follow-up (three of disease, one of treatment-related mortality)[26]. Four other patients had active SSc requiring treatment 6-12 mo post transplant. A phase III trial is now accruing patients in Europe (ASTIS trial), comparing monthly pulse IV cyclophosphamide 750 mg/m2 for 12 mo vs autologous HCT using mobilization with cyclophosphamide/filgrastim and conditioning with cyclophosphamide/ATG. No deaths have been seen in the first 42 patients. Another Phase III trial has been recently opened in the US (SCOT trial), comparing monthly pulse IV cyclophospamide (also 750 mg/m2 for 12 mo) versus autologous HCT using cyclophosphamide/filgrastim mobilization and conditioning with cyclophosphamide, ATG and total body irradiation.
For RA treated with autologous HCT, EBMT registry data revealed no treatment-related mortality in 76 patients who had failed a median of five disease-modifying anti-rheumatic drugs (DMARDS) at a mean follow-up of 16 (3-55) mo[29]. Two thirds of patients obtained American College of Rheumatology score improvements of 50% or better (ACR50), improved function on the HAQ, and tender joints improved for at least 18 mo despite the need for 73% of patients to restart DMARDs within 1 year. In a small study of 14 Dutch patients, 8 of 12 patients responded although 57% required reintroduction of DMARDS at a median of 105 d; three of these heavily treated and refractory patients then responded to DMARDS[30]. Thirty three Australian patients revealed no treatment related mortality with an ACR20 in 70% of patients and ACR20 in 39%, but disease recurred in 29 patients, at a mean of 180 d[31]. Further studies may clarify the role of HCT in rheumatoid arthritis in the context of newer biologic therapies.
Systemic lupus erythematosus has diverse clinical manifestations and an overall survival at ten years of 90%, with a subset of patients refractory to standard immunosuppressive therapy. In a study of 17 patients with refractory SLE given cyclophosphamide for stem cell collection, followed by cyclophosphamide, ATG, and methylprednisolone, 11% died after mobilization of treatment-related toxicity[32]. Follow up at 2 to 66 mo, revealed no deaths in the other 15 patients, with significant reductions in autoantibody titers, prednisone requirements, proteinuria and improved serum complement levels. Two of seven patients followed more than 30 mo relapsed and required further treatment. In the EBMT database, 27 of 51 patients improved, another 14 improved with subsequent relapse, and 7 patients (11%) died[33].
Across all disease groups, mobilization regimens have been associated with toxicity - in the EMBT registry database there was a mortality rate of 1.5%, largely from cyclophosphamide cardiotoxicity and filgrastim- induced disease flares[34].
Treatment-related mortality in the first 470 patients of the EBMT registry who received an autotransplant was 7 to 8% with marked differences between disease groups; although there was a rate of 12.5% in scleroderma patients, only 1 of 70 patients with rheumatoid arthritis died[34]. Death rates are likely related to severity of underlying illness/organ dysfunction and in SSc is associated with pulmonary artery pressure > 50 mm Hg and total body irradiation without lung shielding[35]. Patient selection is key; there have been no deaths in the last 2 years in MS and SSc trials.
BONE MARROW DERIVED CELLS PLAY A CRITICAL ROLE IN IBD
Genetic studies suggest that in patients with inflammatory bowel disease (IBD) there may be several defects in the hemolymphatic (“inflammatory”, hematopoietic cell-derived) cells[36]. Mutations in the NOD2/CARD15 gene increase the risk of developing Crohn’s disease. NOD2/CARD15 is expressed in intestinal epithelial cells and monocytes/macrophages[37,38]. Recognition of intracellular bacteria in intestinal epithelial cells is impaired in 2030insC mutant NOD2[39]. Interestingly presence of NOD2 gene polymorphism, has also been associated with risk of gut GVHD[40,41]. Holler et al[41] found that the presence of single nucleotide polymorphisms of the NOD2/CARD15 gene in both the recipient and donor increased the incidence of GVHD to 56% compared to 18% in cases with no mutations from either recipient or donor.
Interestingly, a 37 years old patient developed CD after receiving an allogeneic marrow transplant from a sibling that had CD[42]. Furthermore, genetic studies showed that another patient that developed severe CD following an allogeneic bone marrow transplant may have developed the disease due to the transfer of known susceptibility genes[43]. In this case, CD developed following receiving marrow from a donor (that did not exhibit CD) but had several haplotype mismatches in HLA class III genes in the IBD3 locus including a polymorphism of NOD2/CARD15 that has been associated with CD[43].
The role of bone marrow-derived cells in the intestine following transplant is presently unclear. Both in animal models as well as in patients it appears that bone marrow derived cells are involved in the healing process following intestinal injury and may contribute to various components of the mucosa including myofibroblasts, endothelium as well as possibly the epithelium[44,45].
Given the above evidence for hematolymphatic cell playing an important role in IBD pathogenesis, a hypothesis has been put forth that HCT may induce remission of IBD. See Figure 2 for the proposed (speculative) mechanism of action. In this scenario macrophages (orange) present peptides (purple diamond) derived from patient cells or intestinal microorganisms (yellow) to T cells (green). The T cells become stimulated and secrete cytokines like tumor necrosis factor alpha (TNF-α) which can directly damage enterocytes and/or amplify the inflammatory response. The macrophages present the peptide by major histocompatibility complex (MHC) class II (e.g., HLA-DR) and the T cells recognize the MHC-peptide complex through T cell receptors. Only macrophages and T cells are depicted here for simplicity, though other hematolymphatic cells (cells derived from hematopoietic stem cells) may participate in the pathogenesis, including neutrophils, eosinophils, dendritic cells and mast cells. Likewise, only enterocytes are depicted here despite other intestinal non-hematolymphatic cells like fibroblasts may participate in the pathogenesis. Genetic predisposition to CD may involve non-hematolymphatic cells (e.g., altered genes needed for the healing of epithelial cells from injury may be missing or altered) or hematolymphatic cells (e.g., altered genes needed for T cells to undergo apoptosis). The latter mechanism may prevail given the ability of HCT to induce durable remission of CD. The genetic predisposition to CD involving the hematolymphatic cells may be due to inherited DNA sequences (e.g., coding for HLA-DR type capable of presenting the pertinent peptide) or DNA sequences generated during ontogeny (e.g., coding for T cell receptor capable of recognizing the peptide). Allogeneic HCT may cure CD by replacing all recipient hematolymphatic cells with donor hematolymphatic cells harboring DNA sequences that do not predispose to CD. Autologous HCT may cure CD by replacing the hematolymphatic cells which (or the ancestors of which) generated the predisposing DNA sequence in ontogeny (e.g., the T cell clone capable of recognizing the peptide) with healthy autologous hematolymphatic cells (e.g., T cell clones generated de novo in the thymus after the HCT that cannot recognize the peptide). The cells that are thought to be replaced by the allogeneic or autologous HCT are pointed out by the red arrows. The pathogenesis of CD is based in part on the following references; [1-4].
Figure 2.
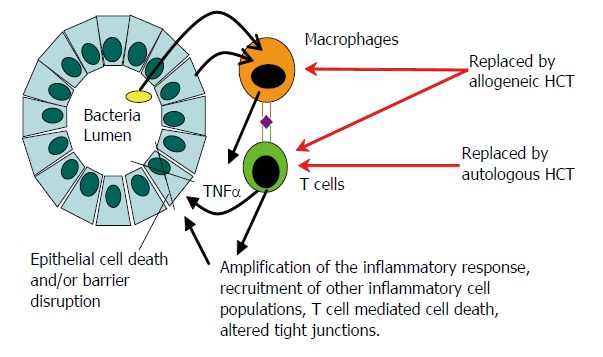
Pathogenesis of CD and proposed mechanism of action of HCT. See text for details.
ALLOGENEIC HEMATOPOIETIC CELL TRANSPLANTATION (HCT) IN CROHN’S DISEASE
To date there are no reported cases of allogeneic bone marrow or HCT performed for the primary treatment of CD. There are 14 patients reported in the literature that had CD and underwent allogeneic HCT for hematological disorders (Table 1). Ditschkowski et al[46] reported a case series in which 10 out of 11 patients remained free of inflammatory bowel disease following allogeneic HCT for hematological malignancy with a median follow-up time of 34 mo. Seven out of the eleven patients had Crohn’s disease. All patients received cyclophosphamide as part of different conditioning regimens. All patients except for two received total body irradiation. The one patient with recurrent IBD symptoms after transplant failed to demonstrate any endoscopic or radiological recurrence of IBD. There was one transplant-related mortality reported at ten months from opportunistic infection. The usefulness of this study for the determination of whether allogeneic HCT may cure CD is limited by the fact that at the last follow up only two patients were off of immunosuppressive drugs.
Table 1.
Allogeneic transplant cases
| Demographics & indication for HCT | Disease activity before HCT | Complications of IBD before HCT | IBD duration (yr) | Donor type | Conditioning therapy | T cell deplet. | |
| Ditschkowski et al[46] Total 11 patients CD-7 UC-4 | Median age 41 Male: 5 Female: 6 CML: 9 AML: 1 MDS: 1 | Inactive: 5 Low Act: 6 | Fistulae: 3 Obstruction: 2 EIM: 2 Surgery: 4 | Median: 10 | HLA- identical sibling - 8 HLA- identical unrelated donor- 2 Mismatched unrelated donor- 1 | TBI + Cy: 6 TBI + Cy + ATG: 1 TBI + Cy + ATG + thiotepa: 1 TBI + Cy + thiotepa:1 Busulfan + Cy: 2 | Yes: 3 No: 8 |
| Lopez-Cubero et al[47] | 27 Male CML | Inactive | EIM | 11 | HLA- match brother | TBI + Cy | No |
| 27 Male CML | Active | Obstruction Fistula | 5 | HLA- match sister | TBI + Cy | No | |
| 41 Male CML | Active | Surgery Fistula EIM | 9 | HLA- match sister | TBI + Cy | No | |
| 41 Male CML | Active | Sclerosing cholangitis | 8 | HLA- match unrelated donor | TBI + Cy | No | |
| 38 Male CML | Active | Surgery EIM, ↑ ALP | 3 | One antigen mismatched brother | TBI + Cy | No | |
| 46 Male AML | Active | Sclerosing cholangitis | 29 | HLA- match sister | TBI + Cy | No | |
| Talbot et al[48] | 35 Male AML | Active | Surgery | 7 | HLA- match brother | TBI + Cy | No |
ALP: Alkaline phosphatase; AML: Acute myeloid leukemia; ATG: Antithymocyte globulin; CD: Crohn’s disease; CML: Chronic myeloid leukemia; Cy: Cyclophosphamide; EIM: Extraintestinal manifestations; HCT: Hematopoietic cell transplantation; MDS: Myelodysplastic syndrome; TBI: Total body irradiation; UC: Ulcerative colitis; Act: Activity; deplet: depletion.
Lopez-Cubero et al[47] reported a case series of six patients with Crohn’s disease who underwent allogeneic marrow transplantation for leukemia. Five of these patients had active CD at the time of transplant. All patients received cyclophosphamide and total body irradiation as conditioning therapy. Two patients had sclerosing cholangitis. One patient died of sepsis at 3 mo post-transplant. The remaining five patients were free of CD related symptoms more than one year post transplant. Four of these five patients had sustained remission of CD at 54 to 183 mo post-transplant. One patient had relapse of CD at d 545 post transplant. Interestingly, the relapsed patient had mixed donor-host hematopoietic chimerism, whereas the four patients who achieved sustained remission were assumed to be complete chimeras as they had GVHD in the first two years after HCT. Immunosuppressive drugs were discontinued at least six months prior to the last follow up in the four patients with sustained CD remission.
An additional case has been reported involving a 35 year old male with CD who was transplanted for AML and at eight years post transplant was free of symptoms and signs of CD[48]. In summary of the 14 published cases of allogeneic bone marrow or stem cell transplant in patients with CD, 11 patients have achieved remission (Table 2). The median follow up time was seven years. Of seven patients followed for CD after they have discontinued immunosuppressive drugs, six patients are in remission of CD. There have been two transplant-related deaths among the 14 cases.
Table 2.
Outcomes of allogeneic transplant cases
| GVHD | Duration of immunosuppressant post HCT (mo) | Length of follow up (mo) | Remission of CD | Ongoing CD medication or immunosuppressant | |
| Ditschkowski et al[46] | Acute: 8 Chronic: 0 | 2 patients off immunosuppressants | 3-117 Median 34 | Yes: 6 No: 1 | Not stated |
| Lopez-Cubero et al[47] | Acute and Chronic | 10 | 183 | Yes | No |
| Acute and Chronic | 70 | 119 | Yes | No | |
| Acute | 6 | 70 Died of suicide | No - recurrent d 545 | Prednisone | |
| Acute and Chronic | 18 | 101 | Yes | No | |
| Acute and Chronic | 48 | 54 Died of myocardial infarction | Yes | No | |
| Acute | Not detected | Died of septic shock d 97 | Not detected | Not detected | |
| Acute | 2 | 96 | Yes | No |
GVHD: Graft versus host disease.
AUTOLOGOUS HEMATOPOIETIC CELL TRANSPLANTATION IN CROHN’S DISEASE
To date there have been 19 patients who have been reported to have both Crohn’s disease and autologous HCT. Patients who underwent transplantation for the primary treatment of Crohn’s disease had previously failed standard medical treatment including infliximab. The first case was published in 1993. A 41 year old female with non-Hodgkin’s lymphoma and CD underwent an autologous bone marrow transplant. The follow-up period was short. However the patient was symptom free at six months[49]. Three additional cases who underwent transplant for malignancies have also remained in clinical remission[50-52].
Recently, Oyama et el[53] and colleagues reported a study in which 12 patients with refractory CD underwent autologous hematopoietic cell transplantation. All of their patients had failed traditional medical treatment including infliximab with a minimal CDAI ≥ 250. Majority of these patients had a history of surgery. Mobilization was with cyclophosphamide and filgrastim, and conditioning with cyclophosphamide and equine ATG. The peripheral blood stem cells were T-cell depleted. There was sustained remission (CDAI ≤ 150) in 11 of 12 patients after a median follow-up of 18.5 mo. One patient experienced CD relapse at 15 mo post-transplant. All patients tolerated the BMT well and there were no transplant-related deaths. One patient died of accidental cause at 37 mo post HCT. Follow-up was at 6 and 12 mo post HCT and then yearly, and included at each visit small bowel radiograph and colonoscopy.
Collectively, 18 out of 19 patients have achieved clinical remission over a median follow up time of 20 mo (Table 3). Only two of these patients are on any ongoing medications again suggesting that HCT may result in CD remission without any need for ongoing medications. Fourteen of these patients underwent autologous HCT for primary treatment of their CD. The majority of these 18 patients had active disease prior to HCT with a history of CD complications including surgery and failure of infliximab therapy in patients where the primary indication for HCT was CD. There has been no mortality related to transplant complications.
Table 3.
Autologous transplant cases
| Demographics & indication for HCT | Disease activity before HCT | Complications of CD | Duration of CD (yr) | Mobilization Conditioning | T cell deplet | Length of follow-up (mo) | Remiss. of CD | Ongoing meds | ||
| Oyama[53] | 21 Female CD | Active | Fistulae, Surgery | 10 | Cy + GCSF | Cy + ATG | Yes | 37 | Yes | No |
| 16 Male CD | Active | Fistulae, Obstruction, GR | 7 | Cy + GCSF | Cy + ATG | Yes | 36 | Yes | No | |
| 38 Female CD | Active | Fissures, Obstruction, Surgery | 20 | Cy + GCSF | Cy + ATG | Yes | 27 | Yes | No | |
| 27 Female CD | Active | Fistulae, EIM, Surgery | 12 | Cy + GCSF | Cy + ATG | Yes | 25 | No | Mtx, pred. | |
| 35 Male CD | Active | Fistulae, Obstruction, Surgery | 12 | Cy + GCSF | Cy + ATG | Yes | 24 | Yes | No | |
| 27 Female CD | Active | Fistula, Surgery | 6 | Cy + GCSF | Cy + ATG | Yes | 20 | Yes | No | |
| 25 Male CD | Active | Perianal disease, Surgery | 13 | Cy + GCSF | Cy + ATG | Yes | 17 | Yes | No | |
| 15 Male CD | Active | EIM, GR, Surgery | 8 | Cy + GCSF | Cy + ATG | Yes | 16 | Yes | No | |
| 31 Female CD | Active | Perianal disease, EIM, Surgery | 10 | Cy + GCSF | Cy + ATG | Yes | 14 | Yes | No | |
| 37 Male CD | Active | Perianal disease | 6 | Cy + GCSF | Cy + ATG | Yes | 14 | Yes | No | |
| 16 Male CD | Active | Fistula, EIM | 1.5 | Cy + GCSF | Cy + ATG | Yes | 7 | Yes | No | |
| 27 Male CD | Active | Perforation, EIM, Surgery | 14 | Cy + GCSF | Cy + ATG | Yes | 7 | Yes | No | |
| Scime[58] | 55 Male CD | Active | EIM | 2 | Cy + GCSF | Cy + ATG | Yes | 5 | Yes | No |
| Kreisel[59] | 36 Male CD | Active | Fistulae, Abscess, Surgery | 14 | Cy + GCSF | Cy | Yes | 10 | Yes | Mtx, pred. |
| Soderholm[52] | 57 Female AML | NS | Obstruction, Fistula, Surgery | NS | NS | Cy + TBI | No | 60 | Yes | No |
| Musso[60] | 30 Male Hodgkin’s Lymph. | Active | Surgery | 10 | Cy + GCSF | Idarubicin Melphalan | NS | 39 | Yes | No |
| Kashyap[51] | 20 Male NHL | NS | Perianal disease, Obstruction, Surgery | 8 | DHAP | Cy + TBI + VP-16 | No | 84 | Yes | No |
| Castro[50] | Age NS, Female Breast ca | Active | NS | 11 | NS | NS | NS | 36 | Yes | Asacol |
| Drakos[49] | 41 female NHL | Inactive | Fistula, Surgery | 22 | NS | BCNU + VP- 16 + Cy + Ara-C + melphalan | NS | 6 | Yes | No |
AML: Acute myeloid leukemia; ATG: Antithymocyte globulin; BCNU: Carmustine; CD: Crohn’s disease; Cy: Cyclophosphamide; D: Duration of Crohn’s disease in years; DHP: Dexamethasone, high dose Ara-C, platinum; EIM: Extraintestinal manifestation; GR: Growth retardation; NHL: Non Hodgkin’s lymphoma; NS: Not stated; TBI: Total body irradiation. VP-16: Etoposide; Mtx: Methotrexate; pred: prednisone; Remiss: Remission; meds: medications; deplet: depletion.
HCT VERSUS IMMUNOSUPPRESSION/GCSF ALONE
Cyclophosphamide and GCSF were used as the mobilization regimen in the study by Oyama et al[54]. Arguably these two agents may explain part of the success of the HCT as cyclophosphamide has been shown to be effective in achieving remission in Crohn’s disease and GCSF may shift the Th1/Th2 balance toward Th2 (whereas Th1 cells predominate in CD)[55]. In pediatric patients with IBD, IBD improved during treatment of glycogen storage disease with GCSF[56]. More recently a randomized placebo control trial showed an improvement in CDAI score and rates of remission with GCSF in CD[57].
However, three cases suggest that sustained clinical remission with HCT is likely not due just to cyclophosphamide and GCSF. Scime et al[58] reported a case of a 55 year old male who underwent HCT for primary treatment of CD. He showed no improvement in colonic lesions one month after mobilization therapy with cyclophosphamide and GCSF. He subsequently achieved both significant endoscopic and histological improvement after autologous HCT. Five months after HCT the patient remains in clinical remission. Kreisel et al[59] describe a 36 year old male who also underwent HCT for the primary treatment of CD. He continued to have persistent histological changes and recurrent CD nine months after mobilization therapy with cyclophosphamide and GCSF (before HCT). Ten mo after HCT the CD was improved, in clinical remission with minimal histological changes. A third case involved a 30 year old male who underwent an autologous transplant for Hodgkin’s lymphoma. He had partial improvement of his CD after mobilization therapy, however, required persistent steroid therapy. At 38 mo after HCT he remains in clinical remission[60].
In Europe a randomized Phase III trial of autologous stem cell transplantation in Crohn’s disease (ASTIC) has been established that should answer the question of whether the autologous transplantation adds significantly to the effect of immunosuppression/GCSF alone. Patients are randomized into those receiving mobilization chemotherapy with GCSF only versus mobilization chemotherapy with GCSF followed by autologous transplantation[61].
SUMMARY OF HCT CASES IN CD
In total there are now 33 cases published of patients with CD who underwent either autologous or allogeneic bone marrow or hematopoietic stem cell transplantation. Twenty nine out of these thirty three patients are considered in remission. There were two cases of transplant related mortality. Importantly of the fourteen cases transplanted primarily for treatment of CD there have been no mortalities related to the transplant. Overall these preliminary data suggest that both allogeneic and autologous HCT may be effective in inducing remission in refractory CD. This supports the hypothesis that the hematolymphatic (inflammatory, bone marrow-derived) cells play a key role in CD and that resetting of the immune system may be a critical approach in the management or cure of CD.
The course of many autoimmune diseases including CD is by nature relapsing and remitting, underscoring the need for control populations with comparable severity of disease to evaluate outcomes in HCT. There is a need for objectively validated disease severity scoring, and for establishing prognostic indicators to identify candidates at high risk for poor disease outcome who might benefit from aggressive treatment. Of the cases where transplant was the primary treatment for CD, there have been no transplant related mortalities. However HCT for CD has to be approached with caution as transplantation for other autoimmune diseases has been associated with a transplant-related mortality of 0 to 25% (Passweg, personal communication on the ASTIS trial)[21,22,26-28,33,34].
Potential candidates for phase II/III trials would include patients who have failed standard medical treatment including infliximab (or can not tolerate it), who are unlikely to benefit from further surgery, and those with severe fistulizing disease not responding to conventional treatment.
Footnotes
S- Editor Pan BR L- Editor Alpini GD E- Editor Liu WF
References
- 1.Loftus EV Jr, Schoenfeld P, Sandborn WJ. The epidemiology and natural history of Crohn's disease in population-based patient cohorts from North America: a systematic review. Aliment Pharmacol Ther. 2002;16:51–60. doi: 10.1046/j.1365-2036.2002.01140.x. [DOI] [PubMed] [Google Scholar]
- 2.Shivananda S, Lennard-Jones J, Logan R, Fear N, Price A, Carpenter L, van Blankenstein M. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD) Gut. 1996;39:690–697. doi: 10.1136/gut.39.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bargetzi MJ, Passweg J, Baertschi E, Schoenenberger A, Gwerder C, Tichelli A, Burger J, Mingrone W, Herrmann R, Gratwohl A, et al. Mobilization of peripheral blood progenitor cells with vinorelbine and granulocyte colony-stimulating factor in multiple myeloma patients is reliable and cost effective. Bone Marrow Transplant. 2003;31:99–103. doi: 10.1038/sj.bmt.1703787. [DOI] [PubMed] [Google Scholar]
- 4.Blondel-Kucharski F, Chircop C, Marquis P, Cortot A, Baron F, Gendre JP, Colombel JF. Health-related quality of life in Crohn's disease: a prospective longitudinal study in 231 patients. Am J Gastroenterol. 2001;96:2915–2920. doi: 10.1111/j.1572-0241.2001.4681_b.x. [DOI] [PubMed] [Google Scholar]
- 5.Cohen RD. The quality of life in patients with Crohn's disease. Aliment Pharmacol Ther. 2002;16:1603–1609. doi: 10.1046/j.1365-2036.2002.01323.x. [DOI] [PubMed] [Google Scholar]
- 6.Bernklev T, Jahnsen J, Aadland E, Sauar J, Schulz T, Lygren I, Henriksen M, Stray N, Kjellevold O, Vatn M, et al. Health-related quality of life in patients with inflammatory bowel disease five years after the initial diagnosis. Scand J Gastroenterol. 2004;39:365–373. doi: 10.1080/00365520310008386. [DOI] [PubMed] [Google Scholar]
- 7.Saibeni S, Cortinovis I, Beretta L, Tatarella M, Ferraris L, Rondonotti E, Corbellini A, Bortoli A, Colombo E, Alvisi C, et al. Gender and disease activity influence health-related quality of life in inflammatory bowel diseases. Hepatogastroenterology. 2005;52:509–515. [PubMed] [Google Scholar]
- 8.Casellas F, López-Vivancos J, Badia X, Vilaseca J, Malagelada JR. Impact of surgery for Crohn's disease on health-related quality of life. Am J Gastroenterol. 2000;95:177–182. doi: 10.1111/j.1572-0241.2000.01681.x. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenstein GR, Yan S, Bala M, Hanauer S. Remission in patients with Crohn's disease is associated with improvement in employment and quality of life and a decrease in hospitalizations and surgeries. Am J Gastroenterol. 2004;99:91–96. doi: 10.1046/j.1572-0241.2003.04010.x. [DOI] [PubMed] [Google Scholar]
- 10.Blomqvist P, Ekbom A. Inflammatory bowel diseases: health care and costs in Sweden in 1994. Scand J Gastroenterol. 1997;32:1134–1139. doi: 10.3109/00365529709002993. [DOI] [PubMed] [Google Scholar]
- 11.Persson PG, Bernell O, Leijonmarck CE, Farahmand BY, Hellers G, Ahlbom A. Survival and cause-specific mortality in inflammatory bowel disease: a population-based cohort study. Gastroenterology. 1996;110:1339–1345. doi: 10.1053/gast.1996.v110.pm8613037. [DOI] [PubMed] [Google Scholar]
- 12.Jess T, Winther KV, Munkholm P, Langholz E, Binder V. Mortality and causes of death in Crohn's disease: follow-up of a population-based cohort in Copenhagen County, Denmark. Gastroenterology. 2002;122:1808–1814. doi: 10.1053/gast.2002.33632. [DOI] [PubMed] [Google Scholar]
- 13.Loftus EV Jr, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Crohn's disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology. 1998;114:1161–1168. doi: 10.1016/s0016-5085(98)70421-4. [DOI] [PubMed] [Google Scholar]
- 14.Jess T, Loftus EV, Harmsen WS, Zinsmeister AR, Tremaine WJ, Melton LJ 3rd, Munkholm P, Sandborn WJ. Survival and cause specific mortality in patients with inflammatory bowel disease: a long term outcome study in Olmsted County, Minnesota, 1940-2004. Gut. 2006;55:1248–1254. doi: 10.1136/gut.2005.079350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longobardi T, Jacobs P, Bernstein CN. Work losses related to inflammatory bowel disease in the United States: results from the National Health Interview Survey. Am J Gastroenterol. 2003;98:1064–1072. doi: 10.1111/j.1572-0241.2003.07285.x. [DOI] [PubMed] [Google Scholar]
- 16.Cohen RD, Larson LR, Roth JM, Becker RV, Mummert LL. The cost of hospitalization in Crohn's disease. Am J Gastroenterol. 2000;95:524–530. doi: 10.1111/j.1572-0241.2000.01779.x. [DOI] [PubMed] [Google Scholar]
- 17.Bassi A, Dodd S, Williamson P, Bodger K. Cost of illness of inflammatory bowel disease in the UK: a single centre retrospective study. Gut. 2004;53:1471–1478. doi: 10.1136/gut.2004.041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn's disease on the need for intestinal surgery. Gut. 2005;54:237–241. doi: 10.1136/gut.2004.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veloso FT, Ferreira JT, Barros L, Almeida S. Clinical out-come of Crohn's disease: analysis according to the vienna classification and clinical activity. Inflamm Bowel Dis. 2001;7:306–313. doi: 10.1097/00054725-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Tyndall A, Saccardi R. Haematopoietic stem cell transplantation in the treatment of severe autoimmune disease: results from phase I/II studies, prospective randomized trials and future directions. Clin Exp Immunol. 2005;141:1–9. doi: 10.1111/j.1365-2249.2005.02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fassas A, Passweg JR, Anagnostopoulos A, Kazis A, Kozak T, Havrdova E, Carreras E, Graus F, Kashyap A, Openshaw H, et al. Hematopoietic stem cell transplantation for multiple sclerosis. A retrospective multicenter study. J Neurol. 2002;249:1088–1097. doi: 10.1007/s00415-002-0800-7. [DOI] [PubMed] [Google Scholar]
- 22.Fassas A, Anagnostopoulos A, Kazis A, Kapinas K, Sakellari I, Kimiskidis V, Smias C, Eleftheriadis N, Tsimourtou V. Autologous stem cell transplantation in progressive multiple sclerosis--an interim analysis of efficacy. J Clin Immunol. 2000;20:24–30. doi: 10.1023/a:1006686426090. [DOI] [PubMed] [Google Scholar]
- 23.Nash RA, Bowen JD, McSweeney PA, Pavletic SZ, Maravilla KR, Park MS, Storek J, Sullivan KM, Al-Omaishi J, Corboy JR, et al. High-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Blood. 2003;102:2364–2372. doi: 10.1182/blood-2002-12-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43:2437–2444. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 25.Clements PJ, Hurwitz EL, Wong WK, Seibold JR, Mayes M, White B, Wigley F, Weisman M, Barr W, Moreland L, et al. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: high-dose versus low-dose penicillamine trial. Arthritis Rheum. 2000;43:2445–2454. doi: 10.1002/1529-0131(200011)43:11<2445::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 26.Farge D, Marolleau JP, Zohar S, Marjanovic Z, Cabane J, Mounier N, Hachulla E, Philippe P, Sibilia J, Rabian C, et al. Autologous bone marrow transplantation in the treatment of refractory systemic sclerosis: early results from a French multicentre phase I-II study. Br J Haematol. 2002;119:726–739. doi: 10.1046/j.1365-2141.2002.03895.x. [DOI] [PubMed] [Google Scholar]
- 27.Farge D, Passweg J, van Laar JM, Marjanovic Z, Besenthal C, Finke J, Peter HH, Breedveld FC, Fibbe WE, Black C, et al. Autologous stem cell transplantation in the treatment of systemic sclerosis: report from the EBMT/EULAR Registry. Ann Rheum Dis. 2004;63:974–981. doi: 10.1136/ard.2003.011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McSweeney PA, Nash RA, Sullivan KM, Storek J, Crofford LJ, Dansey R, Mayes MD, McDonagh KT, Nelson JL, Gooley TA, et al. High-dose immunosuppressive therapy for severe systemic sclerosis: initial outcomes. Blood. 2002;100:1602–1610. [PMC free article] [PubMed] [Google Scholar]
- 29.Snowden JA, Passweg J, Moore JJ, Milliken S, Cannell P, Van Laar J, Verburg R, Szer J, Taylor K, Joske D, et al. Autologous hemopoietic stem cell transplantation in severe rheumatoid arthritis: a report from the EBMT and ABMTR. J Rheumatol. 2004;31:482–488. [PubMed] [Google Scholar]
- 30.Verburg RJ, Kruize AA, van den Hoogen FH, Fibbe WE, Petersen EJ, Preijers F, Sont JK, Barge RM, Bijlsma JW, van de Putte LB, et al. High-dose chemotherapy and autologous hematopoietic stem cell transplantation in patients with rheumatoid arthritis: results of an open study to assess feasibility, safety, and efficacy. Arthritis Rheum. 2001;44:754–760. doi: 10.1002/1529-0131(200104)44:4<754::AID-ANR131>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 31.Moore J, Brooks P, Milliken S, Biggs J, Ma D, Handel M, Cannell P, Will R, Rule S, Joske D, et al. A pilot randomized trial comparing CD34-selected versus unmanipulated hemopoietic stem cell transplantation for severe, refractory rheumatoid arthritis. Arthritis Rheum. 2002;46:2301–2309. doi: 10.1002/art.10495. [DOI] [PubMed] [Google Scholar]
- 32.Traynor AE, Barr WG, Rosa RM, Rodriguez J, Oyama Y, Baker S, Brush M, Burt RK. Hematopoietic stem cell transplantation for severe and refractory lupus. Analysis after five years and fifteen patients. Arthritis Rheum. 2002;46:2917–2923. doi: 10.1002/art.10594. [DOI] [PubMed] [Google Scholar]
- 33.Jayne D, Passweg J, Marmont A, Farge D, Zhao X, Arnold R, Hiepe F, Lisukov I, Musso M, Ou-Yang J, et al. Autologous stem cell transplantation for systemic lupus erythematosus. Lupus. 2004;13:168–176. doi: 10.1191/0961203304lu525oa. [DOI] [PubMed] [Google Scholar]
- 34.Tyndall A, Passweg J, Gratwohl A. Haemopoietic stem cell transplantation in the treatment of severe autoimmune diseases 2000. Ann Rheum Dis. 2001;60:702–707. doi: 10.1136/ard.60.7.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Binks M, Passweg JR, Furst D, McSweeney P, Sullivan K, Besenthal C, Finke J, Peter HH, van Laar J, Breedveld FC, et al. Phase I/II trial of autologous stem cell transplantation in systemic sclerosis: procedure related mortality and impact on skin disease. Ann Rheum Dis. 2001;60:577–584. doi: 10.1136/ard.60.6.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonen DK, Cho JH. The genetics of inflammatory bowel disease. Gastroenterology. 2003;124:521–536. doi: 10.1053/gast.2003.50045. [DOI] [PubMed] [Google Scholar]
- 37.Berrebi D, Maudinas R, Hugot JP, Chamaillard M, Chareyre F, De Lagausie P, Yang C, Desreumaux P, Giovannini M, Cezard JP, et al. Card15 gene overexpression in mononuclear and epithelial cells of the inflamed Crohn's disease colon. Gut. 2003;52:840–846. doi: 10.1136/gut.52.6.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rugtveit J, Brandtzaeg P, Halstensen TS, Fausa O, Scott H. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut. 1994;35:669–674. doi: 10.1136/gut.35.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 40.Dickinson AM, Middleton PG, Rocha V, Gluckman E, Holler E. Genetic polymorphisms predicting the outcome of bone marrow transplants. Br J Haematol. 2004;127:479–490. doi: 10.1111/j.1365-2141.2004.05216.x. [DOI] [PubMed] [Google Scholar]
- 41.Holler E, Rogler G, Herfarth H, Brenmoehl J, Wild PJ, Hahn J, Eissner G, Scholmerich J, Andreesen R. Both donor and recipient NOD2/CARD15 mutations associate with transplant-related mortality and GvHD following allogeneic stem cell transplantation. Blood. 2004;104:889–894. doi: 10.1182/blood-2003-10-3543. [DOI] [PubMed] [Google Scholar]
- 42.Baron FA, Hermanne JP, Dowlati A, Weber T, Thiry A, Fassotte MF, Fillet G, Beguin Y. Bronchiolitis obliterans organizing pneumonia and ulcerative colitis after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1998;21:951–954. doi: 10.1038/sj.bmt.1701198. [DOI] [PubMed] [Google Scholar]
- 43.Sonwalkar SA, James RM, Ahmad T, Zhang L, Verbeke CS, Barnard DL, Jewell DP, Hull MA. Fulminant Crohn's colitis after allogeneic stem cell transplantation. Gut. 2003;52:1518–1521. doi: 10.1136/gut.52.10.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Komori M, Tsuji S, Tsujii M, Murata H, Iijima H, Yasumaru M, Nishida T, Irie T, Kawano S, Hori M. Involvement of bone marrow-derived cells in healing of experimental colitis in rats. Wound Repair Regen. 2005;13:109–118. doi: 10.1111/j.1067-1927.2005.130114.x. [DOI] [PubMed] [Google Scholar]
- 45.Peppelenbosch MP, van Deventer SJ. T cell apoptosis and inflammatory bowel disease. Gut. 2004;53:1556–1558. doi: 10.1136/gut.2004.040824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ditschkowski M, Einsele H, Schwerdtfeger R, Bunjes D, Trenschel R, Beelen DW, Elmaagacli AH. Improvement of inflammatory bowel disease after allogeneic stem-cell transplantation. Transplantation. 2003;75:1745–1747. doi: 10.1097/01.TP.0000062540.29757.E9. [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Cubero SO, Sullivan KM, McDonald GB. Course of Crohn's disease after allogeneic marrow transplantation. Gastroenterology. 1998;114:433–440. doi: 10.1016/s0016-5085(98)70525-6. [DOI] [PubMed] [Google Scholar]
- 48.Talbot DC, Montes A, Teh WL, Nandi A, Powles RL. Remission of Crohn's disease following allogeneic bone marrow transplant for acute leukaemia. Hosp Med. 1998;59:580–581. [PubMed] [Google Scholar]
- 49.Drakos PE, Nagler A, Or R. Case of Crohn's disease in bone marrow transplantation. Am J Hematol. 1993;43:157–158. doi: 10.1002/ajh.2830430223. [DOI] [PubMed] [Google Scholar]
- 50.Castro J, Bentch H, Smith L, Kalter S, Bachier C, Meneghetti C, Moore A, Oliversen S, LeMaistre C. Prolonged clinical remission in patients with inflammatory bowel disease (IBD) after high dose chemotherapy (HDC) and autologous blood stem cell transplantation (ABSCT) Blood. 1996;88:133 A. [Google Scholar]
- 51.Kashyap A, Forman SJ. Autologous bone marrow trans-plantation for non-Hodgkin's lymphoma resulting in long-term remission of coincidental Crohn's disease. Br J Haematol. 1998;103:651–652. doi: 10.1046/j.1365-2141.1998.01059.x. [DOI] [PubMed] [Google Scholar]
- 52.Soderholm JD, Malm C, Juliusson G, Sjodahl R. Long-term endoscopic remission of crohn disease after autologous stem cell transplantation for acute myeloid leukaemia. Scand J Gastroenterol. 2002;37:613–616. doi: 10.1080/00365520252903198. [DOI] [PubMed] [Google Scholar]
- 53.Oyama Y, Craig RM, Traynor AE, Quigley K, Statkute L, Halverson A, Brush M, Verda L, Kowalska B, Krosnjar N, et al. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn's disease. Gastroenterology. 2005;128:552–563. doi: 10.1053/j.gastro.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 54.Stallmach A, Wittig BM, Moser C, Fischinger J, Duchmann R, Zeitz M. Safety and efficacy of intravenous pulse cyclophosphamide in acute steroid refractory inflammatory bowel disease. Gut. 2003;52:377–382. doi: 10.1136/gut.52.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franzke A, Piao W, Lauber J, Gatzlaff P, Könecke C, Hansen W, Schmitt-Thomsen A, Hertenstein B, Buer J, Ganser A. G-CSF as immune regulator in T cells expressing the G-CSF receptor: implications for transplantation and autoimmune diseases. Blood. 2003;102:734–739. doi: 10.1182/blood-2002-04-1200. [DOI] [PubMed] [Google Scholar]
- 56.Visser G, Rake JP, Labrune P, Leonard JV, Moses S, Ullrich K, Wendel U, Groenier KH, Smit GP. Granulocyte colony-stimulating factor in glycogen storage disease type 1b. Results of the European Study on Glycogen Storage Disease Type 1. Eur J Pediatr. 2002;161 Suppl 1:S83–S87. doi: 10.1007/s00431-002-1010-0. [DOI] [PubMed] [Google Scholar]
- 57.Korzenik JR, Dieckgraefe BK, Valentine JF, Hausman DF, Gilbert MJ. Sargramostim for active Crohn's disease. N Engl J Med. 2005;352:2193–2201. doi: 10.1056/NEJMoa041109. [DOI] [PubMed] [Google Scholar]
- 58.Scime R, Cavallaro AM, Tringali S, Santoro A, Rizzo A, Montalbano L, Casa A, Cottone M. Complete clinical remission after high-dose immune suppression and autologous hematopoietic stem cell transplantation in severe Crohn's disease refractory to immunosuppressive and immuno-modulator therapy. Inflamm Bowel Dis. 2004;10:892–894. doi: 10.1097/00054725-200411000-00027. [DOI] [PubMed] [Google Scholar]
- 59.Kreisel W, Potthoff K, Bertz H, Schmitt-Graeff A, Ruf G, Rasenack J, Finke J. Complete remission of Crohn's disease after high-dose cyclophosphamide and autologous stem cell transplantation. Bone Marrow Transplant. 2003;32:337–340. doi: 10.1038/sj.bmt.1704134. [DOI] [PubMed] [Google Scholar]
- 60.Musso M, Porretto F, Crescimanno A, Bondì F, Polizzi V, Scalone R. Crohn's disease complicated by relapsed extranodal Hodgkin's lymphoma: prolonged complete remission after unmanipulated PBPC autotransplant. Bone Marrow Transplant. 2000;26:921–923. doi: 10.1038/sj.bmt.1702621. [DOI] [PubMed] [Google Scholar]
- 61.Hawkey CJ. Stem cell transplantation for Crohn's disease. Best Pract Res Clin Haematol. 2004;17:317–325. doi: 10.1016/j.beha.2004.05.006. [DOI] [PubMed] [Google Scholar]


