Abstract
AIM: To determine the changes of quantitative hepatitis B e antigen (HBeAg) that predicts early detection of non-response or breakthrough to long-term lamivudine (LAM) therapy.
METHODS: Among HBeAg positive chronic hepatitis B patients who failed to achieve HBeAg seroconversion within 12 mo, we retrospectively analyzed 220 patients who had received LAM more than 24 mo.
RESULTS: The mean duration of LAM therapy was 36 (range, 24-72) mo. HBeAg seroconversion after the first 12 mo of LAM therapy was achieved in 53 (24.1%) patients. Viral breakthrough was observed in 105 (47.7%) patients. To find out whether the changing patterns of HBeAg levels can predict the outcome of LAM therapy, we analyzed the reduction rates of HBeAg levels during LAM therapy. Using the decrease more than 90% of pretreatment HBeAg levels, the sensitivity and specificity of response were 96.2% and 70.1%, respectively. Patients were divided into 3 groups according to the reduction patterns of the decrease of quantitative HBeAg: decrescendo, decrescendo-crescendo, no change or fluctuating groups. The optimal time to predict non-response or breakthrough was the first 9 mo of therapy. At 9 mo of therapy, 49 (92.5%) of 53 patients who had achieved HBeAg seroconversion were included in the decrescendo group. On the contrary, in the no change or fluctuating group, only four (7.5%) had achieved HBeAg seroconversion. Among patients who did not show the continuous decrease of HBeAg levels at 9 mo, 95.2% (negative predictive value) failed to achieve HBeAg seroconversion.
CONCLUSION: Almost all patients who failed to show a continuous decrease of HBeAg levels at 9 mo of LAM therapy were non-response or breakthrough. Therefore, monitoring changes of HBeAg levels during LAM therapy in HBeAg positive chronic hepatitis B may be valuable for identifying patients who are at high risk of non-response or breakthrough.
Keywords: Hepatitis B e antigen positive chronic hepatitis B, lamivudine, Quantitative HBeAg levels, Non-response, Breakthrough
INTRODUCTION
Hepatitis B virus (HBV) infection is a serious public health problem worldwide and a major cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma[1]. Of the approximately 2 billion people who have been infected worldwide, more than 400 million are chronic carriers of HBV[2]. The goals of therapy in patients with HBV are to limit or reverse progression of disease through sustained suppression of HBV replication[3]. This is usually achieved through treatment-induced suppression of HBV DNA and through hepatitis B e antigen (HBeAg) seroconversion in HBeAg-positive chronic hepatitis B (CHB) patients[4,5]. Clinical trials for LAM have shown it to be effective in HBV DNA suppression, alanine aminotransferase (ALT) normalization, HBeAg seroconversion and for reducing liver inflammation and the progression of hepatic fibrosis and cirrhosis[4,6-9]. Recently, lamivudine (LAM) treatment has shown to prevent the emergence of hepatocellular carcinoma in patients with HBV[10]. However, the optimal duration of LAM therapy is not well established. In view of the long half-life of covalently closed circular DNA (cccDNA) and the variable turnover of infected hepatocytes[11], long-term treatment beyond 1 year is required to achieve complete HBV elimination[12]. Moreover, the HBeAg seroconversion rates gradually continue to increase with extended LAM therapy[5,8,9,13-15]. Unfortunately, long-term LAM therapy is associated with progressively increasing rates of viral resistance due to mutations within YMDD motif of the HBV DNA polymerase[9,13,14,16]. The incidence of viral resistance to LAM therapy is approximately 20% per year reaching approximately 70% of patients after 5 years of therapy[17]. The initial benefits of treatment with LAM seem to be lost among patients who develop YMDD mutations[4,14]. Nevertheless, acute exacerbation of hepatitis may occur after the emergence of YMDD mutant[18,19]. Fatal hepatic failure after emergence of YMDD mutant has also been reported[20,21]. Currently, the appearance of YMDD mutants during LAM therapy is considered an indication for switching or adding the other approved oral agents, adefovir dipivoxil (ADV) or entecavir.
Earlier identification of non-virologic response or viral breakthrough facilitates the change to other drugs or the addition of a second agent, and could help reduce considerably viral breakthrough and treatment cost in patients who can not get HBeAg seroconversion as well. Moreover, this goal would prevent premature discontinuation of treatment in those who would ultimately have achieved HBeAg seroconversion. However, few investigations have been made about the factors that identify earlier the non-response or breakthrough to LAM treatment in HBeAg positive chronic hepatitis B patients.
Compared with qualitative measurements, quantitative HBeAg levels are sensitive, with a high reproducibility and a wide dynamic range and easy to perform[22,23]. Quantification of HBeAg levels have already been found useful in several studies in monitoring and predicting the outcome of interferon treatment[22-26]. More recently, in our previous study, the changing patterns of quantitative HBeAg levels by serial monitoring during LAM therapy may allow not only the prediction of treatment responses, but also an early recognition of a viral breakthrough[27]. Moreover, viral breakthrough by the turning time in the decrescendo-crescendo group was an earlier detection than that in HBV DNA monitoring by a hybridization assay[27].
The aim of this study was to determine the patient-dependent or laboratory variables that predict earlier identification of virologic non-response or breakthrough in patients who had not achieved seroconversion by 12 mo of therapy. Especially, the change of quantitative HBeAg over time during LAM treatment may be valuable for earlier identifying patients who are at high risk of non-response or breakthrough.
MATERIALS AND METHODS
Patients
Among HBeAg positive naïve CHB patients who had not achieved HBeAg seroconversion by 12 mo of LAM therapy, we retrospectively analyzed 220 patients (176 men and 44 women; mean age, 38.6 ± 10.2 years) who had received LAM for more than 24 mo. All patients had high upper limit of normal (ULN) serum ALT levels, as well as hepatitis B surface antigen (HBsAg), and HbeAg. The HBV DNA in serum was documented for at least 6 mo before the start of LAM therapy. No patient had a history of previous interferon or nucleoside analogues therapy. None of the patients had clinical cirrhosis, and all were excluded from having hepatitis C, D, HIV infection and autoimmune hepatitis. The diagnosis of CHB was based on histological examination for 84 patients. The remaining 136 patients were diagnosed clinically. The clinical criteria for CHB were elevated serum ALT levels over 6 mo, the absence of clinical evidence of portal hypertension and imaging features suggestive of cirrhosis. Written informed consent was obtained from all patients participating in this study, and the study was approved by the Institutional Review Boards at the Ulsan University Hospital.
Methods
Serum HBeAg, anti-HBe, HBV DNA and ALT were measured every 1 or 3 mo until HBeAg seroconversion. HBV DNA levels were measured with a hybridization capture assay with the lower limit of detection at 0.5 ng/L (Digene Hybrid Capture II, Gaithersburg, MD, USA). Serum quantitative HBeAg and anti-HBe levels were measured by microparticle enzyme immunoassay (AxSYM, Abbott, Chicago, IL, USA). The AxSYM assay calculated a result based on the ratio of the sample rate (S) to the cutoff (CO) for each sample and control. Samples with S/CO value less than or equal to 1.0 were considered negative for HBeAg. In this study, HBeAg seroconversion was defined as the loss of HBeAg accompanied with detection of anti-HBe. Response to therapy was defined as a simultaneous HBeAg seroconversion and HBV DNA negativity on two occasions at least one month apart. A non-response to therapy was defined as persistent presence of HBV DNA during treatment. A viral breakthrough was considered as the reappearance of HBV DNA in serum on two or more occasions after its initial disappearance.
Statistical analysis
All data was analyzed using the statistical package SPSS (version 12.0: SPSS Inc., Chicago, IL, USA). To identify favorable factors of response among pretreatment variables, we compared variables for response and non-response or breakthrough using χ2 test or using univariate logistic regression. Cut-off levels of reduction rate of quantitative HBeAg values were determined by univariate receiver operating characteristics (ROC) analysis. The cumulative rates of HBeAg seroconversion and viral breakthrough were calculated by the Kaplan-Meier method. In all cases, a 2-tailed P value less than 0.05 was considered statistically significant.
RESULTS
Treatment outcome
Baseline demographic and clinical features of the 220 patients who had not achieved HBeAg seroconversion by 12 mo of therapy are shown in Table 1. The mean duration of LAM therapy was 36.4 ± 10.5 (range, 24-72) mo. HBeAg seroconversion after 12 mo of LAM therapy was achieved in 53 (24.1%) patients. The cumulative rates of HBeAg seroconversion at 18, 24, 36, 48, and 60 mo were 9%, 15%, 24%, 31%, and 37%, respectively. Viral breakthrough was observed in 105 (47.7%) patients at a mean of 19.8 ± 9.5 (range, 7-60) mo after the start of LAM therapy. The cumulative breakthrough rates at 24, 36 and 48 mo were 39%, 49% and 54%, respectively.
Table 1.
Patient characteristics at baseline and response to LAM therapy (n = 220)
| Patient characteristics | |
| Age (yr) | 38.6 ± 10.2 (16-68) |
| Sex (M/F) | 176:44 |
| ALT (IU/L) | 232 ± 108 (41-1269) |
| < 2 × ULN | 24 (10.9%) |
| ≥ 2- < 5 × ULN | 112 (50.9%) |
| ≥ 5- < 10 × ULN | 52 (23.6%) |
| ≥ 10 × ULN | 32 (14.6%) |
| AST (IU/L) | 145 ± 126 (35-880) |
| HBV DNA (ng/L) | |
| Mean ± SD, log10 | 2.35 ± 1.07 (0.08-5.00) |
| HBeAg level (S/CO) | 256 ± 161 (10.4-1858) |
| Response | 53 (24.1%) |
| Breakthrough | 105 (47.7%) |
| Non-response | 62 (28.2%) |
Data are expressed as mean ± SE (range) unless otherwise stated. ULN: upper limit of normal; S/CO: sample rate/cutoff rate.
Changing patterns of quantitative HBeAg levels during therapy as predictive factors for non-response or breakthrough
To find out whether the changing patterns of HBeAg levels at different times (6, 9, and 12 mo) of therapy can predict non-response or breakthrough, we analyzed the reduction rates of HBeAg levels by serial monitoring during LAM therapy. The cut-off values of reduction rate of HBeAg levels based on ROC curves provided the maximal test efficacy, as defined by the sum of sensitivity and specificity. Therefore, the decrease more than 90% of pretreatment HBeAg levels was chosen to significantly improve the specificity, without reducing the sensitivity, and was adopted as the cut-off level for subsequent subgroup analysis. Using the decrease more than 90% of pretreatment HBeAg levels, the sensitivity and specificity of non-responders were 96.2% and 70.1%, respectively. All the responders, except one, showed the decrease more than 90% of the pretreatment HBeAg values for the maximal reduction rate during LAM therapy. Patients were divided into 3 groups according to reduction patterns, as compared to the pretreatment HBeAg levels, at different times (6, 9, and 12 mo) of LAM therapy: (1) a continuous decrease of HBeAg levels to more than 90% of pretreatment values (decrescendo group), (2) a continuous decrease of HBeAg levels to more than 90% of pretreatment values, and then progressively increasing (decrescendo-crescendo group), (3) no change or fluctuation of HBeAg levels (no change or fluctuating group). The calculation of predictive values of response to therapy was performed using reduction patterns of quantitative HBeAg levels during LAM therapy. A positive predictive value (PPV) at different times of LAM treatment was defined as the proportion of patients achieving HBeAg seroconversion among those with the decrescendo pattern of HBeAg levels each time after the initiation of therapy. A negative predictive value (NPV) was defined as the proportion of patients without HBeAg seroconversion among patients with the decrescendo-crescendo and no change or fluctuating patterns during LAM treatment. Detailed analysis of reduction patterns of quantitative HBeAg at different times had revealed that sensitivity was excellent from 83.0% to 92.5%, while specificity was poor from 46.7% to 49.7% (Table 2). The early prediction of non-responder that optimized capture of potential responders (highest sensitivity) while excluding the largest proportion of non-response or breakthrough (highest negative predictive value) was the changing patterns of HBeAg levels at 9 and 12 mo of treatment (Table 2). Although the changing patterns of HBeAg levels at 9 and 12 mo had similar results, because of increasing rate of LAM mutants and treatment cost, it is desirable to define diagnostic markers that can allow identification of viral non-response or breakthrough as early as possible. Thus, the optimal time to predict non-response or viral breakthrough is month 9 of LAM treatment. According to the changing patterns of HBeAg levels at 9 mo of LAM therapy, the numbers of decrescendo, no change or fluctuating, and decrescendo-crescendo groups were 136, 71, and 13, respectively. Forty-nine (92.5%) of 53 patients who had achieved HBeAg seroconversion were included in the decrescendo group. On the contrary, from the no change or fluctuating group, only 4 (7.5%) had achieved HBeAg seroconversion, and remained patients were non-response or breakthrough. All patients in the decrescendo-crescendo group were non-response or breakthrough. Ultimately, not all patients who had the decrescendo pattern of HBeAg levels achieved HBeAg seroconversion. The PPV at 9 mo was only 36.0%. Among the 136 patients with the decrescendo pattern of HBeAg levels at 9 mo, 106 patients failed to achieve HBeAg seroconversion within 24 mo of treatment. Of these 106 subjects, 60 received repeated quantitative HBeAg level testing at month 36 (Figure 1). According to the changing patterns of HBeAg levels at 36 mo after the start of LAM therapy, there were 42 (70.0%) and 18 (30.0%) patients in the decrescendo and decrescendo-crescendo groups, respectively. HBeAg seroconversion after 24 mo of LAM therapy was achieved in 19 (31.7%). Eighteen (94.7%) of these 19 patients were included in the decrescendo group. Only one of 18 patients in the decrescendo-crescendo group had achieved HBeAg seroconversion.
Table 2.
Comparative results according to changing patterns of HBeAg levels during LAM therapy
| Response | Non- response or reakthrough | Odds atio 95%CI) | Sensitivity | Specificity | PPV1 | NPV2 | |
| At 6 mo | 4. 8 (2.2-13.5) | 83 | 49.7 | 34.4 | 90.2 | ||
| D3 | 44 | 84 | |||||
| N4 | 9 | 83 | |||||
| D-C5 | 0 | 0 | |||||
| At 9 mo | 11.0 (3.8-32.0) | 92.5 | 47.9 | 36 | 95.2 | ||
| D | 49 | 87 | |||||
| N | 4 | 67 | |||||
| D-C | 0 | 13 | |||||
| At 12 mo | 10.5 (3.6-30.5) | 92.5 | 46.7 | 35.5 | 95.1 | ||
| D | 49 | 89 | |||||
| N | 2 | 60 | |||||
| D-C | 2 | 18 |
Positive predictive value. The proportion of patients achieving HBeAg seroconversion among those with decrescendo pattern of HBeAg levels at each time after initiation of therapy;
Negative predictive value. The proportion of patients without HBeAg seroconversion among those with the decrescendo-crescendo and no change or fluctuating patterns during treatment;
Decrescendo pattern. Continuously decreasing HBeAg levels by more than 90% of pretreatment values;
No change or fluctuating pattern. No change or fluctuation of HBeAg levels during treatment;
Decrescendo-crescendo pattern. A continuous decrease of HBeAg levels by more than 90% of pretreatment values, and then progressively increasing.
Figure 1.
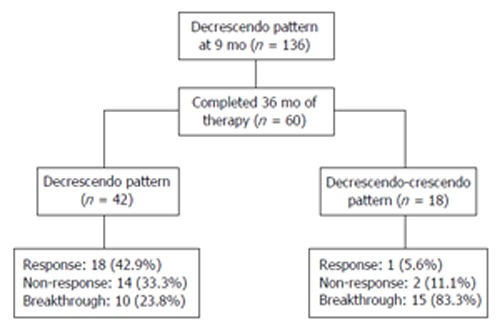
Clinical courses of HBeAg positive chronic hepatitis B patients who were treated more than 36 mo among 136 patients in decrescendo group at 9 mo of lamivudine treatment. Decrescendo pattern, continuously decreasing HBeAg levels by more than 90% of pretreatment values over time; Decrescendo-crescendo pattern: continuous decrease by more than 90% of pretreatment values, and then progressively increasing.
DISCUSSION
Most HBeAg positive patients do not have a virologic response after 1 year of LAM treatment, and prolonged treatment beyond 1 year is often necessary. Treatment may be continued in patients who fail to achieve HBeAg seroconversion and have no evidence of viral breakthrough as HBeAg seroconversion may occur with continued treatment. However, treatment beyond 1 year has not been adequately investigated and the benefits of continued treatment must be balanced against the risks of resistant mutants. The current practice for CHB patients with YMDD mutants is options of continuing or stopping LAM or converting to other antiviral drug when LAM resistance develops, depending on the patient’s clinical state, ALT and HBV DNA levels[3,12,28]. However, recently, resistance against ADV has been observed as well[29]. Moreover, the emergence of ADV mutation appeared to present earlier and more frequent in LAM-resistant patients than in nucleoside/-tide treatment naïve patients and was associated with reduced antiviral efficacy to ADV therapy[30,31]. In addition, LAM resistance leads to selection pressure for HBV with additional mutations in the DNA polymerase gene that reduces the susceptibility to entecavir[32]. Therefore, earlier prediction of non-response or breakthrough in the course of treatment would not only help reduce the incidence of HBV mutants and treatment cost, but would also allow alternative treatment options to be pursued sooner in the course of treatment. Such goal helps maximize the proportion of patients achieving response, which minimizes emergence of mutant virus in patients who might be ultimately non-response during continuation of LAM therapy.
Serum HBeAg, anti-HBe, and HBV DNA are currently the most important markers for assessing the response to antiviral therapy in patients with HBeAg positive CHB. HBeAg is used typically as a qualitative serological marker for diagnosing the virologic response in HBeAg positive CHB. Therefore, serum quantitative HBeAg levels may reflect levels of the cccDNA in hepatocytes because the mRNAs of HBeAg are transcribed from the cccDNA[33-36]. It is conceivable that patients with decreasing HBeAg levels are on the way to HBV clearance, and reflect the process towards HBeAg seroconversion. The absence of decline for quantitative HBeAg levels may indicate the persistence of ongoing viral replication, despite the antiviral treatment. Furthermore, a resurgence of quantitative HBeAg levels during antiviral therapy may be explained by the reappearance of wild type strains or emergence of mutant strain[27]. To date, available information on the association between the changing patterns of HBeAg levels and early prediction of viral non-response or breakthrough to LAV therapy in patients with HBeAg positive CHB is limited. In the present study, we have retrospectively investigated the utility of monitoring of HBeAg level changes to predict treatment outcomes in naive HBeAg positive CHB patients who had not achieved HBeAg seroconversion by 12 mo of therapy. Based upon the predictive values of a positive test for virologic non-response or breakthrough (NPV), the optimal time to earlier predict non-response or breakthrough is recommended to be month 9 of treatment. Forty-nine (92.5%) of 53 patients who had achieved HBeAg seroconversion after 12 mo of LAM therapy were included in the decrescendo group. On the contrary, from the no change or fluctuating group, only four (7.5%) had achieved HBeAg seroconversion and the remained patients were non-response or breakthrough. All patients in the decrescendo-crescendo group were non-response or breakthrough. That is, almost all patients who did not show a continuous decrease of HBeAg levels at 9 mo during LAM therapy were non-response or breakthrough. That is, among patients who did not have the decrescendo pattern of HBeAg levels at 9 mo during LAM therapy, 95.2% (NPV) failed to achieve HBeAg seroconversion. These patients have very little chance of attaining virologic response even if an additional therapy is administered, and thus it is recommended that other nucleoside analogues or the combination with another antiviral agents be used. However, not all patients who had the decrescendo pattern of HBeAg levels at 9 mo of therapy ultimately achieved HBeAg seroconversion. Positive prediction of virologic response during therapy remains disappointing on the basis of the data available. Indeed, the positive predictive value at 9 mo was only 36.0%. In addition, from 60 patients who were treated more than 36 mo among 136 patients of the decrescendo group at 9 mo of treatment, 19 (31.7%) had achieved response. Eighteen patients (94.7%) were included in the decrescendo pattern of HBeAg levels at 36 mo of treatment. Only one of responders was included in the decrescendo-crescendo group at 36 mo of treatment. That is, if patient had the decrescendo pattern of HBeAg levels at 9 mo, further treatment should be continued, and serial monitoring of HBeAg levels during treatment would be necessary.
It is therefore imperative that HBeAg levels and HBV DNA are measured during LAM therapy to monitor the response to therapy and facilitate the early detection of drug-resistant strains. HBV DNA testing by PCR and mutant genotyping are very sensitive methods in detecting viral breakthrough during LAM therapy. Unfortunately, our study did not perform the quantitative PCR and assay for YMDD mutants. Also, HBV DNA monitoring by a hybridization assay has a rather low sensitivity, and it was expected to lag behind in the detection of viral breakthrough, as compared to quantitative PCR. Therefore, further studies are needed to compare the quantitative decrease in HBeAg with the HBV DNA level measurement by the sensitive PCR assay. However, these tests do not predict outcomes of treatment before the emergence of HBV mutants. Nevertheless, this is a very important practical point to clinicians because a quantitative HBeAg level test is easy to measure, less expensive, with a wider dynamic range than other molecular tests. Most importantly, it can identify the changing patterns of HBeAg levels during treatment that have the potential to serve as predictors of long-term drug response to LAM therapy. For patients with the decrescendo pattern of HBeAg levels during LAM therapy, continuous LAM therapy without rescue treatment could be expected for achievement of HBeAg seroconversion. On the contrary, for patients who had the decrescendo-crescendo and the no changing or fluctuating patterns of HBeAg levels at 9 mo of LAM therapy, most patients were viral non-response or breakthrough, and HBeAg seroconversion was rarely developed. Therefore, these patients should be monitored more strictly. In the near future, to prevent the development of breakthrough hepatitis, other antiviral agents should be applied when the decrescendo-crescendo pattern and the no changing or fluctuating of HBeAg levels at 9 mo is observed during LAM therapy.
In conclusion, almost all patients who failed to show the continuous decrease of HBeAg levels at 9 mo of LAM therapy were non-response or breakthrough. On-treatment prediction of no-response or breakthrough to long-term LAM therapy in HBeAg positive CHB can be predicted early at mo 9 of treatment based on the changing patterns of HBeAg levels. That is, monitoring of serum quantitative HBeAg levels, in addition to measurement of HBV DNA by PCR, is helpful for evaluating the response to LAM treatment and for the early detection of non-response or LAM-resistant strains as well.
Footnotes
S- Editor Wang J L- Editor Ma JY E- Editor Ma WH
References
- 1.Cha C, Dematteo RP. Molecular mechanisms in hepatocellular carcinoma development. Best Pract Res Clin Gastroenterol. 2005;19:25–37. doi: 10.1016/j.bpg.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 3.Liaw YF, Leung N, Guan R, Lau GK, Merican I. Asian-Pacific consensus statement on the management of chronic hepatitis B: an update. J Gastroenterol Hepatol. 2003;18:239–245. doi: 10.1046/j.1440-1746.2003.03037.x. [DOI] [PubMed] [Google Scholar]
- 4.Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105–117. doi: 10.1053/gast.2003.50013. [DOI] [PubMed] [Google Scholar]
- 5.Leung NW, Lai CL, Chang TT, Guan R, Lee CM, Ng KY, Lim SG, Wu PC, Dent JC, Edmundson S, et al. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology. 2001;33:1527–1532. doi: 10.1053/jhep.2001.25084. [DOI] [PubMed] [Google Scholar]
- 6.Dienstag JL, Perrillo RP, Schiff ER, Bartholomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657–1661. doi: 10.1056/NEJM199512213332501. [DOI] [PubMed] [Google Scholar]
- 7.Nevens F, Main J, Honkoop P, Tyrrell DL, Barber J, Sullivan MT, Fevery J, De Man RA, Thomas HC. Lamivudine therapy for chronic hepatitis B: a six-month randomized dose-ranging study. Gastroenterology. 1997;113:1258–1263. doi: 10.1053/gast.1997.v113.pm9322520. [DOI] [PubMed] [Google Scholar]
- 8.Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 9.Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, Crowther L, Condreay LD, Woessner M, Rubin M, Brown NA. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256–1263. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 10.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 11.Zeuzem S, de Man RA, Honkoop P, Roth WK, Schalm SW, Schmidt JM. Dynamics of hepatitis B virus infection in vivo. J Hepatol. 1997;27:431–436. doi: 10.1016/s0168-8278(97)80345-5. [DOI] [PubMed] [Google Scholar]
- 12.Lok AS, McMahon BJ. Chronic hepatitis B: update of recommendations. Hepatology. 2004;39:857–861. doi: 10.1002/hep.20110. [DOI] [PubMed] [Google Scholar]
- 13.Dienstag JL, Schiff ER, Mitchell M, Casey DE, Gitlin N, Lissoos T, Gelb LD, Condreay L, Crowther L, Rubin M, et al. Extended lamivudine retreatment for chronic hepatitis B: maintenance of viral suppression after discontinuation of therapy. Hepatology. 1999;30:1082–1087. doi: 10.1002/hep.510300427. [DOI] [PubMed] [Google Scholar]
- 14.Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Chien RN, Dent J, Roman L, Edmundson S, et al. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology. 2000;119:172–180. doi: 10.1053/gast.2000.8559. [DOI] [PubMed] [Google Scholar]
- 15.Song BC, Suh DJ, Lee HC, Chung YH, Lee YS. Hepatitis B e antigen seroconversion after lamivudine therapy is not durable in patients with chronic hepatitis B in Korea. Hepatology. 2000;32:803–806. doi: 10.1053/jhep.2000.16665. [DOI] [PubMed] [Google Scholar]
- 16.Lau DT, Khokhar MF, Doo E, Ghany MG, Herion D, Park Y, Kleiner DE, Schmid P, Condreay LD, Gauthier J, et al. Long-term therapy of chronic hepatitis B with lamivudine. Hepatology. 2000;32:828–834. doi: 10.1053/jhep.2000.17912. [DOI] [PubMed] [Google Scholar]
- 17.Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, Dienstag JL, Heathcote EJ, Little NR, Griffiths DA, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714–1722. doi: 10.1053/j.gastro.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 18.Liaw YF, Chien RN, Yeh CT, Tsai SL, Chu CM. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology. 1999;30:567–572. doi: 10.1002/hep.510300221. [DOI] [PubMed] [Google Scholar]
- 19.Di Marco V, Di Stefano R, Ferraro D, Almasio PL, Bonura C, Giglio M, Parisi P, Cappello M, Alaimo G, Craxì A. HBV-DNA suppression and disease course in HBV cirrhosis patients on long-term lamivudine therapy. Antivir Ther. 2005;10:431–439. [PubMed] [Google Scholar]
- 20.Kim JW, Lee HS, Woo GH, Yoon JH, Jang JJ, Chi JG, Kim CY. Fatal submassive hepatic necrosis associated with tyrosine-methionine-aspartate-aspartate-motif mutation of hepatitis B virus after long-term lamivudine therapy. Clin Infect Dis. 2001;33:403–405. doi: 10.1086/321879. [DOI] [PubMed] [Google Scholar]
- 21.Wang JH, Lu SN, Lee CM, Lee JF, Chou YP. Fatal hepatic failure after emergence of the hepatitis B virus mutant during lamivudine therapy in a patient with liver cirrhosis. Scand J Gastroenterol. 2002;37:366–369. doi: 10.1080/003655202317284309. [DOI] [PubMed] [Google Scholar]
- 22.Perrillo R, Mimms L, Schechtman K, Robbins D, Campbell C. Monitoring of antiviral therapy with quantitative evaluation of HBeAg: a comparison with HBV DNA testing. Hepatology. 1993;18:1306–1312. [PubMed] [Google Scholar]
- 23.Heijtink RA, Kruining J, Honkoop P, Kuhns MC, Hop WC, Osterhaus AD, Schalm SW. Serum HBeAg quantitation during antiviral therapy for chronic hepatitis B. J Med Virol. 1997;53:282–287. [PubMed] [Google Scholar]
- 24.Hayashi PH, Beames MP, Kuhns MC, Hoofnagle JH, Di Bisceglie AM. Use of quantitative assays for hepatitis B e antigen and IgM antibody to hepatitis B core antigen to monitor therapy in chronic hepatitis B. Am J Gastroenterol. 1996;91:2323–2328. [PubMed] [Google Scholar]
- 25.Bernard F, Raymond G, Willems B, Villeneuve JP. Quantitative assessment of serum hepatitis B e antigen, IgM hepatitis B core antibody and HBV DNA in monitoring the response to treatment in patients with chronic hepatitis B. J Viral Hepat. 1997;4:265–272. doi: 10.1046/j.1365-2893.1997.00055.x. [DOI] [PubMed] [Google Scholar]
- 26.Heijtink RA, Janssen HL, Hop WC, Osterhaus AD, Schalm SW. Interferon-alpha therapy in chronic hepatitis B: early monitoring of hepatitis B e antigen may help to decide whether to stop or to prolong therapy. J Viral Hepat. 2000;7:382–386. doi: 10.1046/j.1365-2893.2000.00246.x. [DOI] [PubMed] [Google Scholar]
- 27.Park NH, Shin JW, Park JH, Bang SJ, Kim DH, Joo KR, Kim DH. Monitoring of HBeAg levels may help to predict the outcomes of lamivudine therapy for HBeAg positive chronic hepatitis B. J Viral Hepat. 2005;12:216–221. doi: 10.1111/j.1365-2893.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 28.Lin OS, Keeffe EB. Current treatment strategies for chronic hepatitis B and C. Annu Rev Med. 2001;52:29–49. doi: 10.1146/annurev.med.52.1.29. [DOI] [PubMed] [Google Scholar]
- 29.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med. 2005;352:2673–2681. doi: 10.1056/NEJMoa042957. [DOI] [PubMed] [Google Scholar]
- 30.Fung SK, Chae HB, Fontana RJ, Conjeevaram H, Marrero J, Oberhelman K, Hussain M, Lok AS. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J Hepatol. 2006;44:283–290. doi: 10.1016/j.jhep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM, Lee HC, Chung YH, Lee YS, Yoo W, Kim SO. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006;43:1385–1391. doi: 10.1002/hep.21189. [DOI] [PubMed] [Google Scholar]
- 32.Tenney DJ, Levine SM, Rose RE, Walsh AW, Weinheimer SP, Discotto L, Plym M, Pokornowski K, Yu CF, Angus P, et al. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to Lamivudine. Antimicrob Agents Chemother. 2004;48:3498–3507. doi: 10.1128/AAC.48.9.3498-3507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moraleda G, Saputelli J, Aldrich CE, Averett D, Condreay L, Mason WS. Lack of effect of antiviral therapy in nondividing hepatocyte cultures on the closed circular DNA of woodchuck hepatitis virus. J Virol. 1997;71:9392–9399. doi: 10.1128/jvi.71.12.9392-9399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 35.Summers J, Smith PM, Horwich AL. Hepadnavirus envelope proteins regulate covalently closed circular DNA amplification. J Virol. 1990;64:2819–2824. doi: 10.1128/jvi.64.6.2819-2824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuttleman JS, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986;47:451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]


