Abstract
AIM: To clarify the incidence and nature of posto-perative hyperbilirubinemia in patients after modern extracorporeal circulation, to analyze possible perioperative risk factors, and to elucidate the clinical significance of postoperative hyperbilirubinemia associated mortality and morbidity.
METHODS: Between March 2005 and May 2006, three hundred and eighty six consecutive patients undergoing extracorporeal circulation surgery due to a variety of cardiac lesions were investigated prospectively. The incidence of postoperative hyperbilirubinemia was defined as a serum total bilirubin concentration of more than 51 μmol/L. Several perioperative parameters were compared by logistic regression between hyperbilirubinemia and non-hyperbilirubinemia patients to determine possible risk factors contributing to postoperative hyperbilirubinemia and mortality.
RESULTS: Overall incidence of postoperative hyperbilirubinemia was 25.3% (98/386). In patients with postoperative hyperbilirubinemia, 56.2% reached peak total bilirubin concentration on the first postoperative day, 33.5% on the second day, and 10.3% on the seventh day. Eighty percent of the increase of total bilirubin resulted from an increase of both conjugated and unconjugated bilirubin. Development of postoperative hyperbilirubinemia was associated with a higher mortality (P < 0.01), longer duration of mechanical ventilation (P < 0.05) and longer ICU stay time (P < 0.05). Preoperative total bilirubin concentration, preoperative right atrium pressure, numbers of valves replaced and of blood transfusion requirement were identified as important predictors for postoperative hyperbilirubinemia.
CONCLUSION: Early postoperative hyperbilirubinemia after modern extracorporeal circulation is mainly caused by an increase in both conjugated and unconjugated bilirubin, and is associated with a high mortality. Important contributing factors are the preoperative total bilirubin concentration, preoperative severity of right atrial pressure, numbers of valve replacement procedures, and the amount of blood transfusion requirement during and shortly after surgery. We suggest that postoperative hyperbilirubinemia is a multifactorial process, which is caused by both the impaired liver function of bilirubin transport and the increased production of bilirubin from haemolysis.
Keywords: Extracorporeal circulation, Open-heart surgery, Hyperbilirubinemia, Liver function
INTRODUCTION
It has long been recognized that early hyperbilirubinemia or transient jaundice could occur after extracorporeal circulation surgery. According to earlier studies, overall incidence of postoperative hyperbilirubinemia ranges from about 8.6% to even as high as 40%[1-3]. Despite improvements in perioperative management, as well as in surgical and anaesthetic techniques, early hyperbilirubinemia after modern extracorporeal circulation surgery is still seen quite often. The high frequency of hyperbilirubinemia after cardiac surgery in our department prompted us to start a prospective study recently. It is aimed to clarify the incidence and nature of postoperative hyperbilirubinemia in patients following modern extracorporeal circulation, to analyze possible perioperative risk factors, and to elucidate the clinical significance of postoperative hyperbilirubinemia associated mortality and morbidity.
MATERIALS AND METHODS
Patients’ backgrounds
Three hundred and eighty six patients older than 17 years undergoing extracorporeal circulation surgery from March 2005 to May 2006 in our department were consecutively enrolled into the study. One hundred and eighty were male and 206 were female. Their ages ranged from 17 to 78 years (mean, 47 years). The disease categories comprised valve disease (Valve, 220 cases), congenital heart disease (CHD, 140 cases), coronary atherosclerotic heart disease (CAHD, 16 cases) and valve combined with coronary disease (Valve + CAHD, 10 cases). The protocol was approved by the Committee of Human Study of our institution. Patients were not selected with any other major diseases, such as viral hepatitis, HIV, and syphilis. Patients with preoperative hyperbilirubinemia were defined as a total bilirubin concentration of more than 2 mg/dL (34 μmol/L). Postoperative hyperbilirubinemia was defined as a total bilirubin level over 3 mg/dL (51 μmol/L) within the first postoperative week, and accordingly, patients were divided into two groups: postoperative hyperbilirubinemia group (HB group) and non-postoperative hyperbilirubinemia group (NHB group).
Surgery and parameters tested
Anesthesia was induced with fentanyl (40 to 100 μg/kg), etomidate (0.3 to 0.4 mg/kg) or vecuronium (0.1 to 0.15 mg/kg). All patients had cardiopulmonary bypass (CPB), with isovolemic haemodilution, moderate hypothermia (28°C ± 5°C), roller pump and bubble or membrane oxygenators. The perfusion flow was kept over 2.2 L/m2 during northemia and over 1.8 L/m2 during hypothermia in every patient. The mean arterial pressure (MAP) was kept between 50 to 80 mmHg with dopamine, nitroglycerin, and nitroprusside. Arterial blood gas was monitored routinely every half an hour or on any occasion when considered necessary. The priming solution contained 1.5 to 2.0 L of lactated Ringer’s solution, heparin (2000 U/L) and 1/2 to 1 unit of packed red cells if blood cardioplegic solution was indicated or predicted hematocrit level during CPB was blow 20%. Systemic heparin was given through the right atrium at a dose of 300 U/kg just before cannulation. For valvular replacements operations, either a Carbomedics (Carbomedics, Inc) or a St, Jude Medical (St, Jude Medical, Inc) Mechanical prosthesis was used.
Parameters tested
Preoperative: Blood samples were collected within 2 d before operation by vein puncture. Serum was stored at -70°C for future biochemical and virological studies. Preoperative right atrial pressure was monitored through the preoperative cardiac catheterization recorder. Full blood-count, urea and electrolytes, alanine amino transferase (ALT), aspartate amino transferase (AST), total bilirubin (TB), unconjugated bilirubin(UCB), and conjugated bilirubin (CB) were tested. In all patients, serological viral studies were performed to rule out infection with hepatitis A (HAV IgG + IgM), hepatitis B (HBsAg and HBcAb), hepatitis C (HCV - IgM) and HIV virus.
Perioperative: In every operation, the routine clinical monitors including lead electrocardiogram, the radial arterial line, the pulse oximeter, end-tidal carbon dioxide, nasopharyngeal and rectal temperatures, urine output via Foley catheter, the central venous pressure line, pulmonary artery catheters, the operating time, CPB time, aortic cross-clamp time, types of oxygenator, quantity of packed red cells primed during CPB, and the types and numbers of mechanical valves replaced were recorded.
Postoperative: We recorded right atrial pressure, minimum MAP, and minimum arterial oxygen tension (mPO2) daily. Blood samples were obtained through the central venous line or venopuncture on the first, second and seventh postoperative days and analyzed for concentrations of albumin, globulin, haemoglobin, ALT, AST, TB, UCB, CB, and reticulocyte count by an automated biochemical analyzer (Beckman Array 3000, USA). Serum bile acids (cholyglycine) were tested using stored serum (RIA). Reticulocyte count, plasma haemoglobin, haptoglobin levels, urinary haemosiderin, and urobilinogen were examined to detect haemolysis. The blood transfusions shortly after surgery, the days of hospitalization in the intensive care unit (ICU), mechanical ventilation days, and the numbers of patients who died during hospitalization were registered.
Statistical analysis
Statistical analysis was carried out using SPSS version 10.0 software (SPSS, inc, USA). Data were presented as mean ± SE of the mean. The chi-squared test and Student’s
t test were used to compare categorical variables. Stepwise logistic regression was employed for multivariate analysis. Values of P less than 0.05 were considered significant.
RESULTS
The perioperative changes of liver function are shown in Table 1. The incidence of the postoperative hyperbilirubinemia in these patients is shown in Figure 1.
Table 1.
Comparison of perioperative changes of liver function in two groups (mean ± SD)
| Variable |
HB group |
NHB group |
||||||
| Preop | d1 | d2 | d7 | Preop | d1 | d2 | d7 | |
| TB (mmol/L) | 26.8 ± 2.6d | 72.7 ± 9.1bd | 82.8 ± 10.4bd | 63.8 ± 7.5bd | 14.5 ± 1.4 | 33.8 ± 5.5b | 20.8 ± 4.5 | 17.1 ± 3.7 |
| UCB (mmol/L) | 9.9 ± 1.9 | 47.6 ± 6.5b | 40.9 ± 6.7bd | 32.6 ± 5.6bd | 6.5 ± 1.7 | 18.6 ± 3.9b | 11.9 ± 3.2 | 9.7 ± 1.9 |
| CB (mmol/L) | 16.7 ± 1.5d | 24.7 ± 5.1bd | 39.6 ± 6.1bd | 31.2 ± 5.4bd | 8.4 ± 1.3 | 15.5 ± 3.1b | 9.4 ± 2.1 | 7.6 ± 1.6 |
| ALT (IU/L) | 40.6 ± 3.9d | 112.6 ± 11.6bd | 176 ± 15.6bd | 56.6 ± 5.5d | 28.5 ± 2.6 | 84.6 ± 11.6d | 60.6 ± 5.7 | 50.2 ± 5.1 |
| AST (IU/L) | 40.5 ± 3.6 | 102.6 ± 10.7d | 184 ± 17.1bd | 53.2 ± 5.2d | 42.6 ± 3.3 | 80.5 ± 10.5d | 55.1 ± 5.5 | 48.3 ± 4.9 |
Preop: Preoperative; d1, d2, d7: first, second, and seventh postoperative days, respectively.
P < 0.01 versus Preop:
P < 0.01 HB group versus NHB group at the same time point.
Figure 1.
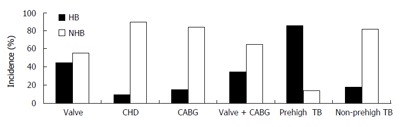
Incidence of postoperative hyperbiliru-binemia among different disease categories, and in patients with and without preoperative hyperbilirubinemia; CHD: Congenital heart disease, CABG: Coronary artery bypass grafting.
Among the 386 patients enrolled in this study, 36 had preoperative hyperbilirubinemia. The overall incidence of the postoperative hyperbilirubinemia was 25.3%. For patients with preoperative hyperbilirubinemia, the incidence of postoperative hyperbilirubinemia was 86.1%.
In patients with postoperative hyperbilirubinemia, 56.2% reached a peak total bilirubin concentration on the first postoperative day, 33.5% on the second day, and 10.3% on the seventh day. Among the patients with preoperative hyperbilirubinemia, 69% had severe postoperative hyperbilirubinemia with highest TB concentration greater than 171 μmol/L; whereas, in other patients, only 18% had a highest TB concentration greater than 171 μmol/L (P < 0.01). The highest TB concentration was significantly greater in patients with preoperative hyperbilirubinemia than in patients without (210.8 ± 28.4 μmol/L vs 62.8 ± 9.6 μmol/L, P < 0.01). On the first postoperative day, the TB, UCB, and CB concentrations increased in both the patients in HB and NHB groups compared with preoperative levels (P < 0.01, Table 1). For the patients with postoperative hyperbilirubinemia, 80.2% of the increased TB was from an increase of the mixture of CB and UCB.
With regard to the effects of the disease and operation category, postoperative hyperbilirubinemia occurred more frequently in patients receiving valvular replacements than in patients undergoing coronary artery bypass grafting (CABG) or operation for CHD(P < 0.01, Figure 1). The incidence of postoperative hyperbilirubinemia was significantly higher in patients with valvular replacements with mechanical prostheses than in the patients without valvular replacements (P < 0.01, Figure 1). The result was similar when patients with preoperative hyperbilirubinemia were excluded.
The comparison of mortality, ventilation time and ICU stay time are shown in Table 2. Only one patient undergoing CABG died in NHB group. He died on 12th d after the operation of respiratory failure. Among four operative mortality cases in the HB group, three had a progressive increase in TB after operation. The highest TB concentration level (over 400 μmol/L) was reached on the seventh postoperative day. One had an increase in TB immediately on the first postoperative day. Two of the four died from hepatic failure, and another two died from multiple organic function failure. Development of postoperative hyperbilirubinemia was associated with a higher mortality (P < 0.01), longer duration of mechanical ventilation (P < 0.05), and longer ICU stay time (P < 0.05).
Table 2.
Comparison of mortality, ventilation time and ICU stay time in two groups (mean ± SD)
| Variable | HB group | NHB group | P value |
| Mortality | 4.10% | 0.3%b | P < 0.01 |
| Ventilation time (h) | 25.5 ± 13.3 | 16.5 ± 9.2a | P < 0.05 |
| ICU stay time (d) | 11.5 ± 6.5 | 4.1 ± 3.9a | P < 0.05 |
P < 0.01 vs HB group;
P < 0.05 vs HB group.
Analysis of possible risk factors for the postoperative hyperbilirubinemia is shown in Table 3. The results of stepwise logistic regression are shown in Table 4. Preoperative total bilirubin concentration, preoperative right atrial pressure, numbers of valves replaced and blood transfusion requirement were identified as important predictors for the postoperative hyperbilirubinemia. Combination of these four perioperative risk factors could predict development of postoperative hyperbilirubinemia in 81.2% of the patients.
Table 3.
Analysis of possible risk factors for the postoperative hyperbilirubinemia (mean ± SD)
| Possible risk factors | HB group | NHB group | P |
| Age (yr) | 47 ± 1.16 | 46. ± 1.02 | > 0.25 |
| Body surface area (m2) | 1.66 ± 0.01 | 1.63 ± 0.03 | > 0.10 |
| Preoperative TB (μmol/L) | 26.8 ± 2.6 | 14.5 ± 2.3 | < 0.01 |
| Preoperative CB (μmol/L) | 16.7 ± 1.4 | 8.4 ± 1.5 | < 0.01 |
| Preoperative TB/CB | 1.69 ± 0.01 | 1.71 ± 0.02 | > 0.30 |
| Preoperative ALT (IU/L) | 40.6 ± 3.9 | 28.5 ± 2.6 | < 0.05 |
| Preoperative AST (IU/L) | 40.5 ± 3.6 | 42.6 ± 3.3 | > 0.10 |
| Right atrial pressure (mmHg) | 9.5 ± 0.6 | 4.9 ± 0.5 | < 0.01 |
| Pulmonary artery pressure (mmHg) | 35.5 ± 1.5 | 23.5 ± 1.3 | < 0.01 |
| Blood transfusion requirement (u) | 3.56 ± 0.02 | 2.15 ± 0.03 | < 0.01 |
| Operation time (min) | 220.5 ± 6.0 | 205.5 ± 5.3 | > 0.15 |
| CPB time (min) | 130.3 ± 4.2 | 112.2 ± 3.1 | < 0.01 |
| Aortic crossclamp time (min) | 76.6 ± 3.1 | 68.6 ± 2.3 | < 0.01 |
| Lowest nasopharyngeal temperature (°C) | 26.7 ± 0.4 | 26.3 ± 0.3 | > 0.25 |
| Lowest MAP (mmHg) | 68.6 ± 1.3 | 65.9 ± 1.5 | > 0.15 |
| Lowest PO2 (mmHg) | 66.6 ± 1.4 | 68.7 ± 1.5 | > 0.20 |
Table 4.
Results of logistic regression for the risk factors of postoperative hyperbilirubinemia
| Variable | β | SE | Significance | R |
| Preoperative TB | 1.274 | 0.312 | 0.000 | 0.205 |
| Right atrial pressure | 0.113 | 0.032 | 0.006 | 0.147 |
| Number of valves replaced | 0.658 | 0.302 | 0.025 | 0.152 |
| Blood transfusion requirement (u) | 0.109 | 0.033 | 0.006 | 0.118 |
| Constant | -3.453 | 0.466 | 0.000 |
β: Regression coefficient; SE: Standard error; R: Partial correlation coefficient.
DISCUSSION
It has been well known that early jaundice and transient liver damage could occur after extracorporeal circulation surgery[1,2]. The mortality can reach 85%-90% once the postoperative jaundice progresses to hepatic failure[4-7]. On the other hand, for patients with preoperative cirrhosis of liver, mortality of CPB surgery can reach 31%[8]. Thus recently, more attention has been paid to the studies of jaundice and liver injuries following CPB.
This prospective study showed that the overall incidence of the postoperative hyperbilirubinemia was 25.3%, similar to the literature report[3]. Despite advances in techniques of perioperative management in cardiac operations, CPB, and cardiac anaesthesia, early hyperbilirubinemia after modern extracorporeal circulation surgery still occurs frequently. With regard to the effects of the disease and operation category, controversies exist about whether incidence differs among different disease category[2,3]. The current results showed that postoperative hyperbilirubinemia occurred more frequently in patients receiving valvular replacements than in patients undergoing CABG or operation for CHD (P < 0.01). The incidence of postoperative hyperbilirubinemia was significantly higher in patients with valvular replacements with mechanical prostheses than in the patients without receiving valvular replacements (P < 0.01). For patients with preoperative hyperbilirubinemia, the incidence of the postoperative hyperbilirubinemia was 86.1%. The decreased hepatic capacities for bilirubin disposal and bile transport, in addition to the haemolysis from CPB, cardiotomy suction, and mechanical prosthesis result in the postoperative hyperbilirubinemia. Both Weber[1] and Lockey[2] proposed that haemolysis contributed to postoperative jaundice; in contrast, Collins[3] and Chu[4] reported that serum TB concentration mainly resulted from increased CB, i.e., hepatic cellular jaundice. However, for the patients with postoperative hyperbilirubinemia in our study, 80.2% of the increased TB was due to an increase of both CB and UCB; 56.2% reached peak TB concentration on the first postoperative day, 33.5% on the second day and 10.3% on the seventh day. Among the patients with preoperative hyperbilirubinemia, 69% had severe postoperative hyperbilirubinemia with highest TB concentration greater than 171 μmol/L, while in other patients only 18% had a highest TB concentration greater than 171 μmol/L (P < 0.01). Development of postoperative hyperbilirubinemia was associated with a higher mortality (P < 0.01), longer duration of artificial ventilation (P < 0.05), and longer ICU stay time (P < 0.05). Furthermore, the mortality of the patients with postoperative hyperbilirubinemia was higher in patients whose TB concentration reached the peak level on the seventh postoperative day than in patients whose TB concentration increased to peak level on the first two days after operation. The occurrence of postoperative hyperbilirubinemia should alert the doctor to the possibility of higher morbidity and mortality, though it might not be considered as a high risk indicator.
Results of the logistic regression showed that preopera-tive total bilirubin concentration, preoperative right atrium pressure, numbers of valves replaced, and blood transfusion requirement were identified as the important predictors for the postoperative hyperbilirubinemia. Combination of these four perioperative risk factors could predict development of postoperative hyperbilirubinemia in 81.2% of all patients. Patients with severe preoperative cardiac failure may have higher right atrial pressure and preoperative hyperbilirubinemia, both reflecting the degree of liver congestion. The capacity of both bilirubin disposal and bile transport may be impaired[6], which also can lead to a higher preoperative TB level. Collins et al had suggested that severe heart failure predisposes the patients to the development of clinical jaundice after CPB[3]. Multiple valve replacements with mechanical prostheses are associated with more haemolysis as a result of CPB, cardiotomy suction, and mechanical prosthesis itself. Haemolysis of transfused blood is a common cause for postoperative jaundice. Our patients who developed postoperative jaundice had received significantly more blood transfusions, which would certainly cause an increased bilirubin load to the liver[9]. The immediate occurrence of postoperative hyperbilirubinemia and rapid decline thereafter may reflect the transient damaging effects on the blood and hepatic function following CPB; whereas, steady progressive increase of TB level to reach its peak on the seventh day indicates liver injury and hepatic dysfunction. CPB can lead to a severe and complicated change of pathophysiology of the liver. Operation stress, reperfusion injury, endotoxemia, and inflammatory reaction may contribute to the liver injury[6,10,11], however, the complex functional and metabolic effects of hepatocyte in CPB require further investigation.
In summary, early postoperative hyperbilirubinemia often occurs after modern extracorporeal circulation. It is mainly caused by an increase in both conjugated and unconjugated bilirubin, and is associated with a higher mortality. Important contributing factors are the preoperative total bilirubin concentration, preoperative severity of right atrial pressure, numbers of valve replacement procedures, and the frequencies of blood transfusion requirement during and shortly after surgery. We suggest that postoperative hyperbilirubinemia is a multifactorial process, which is caused by both the impaired liver function of bilirubin transport and increased production of bilirubin because of haemolysis.
Footnotes
S- Editor Wang J L- Editor Zhu LH E- Editor Ma WH
References
- 1.Welbourn N, Melrose DG, Moss DW. Changes in serum enzyme levels accompanying cardiac surgery with extracorporeal circulation. J Clin Pathol. 1966;19:220–232. doi: 10.1136/jcp.19.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lockey E, McIntyre N, Ross DN, Brookes E, Sturridge MF. Early jaundice after open-heart surgery. Thorax. 1967;22:165–169. doi: 10.1136/thx.22.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins JD, Bassendine MF, Ferner R, Blesovsky A, Murray A, Pearson DT, James OF. Incidence and prognostic importance of jaundice after cardiopulmonary bypass surgery. Lancet. 1983;1:1119–1123. doi: 10.1016/s0140-6736(83)92863-5. [DOI] [PubMed] [Google Scholar]
- 4.Chu CM, Chang CH, Liaw YF, Hsieh MJ. Jaundice after open heart surgery: a prospective study. Thorax. 1984;39:52–56. doi: 10.1136/thx.39.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawahira T, Wakita N, Minami H, Sakata M, Kitano I, Shida T. Lymphatic cardiac tamponade after open-heart surgery with liver dysfunction. Jpn J Thorac Cardiovasc Surg. 2003;51:669–671. doi: 10.1007/s11748-003-0007-6. [DOI] [PubMed] [Google Scholar]
- 6.Michalopoulos A, Alivizatos P, Geroulanos S. Hepatic dysfunction following cardiac surgery: determinants and consequences. Hepatogastroenterology. 1997;44:779–783. [PubMed] [Google Scholar]
- 7.Wark HJ. Hepatic failure after cardiopulmonary bypass is unlikely to be isoflurane hepatitis. Anesthesiology 2002; 97: 1323; author reply 1324 10 Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg. 2002;21:232–244. [Google Scholar]
- 8.Hayashida N, Shoujima T, Teshima H, Yokokura Y, Takagi K, Tomoeda H, Aoyagi S. Clinical outcome after cardiac operations in patients with cirrhosis. Ann Thorac Surg. 2004;77:500–505. doi: 10.1016/j.athoracsur.2003.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Naschitz JE, Slobodin G, Lewis RJ, Zuckerman E, Yeshurun D. Heart diseases affecting the liver and liver diseases affecting the heart. Am Heart J. 2000;140:111–120. doi: 10.1067/mhj.2000.107177. [DOI] [PubMed] [Google Scholar]
- 10.Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg. 2002;21:232–244. doi: 10.1016/s1010-7940(01)01099-5. [DOI] [PubMed] [Google Scholar]
- 11.Byhahn C, Strouhal U, Martens S, Mierdl S, Kessler P, Westphal K. Incidence of gastrointestinal complications in cardiopulmonary bypass patients. World J Surg. 2001;25:1140–1144. doi: 10.1007/BF03215861. [DOI] [PubMed] [Google Scholar]


