Abstract
Aim:
To investigate the roles of acetaldehyde dehydrogenase 2 (ALDH2), the key enzyme of ethanol metabolism, in chronic low to moderate alcohol consumption-induced heart protective effects in mice.
Methods:
Twenty-one male wild-type (WT) or ALDH2-knockout (KO) mice were used in this study. In each genotype, 14 animals received alcohol (2.5%, 5% and 10% in week 1–3, respectively, and 18% in week 4–7), and 7 received water for 7 weeks. After the treatments, survival rate and general characteristics of the animals were evaluated. Serum ethanol and acetaldehyde levels and blood lipids were measured. Metabolomics was used to characterize the heart and serum metabolism profiles.
Results:
Chronic alcohol intake decreased the survival rate of KO mice by 50%, and significantly decreased their body weight, but did not affect those of WT mice. Chronic alcohol intake significantly increased the serum ethanol levels in both WT and KO mice, but KO mice had significantly higher serum acetaldehyde levels than WT mice. Chronic alcohol intake significantly increased the serum HDL cholesterol levels in WT mice, and did not change the serum HDL cholesterol levels in KO mice. After chronic alcohol intake, WT and KO mice showed differential heart and serum metabolism profiles, including the 3 main energy substrate types (lipids, glucose and amino acids) and three carboxylic acid cycles.
Conclusion:
Low to moderate alcohol consumption increases HDL cholesterol levels and improves heart energy metabolism profile in WT mice but not in ALDH2-KO mice. Thus, preserved ALDH2 function is essential for the protective effect of low to moderate alcohol on the cardiovascular system.
Keywords: alcohol, chronic alcohol intake, cardiovascular protection, acetaldehyde dehydrogenase 2, HDL cholesterol, heart energy profile, metabolomics
Introduction
Several epidemiological studies have reported that chronic low to moderate alcohol consumption may provide cardiovascular protective effects and decrease cardiocerebral morbidity1,2,3,4. Regular low to moderate alcohol utilization also enhances cardiomyocyte oxidative stress and is associated with many other extra-cardiac effects5. One of the cardioprotective mechanisms of low to moderate alcohol ingestion is linked to blood lipoprotein profiles and the regulation of cardiac energy utilization6. A meta-analysis confirmed that 1–9 weeks of moderate alcohol consumption could induce the elevation of high-density lipoprotein (HDL), aopA1 and triglycerides7. Alcohol consumption has also been reported to improve insulin resistance and glucose metabolism8.
Aldehyde dehydrogenase 2 (ALDH2), highly expressed in the heart, is the key enzyme of ethanol metabolism and the major intra-mitochondrial cardiac protector9,10. Mochly-Rosen et al reported that ethanol protected the heart from ischemic injury by activating ALDH211. Furthermore, evidence is accumulating that the plasma lipid profile is abnormal under the condition of ALDH2 inactivation12,13. Endo et al reported that overexpression of the ALDH2 inactive allele could enhance cardiac glucose utilization and disturb the metabolic profile of cardiomyocytes14. Taken together, the cardiocerebral protective role of low to moderate alcohol intake might be linked with ALDH2-mediated blood lipid and cardiac lipid-glucose utilization changes.
However, the positive effects are not clear under the condition of ALDH2 inactivation. Here we examined the influence of low to moderate alcohol consumption on lipid and energetic metabolites in ALDH2-knockout (KO) mice.
Materials and methods
Animals and ethanol feeding protocols
Twenty-one adult male C57BL/6 mice (8 weeks old) were obtained from the Shanghai Animal Administration Center (Shanghai, China), and 21 age- and weight-matched ALDH2-knockout mice were produced as described previously15. The mice were housed at room temperature under a 12-h light/dark cycle with free access to drinks and standard laboratory mouse chow. The animals were randomly assigned to receive water (n=7 each genotype) or alcohol (n=14 each genotype). The ethanol feeding protocol was similar to that described previously16 (2.5% for the first week, 5% for the second week, 10% for the third week, and 18% for the 4th to 7th week). Animal experimental protocols were performed according to the guidelines of “The Guide for the Care and Use of Laboratory Animals” Academy Press (NIH Publication No 85–23, revised 1996) and were approved by the Animal Care and Use Committee of Fudan University.
Serum ethanol and acetaldehyde level analysis
After 7 weeks of water or alcohol consumption, all mice were euthanized under deep anesthesia, blood was drawn, and the heart was removed. The levels of serum ethanol and acetaldehyde were determined using commercial kits (BioAssay Systems, Hayward, CA, USA and Roche, Darmstadt, Germany)17,18.
Western blotting
Western blotting was performed as described previously19. The antibody against ALDH2 was purchased from Santa Cruz (Santa Cruz Biotechnology Inc, Dallas, USA), and the horseradish peroxidase-conjugated secondary antibody was purchased from Kangchen (Kangchen Biotechnology, Nanjing, China).
Blood lipid analysis
For the lipid analysis, blood samples were collected following an overnight fast. Total cholesterol (TC), total glycerin (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were measured. The TC and TG levels were determined using commercial kits (Sigma, MO, USA). HDL-C was quantified after the precipitation of apolipoprotein B-containing lipoproteins with an equal volume of a 20% polyethylene glycol solution, as described previously20.
Metabolomics
Left ventricular tissue (50 mg) or serum (100 μL) samples were added to 1 mL or 300 μL of methanol, homogenized for 2 min, and centrifuged at 14 000 r/min for 10 min at 4 °C. Then, a 5-μL aliquot of the supernatant was injected into a C18 column (Agilent, 100 mm×2.1 mm, 1.8 μm) chromatography system (Agilent, USA). The mass spectrometric data were collected using a Q-TOF premier equipped with an electrospray ion source operating in positive ion mode. Centroid data were collected from 100 to 1000 m/z with a scan time of 0.03 s and interscan delay of 0.02 s. Leucine enkephalin was used as the lock mass (m/z 556.2771 in ESI+ mode and 554.2615 in ESI-mode)21. The data were first transformed to mzdata files by Mass Hunter (Agilent, USA) and input into XCMS software for peak picking, peak alignment and peak filtering. The full width at half-maximum (fwhm) was set to 10, and the retention time window was set to 10 (bw=10). The other parameters used were the default parameters. The data were normalized using Excel 2007. The resulting matrix includes the assigned peak index (retention time-m/z pairs), sample names (observations), and normalized peak area (variables), which were then exported for multivariate statistical analysis using PCA and OPLS-DA in the software SIMCA-P+11 (Umetrics, Umea, Sweden). All samples were randomized during the sequence analysis, and the pooled quality-control (QC) was injected every ten samples throughout the entire experiment to evaluate the stability of the system (three QCs). The repeatability of the QC was good.
Statistical analyses
The data shown are presented as the mean±SEM. Student's t-test was used for 2-group comparisons. Multi-group comparisons were performed by one-way ANOVA. The analyses were performed with GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). A value of P<0.05 was considered statistically significant.
Results
General characteristics of the mice consuming low to moderate ethanol
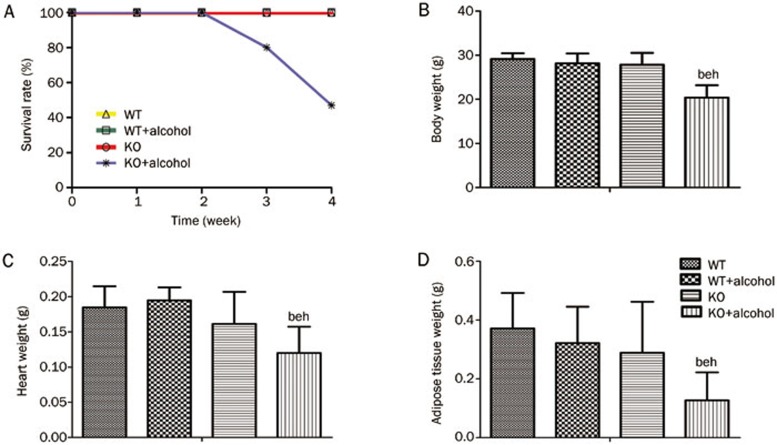
All wild type (WT) mice survived, whereas the survival rate was 50% in the ALDH2-knockout (KO) mice after 7 weeks of ethanol consumption (Figure 1A). There were no significant differences in basic criteria, such as body weight (BW), heart weight (HW) or weight of adipose tissue around the gonads (AW) between the WT and KO mice. After ethanol consumption, BW, HW and AW were all significantly lower in the KO group than in the WT group (BW: 28.2±2.2 vs 20.4±2.8 g, HW: 0.195±0.018 vs0.120±0.037 g, AW: 0.322±0.124 vs 0.127±0.096 g, all P<0.05) (Figure 1B–1D). These results indicated that the ALDH2 knockout induced the deterioration of basic characteristics during alcohol consumption. Echocardiography was performed on all of the mice from the four groups. There were no significant differences in heart function, as shown by ejection fraction (EF) and fractional shortening (FS) measurements, or in structure, as shown by the left ventricular posterior wall diastolic and systolic (LVPW; D&S), left ventricular anterior wall diastolic and systolic (LVAW; D&S) and left ventricular end-diastolic and systolic diameter (LVEDD & ESD) (Figure S1).
Figure 1.
Effect of ALDH2 knockout on survival status post-alcohol consumption in mice. (A) Four week survival curves of mice during ethanol consumption at final concentration (18%). The mice of the two water drinking groups and WT mice treated with alcohol all survived, but half (n=7) of the KO alcohol mice died at the end of experiment. (B, C, and D) BW, HW, and AW were all decreased in the KO alcohol mice compared with the WT alcohol mice. n=7 for each group. Mean±SEM. bP<0.05 vs WT. eP<0.05 vs WT+Alcohol. hP<0.05 vs KO.
ALDH2 deficiency aggravates low to moderate alcohol-induced serum ethanol and acetaldehyde accumulation
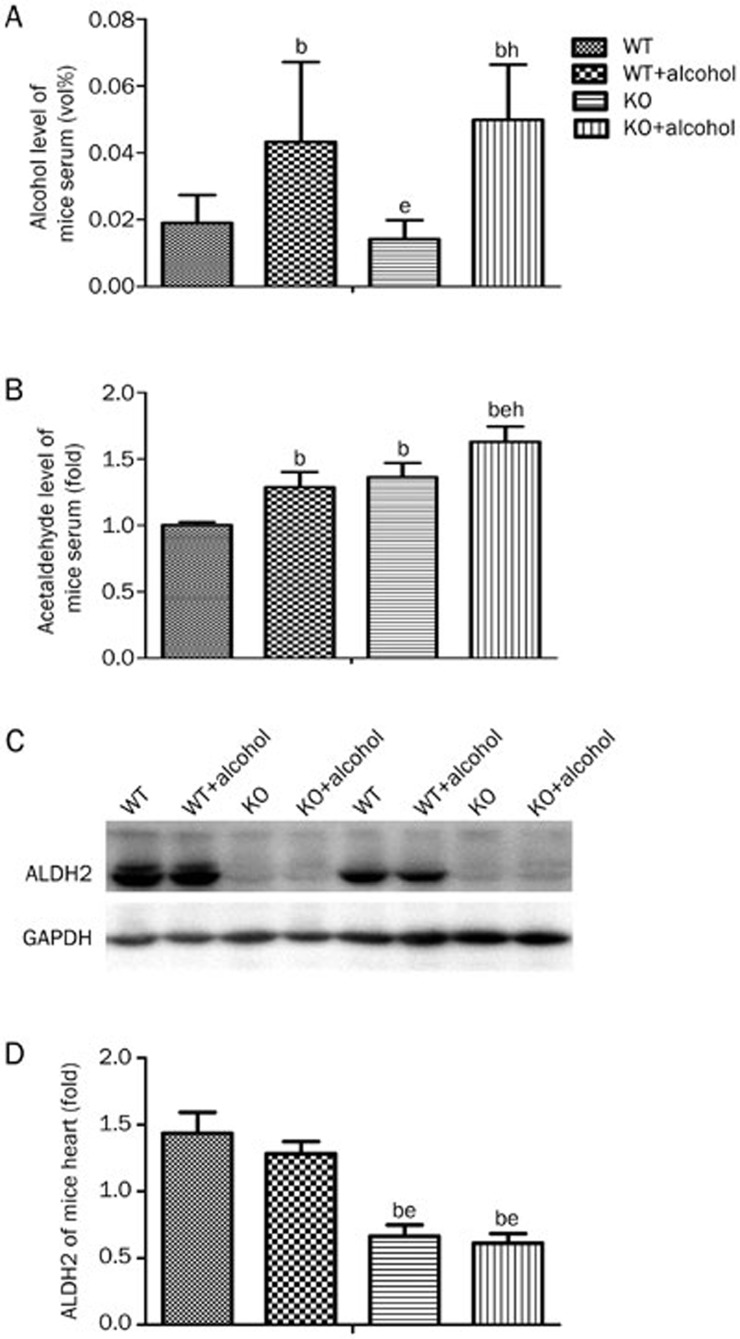
As a key enzyme of alcohol metabolism, ALDH2 dehydrates acetaldehyde to acetic acid, which is the rate-limiting step of alcohol degradation22. Acetaldehyde has been proven to be the most toxic metabolite of alcohol metabolism. The ethanol combined with acetaldehyde level can reflect the degree of alcohol intoxication23. The levels of ethanol and acetaldehyde from WT and ALDH2 KO mouse serum were measured. After consuming alcohol, the serum ethanol and acetaldehyde levels were both significantly elevated. The ethanol level was increased from 0.019%±0.008% to 0.043%±0.024% in the WT mice and from 0.014%±0.006% to 0.050%±0.016% in the KO mice (Figure 2A). The acetaldehyde level was increased to 1.29±0.11-fold in the WT alcohol-drinking mice compared with the non-alcohol-drinking WT mice and to 1.63±0.11-fold in the KO mice after alcohol consumption. Furthermore, the acetaldehyde level was higher in the KO mice than in the WT mice, even without alcohol stimulation (WT: 1.00±0.02 vs KO: 1.36±0.11-fold, P<0.05) (Figure 2B). After 7 weeks of alcohol drinking, ALDH2 protein expression was not significantly different in the WT mice compared with the non-drinking WT mice (Figure 2C and 2D). ALDH2 protein expression was almost non-detectable in the ALDH2-knockout mouse hearts. However, half of the WT heart protein levels in the immunoblot densitometry analysis might be due to the background band sampling technique (Figure 2C, 2D).
Figure 2.
Effect of ALDH2 deficiency on ethanol and acetaldehyde accumulation post-alcohol consumption. (A) Alcohol concentration in mouse serum. At 4 weeks, 18% ethanol consumption significantly increased the level of blood ethanol in both WT and KO mice. (B) Acetaldehyde concentration in mouse serum. The ALDH2 knockout could induce an acetaldehyde elevation in the water group that was aggravated by alcohol stimulation. (C) Heart ALDH2 Western blot lines of each genotype treated with water or alcohol are presented. (D) Protein expression was measured with grayscale analysis of the blots, with GAPDH used as an internal control. n=7 for each group. Mean±SEM. bP<0.05 vs WT. eP<0.05 vs WT+Alcohol. hP<0.05 vs KO.
Low to moderate alcohol induced blood lipid alterations in WT mice but not in ALDH2 KO mice
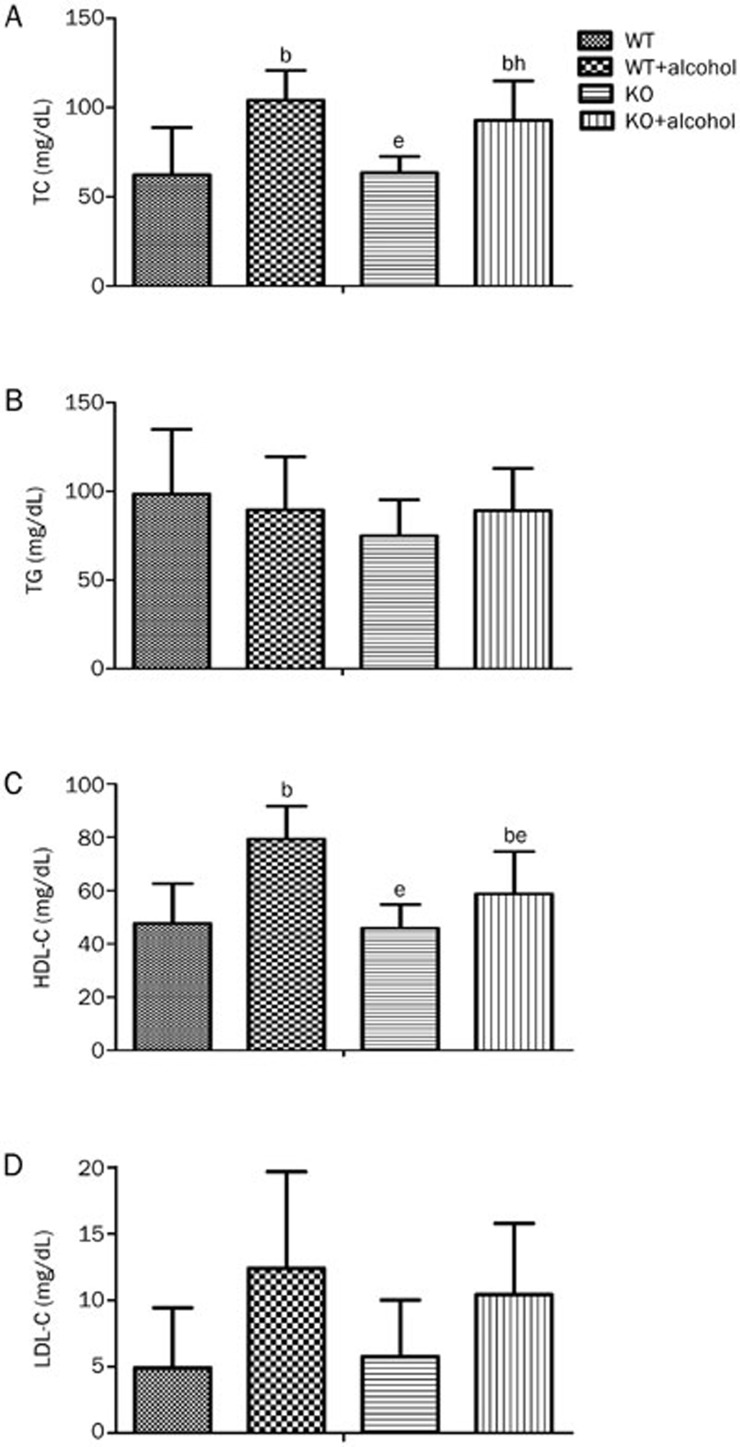
Ethanol has been recognized as an effective blood lipid regulator, and blood lipids are one of the most important risk factors for heart disease6. The baseline lipid levels, including TC, TG, HDL-C, and LDL-C, showed no difference between the WT and KO mice. After alcohol consumption, both TC and HDL-C were increased in the WT mice compared with their non-drinking counterparts (TC: 62.2±26.4 vs 104.0±16.8 mg/dL, P<0.05; HDL-C: 47.6±15.0 vs 79.3±12.4 mg/dL, P<0.05, Figure 3A, 3C). However, in the alcohol drinking KO mice, only TC was increased (63.3±9.2 vs 92.7±22.1 mg/dL, P<0.05, Figure 3A), and HDL-C remained unchanged compared with the non-drinking KO mice (45.9±8.9 vs 58.7±15.9 mg/dL, P=0.1353, Figure 3C). The levels of TG and LDL-C showed no differences in all four groups (Figure 3B, 3D). These results imply that alcohol consumption induced a positive elevation of HDL-C that can be repressed by ALDH2 deficiency.
Figure 3.
Effect of ALDH2 deficiency on blood lipid post-alcohol consumption. All mice were fasted overnight, and serum after 4 weeks of 18% alcohol consumption was collected. (A, B, and C) The serum TC, TG, and HDL-C levels were measured as described in the methods. (D) The LDL levels were calculated using the Friedwald formula. n=7 for each group. Mean±SEM. bP<0.05 vs WT. eP<0.05 vs WT+Alcohol. hP<0.05 vs KO.
Profiling of changed metabolites measured by HPLC-TOF-MS
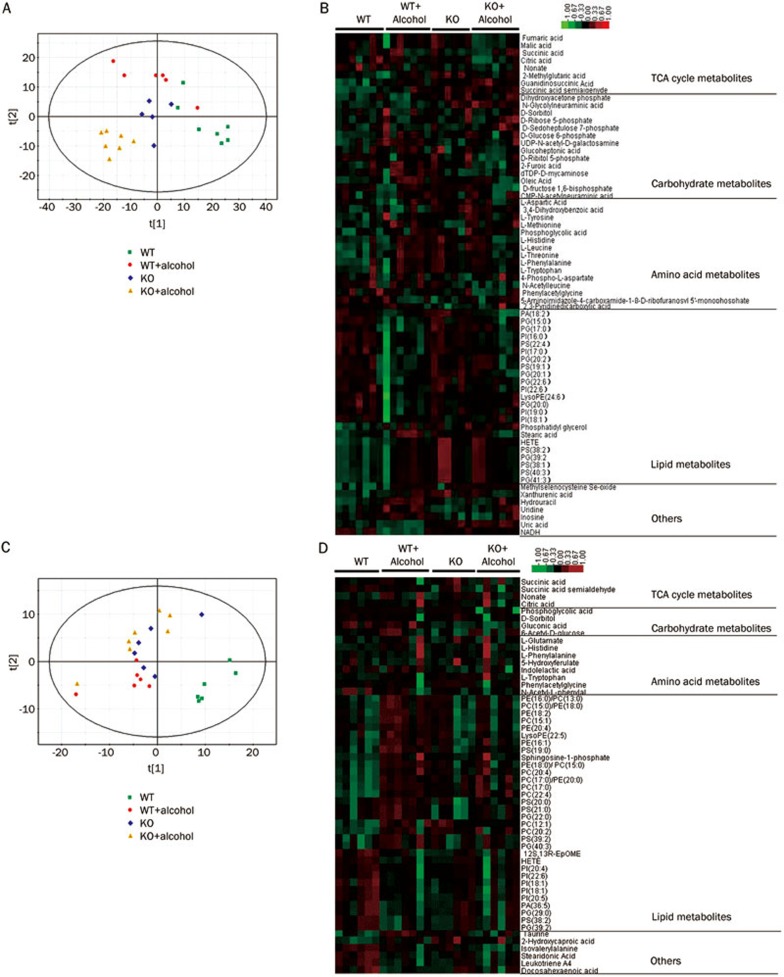
The variations in the cardiac and serum lipid metabolite profiles and in the carbohydrate, amino acid and three carboxylic acid cycle metabolite profiles were measured by metabolomics. Approximately 300 metabolites were identified and quantified by the high-performance liquid chromatography time-of-flight mass spectrometry (HPLC-TOF-MS) method. Principal component analysis (PCA) was performed (Figure 4A, 4C) on both heart and serum data to observe the similarities or differences among the four groups. The PCA score plot for the heart tissue (Figure 4A) showed that the four groups were well separated. In particular, the WT ethanol and KO ethanol groups were separated by the first principal component. These data indicated that the differences in the metabolic parameters are mainly caused by the ALDH2 deficiency. The serum samples were not separated as well as the heart tissue samples. Then a heatmap was generated for metabolites with significant differences between at least two groups (Figure 4B, 4C), and those metabolites were separated into different metabolic pathways. Significant metabolites were selected based on the VIP value (VIP>1) from a typical 7-fold cross-validated orthogonal partial least squares discriminant analysis (OPLS-DA) model and the P-value (P<0.05) of t-test statistics. Differences were found in 23 lipid metabolites from heart tissue and 33 from serum, in 14 carbohydrates from heart and 4 from serum, in 15 amino acids from heart and 8 from serum, and in 8 three-carboxylic acid cycle members from heart and 4 from serum. These data suggest that the metabolite profiles reflect the different metabolic patterns. A detailed list of the differential heart metabolites (WT vs WT±alcohol, KO vs KO±alcohol, WT±alcohol vs KO±alcohol) are shown in Table S1. The differential serum metabolites are shown in Table S2. Both the heart and serum results show that after chronic low to moderate alcohol consumption, lipid metabolites such as stearic acid, oleic acid, and various phospholipids were decreased, and glucose metabolites such as α-D-glucose-6-phosphate, Shikimate-3-phosphate and gluconic acid were increased in ALDH2 KO mice compared with WT mice. These data indicate that ALDH2 deficiency may regulate the lipid-glucose metabolism balance after alcohol consumption. Sketch maps of lipids, glucose and citrate cycle (TCA cycle) members derived from bioinformatic analysis according to the KEGG database are shown in Figure S2.
Figure 4.
Effect of ALDH2 deficiency on the heart and serum metabolism profiles post-alcohol consumption. (A, C) PCA score plot of cardiac and serum metabolite profiles after metabolomics analysis. (B, D) Heatmaps of differentially expressed metabolites, which were classified according to pathway. Red indicates up-regulation, and green indicates down-regulation.
Discussion
In this study, we observed that ALDH2 deficiency weakened the effects of low to moderate alcohol on serum HDL-C levels and the cardiac and serum metabolite profiles, especially the substrates of lipid metabolism observed normally in WT mice. The acetaldehyde accumulation induced by the ALDH2 knockout may partly explain this phenomenon. To the best of our knowledge, this report is the first showing that inactive ALDH2 may affect the beneficial role of alcohol by disturbing the metabolism of lipids and other energy substrates.
As early as 1981, Gordon et al reported that the HDL level was affected by alcohol consumption, which may represent the main mechanism of alcohol-induced cardiac protection24. Wakabayashi et al showed that even low alcohol consumption could lower the serum non-HDL cholesterol level25. Moreover, Krenz and Korthuis previously presented epidemiologic evidence that moderate ethanol ingestion was linked to reduced cardiovascular morbidity5.
In the present study, metabolomics and blood lipid levels were examined in ALDH2 knockout mice after 7 weeks of low to moderate alcohol consumption. Our data showed that half of the KO mice died after 7 weeks of ethanol consumption, indicating the alcohol intolerance of the ALDH2 KO mice. The serum ethanol and acetaldehyde level examination revealed that ALDH2 inactivation significantly enhanced the level of acetaldehyde, even without ethanol stimulation. These data are consistent with a previous study in which the overexpression of ALDH2 significantly reduced the ethanol-induced elevation in blood acetaldehyde levels26. Thus, alcohol intolerance in ALDH2 KO mice might be related to acetaldehyde intoxication. Seven weeks of alcohol consumption induced the deterioration of basic characteristics, including the loss of body weight, emaciation, and loss of fat tissue. The loss of heart weight is another indicator of general deterioration. There are two possible explanations for this deterioration. First, during alcohol administration, food consumption was decreased because ethanol can be used as an energy substrate, which limited the absorption of other nutrients, such as amino acids, and finally led to malnutrition. In the ALDH2 knockout mice, the alcohol metabolism rate and energy supply were lower than those in the WT mice, which aggravated the malnutrition. Second, acetaldehyde accumulation is more severe in the ALDH2 knockout mice, which may lead to a global toxicity reaction, cardiac toxicity injury, enhanced toxicity damage, inflammatory responses, and poor nutritional status that together aggravate the deterioration.
Moreover, the HDL-C level was not increased in the ALDH2 KO mice after alcohol consumption. This finding is consistent with previous studies showing that the blood lipid level was related to the ALDH2 genotype27,28,29. In the case of ALDH2 deficiency, alcohol intake may not play a protective role in the cardiovascular system as the beneficial effect of ethanol intake on HDL disappeared in this context. There is a “U” shaped relationship of alcohol consumption and the incidence of cardiovascular disease. The beneficial effect of low-to-moderate alcohol drinking on atherosclerotic diseases is focused on blood lipid metabolism. In the present study, after alcohol consumption, the HDL-C level of the KO mice was not as high as that of the WT mice, which indicated that the ALDH2 knockout depressed the reaction of HDL-C to alcohol and reduced the beneficial effect of low-to-moderate alcohol drinking on atherosclerotic disease. The absence of the ALDH2 gene does not change the increased TC level induced by alcohol administration. In addition to HDL-C, there are many other types of cholesterol in TC, including very-low-density lipoprotein (VLDL) cholesterols, low-density lipoprotein (LDL-C) cholesterols, intermediate-density lipoprotein (IDL) cholesterols, lipoprotein (a) cholesterols and free cholesterols. The reaction of TC is much more complicated than that of single lipoprotein cholesterols, and the unchanged TC levels in the ALDH2 knockout mice may be due to other cholesterol types. As the ALDH2 mutation is widespread in the East Asian population, our results provide evidence that even low-to-moderate alcohol may be harmful for those individuals carrying a mutant allele. TC and HDL-C are the most commonly used blood lipid indicators in clinical studies. Testing HDL-C and other cholesterol types in habitual alcohol-drinking patients, even when the TC levels are normal, may be useful. The alcohol consumption limits should be stricter in ALDH2 mutation carriers, especially in those at risk of cardiovascular disease.
Recent animal studies showed that the ALDH2*2 mutant allele is a harmful factor for myocardial energy supply and that the balance of cardiac lipid and glucose utilization is disturbed by ALDH2*214. Our results demonstrate that the most significant differences in metabolites between WT and ALDH2 KO mice were observed for metabolites related to lipid metabolism inhibition and glucose metabolism up-regulation, which was consistent in both the heart and serum samples. Several affected signaling pathways were derived from the bioinformatic analysis of these data, including the biosynthesis of unsaturated fatty acids, glycolysis or gluconeogenesis and the citrate cycle (TCA cycle). Long-chain fatty acids are the major energy resource for cardiomyocytes. The lipid metabolic analysis indicated that stearic acid and oleic acid biosynthesis were matched in the database; combined with the metabonomics results, the C18 fatty acid metabolic pathway may be inhibited by ALDH2 knockout. As the major energy substrates are inadequate, the other energy resource — the glucose utilization pathway — is activated in compensation. Two glycolysis pathway metabolites were matched in the KEGG database, D-glucose-6p and D-fructose-1,6-p2, and both were increased after ALDH2 knockout. Chronic energy profile remolding could induce energy starvation at the end stage, and the energy supply ATP and respiratory chain complex activity should be the subject of future studies.
Taken the above data together, a reasonable assumption is that the protective effect of low to moderate alcohol intake may not be present in ALDH2-deficient individuals due to lipid and energy profile changes. There are two possible mechanisms that may be involved in the metabolic effect of ALDH2 deficiency: first, the heart and liver could be injured by acetaldehyde accumulation, which may induce disruptions in lipid and lipoprotein synthesis and utilization; second, ALDH2 has been reported to be a regulator of mitochondrial and metabolic pathways and to directly participate in cardiac fatty acid and glucose oxidation. With respect to these two mechanisms, several potential compensatory mechanisms exist in the absence of ALDH2 to ensure survival. First, among the 19 isozymes of the ALDH family, ALDH2 can most efficiently metabolize acetaldehyde. Under the condition of ALDH2 deficiency, there is a compensatory increase in activation of other ALDHs such as ALDH1A (cytosol), ALDH1B (mitochondria) and ALDH3 (cytosol and nucleus). However, the Km values of other ALDHs are nearly 900-fold higher than ALDH2, and their compensatory function is usually inadequate. In addition, the other key enzyme of alcohol metabolism, alcohol dehydrogenase (ADH), can undergo feedback inhibition by accumulated acetaldehyde, thereby reducing the activity of the entire alcohol metabolic pathway. Then, more serious drunk reactions are induced to stop further alcohol intake. Second, two key molecules of cell metabolic pathways, AMPK and PPAR, can be regulated by ALDH2. Under the condition of ALDH2 deficiency, AMPK phosphorylation and PPAR expression are decreased, and downstream molecules, such as CD36, mCPT-I, ACC2, GLUT-1/4, and PDK/PDH, are modified. Furthermore, energy substrate utilization is regulated, the synthesis of proteins and lipids is inhibited, and high oxygen consumption pathways such as fatty acid oxidation are depressed; in contrast, the efficiency of low oxygen consumption pathways such as glycolytic and ketone oxidation is improved, thus ensuring an adequate energy supply. Although several compensatory mechanisms ensure the survival of ALDH2-knockout animals, pathological changes are still reflected by many indicators, such as abnormal blood acetaldehyde concentrations, dyslipidemia and myocardial energy remodeling.
Ethanol is mainly metabolized by the sequential dehydrogenation enzyme system: first, ADH catalyzes ethanol to acetaldehyde, and then ALDH2 catalyzes acetaldehyde to acetic acid30. Approximately 40% of the East Asian population carries one or two mutant ALDH2*2 alleles, which leads to significantly deficient enzymatic activity10,22. The present study showed that even low to moderate alcohol consumption may be harmful or at least have no benefit in ALDH2 mutant mice. Therefore, individuals with ALDH2 mutations, especially cardiovascular patients with ALDH2 mutations, should avoid alcohol consumption. ALDH2 genotyping may help with the evaluation and prediction of the potential cardiac protective effects of low to moderate alcohol consumption.
Author contribution
Fan FAN, Ai-jun SUN, and Jun-bo GE designed the research; Fan FAN, Quan CAO, and Cong WANG performed the research; Xin MA, Cheng SHEN, and Xiang-wei LIU contributed new reagents and analytical tools; Fan FAN and Cong WANG analyzed the data; and Fan FAN wrote the paper. Li-ping BU, Yun-zeng ZOU, and Kai HU revised the language.
Acknowledgments
This work was supported by the National Basic Research Program of China (No 2011CB503905), National Natural Science Foundation of China (No 30971250, 81300096), and Program for New Century Excellent Talents in University (NCET).
Footnotes
(Supplementary Tables and Figures were available at APS's website.
Supplementary Information
Differences metabolites list of heart.
Differences metabolites list of serum.
References
- Au Yeung SL, Jiang C, Cheng KK, Cowling BJ, Liu B, Zhang W, et al. Moderate alcohol use and cardiovascular disease from Mendelian randomization. PloS One. 2013;8:e68054. doi: 10.1371/journal.pone.0068054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra J, Taylor B, Irving H, Roerecke M, Baliunas D, Mohapatra S, et al. Alcohol consumption and the risk of morbidity and mortality for different stroke types — a systematic review and meta-analysis. BMC Pub Health. 2010;10:258. doi: 10.1186/1471-2458-10-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J. Alcohol consumption and risk of stroke: a meta-analysis. JAMA: J Am Med Assoc. 2003;289:579–88. doi: 10.1001/jama.289.5.579. [DOI] [PubMed] [Google Scholar]
- Krenz M, Korthuis RJ. Moderate ethanol ingestion and cardiovascular protection: from epidemiologic associations to cellular mechanisms. J Mol Cell Cardiol. 2012;52:93–104. doi: 10.1016/j.yjmcc.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaziano JM, Buring JE, Breslow JL, Goldhaber SZ, Rosner B, VanDenburgh M, et al. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. New Engl J Med. 1993;329:1829–34. doi: 10.1056/NEJM199312163292501. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319:1523–8. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson S, Hammar N, Grill V. Alcohol consumption and type 2 diabetes Meta-analysis of epidemiological studies indicates a U-shaped relationship. Diabetologia. 2005;48:1051–4. doi: 10.1007/s00125-005-1768-5. [DOI] [PubMed] [Google Scholar]
- Chen CH, Sun L, Mochly-Rosen D. Mitochondrial aldehyde dehydrogenase and cardiac diseases. Cardiovasc Res. 2010;88:51–7. doi: 10.1093/cvr/cvq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev. 2014;94:1–34. doi: 10.1152/physrev.00017.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–5. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M, Daimon M, Emi M, Iijima H, Sato H, Koyano S, et al. Genetic association between aldehyde dehydrogenase 2 (ALDH2) variation and high-density lipoprotein cholesterol (HDL-C) among non-drinkers in two large population samples in Japan. J Atheroscler Thromb. 2008;15:179–84. doi: 10.5551/jat.e542. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Amamoto K, Tamaki S, Okamura T, Tsujita Y, Ueno Y, et al. Genetic variation in aldehyde dehydrogenase 2 and the effect of alcohol consumption on cholesterol levels. Atherosclerosis. 2002;164:171–7. doi: 10.1016/s0021-9150(02)00059-x. [DOI] [PubMed] [Google Scholar]
- Endo J, Sano M, Katayama T, Hishiki T, Shinmura K, Morizane S, et al. Metabolic remodeling induced by mitochondrial aldehyde stress stimulates tolerance to oxidative stress in the heart. Circ Res. 2009;105:1118–27. doi: 10.1161/CIRCRESAHA.109.206607. [DOI] [PubMed] [Google Scholar]
- Liao J, Sun A, Xie Y, Isse T, Kawamoto T, Zou Y, et al. Aldehyde dehydrogenase-2 deficiency aggravates cardiac dysfunction elicited by endoplasmic reticulum stress induction. Mol Med. 2012;18:785–93. doi: 10.2119/molmed.2011.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HZ, Karliner JS, Gray MO. Moderate alcohol consumption induces sustained cardiac protection by activating PKC-epsilon and Akt. Am J Physiol Heart Circ Physiol. 2002;283:H165–74. doi: 10.1152/ajpheart.00408.2001. [DOI] [PubMed] [Google Scholar]
- Ou XM, Stockmeier CA, Meltzer HY, Overholser JC, Jurjus GJ, Dieter L, et al. A novel role for glyceraldehyde-3-phosphate dehydrogenase and monoamine oxidase B cascade in ethanol-induced cellular damage. Biol Psychiatr. 2010;67:855–63. doi: 10.1016/j.biopsych.2009.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhao W, Chen Y, Guo L, Yang L. A replaceable dual-enzyme capillary microreactor using magnetic beads and its application for simultaneous detection of acetaldehyde and pyruvate. Electrophoresis. 2012;33:2145–51. doi: 10.1002/elps.201200090. [DOI] [PubMed] [Google Scholar]
- Fan F, Sun A, Zhao H, Liu X, Zhang W, Jin X, et al. MicroRNA-34a promotes cardiomyocyte apoptosis post myocardial infarction through down-regulating aldehyde dehydrogenase 2. Curr Pharm Des. 2013;19:4865–73. doi: 10.2174/13816128113199990325. [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhu H, Wang S, Ma X, Liu X, Wang C, et al. Naoxintong protects against atherosclerosis through lipid-lowering and inhibiting maturation of dendritic cells in LDL receptor knockout mice fed a high-fat diet. Curr Pharm Des. 2013;19:5891–6. doi: 10.2174/1381612811319330008. [DOI] [PubMed] [Google Scholar]
- Chen F, Xue J, Zhou L, Wu S, Chen Z. Identification of serum biomarkers of hepatocarcinoma through liquid chromatography/mass spectrometry-based metabonomic method. Analy Bioanal Chem. 2011;401:1899–904. doi: 10.1007/s00216-011-5245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng MY, Luczak SE, Wall TL. ALDH2, ADH1B, and ADH1C genotypes in Asians: a literature review. Alcohol Res Health. 2007;30:22–7. [PMC free article] [PubMed] [Google Scholar]
- Manzo-Avalos S, Saavedra-Molina A. Cellular and mitochondrial effects of alcohol consumption. Int J Environ Res Public Health. 2010;7:4281–304. doi: 10.3390/ijerph7124281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Ernst N, Fisher M, Rifkind BM. Alcohol and high-density lipoprotein cholesterol. Circulation. 1981;64 (3 Pt 2):III 63–7. [PubMed] [Google Scholar]
- Wakabayashi I, Groschner K. Modification of the association between alcohol drinking and non-HDL cholesterol by gender. Clin Chim Acta. 2009;404:154–9. doi: 10.1016/j.cca.2009.03.047. [DOI] [PubMed] [Google Scholar]
- Doser TA, Turdi S, Thomas DP, Epstein PN, Li SY, Ren J. Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation. 2009;119:1941–9. doi: 10.1161/CIRCULATIONAHA.108.823799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ding X, Beier JI, Baldauf KJ, Jokinen JD, Zhong H, Arteel GE. Acute ethanol preexposure promotes liver regeneration after partial hepatectomy in mice by activating ALDH2. Am J Physiol Gastrointes Liver Physiol. 2014;306:G37–47. doi: 10.1152/ajpgi.00085.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long EK, Olson DM, Bernlohr DA. High-fat diet induces changes in adipose tissue trans-4-oxo-2-nonenal and trans-4-hydroxy-2-nonenal levels in a depot-specific manner. Free Radical Biol Med. 2013;63:390–8. doi: 10.1016/j.freeradbiomed.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunner S, Sevane N, Garcia D, Leveziel H, Williams JL, Mangin B, et al. Genes involved in muscle lipid composition in 15 European Bos taurus breeds. Animal Genet. 2013;44:493–501. doi: 10.1111/age.12044. [DOI] [PubMed] [Google Scholar]
- Zakhari S. Alcohol metabolism and epigenetics changes. Alcohol Res: Curr Rev. 2013;35:6–16. doi: 10.35946/arcr.v35.1.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differences metabolites list of heart.
Differences metabolites list of serum.