Abstract
Aim:
The roles of G-protein coupled receptors (GPCRs) in stem cell biology remain unclear. In this study, we aimed to identify GPCRs that might contribute to the self-renewal of mouse embryonic stem cells (mESCs).
Methods:
The expression levels of pluripotent genes and GPCR gene were detected in E14 mESCs using PCR array and RT-PCR. Immunofluorescent staining was used to examine the expression of pluripotent markers and the receptor translocation. Western blot analysis was used to detect phosphorylation of signal proteins. Knock-down of receptor was conducted to confirm its role in pluripotency maintenance.
Results:
In leukemia inhibitory factor (LIF)-free medium, mESCs lost the typical morphology of pluripotency, accompanied by markedly decreases in expression of somatostatin receptor type 2 (SSTR2), as well as the pluripotency biomarkers Oct4, Sox2, Rex1 and Nanog. Addition of the SSTR2 agonist octreotide or seglitide (0.1–30 μmol/L) in LIF-free medium dose-dependently promoted the self-renewal of mESCs, whereas the SSTR2 antagonist S4 (0.03–3 μmol/L) dose-dependently blocked octreotide-induced self-renewal. Knock-down of SSTR2 significantly decreased the self-renewal of mESCs even in the presence of LIF. Addition of LIF (1000 U/mL) or octreotide (1 μmol/L) in LIF-free medium significantly increased both phosphorylation and nuclear ocalization of STAT3.
Conclusion:
The activation of SSTR2 contributes to the self-renewal of mESCs via activation of the STAT3 pathway.
Keywords: stem cell, mouse embryonic stem cell, GPCR, somatostatin receptor type 2, self-renewal, STAT3, leukemia inhibitory factor, octreotide, seglitide
Introduction
Self-renewal and pluripotency are two essential properties of embryonic stem cells (ESCs), which are regulated by a combined action of extracellular signals (such as cytokines and growth factors) and intracellular factors, including their corresponding receptors and downstream signal cascades1,2. Leukemia inhibitory factor (LIF) is one of the most important extracellular signal molecules identified to promote the self-renewal and pluripotency of mESCs3. The binding of LIF to the LIF receptor (LIFR) leads to the recruitment of gp130 and activation of the Jak/Stat3 signaling pathway, which is the major mechanism underlying the effect of LIF in promoting mES cell self-renewal4.
GPCRs are plasma membrane proteins that transduce signals from extracellular ligands to intracellular heterotrimeric GTP-binding proteins (G proteins)5. GPCRs are the third-largest gene family in the human genome, representing over 800 distinct genes and 2% of the human genome. GPCRs are responsible for detecting a diversity of ligands, including biogenic amines, amino acids, ions, lipids and peptides, as well as light, taste and odor stimuli, and coupling these signals to fundamental cellular responses such as growth, death, movement, transcription and excitation. In fact, approximately one-third of all marketed drugs act by modulating GPCR functions6.
In this study, we sought to identify GPCRs that might contribute to the pluripotency of mESCs. Using a PCR array, we found that the expression of SSTR2 decreased significantly in mESCs cultured in differentiation conditions. SSTR2 is one of the five receptors (SSTR1-5) that mediate the physiological functions of somatostatin. SSTR2 is highly expressed in the hypothalamus and regulates the adenohypophyseal release of growth hormone, thyroid-stimulating hormone and prolactin. SSTR2 is also expressed in the frontal cortex and hippocampus, where it modulates many cognitive and vegetative functions7,8. Furthermore, spatially and temporally regulated SSTR2 expression patterns have been observed during embryonic brain and peripheral nerve development, suggesting that somatostatin signaling exerts functions during neurogenesis9. Here, we report a novel function of SSTR2 in maintaining the pluripotency and self-renewal of mESCs and the possible underlying mechanism.
Materials and methods
ESC culture
Murine ESCs (E14TG2a) (CRL-1821; American Type Culture Collection, Manassas, VA, USA) were maintained in mES media (DMEM with 15% FBS (Hyclone 30070.03), 2 mmol/L GlutaMAX, 0.1 mmol/L non-essential amino acids (NEAA), 0.1 mmol/L β-mercaptoethanol, 100 U/mL penicillin, and 100 U/mL streptomycin) supplemented with murine LIF (1000 U/mL, Millipore, Billerica, MA, USA) and 2i (3 μmol/L CHIR99021 and 1 μmol/L PD0325901, Sigma, St Louis, MO, USA). E14 cells were cultured under feeder-free conditions in the presence of LIF and passaged every 3 d. For differentiation, E14 cells were grown in bacteriologic dishes without LIF as control cells for 4 d. LIF, octreotide, seglitide, S4 (GL Biochem, Shanghai, China) and 2i were added in mES media at the indicated concentrations according to the experiments.
Real-time PCR
Total mRNA was isolated using TRIzol (Life Technology, Grand Island, NY, USA), and 2 μg RNA was used to synthesize cDNA using the PrimeScriptTM RT reagent kit (Takara, Otsu, Shiga, Japan) according to the manufacturer's protocol. Real-time PCR was performed using JumpStartTM TaqReadyMixTM (Sigma-Aldrich, St Louis, MO, USA) with Eva Green (Biotium, Hayward, CA, USA) and analyzed with a Stratagene Mx3000P thermal cycler (Agilent). The primer sequences were as follows. The forward primer for Oct4 was 5′-TAGGTGAGCCGTCTTTCCAC-3′, and the reverse primer was 5′-GCTTAGCCAGGTTCGAGGAT-3′. The forward primer for Nanog was 5′-CTCAAGTCCTGAGGCTGACA-3′, and the reverse primer was 5′-TGAAACCTGTCCTTGAGTGC-3′. The forward primer for Sox2 was 5′-AGGGCTGGGAGAAAGAAGAG-3′, and the reverse primer was 5′-CCGCGATTGTTGTGATTAGT-3′. The forward primer for Rex1 was 5′-GACGGATACCTAGAGTGCATCA-3′, and the reverse primer was 5′-GAAGGGAACTCGCTTCCAGAA-3′. The forward primer for SSTR2 was 5′-CGCATGGTGTCCATCGTAGT-3′, and the reverse primer was 5′-GGATTGTGAATTGTCTGCCTTGA-3′.
Alkaline phosphatase and immunofluorescent staining
For alkaline phosphatase (AP) staining, mESCs were fixed with 4% paraformaldehyde (PFA) in PBS for 45 s, rinsed once with PBS and stained using a leukocyte alkaline phosphatase kit (Sigma-Aldrich, St Louis, MO, USA) according to the manufacturer's protocol. For immunofluorescent staining, cells were fixed with 4% PFA for 30 min, then incubated with primary antibodies against SSEA-1 (Santa Cruz, sc-21702), Nanog (Millipore, AB5731), Oct4 (Abcam, ab19857) or Stat3 (CST, 4904) followed by the appropriate secondary antibodies conjugated to Alexa Fluor 555 or Alexa Fluor 488. Nuclei were counterstained with Hoechst 33342. Images were taken with an Olympus IX51 inverted fluorescent microscope or an Olympus FV10i confocal microscope.
RNA interference in mESCs
For lentivirus-mediated SSTR2 knock-down, lentiviral vector FG12 (derived from the pFUGW vector, Addgene) and packaging plasmids pRSV/REV, pMDLG/pRRE and pHCMVG were used. To construct the shRNA expression cassette, complementary DNA oligonucleotides were synthesized, annealed and inserted immediately downstream of the U6 promoter of the pBS/U6 plasmid, and the derived cassette was subcloned into the FG12 vector. Recombinant lentiviruses were produced in HEK-293T cells to express shRNA against coding regions of SSTR2. Virus expressing a scrambled shRNA sequence was prepared as a control. E14 cells were infected overnight with lentiviral supernatants, and GFP+ clones were selected. The RNAi sequence for SSTR2 was 5′-GTAGATGGCATCAATCAGT-3′, and the scrambled sequence was 5′-TTCTCCGAACGTGTCACGTTT-3′.
Western blot analysis
ESCs were lysed and the total protein extracts were sonicated for 5 min and boiled at 95–100 °C for 5 min in sample buffer (50 mmol/L Tris-HCl, 2% w/v SDS, 10% glycerol, 1% β-mercaptoethanol, 0.01% bromophenol blue, pH 6.8). To study the nuclear translocation of STAT3, nuclei and cytoplasm were separated using the Thermo NE-PER Nuclear and Cytoplasmic Extraction Kit according to the manufacturer's instruction. Cell lysates were separated in an SDS-PAGE gel and transferred to polyvinylidene difluoride membranes. The membranes were first incubated with blocking buffer (TBS with 0.05% Tween 20, 10% non fat milk) for 1 h at room temperature and then with antibodies against p-STAT3 (Tyr705) (CST, 9131S), STAT3 (CST, 4904), or GAPDH (CST, 2118) overnight at 4 °C. The membranes were washed thrice with TBST and incubated with proper HRP-conjugated secondary antibodies for 1 h. After washing, the blots were developed using Western Lightning Ultra (Perkin Elmer, Foster City, CA, USA) and visualized using the ChemiDocTM MP System.
Statistical analysis
Values are reported as the mean±SEM. Statistical significance (P value) was determined using the paired Student's t-test. All graphs were plotted using the GraphPad Prism 5 software.
Results
The expression of SSTR2 is decreased in differentiated mESCs
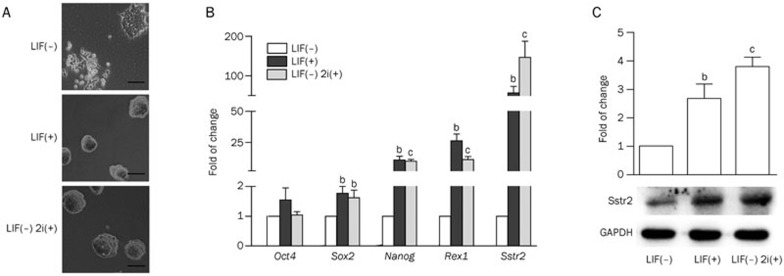
To investigate the role of GPCRs in the maintenance of pluripotency in mESCs, we analyzed the expression profiles of various GPCRs in E14 cells cultured in self-renewal (mES medium with LIF or mES medium without LIF but supplemented with 2i) or differentiation (mES medium without LIF) conditions using a PCR array. As shown in Figure 1A, compared with E14 cells cultured in mES medium supplemented with LIF, cells cultured in LIF-deprived medium lost the typical compact colony morphology of mESCs on d 4. Two small-molecule inhibitors (2i, CHIR99021 and PD0325901) could replace LIF and maintain the typical morphology of the mESCs. Using PCR arrays, we found that some GPCRs that had been reported to be involved in stemness maintenance, such as the Wnt/Frizzled (FZDs)10, were down-regulated in mESCs cultured in LIF-deprived medium and accompanied by a significant decrease in the typical pluripotency biomarkers, including Oct4, Sox2, Rex1, and Nanog. Another GPCR, SSTR2, was also found to be significantly down-regulated after mESC differentiation (data not shown). Because SSTR2 has never been reported to be involved in ESC self-renewal, we decided to further explore its function. The PCR array result was confirmed by quantitative RT-PCR analysis, which also demonstrated a significantly reduced expression of SSTR2 in E14 cells cultured in LIF-deprived mES medium (Figure 1B). The RT-PCR results also confirmed the down-regulation of pluripotency genes, including Sox2, Nanog, and Rex1, after LIF deprivation (Figure 1B). We then analyzed the protein level of SSTR2 in E14 cells cultured in various conditions. As shown in Figure 1C, the protein level of SSTR2 was also reduced in cells cultured in the LIF-deprived medium, while 2i restored the SSTR2 protein level. These data suggest that SSTR2 may play a role in the maintenance of pluripotency.
Figure 1.
Reduced expression of SSTR2 in mESCs cultured in LIF-deprived medium. (A) Morphology of E14 mouse ES cells cultured in basal mES medium (no LIF), or media supplemented with LIF (1000 U/mL) or 2i (1 μmol/L PD0325901 and 3 μmol/L CHIR99021) for 4 d (Scale bar: 50 μm). (B) Quantitative RT-PCR analysis of pluripotency genes and SSTR2 in the E14 cells described in (A). (C) Western blot analysis of SSTR2 in mESCs cultured in basal mES medium (no LIF) or media supplemented with LIF or 2i. The data are the mean±SEM (n=3). bP<0.05, cP<0.01 vs the LIF(−) group.
Activation of SSTR2 maintains mES cell self-renewal in the absence of LIF
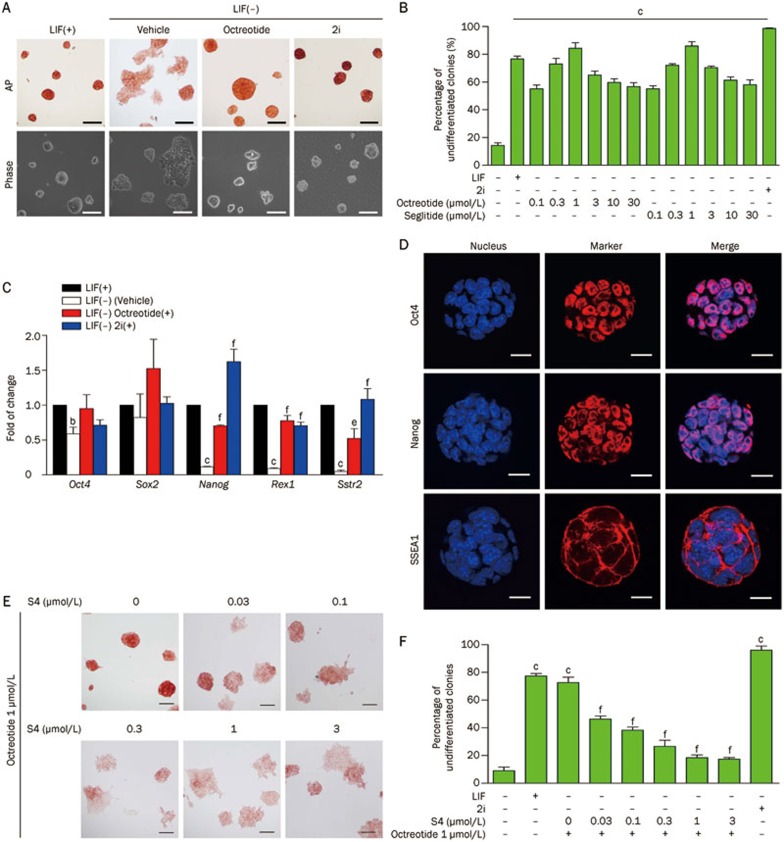
SSTR2 is one of the five somatostatin receptors (SSTR1-5)11. Various somatostatin analogues, such as octreotide and seglitide12, have been developed for clinical applications through specific activation of SSTR2. To investigate the role of SSTR2 in mESC self-renewal, we supplemented the LIF-free medium with various concentrations of octreotide or seglitide. As shown in Figure 2A and 2B, both agonists displayed dose-dependent promotion of mES cell self-renewal in the absence of LIF, with the most effective concentration being 1 μmol/L. In mES cell culture supplemented with 1 μmol/L octreotide, more than 80% of the colonies maintained the typical compact morphology and strong AP staining, even when LIF was removed (Figure 2A and 2B). RT-PCR revealed that octreotide nearly completely reversed the loss of pluripotency genes, including Oct4, Sox2, Nanog, and Rex1, that was induced by LIF withdrawal. Octreotide also prevented the down-regulation of SSTR2 that was induced by LIF deprivation (Figure 2C). Immunofluorescent staining confirmed that mESCs cultured in octreotide-containing, LIF-free medium expressed high levels of pluripotency markers, including Oct4, Nanog, and SSEA1 (Figure 2D).
Figure 2.
Activation of SSTR2 prevents mESC differentiation caused by LIF deprivation. (A) Morphology and alkaline phosphatase (AP) staining of E14 cells cultured in mES media containing LIF (1000 U/mL) or mES media without LIF but supplemented with 2i (1 μmol/L PD0325901 and 3 μmol/L CHIR99021) or the SSTR2 agonist octreotide (1 μmol/L) (Scale bar: 50 μm). (B) The percent of undifferentiated colonies (according to AP staining and morphology) of E14 cells cultured in mES media containing LIF, 2i, octreotide or seglitide (SSTR2 agonist) at various concentrations for 3 d. The data are the mean±SEM (n=3). cP<0.01 vs LIF(−) group. (C) Quantitative RT-PCR analysis of pluripotency genes and SSTR2 in cells corresponding to (A). Data are the mean±SEM (n=3). bP<0.05, cP<0.01 vs LIF(+) condition. eP<0.05, fP<0.01 vs LIF(−) condition. (D) Immunofluorescence staining of pluripotency markers (Oct4, Nanog, and SSEA1) in mESCs cultured in octreotide (1 μmol/L)-containing, LIF-free media. (Scale bar: 10 μm). (E) AP staining of E14 cells pre-incubated with various concentrations of the SSTR2 antagonist S4 for 24 h and then cultured in LIF-free media containing octreotide (1 μmol/L) (Scale bar: 50 μm). (F) The percent of undifferentiated colonies (according to AP staining and morphology) of E14 cells cultured in the indicated media. cP<0.01 vs LIF(−) condition. fP<0.01 vs octreotide alone.
To further confirm that the octreotide-promoted self-renewal of mESCs was indeed via the activation of SSTR2 signaling, a SSTR2 specific antagonist, Ac-4-NO2-Phe-c(o-Cys-Tyr-D-Trp-Lys-Thr-Cys)-D-Tyr-NH2 (S4) was introduced into the culture system13. AP staining data showed that S4 blocked the octreotide-induced self-renewal of mESCs in a dose-dependent manner (Figure 2E and 2F). The percentage of undifferentiated colonies decreased with increasing doses of S4 (Figure 2F). Taken together, these data suggest that activation of SSTR2 plays a positive role in maintaining the self-renewal of mESCs.
Knock-down of SSTR2 leads to loss of pluripotency in mESCs
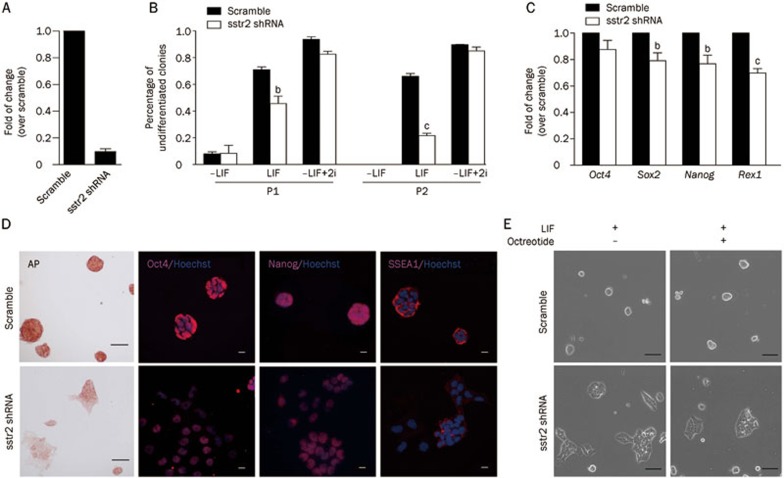
To rule out the possibility of off-target effects by octreotide and S4, we examined the effect of SSTR2 knock-down in mESCs. An shRNA-based technique was employed to specifically knock-down the SSTR2 gene in mESCs cultured in medium containing LIF. Quantitative RT-PCR results revealed that, relative to the scrambled shRNA, the transfection of shRNA targeting SSTR2 led to a dramatic reduction of the SSTR2 mRNA in mESCs 24 h after transfection (Figure 3A). Knock-down of SSTR2 significantly decreased the ratio of undifferentiated E14 cells, even in the presence of LIF (Figure 3B). Cells expressing SSTR2 shRNA displayed a lower expression level of pluripotency genes (Oct4, Sox2, Nanog, and Rex1) (Figure 3C) and showed weak or negative staining for AP and other pluripotent markers, including Oct4, Nanog, and SSEA1 (Figure 3D). Adding LIF or the combination of LIF and octreotide did not rescue the spontaneous differentiation of these SSTR2 knock-down cells (Figure 3E), although the differentiation could be rescued by 2i (Figure 3B). These data confirmed that SSTR2 contributes to mES cell self-renewal and that knock-down of SSTR2 leads to spontaneous differentiation, even in the presence of LIF.
Figure 3.
Knock-down of SSTR2 induces mESC differentiation, even in the presence of LIF. (A) Validation of the knock-down efficiency by shRNA targeting SSTR2 using quantitative RT-PCR. (B) E14 cells (initial density of 10 000 cells/well in a 24-well plate) transfected with SSTR2 shRNA or scramble shRNA were cultured in mES media with or without LIF (1000 U/mL) or LIF-free mES medium supplemented with 2i for 3 d. The percent of undifferentiated colonies was calculated at passages 1 and 2. The data are the mean±SEM (n=3). bP<0.05, cP<0.01 vs scramble shRNA. (C) Quantitative RT-PCR analysis of pluripotency genes in mouse ES cells expressing SSTR3 shRNA or scramble shRNA cultured in mES media with LIF (passage 2). The data are the mean±SEM (n=3). bP<0.05, cP<0.01 vs scramble shRNA. (D) AP and immunofluorescence staining of pluripotency markers (Oct4, Nanog, and SSEA1) of the cells described in (C). (E) Morphology of E14 cells expressing the indicated shRNAs cultured in mES media supplemented with LIF and/or octreotide (1 μmol/L) (Scale bar: 50 μm).
SSTR2 contributes to self-renewal of mESCs via activation of STAT3
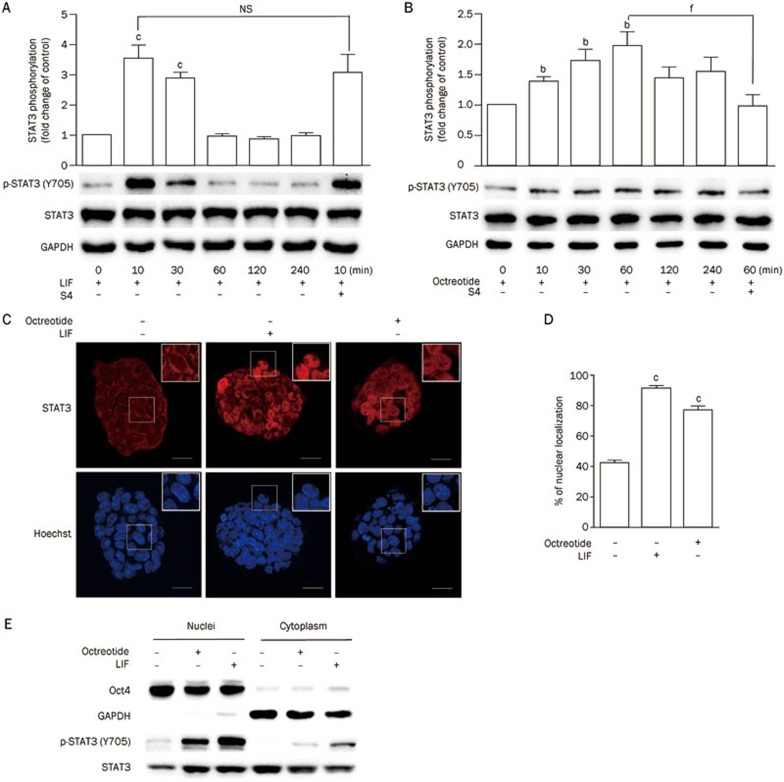
Activation of the JAK/STAT3 pathway is one of the major mechanisms by which LIF promotes the self-renewal of mESCs4. Our study demonstrated that octreotide and seglitide, two SSTR2 agonists, prevented the mESC differentiation induced by LIF deprivation. We wondered whether the activation of SSTR2 leads to phosphorylation and activation of STAT3. Western blot analysis revealed that LIF (1000 U/mL) induced a strong but transient phosphorylation of STAT3 at residue Y705, and p-STAT peaked at 10 min after stimulation. In contrast, octreotide (1 μmol/L) induced a mild but relatively long-lasting phosphorylation of STAT3, and p-STAT peaked at 60 min after stimulation (Figure 4A, 4B). As expected, the SSTR2 antagonist S4 blocked octreotide-induced STAT3 phosphorylation but did not affect LIF-mediated STAT3 phosphorylation (Figure 4A, 4B). Nuclear translocation of STAT3 is another phenomenon indicating activation of the STAT3 pathway14. Indeed, immunofluorescent staining indicated that both LIF and octreotide induced the nuclear translocation of STAT3 in mESCs (Figure 4C, 4D). Next, we measured the total STAT3 and phosphorylated STAT3 levels in the nuclei and cytoplasm of the mESCs. As shown in Figure 4E, stimulation with LIF or octreotide induced phosphorylation of STAT3 and promoted its nuclear translocation. Oct4 and GAPDH were used as markers of nuclear and cytoplasmic proteins, respectively. Taken together, these data suggest that activation of SSTR2 may contribute to the pluripotency and self-renewal of mESCs via activation of the STAT3 pathway.
Figure 4.
Activation of SSTR2 induces phosphorylation of STAT3. (A, B) Representative Western blot and statistical analyses of STAT3 phosphorylation in E14 cells stimulated with LIF (1000 U/mL) (A) or octreotide (1 μmol/L) (B) for various durations. The effect of S4 (1 μmol/L) on LIF- or octreotide-induced phosphorylation was also tested. The data are the mean±SEM (n=3). bP<0.05, cP<0.01 vs control. fP<0.01 vs cells treated with LIF or octreotide. (C) Representative confocal images of immunofluorescent staining of STAT3 in E14 cells stimulated with LIF (1000 U/mL, 10 min) or octreotide (1 μmol/L, 1 h) (Scale bar: 10 μm). (D) Statistical analysis of the percent nuclear localization of STAT3 presented in (C). The data are the mean±SEM (n=10 cells). cP<0.01 vs LIF(−) control. (E) Western blot analysis of STAT3 phosphorylation in the nuclei and cytoplasm of mESCs stimulated with LIF (1000 U/mL, 10 min) or octreotide (1 μmol/L, 1 h). Oct4 was used as a nuclear marker, and GAPDH was used as a cytoplasmic marker.
Discussion
Somatostatin is a hormone produced by many tissues in the body but is predominantly found in the nervous and digestive systems. It regulates a wide variety of physiological functions through inhibiting the secretion of other hormones, including gastrin, secretin, and etc. Somatostatin may also act as a neurotransmitter in the nervous system. Somatostatin acts on multiple cell targets via its receptors, SSTR1–5. Among them, SSTR2 is the best-studied mediator of the antiproliferative action of somatostatin and works as a tumor suppressor15. SSTR2 also plays a key role in neurogenesis9. As with many GPCRs, SSTR2 exerts its functions through inhibiting adenylate cyclase, and this effect depends on Gαi16,17. In addition, SSTR2 stimulates phospholipase C (PLC) and subsequent Ca2+ mobilization18.
Here, we report a novel function of SSTR2 in maintaining the pluripotency and self-renewal of mESCs. We found decreased expression of SSTR2 in mESCs cultured in LIF-deprived conditions. Agonists of SSTR2 rescued the differentiation induced by LIF deprivation, while knock-down of SSTR2 led to differentiation, even in the presence of LIF. As a crucial supplement for mES cell culture19, LIF mediates pluripotent signaling, which is initiated by dimerization of gp130 and LIFR upon LIF binding, activates JAK kinases and then phosphorylates STAT34. Therefore, we wondered whether the activation of SSTR2 also leads to phosphorylation and activation of STAT3. We found that LIF induced a strong but transient phosphorylation of STAT3 at residue Y705, with p-STAT peaking at 10 min after stimulation. In contrast, octreotide induced a mild but relatively long-lasting phosphorylation of STAT3, with p-STAT peaking at 60 min after stimulation, which shows that activation of SSTR2 may contribute to pluripotency and self-renewal of mESCs via activation of STAT3 over different time courses.
GPCRs are integral plasma membrane proteins that transduce signals from extracellular ligands to intracellular heterotrimeric G proteins5. These receptors are responsible for detecting a diversity of ligands and are involved in growth, death, movement, transcription and excitation6. GPCR pathways also play key roles in stem cell pluripotency and self-renewal. Many of the pathways downstream of GPCRs directly regulate or are synergistic with pathways that are critical in regulating stem cell pluripotency. For example, Gαs and Gαq activate Stat-3 signaling20, signaling mediated by the Gαi subfamily of G proteins has been shown to affect the morphology and organization of human iPSC colonies21, and Gs signaling in self-renewing and differentiating mESCs promote proliferation and pluripotency22. The dramatically different expression levels of GPCRs between pluripotent stem cells and differentiated cells show the regulatory roles of GPCRs in pluripotency maintenance. For example, more than 50 GPCRs were expressed exclusively in the human ESC (hESC) population, while another 34 GPCRs were exclusively expressed in the differentiated neurons derived from the hESCs23.
In recent years, the roles of GPCRs in ES cell pluripotency have attracted great attention10. The functions of some GPCRs in embryonic stem cell self-renewal were identified. For example, the FZD signaling pathway has been identified to promote ESC self-renewal by regulating the expression of pluripotency factors24. The binding of Wnt to the FZD receptor leads to the inhibition of glycogen synthase kinase 3 (GSK-3), which, in turn, leads to the accumulation and nuclear translocation of β-catenin and up-regulated expression of pluripotency factors25. FZD7, a receptor for Wnt, has been linked to the expression of Oct4 and Nanog, and knock-down of FZD7 reduces the expression of Oct4 and Nanog and promotes differentiation26,27. The lysophospholipid receptor pathway also contributes to embryonic stem cell pluripotency. In mESCs, lysophospholipid acid (LPA) increases the expression of pluripotency genes and stimulates cell proliferation28. Like LPA, the sphingosine 1-phosphate (S1P) receptor pathway was shown to mediate the proliferation of mESCs through an S1P5-Gi-Erk1/2-dependent pathway29. S1P also mediates hESC survival and regulates the maintenance of human ES cells in the presence of platelet-derived growth factor through Gi and Erk30. In addition, activation of the glutamate receptor mGlu5 promotes mES self-renewal through interaction with a LIF-signaling pathway31,32. In this study, we found that SSTR2 contributes to mES pluripotency and self-renewal through phosphorylation and activation of STAT3. Our data reveal a novel function of SSTR2 in maintaining the pluripotency and self-renewal of mESCs.
Author contribution
Xin XIE designed the research; Xin-xiu XU and Li-hong ZHANG performed the research and analyzed the data; Xin XIE and Li-hong ZHANG wrote the paper.
Acknowledgments
This project was supported by grants from the Chinese Academy of Sciences (XDA01040301), the National Natural Science Foundation of China (No 31371511), Ministry of Science and Technology of China (No 2013ZX09507001, 2012ZX09301001-005), and Shanghai Commission of Science and Technology (No 12XD1402100).
References
- Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002;12:432–8. doi: 10.1016/s0962-8924(02)02352-8. [DOI] [PubMed] [Google Scholar]
- Kristensen DM, Kalisz M, Nielsen JH. Cytokine signalling in embryonic stem cells. APMIS. 2005;113:756–72. doi: 10.1111/j.1600-0463.2005.apm_391.x. [DOI] [PubMed] [Google Scholar]
- Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, et al. Myeloid-leukemia inhibitory factor maintains the developmental potential of embryonic stem-cells. Nature. 1988;336:684–7. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Forrai A, Boyle K, Hart AH, Hartley L, Rakar S, Willson TA, et al. Absence of suppressor of cytokine signalling 3 reduces self-renewal and promotes differentiation in murine embryonic stem cells. Stem Cells. 2006;24:604–14. doi: 10.1634/stemcells.2005-0323. [DOI] [PubMed] [Google Scholar]
- Xu HE, Xiao RP. A new era for GPCR research: structures, biology and drug discovery. Acta Pharmacol Sin. 2012;33:289–90. doi: 10.1038/aps.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Xie X. Tools for GPCR drug discovery. Acta Pharmacol Sin. 2012;33:372–84. doi: 10.1038/aps.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelbaum J, Dournaud P, Fodor M, Viollet C. The neurobiology of somatostatin. Crit Rev Neurobiol. 1994;8:25–44. [PubMed] [Google Scholar]
- Kong H, DePaoli AM, Breder CD, Yasuda K, Bell GI, Reisine T. Differential expression of messenger RNAs for somatostatin receptor subtypes SSTR1, SSTR2 and SSTR3 in adult rat brain: analysis by RNA blotting and in situ hybridization histochemistry. Neuroscience. 1994;59:175–84. doi: 10.1016/0306-4522(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Maubert E, Slama A, Ciofi P, Viollet C, Tramu G, Dupouy JP, et al. Developmental patterns of somatostatin-receptors and somatostatin-immunoreactivity during early neurogenesis in the rat. Neuroscience. 1994;62:317–25. doi: 10.1016/0306-4522(94)90335-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi NR, Hawes SM, Crook JM, Pebay A. G-protein coupled receptors in stem cell self-renewal and differentiation. Stem Cell Rev Reports. 2010;6:351–66. doi: 10.1007/s12015-010-9167-9. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo A, Melmed S. Pituitary somatostatin receptor signaling. Trends Endocrinol Metab. 2010;21:123–33. doi: 10.1016/j.tem.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Herder WW, Hofland LJ, van der Lely AJ, Lamberts SWJ. Somatostatin receptors in gastroenteropancreatic neuroendocrine tumours. Endocrine-Related Cancer. 2003;10:451–8. doi: 10.1677/erc.0.0100451. [DOI] [PubMed] [Google Scholar]
- Bass RT, Buckwalter BL, Patel BP, Pausch MH, Price LA, Strnad J, et al. Identification and characterization of novel somatostatin antagonists. Mol Pharmacol. 1996;50:709–15. [PubMed] [Google Scholar]
- Okumura F, Okumura AJ, Matsumoto M, Nakayama KI, Hatakeyama S. TRIM8 regulates Nanog via Hsp90beta-mediated nuclear translocation of STAT3 in embryonic stem cells. Biochim Biophys Acta. 2011;1813:1784–92. doi: 10.1016/j.bbamcr.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Acunzo J, Thirion S, Roche C, Saveanu A, Gunz G, Germanetti AL, et al. Somatostatin receptor sst2 decreases cell viability and hormonal hypersecretion and reverses octreotide resistance of human pituitary adenomas. Cancer Res. 2008;68:10163–70. doi: 10.1158/0008-5472.CAN-08-1857. [DOI] [PubMed] [Google Scholar]
- Kagimoto S, Yamada Y, Kubota A, Someya Y, Ihara Y, Yasuda K, et al. Human somatostatin receptor, SSTR2, is coupled to adenylyl cyclase in the presence of Gi alpha 1 protein. Biochem Biophys Res Commun. 1994;202:1188–95. doi: 10.1006/bbrc.1994.2054. [DOI] [PubMed] [Google Scholar]
- Law SF, Yasuda K, Bell GI, Reisine T. Gi alpha 3 and G(o) alpha selectively associate with the cloned somatostatin receptor subtype SSTR2. J Biol Chem. 1993;268:10721–7. [PubMed] [Google Scholar]
- Tomura H, Okajima F, Akbar M, Abdul Majid M, Sho K, Kondo Y. Transfected human somatostatin receptor type 2, SSTR2, not only inhibits adenylate cyclase but also stimulates phospholipase C and Ca2+ mobilization. Biochem Biophys Res Commun. 1994;200:986–92. doi: 10.1006/bbrc.1994.1547. [DOI] [PubMed] [Google Scholar]
- Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–7. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Yuen JW, Poon LS, Chan AS, Yu FW, Lo RK, Wong YH. Activation of STAT3 by specific Galpha subunits and multiple Gbetagamma dimers. Int J Biochem Cell Biol. 2010;42:1052–9. doi: 10.1016/j.biocel.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Salomonis N, Tomoda K, Yamanaka S, Conklin BR. G(i)-coupled GPCR signaling controls the formation and organization of human pluripotent colonies. PLoS One. 2009;4:e7780. doi: 10.1371/journal.pone.0007780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layden BT, Newman M, Chen F, Fisher A, Lowe WL., Jr G protein coupled receptors in embryonic stem cells: a role for Gs-alpha signaling. PLoS One. 2010;5:e9105. doi: 10.1371/journal.pone.0009105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada H, Yoshimura N, Tsuji A, Kunisada T. Differentiation of dopaminergic neurons from human embryonic stem cells: modulation of differentiation by FGF-20. J Biosci Bioeng. 2009;107:447–54. doi: 10.1016/j.jbiosc.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Anton R, Kestler HA, Kuhl M. Beta-catenin signaling contributes to stemness and regulates early differentiation in murine embryonic stem cells. FEBS Lett. 2007;581:5247–54. doi: 10.1016/j.febslet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Melchior K, Weiss J, Zaehres H, Kim YM, Lutzko C, Roosta N, et al. The WNT receptor FZD7 contributes to self-renewal signaling of human embryonic stem cells. Biol Chem. 2008;389:897–903. doi: 10.1515/BC.2008.108. [DOI] [PubMed] [Google Scholar]
- Vijayaragavan K, Szabo E, Bosse M, Ramos-Mejia V, Moon RT, Bhatia M. Noncanonical Wnt signaling orchestrates early developmental events toward hematopoietic cell fate from human embryonic stem cells. Cell Stem Cell. 2009;4:248–62. doi: 10.1016/j.stem.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorova MG, Fuentes E, Soria B, Nadal A, Quesada I. Lysophosphatidic acid induces Ca2+ mobilization and c-Myc expression in mouse embryonic stem cells via the phospholipase C pathway. Cell Signal. 2009;21:523–8. doi: 10.1016/j.cellsig.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Rodgers A, Mormeneo D, Long JS, Delgado A, Pyne NJ, Pyne S. Sphingosine 1-phosphate regulation of extracellular signal-regulated kinase-1/2 in embryonic stem cells. Stem Cells Dev. 2009;18:1319–30. doi: 10.1089/scd.2009.0023. [DOI] [PubMed] [Google Scholar]
- Pebay A, Wong RC, Pitson SM, Wolvetang EJ, Peh GS, Filipczyk A, et al. Essential roles of sphingosine-1-phosphate and platelet-derived growth factor in the maintenance of human embryonic stem cells. Stem Cells. 2005;23:1541–8. doi: 10.1634/stemcells.2004-0338. [DOI] [PubMed] [Google Scholar]
- Cappuccio I, Spinsanti P, Porcellini A, Desiderati F, De Vita T, Storto M, et al. Endogenous activation of mGlu5 metabotropic glutamate receptors supports self-renewal of cultured mouse embryonic stem cells Neuropharmacology 200549Suppl 1: 196–205. [DOI] [PubMed] [Google Scholar]
- Spinsanti P, De Vita T, Di Castro S, Storto M, Formisano P, Nicoletti F, et al. Endogenously activated mGlu5 metabotropic glutamate receptors sustain the increase in c-Myc expression induced by leukaemia inhibitory factor in cultured mouse embryonic stem cells. J Neurochem. 2006;99:299–307. doi: 10.1111/j.1471-4159.2006.04038.x. [DOI] [PubMed] [Google Scholar]