Abstract
Objective:
To examine physical activity (PA) thresholds affecting glucose, insulin and lipid concentrations and body fat composition in high-risk patients for type 2 diabetes (T2D).
Intervention:
A total of 113 subjects of both genders having abnormal glucose levels in the oral glucose tolerance test were contacted. A total of 78 subjects with age 58.8±10.4 years and body mass index 31.7±5.3 kg m−2 were randomly assigned to intervention and control groups. Intervention consisted of a supervised walking (60 min three times weekly) for 3 months. All the subjects received standard care for PA and weight reduction and wore an accelerometer during the whole wakeful time.
Results:
Over 80% of the daily steps clustered at an acceleration level of 0.3–0.7 g (2–3 km h−1 of walking) and were 5870 in the intervention and 4434 in the control group (P<0.029). Between 0 and 3 months no significant changes were observed in fasting and 2-h glucose, body weight or maximal oxygen uptake. In contrast, changes in fasting and 2-h insulin (−3.4 mU l−1, P=0.035 and −26.6, P=0.003, respectively), homeostasis model assessment-estimated insulin resistance (−1.0, P=0.036), total cholesterol (−0.55 mmol l−1, P=0.041), low-density lipoprotein (LDL) cholesterol (−0.36 mmol l−1, P=0.008) and visceral fat area (−5.5 cm2, P=0.030) were significantly greater in the intervention than in control subjects. The overall effects of PA were analyzed by quartiles of daily steps of all subjects. There were significant reductions in total and LDL cholesterol and visceral fat area between the highest (daily steps over 6520) and the lowest quartile (1780–2810 daily steps). The changes associated with PA remained significant after adjustments of baseline, sex, age and body weight change.
Conclusion:
Habitual and structured PAs with the acceleration levels of 0.3–0.7 g and daily steps over 6520, equivalent to walking at 2–3 km h−1 for 90 min daily, standing for the relative PA intensity of 30–35% of the maximal oxygen uptake, are clinically beneficial for overweight/obese and physically inactive individuals with a high risk for T2D.
Keywords: overweight, insulin resistance, visceral fat, lipids, accelerometer
Introduction
Lifestyle and genetic factors contribute to the development of type 2 diabetes (T2D). Excessive calorie intake in combination with physical inactivity will lead to decreased body glucose disposal and insulin sensitivity resulting in impaired fasting glucose and/or impaired glucose tolerance. Around 5–10% of people with abnormal blood glucose concentrations become diabetic every year, from which 70% will go on to develop T2D.1 In the United States an estimated 18.8 million people had diagnosed diabetes mellitus, whereas another 7.0 million had undiagnosed diabetes mellitus in 2010.2 In Finland, 280 000 T2D diagnoses were registered in 2009 with an annual increase of 5–7% (http://www.diabetes.fi/en/finnish_diabetes_association/diabetes_in_finland).
Health-related risks of T2D have been investigated in several clinical trials and prospective studies.3, 4, 5 Lifestyle changes such as increased physical exercise and weight reductions were shown to reduce the risk of T2D by ∼58%.6, 7
Recent recommendations for T2D prevention include a minimum of 150 min per week of moderate-to-vigorous PA.8, 9 Taking into account the intensity of the moderate PA (>3 metabolic equivalents, METs) the recommendations present a relative intensity >46% of the maximal oxygen uptake in healthy adults.10 This recommendation9 states also that people unable to meet these minimums can still benefit from some activity, but the intensity levels producing health benefits are not known.
Walking is the most common and most acceptable form of PA in people with T2D10 and a walking speed of 4.8 km h−1 (3 miles h−1) represents moderate PA.11 In the pioneering studies6, 7 regarding the effects of lifestyle changes in the prevention of T2D, the above guidelines for PA (moderate exercise 150 min weekly) were followed. Significant clinical results were observed, yet the exact amounts and intensities of PA that the participants performed in the studies6, 7 were unknown.12 Thus, no threshold intensity of exercise needed to reduce metabolic risks could be attained. Furthermore, PA measurements by motion sensors have demonstrated that only very few adults meet the PA guidelines. Johnson and colleagues13 used pedometers for 3 days in T2D subjects demonstrating a habitual average walking speed of 3.3 km h−1, which is clearly below the recommended guidelines. In other studies, the ambulatory activity was recently studied using motion sensors in a large and healthy adult USA population (NHANES). Surprisingly, less than 5% of the subjects performed PA at a moderate-intensity level.14, 15 Therefore accurate and precise measures of PA are required to elucidate lifestyle modifications decreasing T2D risks.12
An alternative PA recommendation suggests to aim at 10 000 steps per day16 but concerns have been expressed as health benefits may not be achieved owing to the low-intensity level of walking.13 In subjects with impaired glucose tolerance, 6000–9000 steps per day of moderately intense walking resulted in decreased fasting and 2-h glucose concentrations during the 3–12-month intervention period.17 Similar increases in daily step counts were observed in T2D subjects during a 16-week intervention, but no changes in fasting or 2-h glucose were apparent.18 Unfortunately, walking intensity was not recorded, leading investigators to conclude that PA intensity was insufficient.13 A recent cross-sectional study showed that the percentage of normal body mass index subjects having PA volume of over 10 000 steps per day was higher than that of overweight/obese subjects.19
The growing prevalence of T2D and lack of objectively measured amounts and intensities of PA to improve the glycemic status in high-risk subjects have led us to perform this randomized controlled trial. Our primary aim was to investigate the effects of a 3-month structured aerobic walking exercise on fasting and 2-h glucose and insulin concentrations and lipid homeostasis in sedentary overweight subjects with impaired fasting glucose and/or impaired glucose tolerance. To achieve our aims, we recorded body weight/composition changes and nutrient intakes and measured total (habitual and structured) PA. We hypothesized that at certain PA levels of daily steps and accelerations, glucose and lipid metabolism and insulin sensitivity will improve.
Methods
Trial design
This trial was a parallel study with age and gender stratification in allocation ratio 1:1, performed in three centers in the city of Oulu, Finland.
Participants
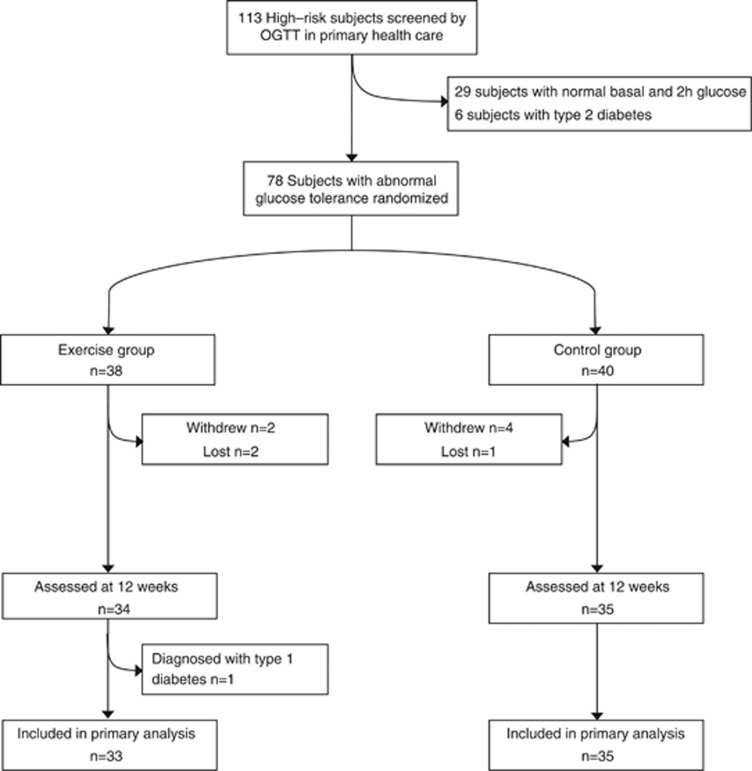
For this randomized controlled trial (acronym: PreDiabEx) between November 2009 and January 2010 Caucasian subjects from the outpatient diabetic clinics in the Oulu Deaconess Hospital and the City of Oulu were evaluated with the FINDRISC questionnaire for T2D (http://www.idf.org/webdata/docs/FINDRISC_English.pdf20). Those subjects receiving scores >15 were selected for the study. A total of 113 subjects with a median FINDRISC score of 20 (range 15–26) were further evaluated by an oral glucose tolerance test. We used the WHO criteria for impaired fasting glucose (⩾5.6 and <7.0 and 2-h glucose <7.8 mmol l−1) or impaired glucose tolerance (fasting glucose <7.8 and 2-h glucose ⩾7.8 and <11.1 mmol l−1). Seventy-eight subjects fulfilled the criteria and were willing to participate in the 3-month study (Figure 1). They were randomized based on age and gender using a parallel block design to distinguish intervention and control groups with the allocation ratio of 1:1. We used computerized random number generation. The estimated sample size to detect a 1.2-mmol l−1 (s.d.=2.5) change between intervention and control groups in 2-h glucose and 40-mU l−1 (s.d.=80) change in 2-h insulin with a significance level of 5% and a power of 80% was 65 and 62, respectively. Exclusion criteria were any functional limitation or chronic disease that might have limited the training and testing of the cardiovascular and respiratory systems, any medication for diabetes or current vigorous PA for more than 75 min per week as revealed by a questionnaire or physician's examination. The study protocol was approved by the institutional ethics committee (Northern Ostrobothnia Hospital District). All subjects gave their informed written consent. The Municipal Board of the Northern Ostrobothnia Hospital District approved the trial under the registration number 113/2009. The trial was also registered under NCT01649219 (clinicaltrial.gov). The CONSORT checklist is available as Supplementary Information (Checklist S1).
Figure 1.
Flow chart of the PreDiabEx study participants and dropouts from the study.
Questionnaires and measurements
Clinical examinations and measurements were performed before (baseline) and at the end of a 3-month intervention. Height, weight, hip and waist circumference, electrocardiogram and blood pressure were measured and body mass index was calculated. Body composition was measured by bioelectrical impedance analysis InBody 720 (Mega Electronics, Kuopio, Finland). For visceral fat area a highly significant correlation (R=0.905) between the bioimpedance and computed tomography methods has been previously documented.21
A self-administered questionnaire22 with questions on PA health condition, medications, smoking, alcohol use, profession and education was distributed to all subjects before the experiments. The questionnaire has previously been validated.23 For the leisure-time PA, the daily durations of PA (⩾15 min, 15–30 min or ⩾30 min) and intensities (light: slow walking; moderate: brisk walking; vigorous: running and jogging) were asked and expressed as metabolic units (MET). Light PA was 0–3, moderate 3–6 and vigorous 6–9 METs. They were combined to MET h per week using means of the physical activities of 1.5, 4.5 and 7.5 METs, respectively. A 3-day dietary questionnaire with a colored dietary atlas of usual food and drink items24 was distributed to the subjects before and after the intervention. A professional dietician counseled the subjects in filling up the questionnaire. An oral glucose tolerance test was performed in the morning after an overnight fast according to WHO instructions. Fasting and 2-h plasma glucose and insulin were measured by direct methods using an Advia 1800, Siemens (Tarrytown, NY, USA). Plasma total cholesterol, high-density lipoprotein (HDL-) and low-density lipoprotein (LDL) cholesterols and triglycerides were determined by direct methods using an Advia Centaur XP, Siemens. Homeostasis model assessment-estimated insulin resistance (HOMA-IR) was calculated from fasting serum levels of glucose and insulin as follows: HOMA-IR=insulin (μIU ml−1) × glucose (mmol l−1)/22.5.
Indirect submaximal oxygen uptake tests were performed using a cycle ergometer (Schiller ergometer 911S, Baar, Switzerland and Monark ergometer 828E, Varberg, Sweden).25 Heart rate was recorded using a heart rate monitor (M61, Polar Electro, Kempele, Finland) at three resistance levels (30, 60 and 90 W) each lasting for 4 min. Resistances of 30, 50 and 70 W were used, if the subject had uncomfortable feelings or exhaustion at the 30-W level. Blood pressure and blood lactic acid (Lactate Pro, Argray Inc., Kojo, Japan) were measured at each resistance level.
Intervention
We wanted to follow the ACSM 2011 recommendation9 for the exercise for healthy adults (150 min per week of moderate PA). The training sessions of the intervention group were carried out in an indoor sports hall three times a week over a period of 3 months and were supervised by an exercise instructor and a physician. Each session lasted for 60 min and included a 5-min warm-up and stretching, 20-min walk at a speed of ∼3–4 km h−1 on plane area (depending on the participant's physical condition). Most subjects were not able to walk at a speed of 5 km h−1 required for moderate PA. Walking was followed by a 5-min stretching and balance training, a 20-min walk and a 10-min stretching and balance exercise. After 1.5 months the 5-min stretching and balance training between the two 20-min walks were eliminated, and walking time was increased to 45 min. During the 3-month intervention period, 32 sessions were held with a median adherence of 22 times (67% range 11–31 times). Before the intervention, all subjects together with the investigators, a dietician and nurses attended two sessions of 60 min in which the exercise intervention program, the identification of abnormal glucose tolerance and the significance of regular PA and weight reduction to prevent the development of T2D as the best standard care were addressed. The intervention participants met the athletic instructor three times a week during the structured exercises. The control participants group met research assistants once a week for downloading the data from the accelerometers.
Objective measurement of PA
All subjects carried an accelerometer (Newtest Exercise Monitor, Newtest, Oulu, Finland), except during bathing and other aquatic activities, during the waking hours, every day for 3 months to avoid confounding effects by weekends, holidays and weather. The device was worn on a belt close to the right iliac crest or clipped to trousers. Before the trial, the subjects familiarized themselves with the use of the accelerometer, but no pre-intervention data were collected.
The device recorded the number of vertical accelerations at a sampling rate of 400 s−1 and classified them according to peak acceleration to 33 acceleration levels from 0.3–9.9 g (g=gravity acceleration 9.81 m−2; standing is 0 g). The reproducibility of the accelerometer to register acceleration peaks and their correlations with force plate accelerations have been described earlier.26, 27 The variation in the weighted average of accelerations in walking/running speeds of 3, 6 and 6 km h−1 accounted for 92% of the variance in the energy expenditure (Supplementary Figure 1).
The device was sealed in order not to give any feedback to the user. Average daily distribution of steps was analyzed with a custom-made computer software (Matlab, Mathworks Inc., Natick, MA, USA), and a valid day consisted of more than 1000 steps. The daily wear-time was checked 1–2 times weekly during downloading the data. The median for the whole-day wear-time was 78 days (range 26–93 in the intervention and 24–90 in the control group). The mean total number of steps per day and number of steps at different acceleration classes (0.3–0.5, 0.5–0.7, 0.7–0.9, 0.9–1.1, 1.1–3 and ⩾3 g) were used to describe PA during the whole 12-week trial. We used steps with intensities between 0.3–9.9 g as markers of steps during walking and running. Sitting or very slow walking (below 1 km h−1 or a step ∼every 3–4 s) induces accelerations below 0.3 g, which were not registered. Walking at a speed of 3 km h−1 induces steps with intensities of 0.7–0.9 g and at 5 km h−1 intensities of 0.8–1.2 g.28 We have previously observed that beneficial health effects in maximal oxygen uptake and cholesterol levels in healthy women appear only when the step intensities exceed 1.1 g.29
Outcome
The primary outcome measures were fasting and 2-h glucose and insulin. The secondary outcomes were maximal oxygen uptake, daily steps, lipids and body weight and fat distributions.
Statistical analyses
The data were analyzed using the SPSS statistical package (PASW statistics 18 for Windows, SPSS Inc., Chicago, IL, USA). Unpaired two-tailed t-test and χ2-test were used to compare participant characteristics at baseline. The efficacy of the intervention on primary and secondary end points was assessed using a unpaired t-test based on the original values or on logarithmic transformations, or a nonparametric test (Mann–Whitney, details in Tables and Figures). Repeated measures analysis of variance was used to determine the effect of the intervention on glucose, insulin and lipid concentrations, body constituents and macronutrients and controlled for potential confounding variables like baseline (model 1), sex, age (model 2, including baseline) and body weight changes (model 3 including baseline, sex and age). Mixed models for repeated measurements were used to analyze weekly impact data. Group randomization was used as a covariant in the analysis of insulin, LDL cholesterol and visceral fat data. We also evaluated the effects of daily total PA on our outcome variables by combining them together from both groups, dividing to quartiles based on the average number of steps per day and comparing the variables between the least and most active quartiles using Dunnett's test.
Results
The characteristics of the study groups were similar at baseline in anthropometric, fitness and educational data, smoking, alcohol consumption and medication (Table 1). Systolic blood pressure was higher in the control than in the intervention group.
Table 1. Clinical characteristics, physical activity and medication of study participants by group at baseline.
| Variable | Intervention | Control | P-value |
|---|---|---|---|
| Number of subjects | 33 | 35 | |
| Age (years) | 58.1 (9.9) | 59.5 (10.8) | 0.565 |
| Women | 24 (72.7%) | 26 (74.3%) | 0.885 |
| Weight (kg) | 92.4 (19.4) | 84.6 (14.4) | 0.065 |
| BMI (kg m−2) | 32.6 (5.7) | 30.9 (4.8) | 0.191 |
| Waist (cm) | 98.4 (16.9) | 94.4 (11.1) | 0.263 |
| VO2 max (ml kg-1 min−1) | 22.5 (4.7) | 23.8 (4.8) | 0.284 |
| Systolic BP (mm Hg) | 138.5 (16.4) | 150.4 (20.2) | 0.026 |
| Diastolic BP (mm Hg) | 83.8 (8.0) | 85.4 (9.5) | 0.352 |
| Self-reported physical activity | |||
| Light, MET (h per week) | 1.5 (0.5) | 1.4 (0.5) | 0.794 |
| Moderate, MET (h per week) | 2.7 (1.8) | 2.6 (1.8) | 0.826 |
| Vigorous, MET (h per week) | 1.8 (2.4) | 1.8 (2.7) | 1 |
| Self-reported education | |||
| Basic | 8 (25.0%) | 12 (38.7%) | 0.246 |
| Secondary | 17 (53.1%) | 14 (45.2%) | 0.531 |
| Tertiary | 7 (21.9%) | 5 (16.1%) | 0.565 |
| Self-reported medication by prescription | |||
| Nitrates | 1 (3.0%) | 4 (11.4%) | 0.188 |
| Antihypertensives | 18 (54.5%) | 18 (51.4%) | 0.798 |
| Diuretics | 4 (12.1%) | 4 (11.4%) | 0.93 |
| Aspirin anti-coagulants | 6 (18.2%) | 7 (20.0%) | 0.85 |
| Lipid-lowering drugs | 12 (36.4%) | 12 (34.3%) | 0.859 |
| Bronchodilatators | 3 (9.1%) | 4 (11.4%) | 0.753 |
| Thyroxine | 3 (9.1%) | 5 (14.3%) | 0.509 |
| Antiulcer-drugs | 4 (12.1%) | 4 (11.4%) | 0.93 |
Abbreviations: BMI, body mass index; BP, blood pressure; MET, metabolic equivalent.
Means and s.d. or percentages are given. P-values from t-tests and from χ2-test (education and medication).
The changes in the outcome parameters at baseline and at 3 months and differences between the groups are shown in Table 2. In the intervention group fasting and 2-h glucose decreased significantly, but in the control group no significant changes were found. The fasting and 2-h glucose between the intervention and control groups were not significant. In the intervention group fasting and 2-h insulin decreased significantly, whereas in the control group no significant changes were observed. The differences in the changes of the unadjusted fasting and 2-h insulin concentrations between the intervention and control groups were 3.4 mU l−1 (P=0.047) and 26.6 mU l−1 (P=0.003), respectively. After the adjustment for baseline, age, gender and weight change, the P-values remained at the same level. HOMA-IR decreased by 1.1 (P=0.003) in the intervention group, but no significant change was detected in the control group. The difference of unadjusted HOMA-IR values between the groups was significant (P=0.050), whereas the adjustment changed the P-value slightly (P=0.036).
Table 2. Metabolic parameters and nutrient intakes before and after a 3-month physical activity intervention in subjects with high-T2D risk.
|
Intervention (N=33) |
P |
Control (N=35) |
P | Differencea |
ANOVAb (P-value) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 3 months | Baseline | After 3 months | Model 1 | Model 2 | Model 3 | ||||
| Fasting glucose (mmol l−1) | 6.6 (0.8) | 6.4 (0.8) | 0.019 | 6.5 (0.7) | 6.6 (1.8) | 0.659 | 0.3 (−0.2 to 0.9) | 0.256 | 0.2183 | 0.247 |
| 2-h glucose (mmol l−1) | 8.9 (2.6) | 7.8 (2.6) | 0.017 | 8.7 (2.6) | 7.9 (3.0) | 0.061 | 0.2 (−0.9 to 1.3) | 0.915 | 0.831 | 0.789 |
| Fasting insulin (mU l−1) | 18.7 (11.0) | 15.6 (10.3) | 0.008 | 16.3 (11.9) | 16.9 (15.9) | 0.757 | 3.4 (−0.8 to 7.6) | 0.047 | 0.041 | 0.035 |
| 2-h insulin (mU l−1) | 116.9 (70.8) | 75.6 (62.7) | <0.001 | 94.8 (72.8) | 91.4 (60.6) | 0.794 | 26.6 ( 1.1 to 51.8) | 0.003 | 0.003 | 0.003 |
| Homa-IR | 5.7 (3.8) | 4.6 (3.3) | 0.003 | 4.8 (3.7) | 4.9 (4.4) | 0.939 | 1.0 (−0.2 to 2.2) | 0.050 | 0.043 | 0.036 |
| Total cholesterol (mmol l−1) | 5.3 (0.8) | 5.0 (0.8) | 0.001 | 5.7 (1.3) | 5.5 (1.1) | 0.298 | 0.3 (−0.0 to 0.7) | 0.064 | 0.062 | 0.041 |
| HDL cholesterol (mmol l−1) | 1.4 (0.3) | 1.4 (0.4) | 0.514 | 1.5 (0.3) | 1.5 (0.3) | 0.544 | 0.0 (−0.1 to 0.1) | 0.674 | 0.660 | 0.553 |
| LDL cholesterol (mmol l−1) | 3.0 (0.8) | 3.0 (0.8) | 0.771 | 3.2 (1.2) | 3.6 (1.0) | 0.018 | 0.4 ( 0.1 to 0.7) | 0.014 | 0.012 | 0.008 |
| Triglycerides (mmol l−1) | 1.7 (0.6) | 1.5 (0.4) | <0.001 | 1.8 (0.7) | 1.7 (0.9) | 0.391 | 0.2 (−0.0 to 0.5) | 0.239 | 0.251 | 0.179 |
| Systolic blood pressure (mm Hg) | 138.5 (16.4) | 131.3 (15.2) | 0.005 | 150.4 (20.2) | 139.0 (16.0) | <0.001 | 1.0 (−5.4 to 7.5) | 0.688 | 0.653 | 0.742 |
| Diastolic blood pressure (mm Hg) | 83.8 (8.0) | 79.1 (8.2) | 0.004 | 85.4 (9.5) | 80.1 (8.8) | 0.007 | 0.4 (−3.5 to 4.4) | 0.820 | 0.479 | 0.392 |
| Max. O2 uptake (ml kg−1 min−1) | 22.7 (4.6) | 26.3 (6.8) | 0.002 | 23.6 (4.8) | 26.4 (5.5) | 0.002 | −0.9 (−3.0 to 1.2) | 0.394 | 0.450 | 0.428 |
| Weight (kg) | 92.4 (19.4) | 91.5 (20.3) | 0.028 | 84.6 (14.4) | 83.3 (15.3) | 0.047 | −0.1 (−1.6 to 1.4) | 0.899 | 0.970 | |
| BMI (kg m−2) | 32.6 (5.7) | 32.3 (6.1) | 0.038 | 30.9 (4.8) | 30.2 (4.9) | 0.011 | −0.3 (−0.8 to 0.3) | 0.379 | 0.402 | |
| Waist circumference (cm) | 97.4 (17.2) | 95.1 (14.1) | 0.061 | 91.4 (9.7) | 88.3 (8.4) | 0.005 | −2.3 (−4.9 to 0.4) | 0.100 | 0.123 | 0.132 |
| Body fat (%) | 40.0 (7.6) | 37.9 (9.0) | 0.018 | 38.6 (9.0) | 37.5 (9.3) | 0.020 | 0.9 (−1.0 to 2.8) | 0.279 | 0.284 | 0.199 |
| Visceral fat area (cm2) | 163.7 (29.8) | 155.9 (31.4) | 0.013 | 156.3 (28.0) | 154.0 (32.7) | 0.185 | 5.5 (−1.4 to 12.3) | 0.073 | 0.082 | 0.030 |
| Skeletal mass (kg) | 29.6 (8.4) | 30.4 (9.3) | 0.140 | 28.5 (5.5) | 28.6 (5.3) | 0.867 | −0.7 (−1.9 to 0.4) | 0.443 | 0.328 | 0.214 |
| Energy intake (kcal per day) | 1766 (568) | 1831 (609) | 0.486 | 1725 (466) | 1719 (550) | 0.958 | −85 (−335 to 165) | 0.683 | 0.735 | 0.764 |
| Protein intake (kcal per day) | 77.8 (25.6) | 81.5 (24.1) | 0.354 | 73.5 (25.0) | 73.4 (24.5) | 0.978 | −5.3 (−14.7 to 4.2) | 0.335 | 0.260 | 0.295 |
| Carbohydrate intake (kcal per day) | 203.4 (73.8) | 191.6 (65.9) | 0.309 | 203.4 (66.4) | 193.1 (60.7) | 0.476 | 1.5 (−28.1 to 31.0) | 0.748 | 0.660 | 0.560 |
| Fat intake (kcal per day) | 63.7 (27.2) | 74.0 (33.4) | 0.063 | 63.7 (20.7) | 68.1 (27.6) | 0.339 | −5.9 (−19.7 to 7.8) | 0.407 | 0.521 | 0.497 |
| Gluose intake (kcal per day) | 93.6 (31.4) | 85.2 (37.1) | 0.154 | 89.3 (34.0) | 81.6 (34.9) | 0.377 | −1.9 (−19.5 to 15.8) | 0.878 | 0.923 | 0.953 |
Abbreviations: ANOVA, analysis of variance; Homa-IR, homeostasis model assessment-estimated insulin resistance; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Max., maximum; T2D, type 2 diabetes.
Data are mean and standard deviation (s.d.) or 95% confidence interval. P is paired t-test probability.
Model 1=controlled with baseline value. Model 2=Model 1+sex+age. Model 3=Model 2+weight change during intervention.
Based on log transformation.
Total plasma cholesterol and triglycerides decreased significantly during the 3 months in the intervention group, but no changes were seen in the control group. The difference between the changes of LDL cholesterol in the intervention and control group was significant (P=0.014) and the adjustment (baseline, age, gender and body weight change) changed the P-value (P=0.008). The difference between changes of total cholesterol was not significant, but became significant after the adjustment had been made (P=0.041).
During the 3 months intervention, body weight and body mass index decreased significantly in both groups and waist circumference (P=0.005) in the control group, but no significant differences in the decreases between the groups were observed. Systolic and diastolic blood pressures decreased significantly in both groups, but the difference between the intervention and control groups was not significant.
Visceral fat area significantly decreased during the 3 months in the intervention (P=0.013), but not in the control group. The differences of unadjusted values between the groups were not significant (P=0.073), but became significant (P=0.030) after the adjustment of baseline, age, gender and body weight change. The total energy intake, based on protein, carbohydrate and fat intakes, did not show any significant changes during the 3 months in both groups and the differences between the groups were not significant.
Between 0 and 3 months, there were significant increases in maximal oxygen uptake values in both intervention and control groups (Table 2). However, these increases were not different between the groups (P⩾0.05).
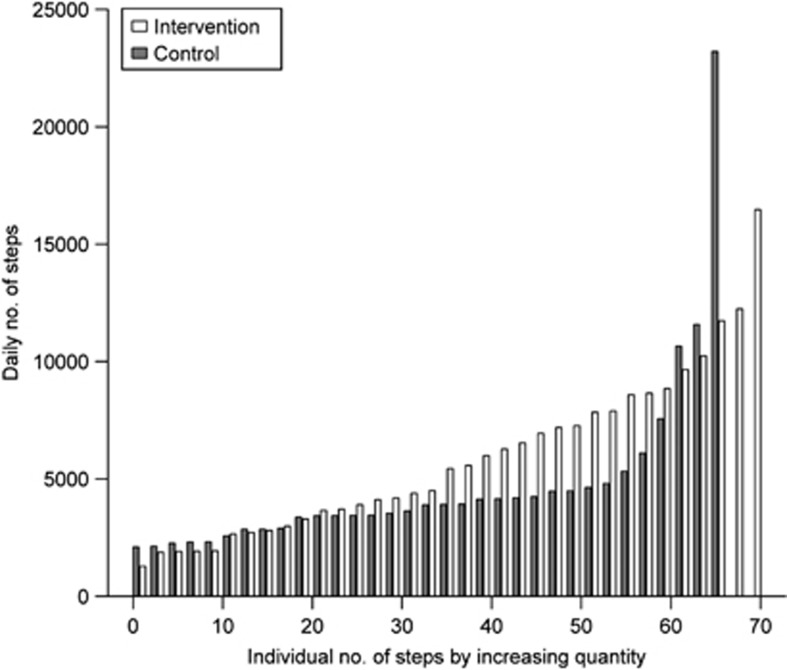
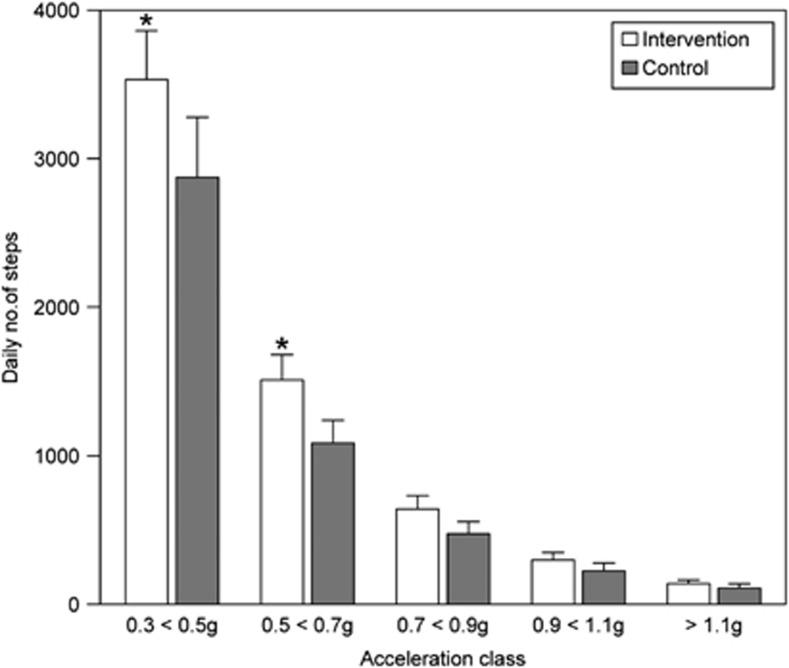
Accelerometer data were recorded through the whole 3-month study. Mean numbers of the daily steps showed large variations between the subjects (Figure 2). In both groups (medians of weekly steps; Supplementary Figure 2) there were no significant step differences between the intervention vs control group in any week during the entire study time nor was there a difference in between the weeks. There was no group × time interaction (P=0.214). The total daily number of steps (average daily step count summarized across all time points; acceleration classes 0.3–⩾1.1) was higher in the intervention than in the control group, median 5576 (range 1196–15 687 vs 4004 (range 2119–23 088) steps. Over 80% of the daily steps clustered at the acceleration classes 0.3–0.7 g and were 5870±3277 (mean±s.d.) in the intervention and 4434±3460 in the control group (P<0.029, Figure 3).
Figure 2.
Individual distribution of mean daily steps of all the subjects (n=33 intervention and 35 controls) by magnitude order (control subjects, gray; intervention subjects, white). Please note the intervention effects in subjects 20–60 and two highly active subjects.
Figure 3.
Number of daily steps (means and s.e.m.) in the five acceleration classes in intervention and control subjects. An asterisk denotes P<0.05 between intervention and control groups.
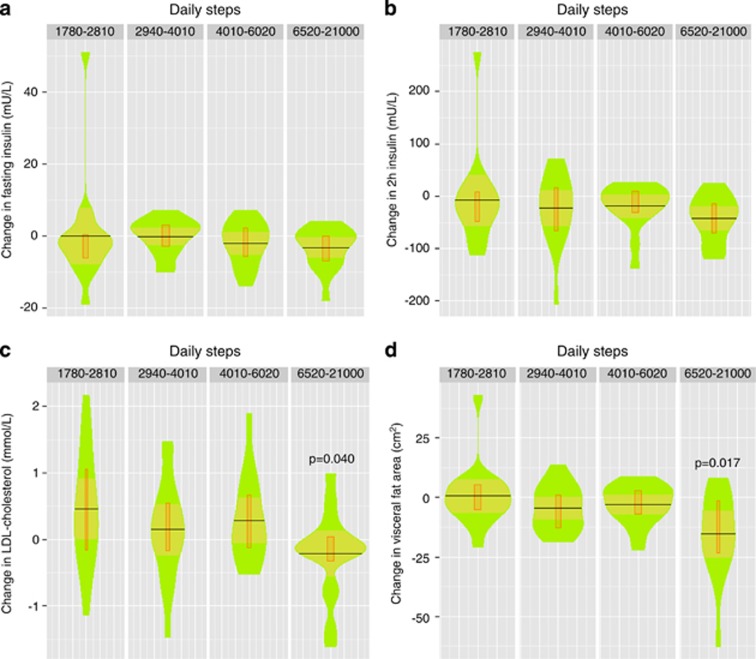
In order to analyze closer the observed significant effects of the intervention on insulin, LDL cholesterol and visceral fat, we pooled the variables in the control and intervention groups into one group, adjusted them by body weight change and analyzed their differences between the quartiles of the daily step numbers. There were no significant differences between the quartiles of fasting glucose/insulin, 2-h glucose/insulin or HOMA-IR (only insulin data are shown, Figures 4a and b). However, in the highest active quartile there was a highly significant reduction in the adjusted LDL cholesterol and visceral fat area when compared with the lowest one (Figures 4c and d). The reductions when the number of daily steps exceeded 6520 were for LDL cholesterol 0.7 mmol l−1 (95% confidence interval 0.1–1.2 mmol l−1) and 16 cm2 (7–25 cm2) for visceral fat area.
Figure 4.
Changes in fasting (a) and 2-h insulin (b), LDL cholesterol (c) and visceral fat area (d) in the physical activity quartiles: I 1780–2810, II 2940–4010, III 4010–6020 and IV 6520–21 000 daily steps. Changes in P-values represent significance between the lowest and highest quartiles. Step data were taken from the acceleration levels 0.3–0.7 g. Data were log-transformed and adjusted to baseline, sex, age and weight changes. Original values are used in the figures. In the violin box plot the black horizontal line is mean, orange area 95% confidential limits, narrow box II and III quartiles and green area dispersion of the observations (n=17 in each quartile).
Discussion
This randomized controlled trial in high-risk subjects demonstrated that a 3-month structured walking exercise of light intensity (that is, walking at ∼3 km h−1 corresponding to ∼two MET), resulted in significant decreases in fasting and 2-h insulin levels, HOMA-IR, total and LDL cholesterol concentrations and visceral fat area. The weekly duration of our structured exercise was 120–150 min, which was closely similar to the PA guidelines recommending a minimum of 150 min per week of moderate-intensity exercise.8, 9 However, our subjects were able to perform only light intensity PA, evidently owing to their overweight/obesity status and low-maximal oxygen uptake capacity, a common problem in this high-risk subject group. Their PA level two METs present about 30% of their maximal oxygen uptake, and only 2.6% of their daily steps were above the acceleration class of ⩾1.1 g, presenting the lowest level of the three-MET activity. In two randomized studies in which objective measurements of PA were used, prediabetic subjects reached daily step numbers of 9000–10 000 and their 2-h glucose decreased at 3 and 12 months.17, 30 The median daily step counts in our subjects were 5576 (intervention group) and 4004 (control group), clearly lower than those in the two previously mentioned studies.17, 30 This is the likely reason we did not find any significant intervention effects on the fasting and 2-h glucose after 3 months. Insulin was not measured in these studies, but there was a 44% risk reduction in T2D development in the latter one.30 Both studies did not record the exercise intensity and used pedometers only for compliance monitoring. Achieving 10 000 daily steps may be quite difficult for most subjects. Only seven of 68 of our subjects were able to reach 10 000 steps per day.
The effects of lifestyle modifications on insulin sensitivities have previously demonstrated a significant association with improved insulin sensitivity and body weight reduction. In the AusDiab Study, daily step counts were measured in 592 adults.31 Higher step counts were associated with improved insulin sensitivity, but the effect was mediated by lower adiposity. In the Finnish Diabetes Prevention Study, a 4-year lifestyle intervention with dietary and PA counseling significantly reduced the risk of developing T2D with improvements in insulin sensitivity and weight loss.32 In our study, the intervention effects on insulin were statistically significant regardless of changes in body weight and other confounding factors. The improvement in the insulin sensitivity of our subjects appeared to be mostly an independent outcome and may reflect the effects of PA on insulin signaling in the skeletal muscle.33 This is in agreement with the preset finding in which the change in fitness (PA) in response to lifestyle modifications was found to have an independent effect without body weight changes on health-related outcomes in T2D patients.34
The reduction in the visceral fat area we observed in our subjects between the highest and lowest active quartile was 16 cm2, corresponding to 10% of the total visceral fat. This is in agreement with the findings that 4 weeks of aerobic cycling exercise in obese individuals significantly reduced visceral adipose tissue volume by 12% without an effect on basal insulin or HOMA-IR.35 Thus, our novel results demonstrate a clinically relevant observation, namely that even very low-PA intensity starts a chain of events reducing visceral fat mass, blood cholesterol and insulin resistance, all known to diminish the risk of developing T2D.36
Henson et al.37 recently measured sedentary time (<25 actigraph counts per 15 s), moderate-to-vigorous intensity physical exercise activity (⩾488 counts per 15 s) and total PA (total counts) demonstrating that time spent sedentary is adversely associated with elevated 2-h glucose and triglycerides in subjects with known risk factors for T2D.32 The authors concluded that sedentary behavior is a more important indicator for poor health than moderate-to-vigorous activity. Our results support these findings as an increasing number of daily steps ameliorated insulin resistance and lipid status.
The accelerometer used in the present study records the number of acceleration peaks induced by walking (steps) and their accelerations (intensities), which had not been previously used in subjects with high-T2D risk. We have used these accelerometers in previous studies to establish precise exercise threshold levels for beneficial health effects in healthy women including increased bone mass, muscle strength, maximal oxygen uptake and decrease in total and LDL cholesterol.28, 29 For the present study we also pooled accelerometer data from the intervention and control group and compared each variable between quartiles. We observed dose–response relations between LDL cholesterol and visceral fat area. Significant reductions occurred at the highest active quartiles above 6520 daily steps. Interestingly, there was no significant dose–response relation between the changes in the glucose or insulin levels between the quartiles. The difference in 2-h insulin changes between the highest and lowest active quartile was as high as 36 mU l−1 but did not reach statistical significance, owing to the large variation in the least active quartile.
The present study does have limitations. The intervention was short and the number of subjects relatively small. However, whole-day recordings of step-induced steps and accelerations throughout the 3-month intervention made it possible to measure accurately the amount and intensity of PA and allowed us to determine quantitatively their effects on glucose and insulin levels as well as lipid homeostasis.
Before starting the intervention, we informed all the participating subjects about their abnormal blood glucose levels and the beneficial effects of increased PA and weight reduction according to the best standard of care. In addition, the weekly downloading of the data could possibly have affected the subjects. This led to ‘co-intervention' in the control group in which high physical activities and large weight reductions were also observed in several subjects. To avoid the interference of ‘co-intervention' we analyzed associations of daily step numbers with outcome variables from all the subjects.
Our novel finding was that we were able to translate steps and accelerations to practical PA guidelines in sedentary and obese subjects with high-T2D risk. At the intensity level of 0.3–0.7 g, which is equivalent to walking 2–3 km h−1 or ∼two METs,11 the daily number of steps needed to reduce insulin and lipid levels and visceral fat area was at least 6500, requiring ∼90 min of ambulatory activities. This goal can be achieved by walking at a speed of 2–3 km h−1 and/or leisure-time-, household-, commuting- and occupation-related activities.
We have previously observed in healthy adult females, who were able to exercise at acceleration levels beyond 1.1 g, that significant improvements in maximal oxygen uptake, skeletal bone masses and blood cholesterol levels were possible with daily exercises lasting for at least 15 min.28, 29 Thus, a common PA guideline is difficult to apply to subjects with different physical fitness levels, so that tailored guidelines are rather needed. Given the sedentary lifestyles that many people nowadays lead, objective measurements are necessary to establish specific PA guidelines to provide adequate protection against metabolic disorders.
Acknowledgments
Funding sources: The Finnish Diabetes Foundation and EVO funds from Pohjois-Pohjanmaa Hospital District and Oulu University Hospital. The work was supported in part by the Finnish Diabetes Foundation and EVO funds from Pohjois-Pohjanmaa Hospital District and Oulu University Hospital.We kindly acknowledge the most valuable assistance and cooperation of Pirjo Härkönen & Marjo Mannelin (Unit of General Practice, Oulu University Hospital), Tiina Kukonlehto, Jenni Isotalo, Erja Metsämarttila, Helena Savolainen and Meeri Kröger (Institute of Biomedicine, University of Oulu).
TJ is a patent holder (Newtest, Finland). The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on International Journal of Obesity website (http://www.nature.com/ijo)
Supplementary Material
References
- Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Increasing prevalence of diagnosed diabetes—United States and Puerto Rico, 1995-2010. MMWR Morb Mortal Wkly Rep. 2012;61:918–921. [PubMed] [Google Scholar]
- Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS. Physical activity and reduced occurrence of non-insulin dependent diabetes mellitus. N Engl J Med. 1991;325:147–152. doi: 10.1056/NEJM199107183250302. [DOI] [PubMed] [Google Scholar]
- Manson JE, Rimm EB. Physical activity and incidence of non-insulin dependent diabetes mellitus in women. Lancet. 1991;338:774–778. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- Pan X-R, Li G-W, Hu Y-H, Wang J-X, An Z-X, Hu Z-X, et al. Effect of diet and exercise in preventing NIMDD in people with impaired glucose tolerance. The Da Quing IGT and diabetes study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachlin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig JL, Ferannini E, Grudy SM, Haffner SM, Heine JR, Horton ES, et al. Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3671–3689. doi: 10.1210/jc.2008-0222. [DOI] [PubMed] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee I-M, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescripting exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- Sigal RJ, Kenny GP, Wasserman DH, Castandea-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes. Diabetes Care. 2006;29:1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Med. 2000;32 (9 Suppl:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Pomeroy J, Brage S, Curtis JM, Swan PD, Knowler WC, Franks WP. Between-monitor differences in step counts are related to body size: implications for objective physical activity measurement. PLoS One. 2011;6:e18942. doi: 10.1371/journal.pone.0018942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Tudor-Locke C, McCarger LJ, Bell RC. Measuring habitual walking speed of people with type 2 diabetes. Diabetes Care. 2005;28:1503–1504. doi: 10.2337/diacare.28.6.1503. [DOI] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- Tucker JM, Welk GJ, Beyler NK. Physical activity in US adults: compliance with the physical activity guidelines for Americans. Am J Prev Med. 2011;40:454–461. doi: 10.1016/j.amepre.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Pate RR, Pratt M, Blair SN, Haskel WL, Macear CA, Bouchard C, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and the American College of Sports Medicine. JAMA. 2005;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- Yates T, Davies M, Gorely T, Bull F, Khunti K. Effectiveness of a pragmatic education program designed to promote walking activity in individuals with impaired glucose tolerance. Diabetes Care. 2009;32:1404–1410. doi: 10.2337/dc09-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor-Locke C, Bell RC, Myers AM, Harris SB, Ecclestone NA, Lauzon N, et al. Controlled outcome evaluation of the first step program: a daily physical activity intervention for individuals with type II diabetes. Int J Obes. 2004;28:113–119. doi: 10.1038/sj.ijo.0802485. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Brashear MM, Johnson WD, Katzmarzyk PT. Accelerometer profiles of physical activity and inactivity in normal weight, overweight, and obese U.S. men and women. Int J Behav Nutr Phys Sci. 2010;7:60. doi: 10.1186/1479-5868-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care. 2003;26:725–731. doi: 10.2337/diacare.26.3.725. [DOI] [PubMed] [Google Scholar]
- Nagai M, Komiya H, Mori Y, Ohta T, Kasahar Y, Ikeda Y. Estimating visceral fat area by multifrequency bioelectrical impedance. Diabetes Care. 2010;33:1077–1079. doi: 10.2337/dc09-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglal SB, Kreiger N, Darlington GA. Lifetime occupational physical activity and risk of hip fracture in women. Ann Epidemiol. 1995;5:321–324. doi: 10.1016/1047-2797(94)00100-8. [DOI] [PubMed] [Google Scholar]
- Slattery ML, Abd-Elghany, Kerber N, Schumacher MC. Physical activity and colon cancer: a comparison of various indicators of physical activity to evaluate the association. Epidemiology. 1990;1:481–485. [PubMed] [Google Scholar]
- Tuppurainen M, Kröger H, Saarikoski S, Honkanen R, Alhava E. The effects of gynecological risk factors on lumbar and femoral bone mineral density in peri- and postmenopausal women. Maturitas. 1995;21:137–145. doi: 10.1016/0378-5122(94)00878-b. [DOI] [PubMed] [Google Scholar]
- Lange Andersen K, Basironi R, Rutenfranz J, Seliner V.(eds) Habitual Physical Activity and Health WHO Regional Publications: Copenhagen, Denmark; 1978 [Google Scholar]
- Jämsä T, Vainionpää A, Korpelainen R, Vihriälä E, Leppäluoto J. Effect of daily physical activity on proximal femur. Clin Biomech. 2006;21:1–7. doi: 10.1016/j.clinbiomech.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Vihriälä E, Oksa J, Karkulehto J, Korpelainen R, Myllylä R, Jämsä T. Reliability of an accelerometer in the assessment of body movements. Technolog Health Care. 2004;12:122–124. [Google Scholar]
- Vainionpää A, Korpelainen R, Vihriälä E, Rinta-Paavola A, Leppäluoto J, Jämsä T. Intensity of exercise is associated with bone density change in premenopausal women. Osteoporos Int. 2006;17:455–463. doi: 10.1007/s00198-005-0005-x. [DOI] [PubMed] [Google Scholar]
- Vainionpää A, Korpelainen R, Kaikkonen H, Knip M, Leppäluoto J, Jämsä T. Effect of impact exercise on physical performance and cardiovascular risk factors. Med Sci Sports Exerc. 2007;39:756–763. doi: 10.1249/mss.0b013e318031c039. [DOI] [PubMed] [Google Scholar]
- Saito T, Watanabe M, Hishida J, Izumi T, Omura M, Takagi T, et al. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose. Ann Intern Med. 2011;171:1352–1360. doi: 10.1001/archinternmed.2011.275. [DOI] [PubMed] [Google Scholar]
- Dwyer T, Ponsonby A-L, Ukoumunne OC, Pezic A, Venn A, Dunstan D, et al. Association of change in daily step count over five years with insulin sensitivity and adiposity: population based cohort study. BMJ. 2010;341:c7249. doi: 10.1136/bmj.c7249. [DOI] [PubMed] [Google Scholar]
- Mello VDF, Lindström J, Eriksson J, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Sundvall J, et al. Insulin secretion and its determinant in the progression of impaired glucose tolerance to type 2 diabetes in impaired glucose-tolerant individuals. Diabetes Care. 2012;35:211–217. doi: 10.2337/dc11-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després J-P, Lemieux I, Prud'homme D. Treatment of obesity: need to focus on high risk abdominally obese patients. BMJ. 2001;322:716–720. doi: 10.1136/bmj.322.7288.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakicic LM, Jaramillo SA, Balasupramanyam A, Bancroft B, Curtis JM, Mathews A, et al. Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: results from the Look AHEAD Study. Int J Obesity. 2009;33:305–316. doi: 10.1038/ijo.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105–1112. doi: 10.1002/hep.23129. [DOI] [PubMed] [Google Scholar]
- O'Leary VB, Marchetti CM, Krishnan RK, Bradley P, Stetzer FC, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol. 2006;100:1584–1589. doi: 10.1152/japplphysiol.01336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson J, Yates T, Biddle SJH, Edwardson CL, Khunti K, Wilmont EG, et al. Associations of objectively measured sedentary behavior and physical activity with markers of cardiometabolic health. Diabetologia. 2013;56:1012–1020. doi: 10.1007/s00125-013-2845-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.