Abstract
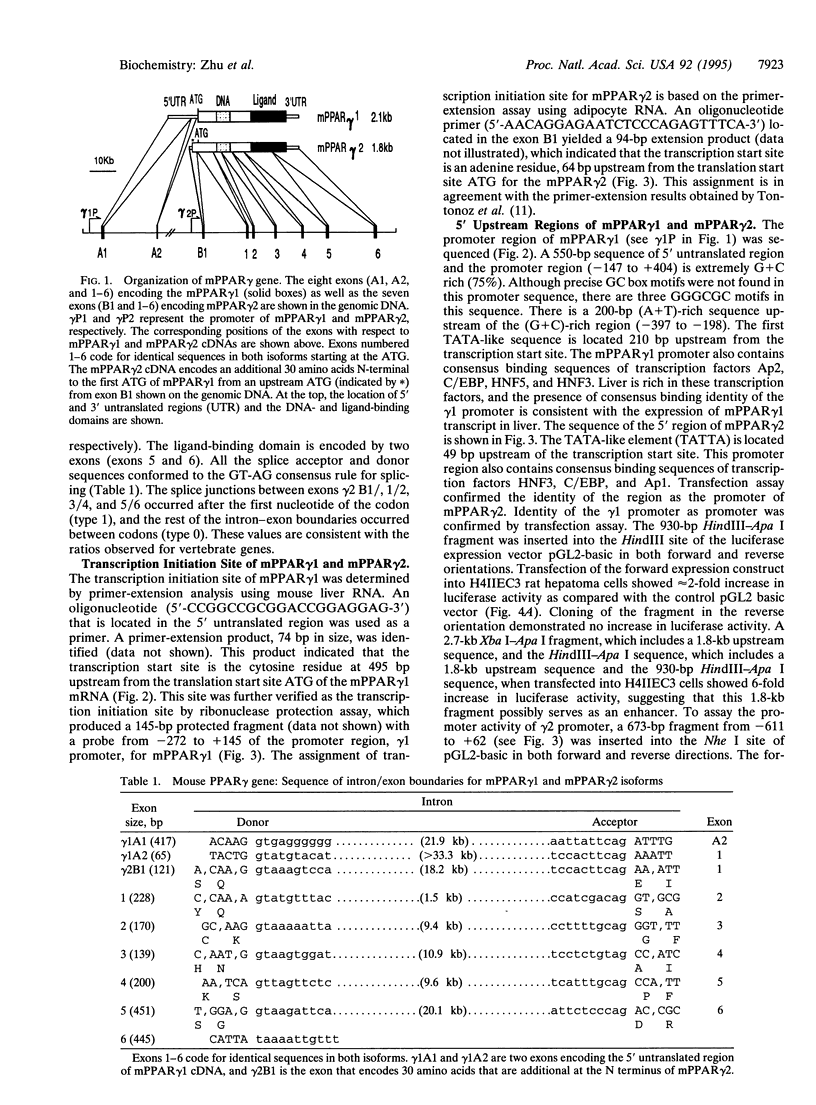
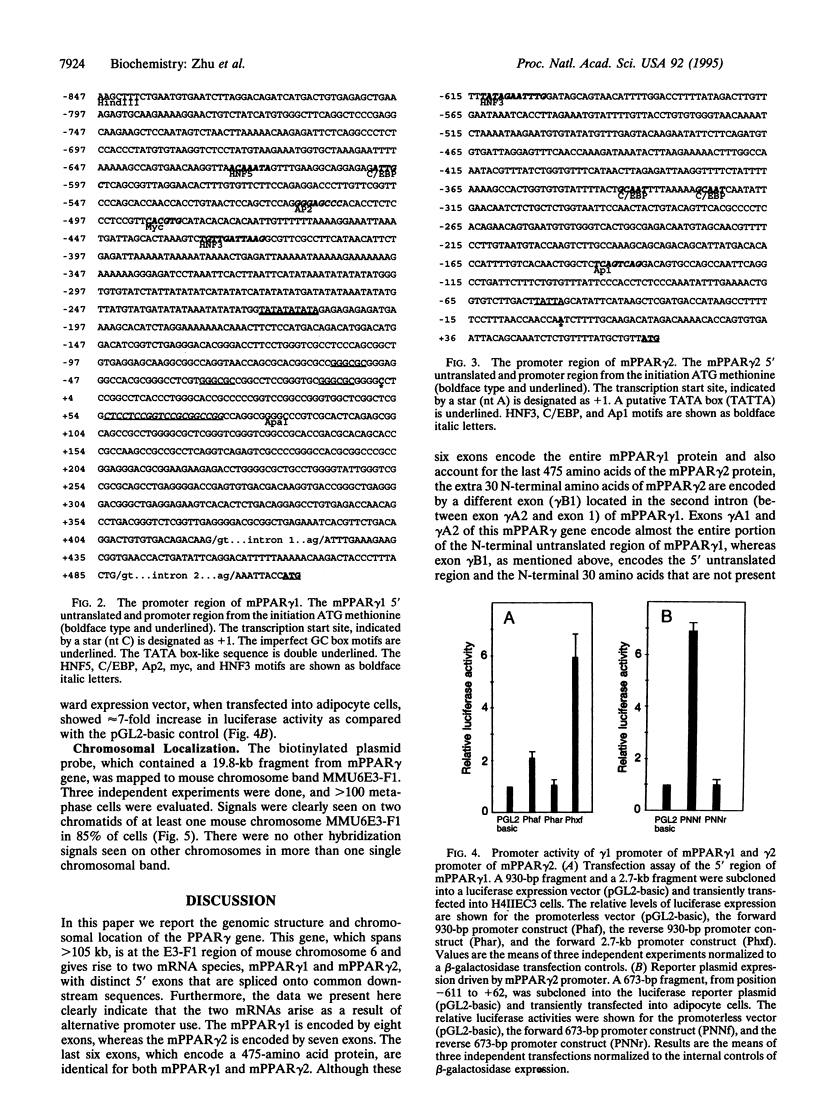
To gain insight into the regulation of expression of peroxisome proliferator-activated receptor (PPAR) isoforms, we have determined the structural organization of the mouse PPAR gamma (mPPAR gamma) gene. This gene extends > 105 kb and gives rise to two mRNAs (mPPAR gamma 1 and mPPAR gamma 2) that differ at their 5' ends. The mPPAR gamma 2 cDNA encodes an additional 30 amino acids N-terminal to the first ATG codon of mPPAR gamma 1 and reveals a different 5' untranslated sequence. We show that mPPAR gamma 1 mRNA is encoded by eight exons, whereas the mPPAR gamma 2 mRNA is encoded by seven exons. Most of the 5' untranslated sequence of mPPAR gamma 1 mRNA is encoded by two exons, whereas the 5' untranslated sequence and the extra 30 N-terminal amino acids of mPPAR gamma 2 are encoded by one exon, which is located between the second and third exons coding for mPPAR gamma 1. The last six exons of mPPAR gamma gene code for identical sequences in mPPAR gamma 1 and mPPAR gamma 2 isoforms. The mPPAR gamma 1 and mPPAR gamma 2 isoforms are transcribed from different promoters. The mPPAR gamma gene has been mapped to chromosome 6 E3-F1 by in situ hybridization using a biotin-labeled probe. These results establish that at least one of the PPAR genes yields more than one protein product, similar to that encountered with retinoid X receptor and retinoic acid receptor genes. The existence of multiple PPAR isoforms transcribed from different promoters could increase the diversity of ligand and tissue-specific transcriptional responses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Cheng S., Fockler C., Barnes W. M., Higuchi R. Effective amplification of long targets from cloned inserts and human genomic DNA. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5695–5699. doi: 10.1073/pnas.91.12.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer C., Krey G., Keller H., Givel F., Helftenbein G., Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992 Mar 6;68(5):879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990 Oct 18;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Forman B. M., Blumberg B., Ong E. S., Borgmeyer U., Mangelsdorf D. J., Umesono K., Evans R. M. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenberg J. R., Chen X. N. Human cDNA mapping using a high-resolution R-banding technique and fluorescence in situ hybridization. Cytogenet Cell Genet. 1995;69(3-4):196–200. doi: 10.1159/000133962. [DOI] [PubMed] [Google Scholar]
- Krey G., Keller H., Mahfoudi A., Medin J., Ozato K., Dreyer C., Wahli W. Xenopus peroxisome proliferator activated receptors: genomic organization, response element recognition, heterodimer formation with retinoid X receptor and activation by fatty acids. J Steroid Biochem Mol Biol. 1993 Dec;47(1-6):65–73. doi: 10.1016/0960-0760(93)90058-5. [DOI] [PubMed] [Google Scholar]
- Laudet V., Hänni C., Coll J., Catzeflis F., Stéhelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992 Mar;11(3):1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima N., Horiuchi R., Shibuya Y., Fukushige S., Matsubara K., Toyoshima K., Yamamoto T. Two erbA homologs encoding proteins with different T3 binding capacities are transcribed from opposite DNA strands of the same genetic locus. Cell. 1989 Apr 7;57(1):31–39. doi: 10.1016/0092-8674(89)90169-4. [DOI] [PubMed] [Google Scholar]
- Pierce J. C., Sternberg N., Sauer B. A mouse genomic library in the bacteriophage P1 cloning system: organization and characterization. Mamm Genome. 1992;3(10):550–558. doi: 10.1007/BF00350620. [DOI] [PubMed] [Google Scholar]
- Ponglikitmongkol M., Green S., Chambon P. Genomic organization of the human oestrogen receptor gene. EMBO J. 1988 Nov;7(11):3385–3388. doi: 10.1002/j.1460-2075.1988.tb03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J. K., Azarnoff D. L., Hignite C. E. Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature. 1980 Jan 24;283(5745):397–398. doi: 10.1038/283397a0. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Goel S. K., Nemali M. R., Carrino J. J., Laffler T. G., Reddy M. K., Sperbeck S. J., Osumi T., Hashimoto T., Lalwani N. D. Transcription regulation of peroxisomal fatty acyl-CoA oxidase and enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase in rat liver by peroxisome proliferators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1747–1751. doi: 10.1073/pnas.83.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J. K., Lalwai N. D. Carcinogenesis by hepatic peroxisome proliferators: evaluation of the risk of hypolipidemic drugs and industrial plasticizers to humans. Crit Rev Toxicol. 1983;12(1):1–58. doi: 10.3109/10408448309029317. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Mannaerts G. P. Peroxisomal lipid metabolism. Annu Rev Nutr. 1994;14:343–370. doi: 10.1146/annurev.nu.14.070194.002015. [DOI] [PubMed] [Google Scholar]
- Student A. K., Hsu R. Y., Lane M. D. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980 May 25;255(10):4745–4750. [PubMed] [Google Scholar]
- Tolbert N. E. Metabolic pathways in peroxisomes and glyoxysomes. Annu Rev Biochem. 1981;50:133–157. doi: 10.1146/annurev.bi.50.070181.001025. [DOI] [PubMed] [Google Scholar]
- Tontonoz P., Hu E., Graves R. A., Budavari A. I., Spiegelman B. M. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994 May 15;8(10):1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- Tontonoz P., Hu E., Spiegelman B. M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994 Dec 30;79(7):1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Alvares K., Huang Q., Rao M. S., Reddy J. K. Cloning of a new member of the peroxisome proliferator-activated receptor gene family from mouse liver. J Biol Chem. 1993 Dec 25;268(36):26817–26820. [PubMed] [Google Scholar]