Abstract
It has been known that the process of leaf senescence is accompanied by programmed cell death (PCD), and the previous study indicated that dark-induced senescence in detached leaves from rice led to the release of cytochrome f (Cyt f) from chloroplast into the cytoplasm. In this study, the effects of Cyt f on PCD were studied both in vitro and in vivo. In a cell-free system, purified Cyt f activated caspase-3-like protease and endonuclease OsNuc37, and induced DNA fragmentation. Furthermore, Cyt f-induced caspase-3-like activity could be inhibited by MG132, which suggests that the activity was attributed to the 26S proteasome. Conditional expression of Cyt f in the cytoplasm could also activate caspase-3-like activity and DNA fragmentation. Fluorescein diacetate staining and annexin V-FITC/PI double staining demonstrated that Cyt f expression in cytoplasm significantly increased the percentage of PCD protoplasts. Yeast two-hybrid screening showed that Cyt f might interact with E3-ubiquitin ligase and RPN9b, the subunits of the ubiquitin proteasome system (UPS), and other PCD-related proteins. Taken together, these results suggest that the released Cyt f from the chloroplast into the cytoplasm might activate or rescue caspase-3-like activity by interacting with the UPS, ultimately leading to the induction of PCD.
Programmed cell death (PCD) is a genetically regulated physiological process that is of irreplaceable importance for the development and homeostasis of multicellular organisms. In animals, the mechanisms of PCD, also known as apoptosis, have been well documented, while the mechanisms of plant PCD are still under investigation1. Plant PCD shares several cellular and biochemical hallmarks with animal apoptosis, such as cytoplasm shrinkage and DNA fragmentation. In our previous study, OsNuc37 was identified as an endonuclease in nuclei, which was responsible for DNA fragmentation during PCD in rice2. Similar to endonucleases, caspases also play an important role in PCD. Although the sequence homologues of caspases were not found in the plant genome, different types of proteases in plants, such as vacuolar processing enzymes3, metacaspases4, and subtilases5, were demonstrated to exhibit caspase-like activity. As one of the executive caspases, caspase-3 plays a crucial role in the execution of apoptosis in animal cells. Recently, PBA1, a catalytic subunit of the 20S proteasome, was identified as a caspase-3-like protease in Arabidopsis and in Populus tomentosa6,7. In addition to proteasome, Fernández et al. reported that a serine protease in the apoplast of potato is also responsible for caspase-3-like activity8. These results suggested that there may be more than one kind of caspase-3-like protease in plant cells.
In addition to the putative PCD regulators conserved throughout the animal and plant kingdoms, there are several specific mediators in plant PCD, probably involving chloroplast. In analogy to impaired mitochondria, a rapid loss of chloroplast integrity may be the source of a plant specific PCD signaling pathway9. It has been demonstrated that the reactive oxygen species (ROS), especially singlet oxygen (1O2), mediated the disintegration of the chloroplast and the release of chloroplast proteins10,11. However, it is not clear whether the release of chloroplast proteins into the cytoplasm has any effect on the PCD process.
Cytochrome b6f complex, located in the electron transport system between PSII and PSI, is responsible for the transfer of electrons from plastoquinol to plastocyanin in photosynthetic organisms. As one of the main subunits of Cyt b6f, cytochrome f (Cyt f) was encoded by the chloroplast petA gene with a molecular weight of 30 KDa, and was specifically anchored to the thylakoid membrane with its C terminus12. Similar to cytochrome c in mitochondria, Cyt f was also a c-type cytochrome for covalent attachment of the heme c to the polypeptide chain, and its biological function in photosynthesis has been well studied13.
It has been reported that Cyt f might be involved in the cell death of eggplants induced by palmitoleic acid via a release of Cyt f from the chloroplast into the cytoplasm14. Similar to this observation, chloroplast in Chlorella saccharophila underwent deep alterations of the thylakoid membrane structure in response to heat shock, accompanied by the release of Cyt f into the cytoplasm15. The release of trace amounts of Cyt f from the thylakoid membrane to the cytoplasm was also observed during the PCD process in the protoplasts of the conditional fluorescent (flu) mutant of Arabidopsis thaliana11. However, the detailed functions of Cyt f in the PCD pathway remain to be elucidated. In this study, in senescent rice leaves we observed the release of Cyt f from the chloroplasts prior to PCD. In order to test if Cyt f has any direct role in activating PCD we tested its ability to activate caspase-like molecules or DNA degradation in protoplasts and cell free systems. Our results show that in protoplasts and cell free systems Cyt f can activate or rescue cytosolic caspase-3-like activity by interacting with the ubiquitin-proteasome system.
Results
Activation of endonuclease OsNuc37 and cytosolic DEVDase by Cyt f and subsequent induction of DNA fragmentation in vitro
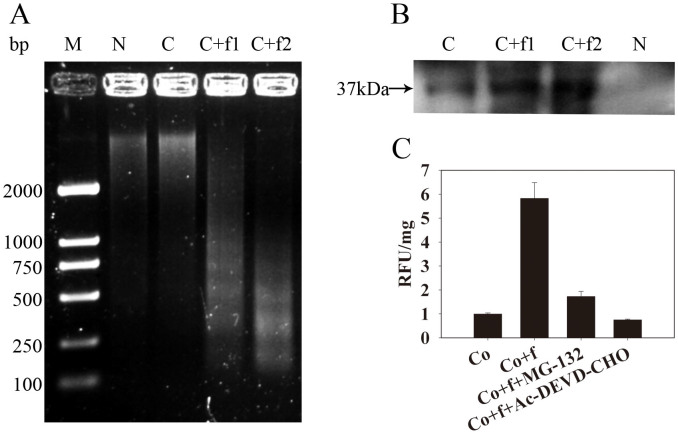
Degradation of the thylakoid membrane and photosynthetic complexes has been demonstrated in dark-induced leaf senescence in rice16. In our previous study, we also found that Cyt f was released to the cytoplasm prior to PCD in senescent rice leaves (data not shown). As Cyt f is released in senescent tissue prior to death it is of interest to evaluate if it could be involved in the actual induction of the cell death program. It has been known that DNA laddering is an important biochemical hallmark in plant PCD. In order to study the effect of Cyt f on DNA fragmentation, we established a cell-free system consisting of cytosolic fraction and nuclei from rice suspension cells. Purified Cyt f was incubated in the cell-free system, and DNA and proteins were extracted from the nuclei. Compared to a cell-free system only, Cyt f induced DNA laddering in nuclei (Fig. 1A). The nuclear protein extracts were further subjected to SDS-PAGE for the detection of OsNuc37 (Oryza sativa nuclear endonucleases of 37 kDa) activity, which was responsible for DNA fragmentation in rice2. As shown in Fig. 1B, Cyt f also induced OsNuc37 activity, which was identified as 37 kDa dark bands reflecting the absence of denatured salmon sperm DNA, indicating that Cyt f could induce DNA fragmentation through activating OsNuc37. Since nuclear alteration is one of the later stage events in the PCD process, we asked whether this event is dependent on the caspase-like proteases, and detected caspase-3-like activity in the cytosolic fractions of the cell-free system. As shown in Fig. 1C, exogenous Cyt f activated a DEVDase activity, and this activity could be inhibited by the caspase-3 specific inhibitor N-acetyl-Asp-Glu-Val-Asp-CHO (Ac-DEVD-CHO). Interestingly, it was also inhibited by MG132, the specific 26S proteasome inhibitor, which indicated that Cyt f-induced cytosolic DEVDase activity was related to 26S proteasome in rice.
Figure 1. Induction of Cyt f PCD hallmarks in a cell-free system.
(a) DNA laddering and (b) endonuclease activity in the nuclear extracts, which were induced by different concentrations of Cyt f. M, marker; N, isolated nucleus; C, cell-free system including nucleus and cytosolic fraction; C + f1, cell-free system plus 0.2 μM Cyt f; C + f2, cell-free system plus 1 μM Cyt f. Arrowhead points to a 37 kDa dark bands, which reflected the absence of denatured salmon sperm DNA due to DNase activity. (c) Caspase-3-like activity in cytosolic fraction. Co, cell-free system without treatment. Co + f, cell-free system plus 1 μM Cyt f. Co + f + MG-132, cell-free system plush 1 μM Cyt f and 10 μM MG-132. Co + f + Ac-DEVD-CHO, cell-free system plus 1 μM Cyt f and 100 μM Ac-DEVD-CHO. RFU/mg, Relative fluorescence units per mg protein.
Induction of PCD in mesophyll protoplasts by conditional expression of C-terminus lacking Cyt f in cytoplasm
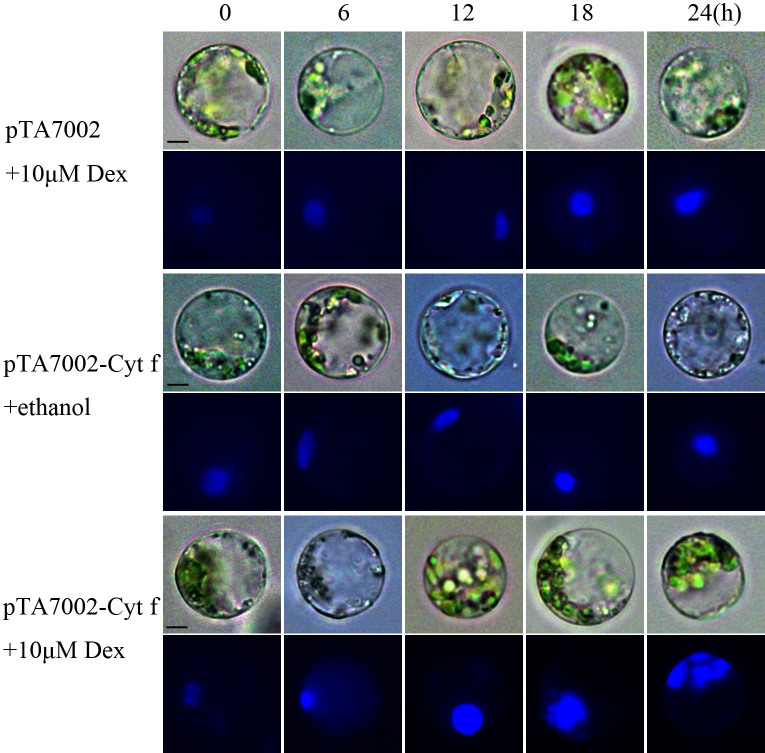
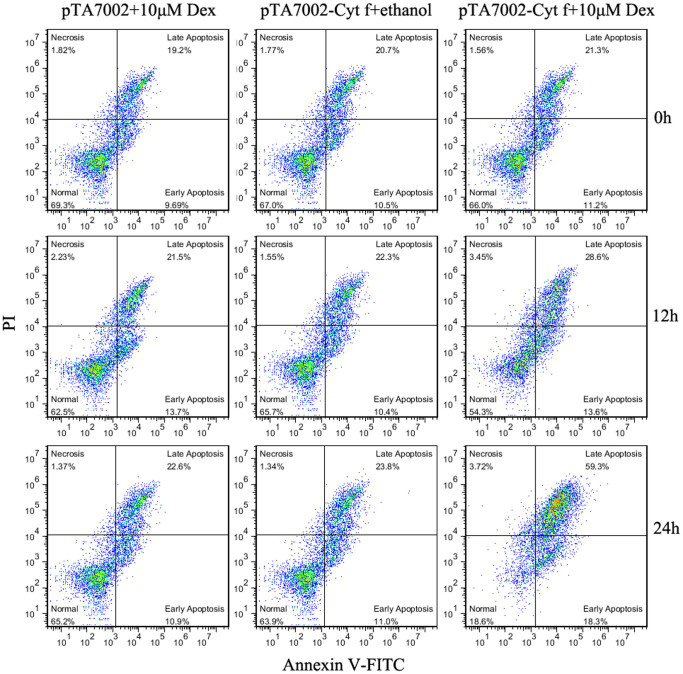
Since Cyt f is located in the chloroplast under normal growth conditions, we investigated whether PCD occurs if Cyt f is expressed in the cytoplasm. In order to test this hypothesis, conditional expression of C-terminus lacking Cyt f in the cytoplasm was established in the mesophyll protoplast (see details in Methods). Protoplasts bearing pTA7002-Cyt f were treated with dexamethasone (Dex) at different times, and immunoblot analysis revealed that Cyt f could be detected in the cytoplasm as early as 6 h after 10 μM Dex induction. In the following 6 h, the expression level increased significantly (Fig. 2). Following Cyt f expression in the cytoplasm, nuclei expansion occurred at 12 h and DNA fragmentation was observed at 18 h, after Dex treatment. After 24 h, the generation of large debris indicated the disintegration of the nuclei (Fig. 3). At the same time, if the Dex was substituted by an equal volume of ethanol, the solvent of Dex, no changes in nuclei were visible (Fig. 3). It should be noted that transient expression of the reference construct showed that the transformation efficiency was as high as 71% (data not shown), which inferred that Cyt f was expressed in most of the protoplasts. Similar to the observation in a cell-free system, conditional expression of Cyt f in mesophyll protoplasts induced caspase-3-like activity up to 10-fold over than that of the vector control (Fig. 4). Viability of transformed protoplasts was assessed using fluorescein diacetate (FDA) staining. As shown in Fig. 5, within 6 h after Dex induction, both the transgenic and control protoplasts exhibited bright fluorescence and normal morphology (Fig. 5A). In the following 18 h, the fluorescence intensity was gradually reduced. However, the reduction of fluorescence intensity in transgenic protoplasts was more significant than that in the vector control, and at 24 h after Dex induction, the average RFU was reduced 70% in transgenic protoplasts, compared with a 40% reduction in the vector control (Fig. 5B). Flow cytometry analysis showed that the percentage of apoptotic-like protoplasts (Annexin V-FITC+/PI+) in the transgenic group were also increased than that in the vector control (Fig. 6), which indicates that in addition to caspase-3 activation and DNA fragmentation, expression of Cyt f in cytoplasm induced PCD in mesophyll protoplasts in rice.
Figure 2. Conditional expression of N-terminus-lacking Cyt f in mesophyll protoplasts from rice.
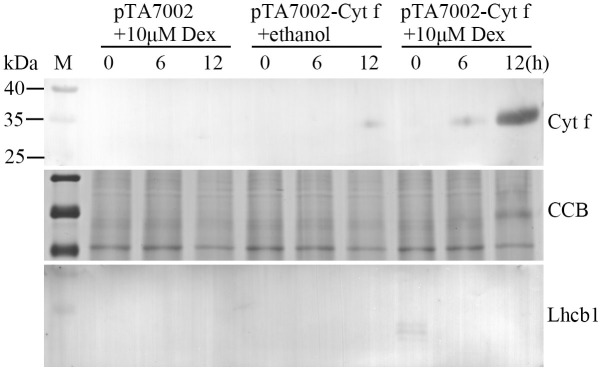
(a) Expression of Cyt f in cytosolic fraction from mesophyll protoplasts by immunoblot analysis. pTA7002 + 10 μM Dex, protoplasts with empty vector which were induced by 10 μM Dex. pTA7002-Cyt f + 10 μM ethanol, protoplasts with pTA7002-Cyt f which were incubated with equal amount of ethanol. pTA7002-Cyt f + 10 μM Dex, protoplasts with pTA7002-Cyt f which were induced by 10 μM Dex. Cytosolic proteins were prepared from the protoplasts at each time point (0, 6, 12 h) after treatment. (b) Immunoblotting of Lhcb1 for the same cytosolic fractions. Relative protein amounts of cytosolic proteins from different samples were normalized against protein loading. CCB, colloidal coomassie blue staining.
Figure 3. Chromatin fragmentation in mesophyll protoplasts under the expression of Cyt f.
The protoplasts were treated with Dex or ethanol for different times, and then stained with Hoechst-33342, and observed under fluorescence microscope.
Figure 4. Caspase-3 like activity in mesophyll protoplasts under the expression of Cyt f.
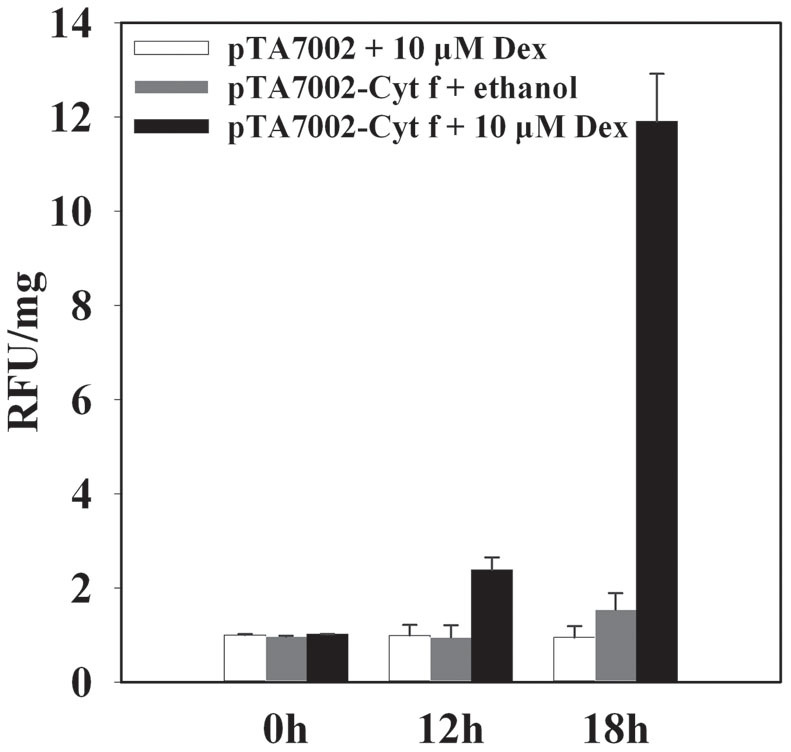
Protoplasts were treated with Dex or ethanol for different times, and equal amount of cytosolic fractions were incubated with Ac-DEVD-AMC. The AMC fluorescences were measured by a Microplate reader at 30°C. The RFU/mg was relative fluorescence units per mg protein.
Figure 5. Viability of mesophyll protoplasts under the expression of Cyt f by FDA staining.
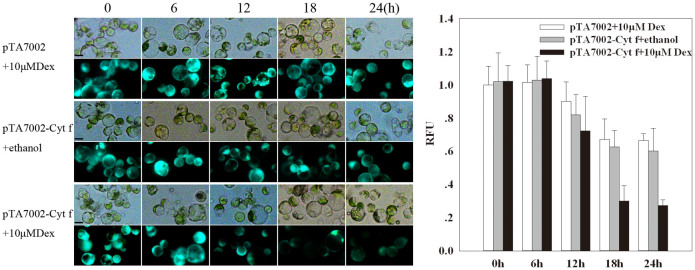
(a) Representative images illustrating protoplasts viability following different treatments at 0, 6, 12, 18, and 24 h, as estimated by FDA staining under bright (upper panel) and fluorescence field (lower panel) for the control (pTA7002 + 10 μM Dex and pTA7002-Cyt f + 10 μM ethanol) and experimental groups (pTA7002-Cyt f + 10 μM Dex). Scale bar = 20 μm. (b) Count of dead cells in (a). Fluorescence intensity was analyzed with Image-Pro Plus, 200 protoplasts for each time point of each treatment were counted and analyzed to indicate the Relative Fluorescence units (RFU). Counting was repeated three times.
Figure 6. Quantification analysis of PCD mesophyll protoplasts under the expression of Cyt f by flow cytometry.
Representative cytograms illustrating protoplast viability following different treatments at 0, 12, and 24 h, as estimated by annexin V-FITC and PI staining. Late apoptotic-like protoplasts (annexin V+/PI+) are positioned in the upper right quadrant; Early apoptotic-like protoplasts (annexin V+/PI−) are positioned in the lower right quadrant; Normal protoplasts (annexin V−/PI−) are present in the lower left quadrants; Necrotic protoplasts (annexin V−/PI+) are present in the upper left quadrants. To depress the background fluorescence, all the protoplasts for transient expression were prepared from etiolated seedlings.
Interaction of Cyt f with the subunits of the ubiquitin-proteasome system and PCD-related proteins
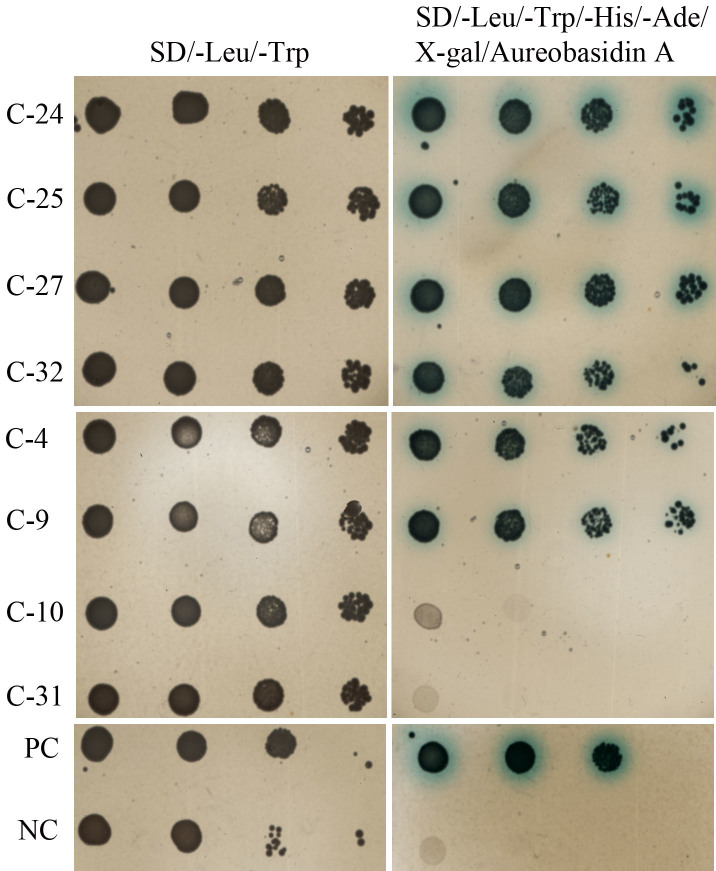
In order to reveal the mechanisms of the activation of caspase-3-like activity by Cyt f, a yeast two-hybrid system (Y2H) was used to screen the possible Cyt f-interacting proteins in the cytoplasm. The C-terminus lacking Cyt f was fused to the GAL4 DNA-binding domain (BD) in pGBKT7, and used as a bait to screen the cDNA library of Arabidopsis. We got five mRNAs as putative Cyt f-interacting proteins, and all of them are PCD-related (Fig. 7 and Table 1). Among these putative interacting proteins, RPN9b and RING-H2 finger protein ATL72 (E3-ubiquitin ligase) are the subunits of the Ubiquitin-Proteasome-System (UPS), and CDPK32 might be involved in the phosphorylation of UPS subunits (Table 1). Combined with the results that Cyt f activated caspase-3-like activity could be inhibited by MG132 (Fig. 1C), our results suggested that the 26S proteasome might be responsible for the caspase-3-like activity in rice, and that Cyt f induced this activity in cytoplasm by interacting with the regulatory and/or catalytical subunits of UPS.
Figure 7. Screening for the putative interacting proteins of Cyt f in Arabidopsis cDNA library through the Y2H.
C4-32 represents different clones in the cDNA library, and the mRNA information in these clones is shown in Table 1. PC, positive control; NC, negative control.
Table 1. Putative Cyt f-interacting proteins which were screened by Y2H in Fig. 7.
| No. | Arabidopsis gene locus | Protein | Function in PCD |
|---|---|---|---|
| C-4 | NM_118938 | P-loop containing nucleoside triphosphate hydrolases | R-protein, the key components of plant immune system, and animal apoptosis regulators CED4/Apaf-1, are P-loop NTPase members32,33 |
| C-9 | AY230842 | 26S proteasome subunit RPN9b | Deletion of RPN9b led to PCD in tobacco26 |
| C-24/25 | NM_111928 | RING-H2 finger protein ATL72 | E3-ubiquitin ligase activity is needed to suppress plant PCD27 |
| C-27 | NM_001036850 | Nuclear factor Y subunit B13 (NF-YB13) | NF-YB was imported into nucleus to form an intact NF-Y complex, and the latter could induce the expression of anti-apoptotic genes30,31 |
| C-32 | NM_121950 | Calcium-dependent protein kinase 32 (CDPK32) | CDPK1 interacted with Rpn3 in 26S proteasome, silence of CDPK1 led to premature cell death29 |
Discussion
In plant PCD studies, more and more DEVDase activities were detected in different sources including tissues17,18,19, or suspension cells20,21. Since different DEVDase were identified, we believed that there is probably more than one DEVDase activity in plant cells, which may have different sub-cellular localization. In fact, when previously attempting to purify DEVDase from rice in the lab, we observed two peaks which possess DEVDase activity after chromatography, from whole cell lysate in rice suspension cells. Based on these observations, in this study, we focused on cytosolic fractions both in vitro and in vivo, and provided evidence for the first time that Cyt f is a positive regulator of plant PCD. We proposed that during leaf senescence in rice, Cyt f is released from the chloroplast into the cytoplasm, and directly activated cytosolic caspase-3 like activity. Caspase proteolytic cascades induced endonuclease OsNuc37 in nuclei, which cleaved chromatin DNA and led to cell death.
Consistent with the reports in Arabidopsis11, eggplants14, and Chlorella saccharophila15, we found that Cyt f was also released from the chloroplast into the cytoplasm during dark-induced leaf senescence in rice. However, the mechanism of Cyt f release is not fully understood. Recently, a possible pathway for Cyt f release has been proposed, which includes a specific channel complex in the chloroplast inner membrane. Similar to the interaction between CidA and LrgA, the homologues of holins and antiholins in bacteria22, plant CidAB might interact with LrgAB in the inner membrane of the chloroplast and form a channel complex, leading to membrane disruption and caspase-like protease activation23. It will be interesting to elucidate whether Cyt f release has a relationship with CidAB/LrgAB complex formation in a future study.
Although Cyt f release from chloroplast to cytoplasm has been known in different PCD models, it is not clear whether the involvement of Cyt f in the PCD process is related to its loss of function or if the protein by itself is an important regulatory factor in PCD. In this study, we demonstrated that Cyt f serves as a pro-PCD factor (Figs. 3 and 5) through activating cytosolic caspase-3-like activity (Fig. 4). Cyt f possibly interacts with five proteins in Arabidopsis, and all of them are PCD-related (Table 1). Several studies indicated that UPS might play an important role in plant PCD. For example, it has been demonstrated that PBA1, the subunit of 20S proteasome, is responsible for caspase-3-like activity in Arabidopsis and Populus tomentosa6,7. Although we do not know whether PAB1 exhibited caspase-3-like activity in rice, our results suggested that Cyt f might activate 26S proteasome-related DEVDase (Fig. 1C) through interacting with some regulatory and catalytical subunits of UPS such as RING-H2 finger protein ATL72 and RPN9b. RING-H2 finger protein ATL72 represents a E3-ubiquitin ligase, and intrinsic E3-ubiquitin ligase activity was needed to suppress plant PCD24,25. In animal cells, inhibitor of apoptosis proteins (IAPs) blocked apoptosis by binding to active caspases and suppressing their function26. It has been demonstrated that certain IAPs displayed ubiquitin ligase activity and mediated the ubiquitination of caspases27. During apoptosis, mitochondrial protein Smac/Diablo released into cytoplasm and interacted with IAP28. By disrupting IAP-caspase interaction and repressing the ubiquitin ligase activities of IAPs, Samc/Diablo may rescue caspase activity from ubiquitination and then promote apoptosis29. Since Cyt f has the strongest interaction with RING-H2 finger protein ATL72 in this study (Fig. 7), we proposed that released Cyt f might rescue the cytosolic caspase-3-like activity through interacting with ATL72 and repressing its ubiquitin ligase activity. It will be very interesting to investigate whether ATL72 inhibits the caspase-3-like activity in plant cells, like IAP does in animal cells.
With regards to other possible interacting proteins, RPN9 is a subunit of regulatory 19S complex in the 26S proteasome. It has been demonstrated that deletion of RPN9b led to PCD in Nicotiana benthamiana30. Similar with the UPS subunits itself, calcium-dependent protein kinase 32 (CDPK32), a close homologue of CDPK1, was also identified as a putative interacting protein of Cyt f. It has been reported that CDPK1 could interact with the RPN3 subunit of the 26S proteasome, and led to the phosphorylation of the latter, and silence of CDPK1 resulted in premature cell death in tobacco31. Besides UPS related proteins, nuclear factor Y (NF-Y) was an important transcription factor which could induce the expression of anti-PCD genes as a heterologous NF-YA/YB/YC trimer32. In contrast to the nuclear location of NF-YA and -YC, NF-YB was localized to cytoplasm, and imported into the nucleus for the assembly of the heterotrimeric complex33. It has long been known that several pro-PCD factors, such as R-protein in plants and Apaf-1 in animals, belong to the P-loop NTPase superfamily34,35. As the substrate of NTPase, NTPs, especially ATP and GTP, was reported as a natural inhibitor of apoptosome formation by binding directly with Cyt c in animal cells, and caspase activation was accompanied by the decrease of the intracellular NTP pool36. Since all five proteins in Table 1 are PCD-related, we supposed that Cyt f interaction mimics the interference or activation of these proteins and promotes PCD in rice. These results support the conclusion that, as a dual function protein, Cyt f is a pro-PCD factor itself, and also indicate that the Cyt f-dependent pathway is a complex network which involves UPS, the NF-Y transcription factor, and the level of the intracellular NTP pool.
Taken together, we have shown in rice leaves that Cyt f was released from the chloroplast into the cytoplasm at an early stage of dark-induced senescence. Using cell free systems and protoplasts in this study, we have further shown that Cyt f could interact with several anti-PCD proteins, including UPS, and can active or rescue 26S proteasome-related caspase-3-like activity. As an executor of PCD, activated caspase-3 might induce several PCD later-stage events, including the activation of endonuclease OsNuc37, and finally lead to DNA fragmentation and cell death.
Methods
Vector construction
Total RNA was isolated from rice (Oryza sativa L. cv. Nipponbare) seedlings using the Trizol reagent (Invitrogen, USA). Reverse transcription polymerase chain reaction (RT-PCR) was used to obtain Cyt f cDNA (GenBank: M15955.1) lacking C-terminal trans-membrane region. The product was directionally cloned into the dexamethasone (Dex)-inducible binary vector pTA7002. The resulting constructs pTA7002-Cyt f and the vector control were introduced into rice mesophyll protoplasts respectively for induced transient expression.
Cell culture and cell-free system preparation
Rice suspension cells of wild type Nipponbare calli were routinely propagated and cultured at 28°C. Protoplasts were isolated from 3–4 d suspension cells37, with CPW-9M enzyme solution (1.5% cellulase R-10, 0.5% macerozyme R-10, pH5.80). The protoplasts were collected through a 35 μm nylon mesh filter, followed by centrifugation at 800 rpm for 5 min, then purified by 20% sucrose density gradient centrifugation.
The purified protoplasts were collected and washed with 50 mM PBS (pH 5.8), and suspended in lysis buffer (50 mM HEPES pH 7.4, 100 mM NaCl, 250 mM sucrose, 0.1% CHAPS, 1 mM DTT, 0.1 mM EDTA, 1 μg/mL pepstatinin, 8 μg/mL aprotinin, 10 μg/mL leupeptin). The suspensions were homogenized on ice and centrifugated at 100 000 g for 1 h at 4°C, the soluble cytosol fraction was collected and adjusted to 2 mg/ml. Nuclei were isolated according to Saxena et al (1985)38, and suspended in nuclear storage buffer [10 mM 2-(N-morpholino) ethanesulfonic acid (MES), pH 5.2, 250 mM sucrose, 1 mM EDTA, 2.5 mM DTT, 0.1 mM spermidine, 10 mM NaCl, 10 mM KCl, and 50% glycerol] to 107 nuclei/ml and stored at −80°C in multiple aliquots until use.
The cell-free system contained nearly 2.5 × 105 nuclei and 200 μl cytosol (2 mg proteins/ml. Different concentrations (0.2 or 1 μM) of cytochrome f from spinach (Sigma-Aldrich, USA) was added into the cell-free system, and incubated at 25°C for 5 h.
Plant material and treatments
Seeds of rice were surface sterilized with a 3% NaClO solution for 30 min followed by wash with distilled water. After germination, seeds were sown on porous plates that could float above the nutrient solution from International Rice Research Institute (IRRI), and illuminated with white fluorescent tubes under a 16 h photoperiod and 8 h dark. For rice seedlings etiolation, the germinated seeds grew under light for 2 d, and then moved to the dark for another 4–5 d.
Cytosolic protein preparation
Cytosolic protein extracts from rice leaves were prepared as described39. Protein contents of the samples were determined by the Bradford assay using bovine serum albumin as a standard. The quality of the cytosolic protein extracts were verified by immuno-blot using anti-Lhcb1 antibody.
SDS-PAGE and immunoblotting
The prepared sample of 30 μg proteins were subjected to 12% SDS-PAGE and transferred to PVDF membrane. Following transfer to PVDF, the proteins were visualized by modified colloidal coomassie brilliant blue to verify the protein loading. Membrane blotting was performed with the Wet-system (Bio-Rad, USA) according to the manufacturer's instructions, with a Chlamydomonas reinhartii anti-cytochrome f antibody (1:5000 dilution, Agrisera AB, Swedish) and a plant anti-Lhcb1 antibody (1:5000 dilution, Agrisera AB, Swedish). After incubation with HRP-Goat Anti-Rabbit IgG (H + L) (1:5000 dilution, BOSTER, China), HRP signal was detected with Enhanced HRP-DAB chromogenic substrate kit (TIANGEN, China).
Endonuclease activity assay
Endonuclease activity assay was performed according to Jiang et al (2008)2. Nuclear extracts (20 μg protein) harvested from cell-free system supplemented with Cyt f or not as described above were subjected to SDS-PAGE gels containing 50 μg/mL denatured salmon sperm DNA. The endonuclease activities were identified as dark bands reflecting absence of DNA due to DNase activity where endonuclease was present.
DNA laddering analysis
DNA samples were extracted from the nuclei in cell-free system by DNA extraction buffer (100 mM Tris-HCl, pH 8.0, 5 mM EDTA, 0.2 mM NaCl, 0.4% sodium dodecyl sulfate, and 0.2 mg/mL protease K) at 37°C for 12 h. After phenol/chloroform (25:24) extraction followed by ethanol precipitation, the DNA was transferred to TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0) with DNase-free RNase. Finally, the DNA from each sample was subjected to electrophoresis on 1.6% agarose gel, and then visualized by ethidium bromide staining.
Caspase-3 like activity assay
Caspase 3-like activity was analysed in cytosolic protein from cell-free system and mesophyll protoplasts as described12. Fractions of 50 μl cytosol from Dex-induced transfected protoplasts and Cyt f-induced cell-free system in presence or absence of protease inhibitors, were incubated in 150 μl assay buffer (50 mM Hepes-KOH, pH 7.5, 10% glycerol, 50 mM KCl, 2.5 mM MgCl2 and 1 mM DTT) supplemented with 70 μM of N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (Ac-DEVD-AMC, EnzoLife Sciences, USA). The reaction system was incubated in darkness at 30°C for 1 h. The fluorescence was measured in a Microplate reader (TECAN, USA) at 380/460 nm. The difference of fluorescence unit before and after incubation was normalized against the corresponding protein content. All assays were performed on three independent samples. For inhibitor assays, the proteasome specific inhibitor MG-132 (10 μM) and the specific caspase-3 protease inhibitor Ac-DEVD-CHO (100 μM), were added respectively.
Protoplast isolation from rice green tissue
Rice seedlings were grown in the nutrient solution 28°C for 7–10 days. Nearly 10 cm high seedlings were used for protoplast isolation. Tissues of stem and leave sheath were cut into approximately 0.5 mm strips and incubated in K3 medium supplemented with 1.5% cellulase R-10 (Yakult Honsa, Japan) and 0.3% macerozyme R-10 (Yakult Honsha, Japan). The segments were vacuum-infiltrated for 1 h in darkness and digested at 26°C for 4 h with gentle shaking at 40 rpm. Then the enzyme medium was gently removed and replaced by the same volume of W5 solution (154 mM NaCl, 125 mM CaCl2, 5 mM KCl and 2 mM MES, adjusted to pH 5.70 with 1 M KOH). The mixed solution was further shaking for 1 h at 80 rpm to release the protoplasts, followed by filtering through a 35-μm nylon mesh. Protoplasts were collected by centrifugation at 150 g for 4 min at 4°C, suspended in W5 solution and adjusted to 2 × 106/ml.
Protoplast transformation and fluorescence microscopy
Protoplasts (2 × 106/ml) in W5 solution were incubated on ice for 30 min, and collected at 150 g for 4 min and resuspended in equal volume of suspension buffer (0.4 M D-mannitol, 20 mM CaCl2 and 5 mM MES, adjusted to pH 5.70 with KOH). Plasmids DNAs of empty vector pTA7002 or pTA7002-Cyt f (20–25 ug) were added to 200 μl protoplasts respectively, then the mixture were added gradually to an equal volume of 40% PEG solution (40% PEG4000, 0.4 M D-mannitol and 0.1 M Ca(NO3)2, pH 7.0, 1 M KOH). After incubation for 20 min at room temperature in darkness, 1.0 ml W5 solution was added, then washed twice with W5 solution. Protoplasts with vector control and pTA7002-Cyt f were resuspended in W5 medium supplemented with 10 μM Dex and incubated in a six-well culture plate at 28°C in darkness for the indicated time. The same amount of ethanol used to dissolve Dex was applied as control. Transformed protoplasts for different induction time were stained with 10 μg/ml bisBenzimide H33342 trihydrochloride (Hoechst 33342, Sigma-Aldrich, USA) for 10 min at room temperature, washed twice and analyzed with a fluorescence microscope (ZEISS, Germany). To assess the viability of the transformed protoplasts, protoplasts at different induction times were stained with 0.01% fluorescein diacetate (FDA) for 10 min at room temperature, washed with W5 solution and then visualized under fluorescence microscope. Round protoplasts with evident green fluorescence indicated viable protoplasts. Fluorescence and bright field image for each sample were shown, fluorescence intensity were analysed with Image-Pro Plus, at least 200 protoplasts for each time point of each treatment were counted and analyzed to indicate the relative fluorescence units (RFU).
Flow cytometry
Protoplasts from etiolated rice seedlings were transformed with empty vector pTA7002 or pTA7002-Cyt f respectively, as described above. After Dex induction, 5 μl Annexin V-FITC and 10 μl PI from Annexin V-FITC Apoptosis detection Kit (eBioscience, USA) were used to label the protoplasts. Protoplasts were then analyzed with a flow cytometer (BD Accuri C6). 10,000 cells were counted for each assay, and each samples had at least two independent repeats.
Yeast two hybrid analysis
Arabidopsis Cytf lacking C-terminal transmembrane region and fused to GAL4 DNA-binding domain (pGBKT7 from Matchmaker™ Gold Yeast Two-Hybrid System, Clontech, USA). The yeast transformation was performed following BD Matchmaker™ Library Construction & Screening Kits User Manual. The Arabidopsis cDNA libraries were used for screening (Mate & Plate™ Library-Universal Arabidopsis, Clontech, USA). The yeast clones with recombined prey vector which can grow on QDO/X/A (SD/-Leu/-Trp/-His/-Ade/X-α-gal/Aureobasidin A) plates and turn blue were identified as positive clones, and the prey vectors in the clones were sequenced to get the inserting fragment sequences that were used to search Arabidopsis thaliana reference RNA Database.
Statistical analysis
Data are presented as mean ± S.D. of three independent experiments, using Student's t test to compare the significance of differences between data point (P < 0.05).
Author Contributions
H.W., X.C. and W.Z. wrote the main manuscript text and H.W., X.Z., H.L., J.C., C.L. and W.Z. performed the experiments. All authors reviewed the manuscript.
Acknowledgments
This work was support by the National Natural Science Foundation of China 31171326 (to W.Z.).
References
- van Doorn W. G. Classes of programmed cell death in plants, compared to those in plants. J. Exp. Bot. 62, 4749–4761 (2011). [DOI] [PubMed] [Google Scholar]
- Jiang A., Cheng Y., Li J. & Zhang W. A zinc-dependent nuclear endonuclease is responsible for DNA laddering during salt-induced programmed cell death in root tip cells of rice. J. Plant Physiol. 165, 1134–1141 (2008). [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura I., Hatsugai N., Nakaune S., Kuroyanagi M. & Nishimura M. Vacuolar processing enzyme: an executor of plant cell death. Curr. Opin. Plant Biol. 8, 404–408 (2005). [DOI] [PubMed] [Google Scholar]
- Coll N. S. et al. Arabidopsis type I metacaspases control cell death. Science 330, 1393–1397 (2010). [DOI] [PubMed] [Google Scholar]
- Chichkova N. V. et al. Phytaspase, a relocalisable cell death promoting plant protease with caspase specificity. EMBO J. 29, 1149–1161 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsugai N. et al. A novel membrane fusion-mediated plant immunity against bacterial pathogens. Genes Dev. 23, 2496–2506 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. J. et al. The proteasome is responsible for caspase-3-like activity during xylem development. Plant J. 72, 129–141 (2012). [DOI] [PubMed] [Google Scholar]
- Fernández M. B., Daleo G. R. & Guevara M. G. DEVDase activity is induced in potato leaves during Phytophthora infestans infection. Plant Physiol. Biochem. 61, 197–203 (2012). [DOI] [PubMed] [Google Scholar]
- Mullineaux P. & Karpinski S. Signal transduction in response to excess light: getting out of the chloroplast. Curr. Opin. Plant Biol. 5, 43–48 (2002). [DOI] [PubMed] [Google Scholar]
- Doyle S. M., Diamond M. & McCabe P. F. Chloroplast and reactive oxygen species involvement in apoptotic-like programmed cell death in Arabidopsis suspension cultures. J. Exp. Bot. 61, 473–482 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. et al. Chloroplasts of Arabidopsis are the source and a primary target of a plant-specific programmed cell death signaling pathway. Plant Cell 24, 3026–3039 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. C., Rochford R. J. & Packman L. C. Proteolytic removal of the C-terminal transmembrane region of cytochrome f during extraction from turnip and charlock leaves generates a water-soluble monomeric form of the protein. Eur. J. Biochem. 223, 481–488 (1994). [DOI] [PubMed] [Google Scholar]
- Gray J. C. Cytochrome f: structure, function and biosynthesis. Photosynthesis Res. 34, 359–374 (1992). [DOI] [PubMed] [Google Scholar]
- Peters J. S. & Chin C. Evidence for cytochrome f involvement in eggplant cell death induced by palmitoleic acid. Cell Death. Differ. 12, 405–407 (2005). [DOI] [PubMed] [Google Scholar]
- Zuppini A., Gerotto C., Moscatiello R., Bergantino E. & Baldan B. Chlorella saccharophila cytochrome f and its involvement in the heat shock response. J. Exp. Bot. 60, 4189–4200 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaba M. et al. Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence. Plant Cell 19, 1362–1375 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. G. & Franklin-Tong V. E. Self-incompatibility triggers programmed cell death in Papaver pollen. Nature 429, 305–309 (2004). [DOI] [PubMed] [Google Scholar]
- Coffeen W. C. & Wolpert T. J. Purification and characterization of serine proteases that exhibit caspase-like activity and are associated with programmed cell death in Avena sativa. Plant Cell 16, 857–873 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A., Rotari V. I., Gordon A., Mailhac N. & Gallois P. Ultraviolet-C overexposure induces programmed cell death in Arabidopsis, which is mediated by caspase-like activities and which can be suppressed by caspase inhibitors, p35 and Defender against Apoptotic Death. J. Biol. Chem. 279, 779–787 (2004). [DOI] [PubMed] [Google Scholar]
- Korthout H. A., Berecki G., Bruin W., van Duijn B. & Wang M. The presence and subcellular localization of caspase 3-like proteinases in plant cells. FEBS Lett. 475, 139–144 (2000). [DOI] [PubMed] [Google Scholar]
- Mlejnek P. & Procházka S. Activation of caspase-like proteases and induction of apoptosis by isopentenyladenosine in tobacco BY-2 cells. Planta 215, 158–166 (2002). [DOI] [PubMed] [Google Scholar]
- Ranjit D. K., Endres J. L. & Bayles K. W. Staphylococcus aureus CidA and LrgA proteins exhibit holin-like properties. J. Bacteriol. 193, 2468–2476 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. & Bayles K. W. Programmed cell death in plants: lessons from bacteria? Trends Plant Sci. 18, 133–139 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramovitch R. B., Janjusevic R., Stebbins C. E. & Martin G. B. Type III effector AvrPtoB requires intrinsic E3 ubiquitin ligase activity to suppress plant cell death and immunity. Proc. Natl. Acad. Sci. USA 103, 2851–2856 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjusevic R., Abramovitch R. B., Martin G. B. & Stebbins C. E. A bacterial inhibitor of host programmed cell death defenses is an E3 ubiquitin ligase. Science 311, 222–226 (2006). [DOI] [PubMed] [Google Scholar]
- Deveraux Q. L. et al. IAPs block apoptosis events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 17, 2215–2223 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Nakabayashi Y. & Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc. Natl. Acad. Sci. USA. 98, 8662–8667 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C., Fang M., Li Y., Li L. & Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102, 33–42 (2000). [DOI] [PubMed] [Google Scholar]
- Creagh E. M., Murphy B. M., Duriez P. J., Duckett C. S. & Martin S. J. Smac/Diablo antagonizes ubiquitin ligase activity of inhibitor of apoptosis proteins. J. Biol. Chem. 279, 26906–26914 (2004). [DOI] [PubMed] [Google Scholar]
- Kim M. et al. Activation of the programmed cell death pathway by inhibition of proteasome function in plants. J. Biol. Chem. 278, 19406–19415 (2003). [DOI] [PubMed] [Google Scholar]
- Lee S. S. et al. Interaction of NtCDPK1 calcium-dependent protein kinase with NtRpn3 regulatory subunit of the 26S proteasome in Nicotiana tabacum. Plant J. 33, 825–840 (2003). [DOI] [PubMed] [Google Scholar]
- Benatti P., Basile V., Merico D., Fantoni L. I., Tagliafico E. & Imbriano C. A balance between NF-Y and p53 governs the pro- and anti-apoptotic transcriptional response. Nucleic. Acids Res. 36, 1415–1428 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenberg D. et al. Studies on differential nuclear translocation mechanism and assembly of the three subunits of the Arabidopsis thaliana transcription factor NF-Y. Mol. Plant 5, 876–888 (2012). [DOI] [PubMed] [Google Scholar]
- Fenyk S., Campillo Ade S., Pohl E., Hussey P. J. & Cann M. J. A nucleotide phosphatase activity in the nucleotide binding domain of an orphan resistance protein from rice. J. Biol. Chem. 287, 4023–4032 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipe D. D., Koonin E. V. & Aravind L. STAND, a class of P-loop NTPases including animal and plant regulators of programmed cell death: multiple, complex domain architectures, unusual phyletic patterns, and evolution by horizontal gene transfer. J. Mol. Biol. 343, 1–28 (2004). [DOI] [PubMed] [Google Scholar]
- Chandra D. et al. Intracellular nucleotides act as critical prosurvival factors by binding to cytochrome C and inhibiting apoptosome. Cell 125, 1333–1346 (2006). [DOI] [PubMed] [Google Scholar]
- Maas C., Reichel C., Schell J. & Steinbiss H. H. Preparation and transformation of monocot protoplasts. Method. Cell Biol. 50, 383–399 (1995). [DOI] [PubMed] [Google Scholar]
- Saxena P. K., Mii M., Liu Y., Fowke L. C. & King J. High Nuclear Yields from Protoplasts of Several Plants. J. Plant Physiol. 121, 193–197 (1985). [Google Scholar]
- Debnam P. M. & Emes M. J. Subcellular distribution of enzymes of the oxidative pentose phosphate pathway in root and leaf tissues. J. Exp. Bot. 50, 1653–1661 (1999). [Google Scholar]