Abstract
Ivermectin (IVM), an FDA approved anthelmintic agent, can significantly reduce ethanol intake in mice following acute administration. The current study evaluates the sustainability and safety of multi-day IVM administration in reducing 10E intake in mice at a dose shown to be safe in humans. We tested the effect of 10-day administration of IVM (3.0 mg/kg/day; i.p.) on reducing 10% v/v alcohol (10E) intake in C57BL/6J mice using a 24-h, two-bottle choice paradigm. On the 10th day of IVM administration, mice were sacrificed at 0, 0.5, 2, 8, 32, 48 and 72 hours post-injection. Brain tissue and plasma samples were collected and analyzed using liquid chromatography with tandem mass spectrometry (LC-MS/MS). Analysis of variance (ANOVA) was used to assess the effect of 10-day IVM administration on 10E intake, 10E preference, water intake and total fluid intake with Dunnett’s Multiple Comparison post-hoc test. Individual student’s t-tests were also used to further quantify changes in these dependent variables. IVM significantly decreased 10E intake over a 9-day period (p<0.01). Pre IVM 10E intake was 9.1 ± 3.2 g/kg/24-h. Following the 9th day of IVM injections, intake dropped by almost 30% (p<0.05). IVM had no effect on total water intake or mouse weight throughout the study; however, there was a significant decrease in both preference for 10E (p<0.01) and total fluid intake (p<0.05). Multi-day administration of IVM significantly reduces 10E intake and preference in animals without causing any apparent adverse effects at a dose shown to be safe in humans.
Keywords: Alcohol use disorders, medications development, Alcoholism therapy, drug repositioning
INTRODUCTION
Alcohol use disorders (AUDs) rank third on the list of preventable causes of morbidity and mortality in the United States, having a national impact affecting over 18 million people and causing over 100,000 deaths annually [1–3]. The economic burden to society for AUDs is in excess of $200 billion/year [3], which exceeds the costs of other leading preventable causes of death such as cigarette smoking and physical inactivity [4]. According to the Global Status Report on Alcohol and Health 2011, nearly 4% of all deaths worldwide were alcohol-related [5]. Current FDA approved clinical treatment options include three FDA-approved oral medications and one FDA-approved injectable agent, which include disulfiram, naltrexone (both oral and injectable), and acamprosate [6, 7]. Data collected from The National Survey on Drug Use and Health (2006) and the National Epidemiologic on Alcohol and Related Conditions (2001–2002) found only 8.2% of subjects with 12-month AUDs had received treatment for AUDs [8]. These statistics reflect, in part, the fact that current therapeutic strategies are largely inadequate for the management of AUDs. Despite considerable efforts focusing on drug development to reduce ethanol abuse, high rates of uncontrolled heavy alcohol drinking persist.
We propose that ivermectin (IVM), a drug used by millions of humans for treatment of parasites [9, 10] can be repositioned as a novel pharmacotherapy to treat and/or prevent excess alcohol consumption and abuse. In P2×4 receptors (P2×4Rs), IVM is used to selectively identify the participation of P2×4Rs from other P2× family members in adenosine 5’-triphosphate (ATP)-mediated processes (Jelinkova et al., 2006; Khakh et al., 1999; Silberberg et al., 2007). Interestingly, sites of action of IVM are proximal to regions that we have previously reported as being important for ethanol modulation (Asatryan et al., 2008; Popova et al., 2010). Studies from our lab demonstrated that IVM is able to antagonize the inhibitory effect of ethanol in vitro (Asatryan et al., 2010).
Further support for the repositioning of IVM is drawn from a number of studies showing that IVM significantly reduces ethanol intake and preference in mice as determined across several validated alcohol drinking paradigms [11–13]. This work found that IVM doses ranging from 1.25 to 10.0 mg/kg can be safely administered and can significantly reduce alcohol intake using a 24-h access model [5, 11] that mimics “social” or non-intoxicating levels of alcohol drinking [14].
We also found that acute administration of IVM can significantly reduce higher levels of alcohol drinking using the intermittent limited-access model, which mimics binge-like drinking [11]. Importantly, in humans, young adults who participate in binge or heavy drinking are more likely to progress to alcohol abuse or dependence than age-matched counterparts [1]. Further, individuals participating in binge-like drinking behavior and/or drinking to intoxication is associated with significant increases in vehicle accidents, injuries, date rape and other types of violence, pregnancy, and blackouts (for review see [1]). Our findings that IVM significantly reduces binge-like drinking in mice [11] further supports the development of IVM as a new pharmacotherapeutic agent for treatment and/or prevention of AUDs.
The current approved dosing and administration regimen for IVM is based on acute use of the drug in human subjects. However, chronic administration would be anticipated in patients for treatment of AUDs. Several pieces of information support the safety of the chronic administration of IVM. First, doses up to 10 times that of the recommended dosage (i.e., 2.0 mg/kg/day) have been safely tested in human clinical trials [15]. Second, in rodents, doses less than 10 mg/kg IVM do not cause detectable CNS depression [16], and is more than 2.5 fold lower than the LD50 (25–50 mg/kg) [17]. Third, allometric scaling identified a dose of 3.1 mg/kg/day IVM in mice that corresponds to an oral dose (30 mg or approximately 0.5 mg/kg) already shown to be safe in humans [15]. Fourth, a case-control study reported that there were no significant increases in severe adverse events (SAEs) for patients that had self-reported consuming alcoholic beverages at the time of IVM administration [18]. Collectively, these findings point to IVM as an attractive agent for the treatment of AUDs, with good margin of safety and tolerability. The present study tests the hypothesis that multi day dosing of IVM is safe and has sustainable pharmacological AUDs activity.
MATERIALS AND METHODS
Animals
Studies were performed on drug naïve C57BL/6J male mice that were 8 weeks old upon purchase (Jackson Laboratory, Bar Harbor, ME, USA). Mice were singly housed in polycarbonate/polysulfone cages at a 12 h light/dark cycle with lights off at 12:30PM. The holding room was maintained at approximately 22°C. All procedures in this study were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and all efforts were made to minimize animal suffering. The USC Institutional Animal Care and Use Committee approved the protocols.
Drugs
IVM was administered via daily intraperitoneal (IP) injections. Noromectin (10 mg/ml in 60% propylene glycol) (Norbrook Inc, Lenexa, KS) was used for IVM injections. The noromectin was diluted using a 0.9% sodium chloride solution (saline) to a concentration that would allow for an injection volume of 0.01 ml/g of body weight. Gold Shield Alcohol (200 proof USP solution, Gold Shield Chemical Company, Hayward, CA) was diluted in water to achieve a 10% v/v ethanol solution (10E).
Alcohol Intake Study
The 24-h access model [e.g., [19–23] is widely used to assess changes in drinking behaviors. We used a modification of the procedure employed by Yoneyama et al. [23]. Briefly, using a within subjects design, 24 male mice had 24-h access to two inverted bottles of water with metal sippers placed on the cage tops for one day. One bottle of water was replaced with a bottle containing 10E. Food was distributed near both bottles to avoid food associated tube preferences. Alcohol determinations followed previously described procedures [11]. Briefly, daily 10E intake was measured until it stabilized (± 10% variability from the mean dose over 3 consecutive days). After establishing stable alcohol drinking level (after 7 days) mice received daily saline injections (intraperitoneal; i.p.) until 10E intake stabilized again (after 3 days). Mice then received one injection (i.p.) of IVM (3.0 mg/kg) daily for 10 consecutive days. The data reflects 9 days because mice were sacrificed post day 10 injections therefore, no drinking data was obtained after day 10 injections.
Analysis of brain concentrations of IVM using liquid chromatography with tandem mass spectrometry (LC-MS/MS)
Brain samples were collected just prior to day 10 injections (t=0), and 0.5, 2, 8, 32, 48 and 72 hours (n=3–4/time point) after day 10 IVM injections. Brain samples were analyzed as previously described [11, 12]. To 150 mg of brain, 2 µg of abamectin was added as internal standard. To each brain sample, 1 scoop of 1mm zirconium beads was added with 1 mL of acetonitrile, and sample was vigorously homogenized using the bullet blender for 5 minutes. Samples were then centrifuged at 10,000 rpm for 5 minutes and supernatant was collected. This was repeated with another 1 mL of fresh acetonitrile, and the combined 2 mL supernatant were evaporated to dryness using a steady stream of dried air. Evaporated residues of brain samples were reconstituted in 100uL of acetonitrile : water (90:10 v/v). A 40 µ L aliquot was injected into the Agilent 1100 HPLC System linked to an AB Sciex API 3000 Turboion spray mass spectrometer. The analytes were separated using an ACE C18 column with an isocratic mobile phase consisting of 0.1% formic acid in acetonitrile : 0.1% of acid in water (90:10 v/v). IVM and ABM was quantified in positive mode with multiple reaction monitoring using parent to transition ions of 888.8 → 551.5 and 890.1 → 449.6 respectively.
Data Analyses
Alcohol Intake Study
Ethanol dose (g/kg) and ethanol preference ratio (mls ethanol/total mls) were calculated daily. The dependent variables included 10E intake (g/kg), 10E preference (%), water (ml) and total fluid intake (ml). One-way analysis of variance (ANOVA) was used to assess the effect of 10-day IVM administration on the 4 dependent variables with Dunnett’s Multiple Comparison post-hoc test. Outliers were defined as data points ± 2 standard deviations from the daily mean of each dependent variable and were removed from the analysis. One-tailed, unpaired, individual Student’s t-test was used to analyze 10E intake and preference for 10E. Two-tailed, unpaired, individual Student t-test was used to analyze total fluid intake. The significance level was set at p ≤ 0.05.
Pharmacokinetic Analysis
The pharmacokinetics (PK) of IVM brain were analyzed using non-compartmental PK modeling. Serial blood and tissue IVM quantification was used to calculate PK parameters such as maximum drug concentration (Cmax), time to achieve maximal drug exposure (Tmax), half-life, elimination constant and area under the curve (AUC).
RESULTS
Multi-day IVM administration significantly reduced alcohol intake and preference in male mice
Alcohol Intake Study
The effect of 3.0 mg/kg/day IVM administered for 10 consecutive days using a 24-h access two-bottle choice alcohol paradigm (10E versus tap water) in C57BL/6J mice was evaluated. This study was designed based on our previous investigation that tested the effects of IVM on ethanol intake and preference [11]. Additionally, our PK modeling using allometric scaling study identified 3.1 mg/kg as the dosage that corresponds to a dose of IVM used safely in humans [15].
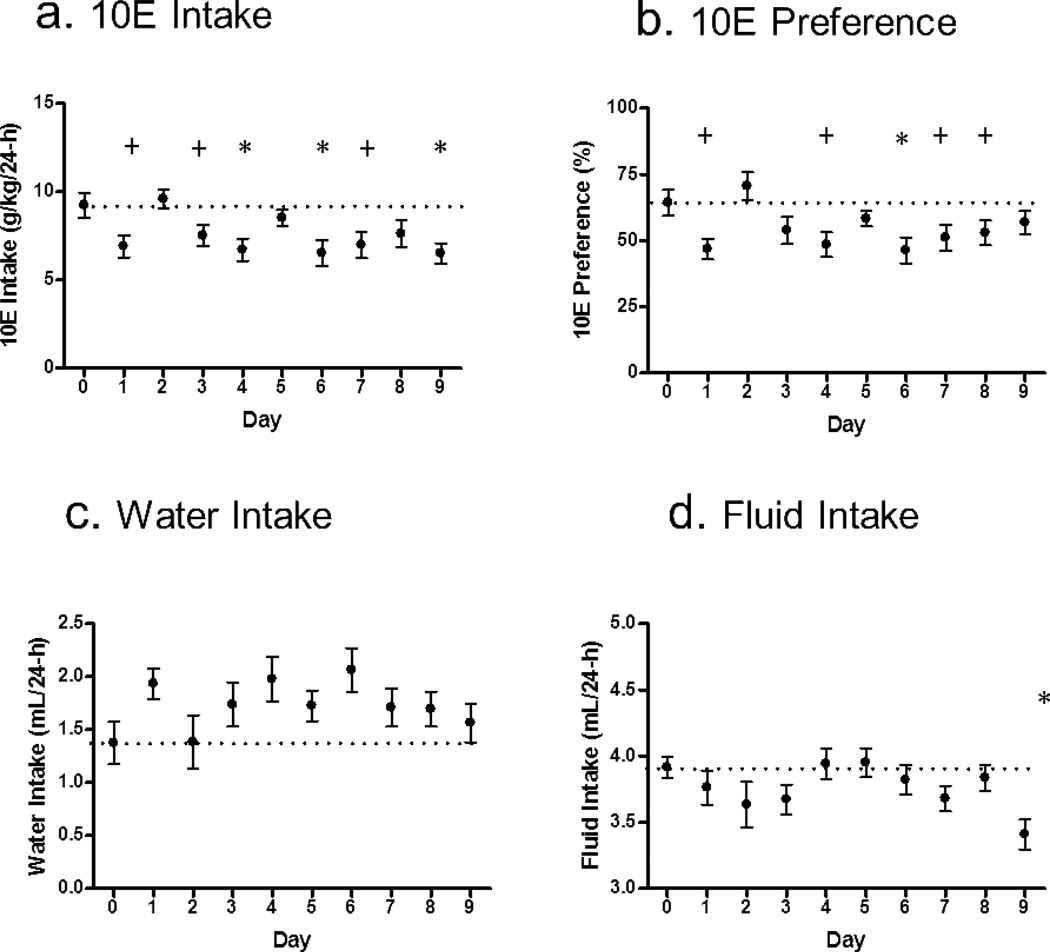
Prior to the initiation of saline injections, the 3-day average 10E intake was 9.0 ± 3.7 g/kg/24-h in the absence of vehicle or drug injections. The 3-day average 10E intake with saline injections was 9.1 ± 3.2 g/kg/24-h. A one-way ANOVA revealed that IVM (3.0 mg/kg/day×10 days) significantly decreased 10E intake over a 9-day period (p<0.01; Fig. 1a). Dunnett's Multiple Comparison Test revealed a significant reduction in ethanol intake on days 4 (p<0.05; reduction of 27% in 10E intake), 6 (p<0.05; reduction of 28%) and 9 (p<0.05; reduction of 30%) compared to the day immediately prior to the start of IVM injections (pre IVM; day 0). Using one-tailed, unpaired, individual student’s t-tests, identified 3 additional days where ethanol intake was significantly reduced. These included days 1 (t=2.488, p<0.05; reduction of 26%), 3 (t=1.847, p<0.05; reduction of 19%) and 7 (t=2.190, p<0.05; reduction of 25%), with a trend for decreased 10E intake on day 8 (p=0.06; reduction of 17%) compared to pre IVM levels. 48-h after the final administration of IVM, 10E intake increased to 8.4 ± 3.3 g/kg/24-h (n=6). 72-h after the final administration of IVM, 10E intake returned to baseline levels with an average of 10E intake of 9.7 ± 3.4 g/kg/24-h 72-h (n=3).
Figure 1. IVM significantly reduces 10E intake in C57BL/6J mice.
The graph illustrates the effects of IVM (3.0 mg/kg) administration in male mice using 24-h access two-bottle choice paradigm. Day 0 represents the saline control the day prior to the initiation of IVM administration for (a) 10E intake, (b) preference for 10E, (c) water intake, and (d) total fluid intake. Values represent the mean ± SEM for 24 mice. *p<0.05 vs. respective day 0 value (represented by the dotted line) as determined by Dunnett’s Multiple Comparison Test and +p<0.05 as determined by individual Student’s t-test.
Preference for 10E was significantly affected by IVM treatment (p<0.01; Fig.1b). Dunnett's Multiple Comparison Test showed a significant decrease in 10E preference on day 6 only (p<0.05). Similar to the above 10E intake results, using the one-tailed, unpaired, individual student’s t-tests identified additional days of significant decrease in 10E preference including days 1 (t=2.801, p<0.01), 4 (t=2.346, p<0.05), 7 (t=1.883, p<0.05) and 8 (t=1.696, p<0.05) with a trend for decreased 10E preference on day 3 (p=0.07).
There was no significant effect of treatment on water intake (Fig. 1c). There was a significant effect of treatment on fluid intake (p<0.05) with Dunnett's Multiple Comparison Test revealing a significant decrease in fluid intake on day 9 of IVM injections compared to pre IVM fluid intake (p<0.05; Fig.1d). Additionally, two-tailed, unpaired, individual student’s t-tests revealed a trend for decreased fluid intake on days 3 (p=0.09) and 7 (p=0.07).
Pharmacokinetics of IVM
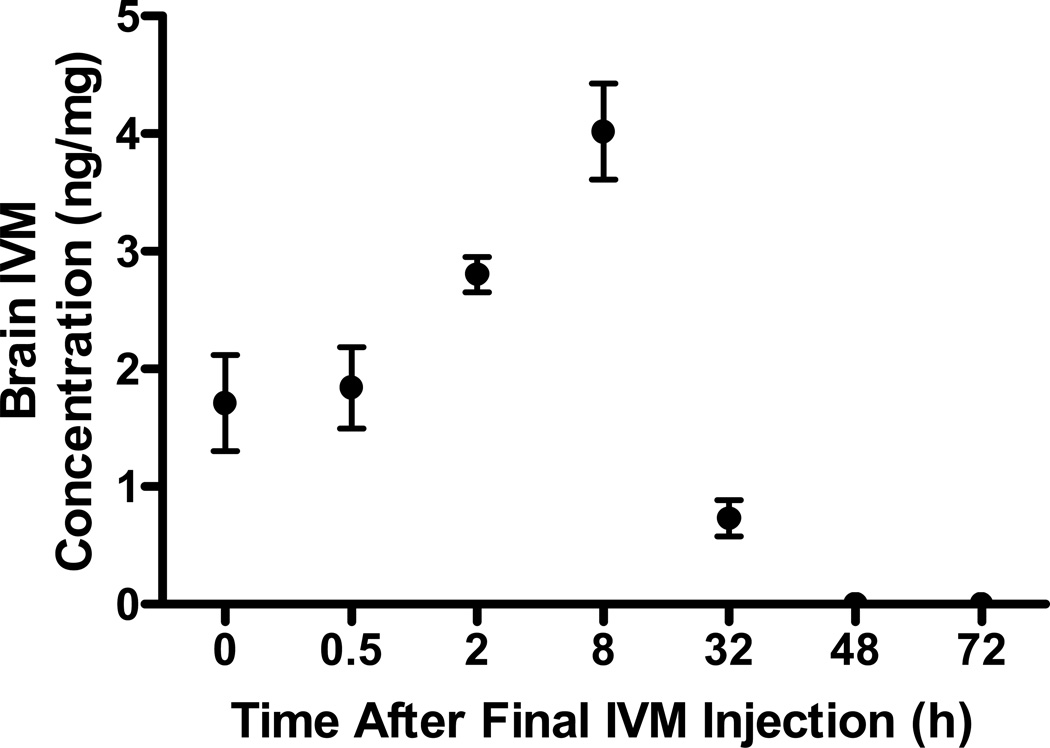
Brain samples were collected at designated time points of 0, 0.5, 2, 8 32, 48, and 72 h where time zero was defined as just prior to administration of the last dose. The pharmacokinetic parameters from this multi-day dosing study are summarized in Table 1. The results indicate there is accumulation of IVM in the brain with chronic dosing, however, most of the drug is cleared within 2–3 days after the last dose, as IVM was undetected at 48 and 72 h (Fig. 2).
Table 1.
IVM disposition in the brain was analyzed at 7 time points following the 10 days of injections: 0 (just prior to day 10 injections), 0.5, 2, 8, 32, 48 and 72.
| Brain Pharmacokinetic | ||
|---|---|---|
| Parameters | Mean | SD |
| Cmax (ng/mg of tissue) | 4.02 | 0.35 |
| Tmax (h) | 8 | n/a |
| Half-life (h) | 9.75 | 1.57 |
| AUC0-t(ng × h/mg of tissue) | 87.7 | 9.51 |
Figure 2. IVM accumulates in the CNS in a time dependent manner.
The graph illustrates the IVM levels in the brain tissue at the indicated time points following 10 consecutive days of IVM administration correlated with time to effect. Time 0 represents the concentration levels 24-h post day 9 IVM administration. Values represent the mean ± SEM for 3–4 mice/ time point. The Cmax correlated to onset of IVM activity. Additionally IVM half-life in the brain is 9.7 hours, where correlates with the reduction in brain concentration after 32 hours.
DISCUSSION
The present study tested the hypothesis that multi day dosing of IVM is safe and sustainable in reducing alcohol intake in mice. The dose of IVM that we tested in mice corresponds to a dose already shown to be safe in humans. In support of this hypothesis, we found that administration of IVM for 9 days, reduced 10E intake by approximately 25% compared to baseline measures (Fig. 1a). There were no overt changes in behavior or significant changes in body weight over the 9-day period suggesting that longer term IVM administration is well tolerated in mice. In support of this latter notion, we found that IVM administration over a period of 9 days did not significantly affect water intake. This finding further supports the contention that the effect of IVM is not a result of a generalized hypodipsia, which is consistent with previous studies testing the effect of acute IVM administration [11, 24]. The significant decrease in total fluid intake measured on day 9 of the study may have been due, in part, to the significant decrease in alcohol intake alone. This finding is consistent with previous results [11]. Pharmacokinetic studies revealed the average brain Cmax of IVM 32-h post day 10 injections was 0.73 ng/mg almost 3 times greater than the brain Cmax of IVM after a single dose of 2.5 mg/kg (0.261 ng/mg), the minimum effective single dose necessary to significantly decrease 10E intake in a 24-h two bottle choice paradigm [11].
IVM is a semi-synthetic macrocyclic lactone that is currently used worldwide as a broad-spectrum antiparasitic agent. The safety of IVM has been demonstrated for over 30 years where millions of humans have been treated successfully with good tolerability and few severe adverse events (for review see [25, 26]). IVM is also widely used for veterinary purposes in animals that are raised for food thus requiring significant studies reviewing the long-term safety of oral exposure to IVM as a food additive. This latter effort, based on available toxicological data by the World Health Organization (WHO), identified an acceptable daily intake (ADI) of 1–10 µg/kg/day based upon a No Observed Effect Level (NOEL) for maternal toxicity in reproductive toxicology studies in the most sensitive species (CD1 mouse) as well as a safety margin of 50–500 fold for the absence of neurological effects in dogs.
Importantly, single doses of up to 120 mg, a dose 4 times greater than the dose tested in the current study, have been safely administered to human subjects with no reported adverse experience for other indications [15]. In this same study, a 30 mg dose in humans (equivalent to a 3.1 mg/kg dose in mice), was administered three times in the first week (days 1, 4, and 7) followed by a washout period of 1 week and then a single dose of IVM (30 mg). Of these patients receiving IVM, 33.3% (n=5) had one or more adverse experience compared to 35.3% of the placebo group (n=6). There was no apparent evidence of CNS toxicity associated with IVM administration in this study at either dose measured assessed using quantitative pupillometry. This suggests that IVM has a good therapeutic index as a treatment for AUDs since the minimum effective dose necessary to decrease alcohol intake is far lower than the lethal dose 50 (LD50) of IVM which is approximately 25–50 mg/kg in mice [17].
The potential for repositioning IVM as an anti-alcohol therapy is further supported by previous investigations that reported that IVM at doses needed to produce the anti-alcohol effects in C57BL6 mice did not induce overt signs of toxicity across a wide range of well-validated behavioral paradigms for the assessment of sensory, motor and cognitive competence [24]. IVM has been reported to elicit anxiolytic-like effects when tested using the elevated plus maze and marble burying assays. However unlike other anxiolytic therapies such as benzodiazepines, the same IVM regimen did not exert rewarding properties in the conditioned place preference test. The latter strongly suggested that the psychotropic effects of IVM are dissociated from any addiction liability or potential issues of therapeutic compliance.
CONCLUSION
Findings from the present investigation demonstrate that IVM, administered for 10 days using a dose of IVM that consistently reduces ethanol intake and preference is well tolerated. Importantly, this dose of IVM corresponds to a dose already shown to be safe in humans. Collectively, current and previous findings support the contention that IVM administered at doses required to produce the anti-alcohol effects in mice should be safe and effective in humans for the treatment of AUDs. Future studies will focus on the safety and efficacy of chronic, oral delivery of IVM as a therapy to prevent and/or treat AUDs.
ACKNOWLEDGEMENTS
This work was conducted as partial fulfilment of the requirements for the PhD degree in Molecular Pharmacology and Toxicology, University of Southern California (M.M.Y.).
This work was supported in part by Research grants SC CTSI (NIH/NCRR/NCATS --TL1TR000132 [M.M.Y.]) and UL1TR000130 (D.L.D.), and by NIAAA/NIH KO1 AA017243 (L.A.), AA022448 (D.L.D.), and the USC School of Pharmacy.
Footnotes
The authors declare no conflict of interest and are entirely responsible for the scientific content of the paper.
REFERENCES
- 1.Johnson B. Medication treatment of different types of alcoholism. Am J of Psychiatry. 2010;167:630–9. doi: 10.1176/appi.ajp.2010.08101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant BF, Dawson DA, Stinson FS, Chou P, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic Costs of Excessive Alcohol Consumption in the U.S., 2006. Am J Prev Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 4.Naimi TS. The cost of alcohol and its corresponding taxes in the U.S.: A massive public subsidy of excessive drinking and alcohol industries. Am J Prev Med. 2011;41:546–547. doi: 10.1016/j.amepre.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Global status report on alcohol and health. 2011 [Google Scholar]
- 6.Harris AH, Kivlahan DR, Bowe T, Humphreys KN. Pharmacotherapy of alcohol use disorders in the Veterans Health Administration. Psychiat Serv. 2010;61:392–398. doi: 10.1176/ps.2010.61.4.392. [DOI] [PubMed] [Google Scholar]
- 7.Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML, et al. Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol. 2012;17:513–527. doi: 10.1111/j.1369-1600.2012.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edlund MJ, Booth BM, Han X. Who seeks care where? Utilization of mental health and substance use disorder treatment in two national samples of indviduals with alcohol use disorders. J Studies Alcohol Drugs. 2012;73:12. doi: 10.15288/jsad.2012.73.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geary TG. Ivermectin 20 years on: maturation of a wonder drug. Trends Parasitol. 2005;21:530–532. doi: 10.1016/j.pt.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Molinari G, Soloneski S, Larramendy ML. New ventures in the genotoxic and cytotoxic effects of macrocyclic lactones, Abamectin and Ivermectin. Cyogenet Genome Res. 2010;128:37–45. doi: 10.1159/000293923. [DOI] [PubMed] [Google Scholar]
- 11.Yardley M, Wyatt L, Khoja S, Asatryan L, Ramaker MJ, Finn DA, et al. Ivermectin reduces alcohol intake and preference in mice. Neuropharmacol. 2012;63:190–201. doi: 10.1016/j.neuropharm.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asatryan L, Yardley MM, Khoja S, Trudell JR, Huynh N, Louie SG, et al. Avermectins differentially affect ethanol intake and receptor function: Implications for developing new therapeutics for alcohol use disorders. Int J Neuropsychopharmacol. doi: 10.1017/S1461145713001703. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wyatt LR, Finn DA, Khoja S, Yardley MM, Asatryan L, Alkana RL, et al. Contribution of P2×4 receptors to ethanol intake in male C57BL/6 mice. Neurochem Res. doi: 10.1007/s11064-014-1271-9. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blednov YA, Ozburn AR, Walker D, Ahmed S, Belknap JK, Harris RA. Hybrid mice as genetic models of high alchol consumption. Behav Genet. 2010;40:93–110. doi: 10.1007/s10519-009-9298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guzzo CA, Furtek CI, Porras AG, Chen C, Tipping R, Clineschmidt CM, et al. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol. 2002;42:1122–1133. doi: 10.1177/009127002401382731. [DOI] [PubMed] [Google Scholar]
- 16.Trailovic AM, Trailovic N. Central and Peripheral Neurotoxic effects of ivermectin in rats. J Met Med Sci. 2010;73:591–599. doi: 10.1292/jvms.10-0424. [DOI] [PubMed] [Google Scholar]
- 17.Merck, Sharp, Dohme . Ivermectin. Pennsylvani: Div of Merck & Co Ltd WP; 1988. Poision control monograph. [Google Scholar]
- 18.Takougang I, Ngogang J, Sihom F, Ntep M, Kamgno J, Eyamba A, et al. Does alcohol consumption increase the risk of severe adverse events to ivermectin treatment? Afr J Pharm Pharmacol. 2008;2:77–82. [Google Scholar]
- 19.McClearn GE. The genetics of mouse behavior in novel situations. J Compar Physiol Psychol. 1959;52:62–67. doi: 10.1037/h0044664. [DOI] [PubMed] [Google Scholar]
- 20.Rodgers DA. Research activities related to treatment of alcoholism. Compr Psychiat. 1966;7:57–67. doi: 10.1016/s0010-440x(66)80007-x. [DOI] [PubMed] [Google Scholar]
- 21.Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacol (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- 22.Middaugh LD, Kelley BM, Bandy AL, McGroarty KK. Ethanol consumption by C57BL/6 mice: Influence of gender and procedural variables. Alcohol. 1999;17:175–183. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- 23.Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bortolato M, Yardley M, Khoja S, Godar SC, Asatryan L, Finn DA, et al. Pharmacological insights into the role of P2×4 receptors in behavioral regulation: Lessons from ivermectin. Int J Neuropsychopharmacol. 2013;16:1059–1070. doi: 10.1017/S1461145712000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omura S. Ivermectin: 25 years and still going strong. Int J Antimicrobial Agents. 2008;31:91–98. doi: 10.1016/j.ijantimicag.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Crump A, Omura S. Ivermectin, 'wonder drug' from Japan: The human use perspective. P Jpn Acad B-Phys. 2011;87:13–28. doi: 10.2183/pjab.87.13. [DOI] [PMC free article] [PubMed] [Google Scholar]