Abstract
Imaging mass spectrometry (IMS) studies increasingly focus on endogenous small molecular weight metabolites and consequently bring special analytical challenges. Since analytical tissue blanks do not exist for endogenous metabolites, careful consideration must be given to confirm molecular identity. Here we present approaches for the improvement in detection of endogenous amine metabolites such as amino acids and neurotransmitters in tissues through chemical derivatization and matrix-assisted laser desorption/ionization (MALDI) IMS. Chemical derivatization with 4-hydroxy-3-methoxycinnamaldehyde (CA) was used to improve sensitivity and specificity. CA was applied to the tissue via MALDI sample targets precoated with a mixture of derivatization reagent and ferulic acid (FA) as a MALDI matrix. Spatial distributions of chemically derivatized endogenous metabolites in tissue were determined by high-mass resolution and MSn imaging mass spectrometry. We highlight an analytical strategy for metabolite validation whereby tissue extracts are analyzed by high-performance liquid chromatography (HPLC)-MS/MS to unambiguously identify metabolites and distinguish them from isobaric compounds.
Keywords: MALDI imaging, In-tissue derivatization, Amine metabolites, Amino acids, Neurotransmitters, Identification, Reagent precoated MALDI targets
INTRODUCTION
An understanding of the distribution of endogenous metabolites within mammalian tissues can provide insight into the mechanism and location of biological and metabolic processes. Neurotransmitters (NTs) and amino acid (AA) and metabolites such as dopamine, serotonin, norepinephrine and γ-aminobutyric acid (GABA) have been implicated in processes such as behavior, sleep, body temperature regulation, exercise fatigue, as well as cognition and motor skills [1–2]. Improper neural system function related to abnormal concentrations of NTs in the brain has been associated with anxiety and depression, as well as diseases such as Parkinson’s [2] and Alzheimer’s [3]. Most information about the localization of these compounds in the brain is inferred based on the location of related molecules (e.g., their transporters); however, due to the importance of these compounds for homeostasis and in neurobiological diseases, the ability to directly measure the spatial localization of these molecules in tissue would be immensely beneficial.
Among the tools available for the measurement of AAs and NTs, imaging mass spectrometry (IMS) is well suited to provide information to aid in this understanding. IMS involves acquiring mass spectral data at predefined locations (pixels) across the tissue surface. Spectral data can be mined to produce two-dimensional molecular maps representing the spatial expression of any analyte (m/z) recorded across the surface of the tissue section. The molecular specificity of mass spectrometry along with its ability to map locations of compounds provides information that is not easily acquired by other means[4]. Specifically, matrix-assisted laser desorption/ionization (MALDI) IMS has been used to study the spatial localization of a variety of small molecules directly from tissue sections, including lipids [5–8], xenobiotics [9–11], and metabolites [12].
MALDI IMS of AAs or NTs has previously been performed on plants [13–15], fingerprints [16], tissues associated with the central nervous system [12, 17] and cancer xenografts following derivatization[18]. Additionally, desorption electrospray ionization (DESI) mass spectrometry has been used to image NTs in adrenal gland tissue [19]. However, most of these approaches did not definitively identify the AAs or NTs reported. Considerations were not made for structurally similar exactly isobaric species which could have been responsible for or contributed to the measured signals. Doing so is crucial for maintaining the scientific accuracy of the data and ensuring that any conclusions drawn are valid.
In addition to isobaric interferences, trace level measurements of NTs and AAs can be challenging due to background spectral interferences from MALDI matrix or tissue components, analyte in-source fragmentation, poor ionization, or ion suppression effects. However, the selection of the optimal MALDI matrix [20–22] or chemical derivatization of the analytes [18, 23–24] in tissue may be used to minimize molecular interferences and improve sensitivity. The use of high-resolution FT-MS analysis [12, 25] or MS/MS analysis [7, 10–11] may aid in the detection of some compounds when simple sample preparation procedures alone are insufficient. The examples shown in the current study will demonstrate that certain situations require a combination of techniques for unequivocal identification.
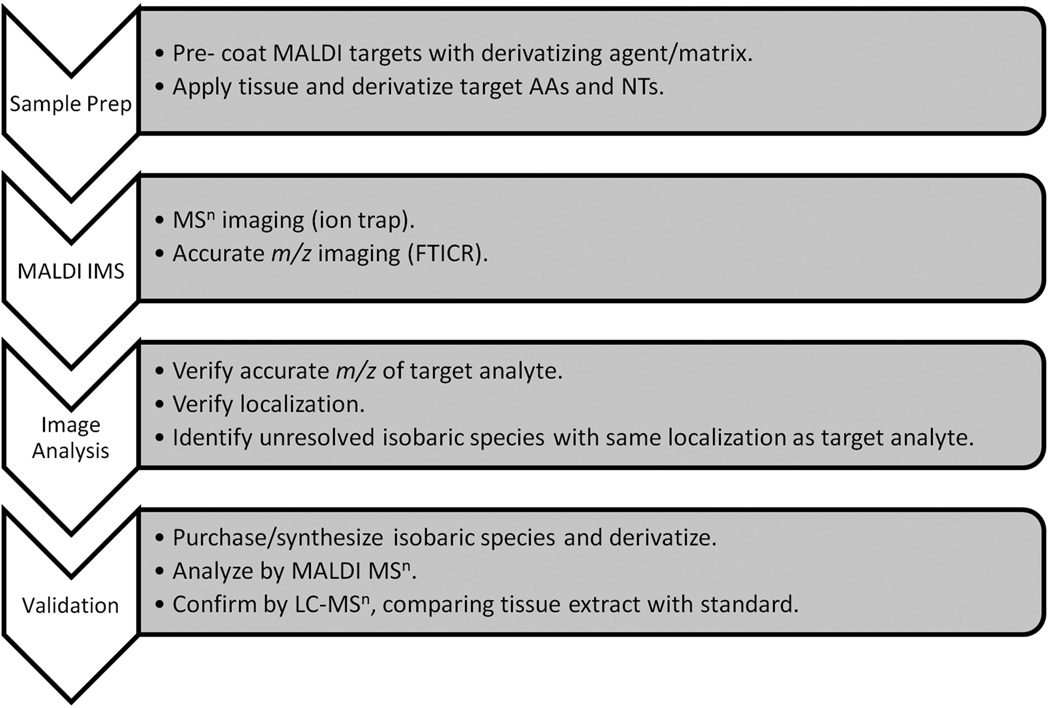
Here we present an analysis workflow that allowed us to achieve the necessary sensitivity and structural specificity for MALDI imaging of endogenous amine metabolites directly in tissues by utilizing a combination of in-tissue chemical derivatization, accurate mass measurement, and MSn technology. Since there are many isobaric compounds that have similar structures to the investigated AAs and NTs, it is critical that the molecular validation is rigorously performed so that accurate information is obtained. The overall workflow described in the present article for analysis and identification is shown in Figure 1. Detection sensitivity was improved through derivatization, and compound identity was confirmed by MS/MS (or MSn) and FTICR MS high resolution/accurate mass analysis. Isobaric metabolites were derivatized and their MSn fragmentation spectra compared to those of the target analytes. HPLC-MS/MS or MSn was performed on extracted tissue homogenates as a final validation.
Figure 1.
Amino acid and neurotransmitter analysis and identification workflow.
EXPERIMENTAL
Materials
Nine AAs and NTs were evaluated (tryptophan, tyrosine, glutamic acid, γ-aminobutyric acid, dopamine, serotonin, melatonin, epinephrine, norepinephrine) and were purchased as neat compounds or the hydrochloride salts from Sigma-Aldrich Chemical Co. (St. Louis, MO). The following reagents were also purchased from Sigma-Aldrich and used for initial derivatization evaluation of the AAs and NTs: 1) 4-hydroxy-3-methoxycinnamaldehyde 2) trans-4-(diethylamino)cinnamaldehyde 3) α-bromocinnamaldehyde 4) trans-cinnamaldehyde 5) β-phenylcinnamaldehyde 6) 4-nitrocinnamaldehyde 7) (N-succinimidyloxycarbonylmethyl)tris(2,4,6-trimethoxyphenyl)phosphonium bromide (TMPP) 8) hydrocortisone 9) dansyl chloride and 10) dabsyl chloride. Compounds that are exactly isobaric with some of the AAs and NTs that could interfere if present in tissues were purchased, including: for γ-aminobutyric acid (GABA): dl-2-aminobutyric acid, 2-aminoisobutyric acid, dl-3-aminoisobutyric acid, N-ethylglycine, N-methyl-L-alanine, for dopamine: 4-hydroxy-3-methoxybenzylamine, for norepinephrine: 5-hydroxydopamine, 6-hydroxydopamine and for epinephrine: dl-normetanephrine. These were also purchased from Sigma-Aldrich as neat compounds or as the hydrochloride or hydrobromide salts. Methanol, acetonitrile (both HPLC grade) and formic acid were obtained from Fisher Scientific (Pittsburgh, PA). All evaluated MALDI matrices including α-cyano-4-hydroxycinnamic acid (CHCA) and trans-ferulic acid (FA), as well as trifluoroacetic acid (TFA), were obtained from Sigma-Aldrich. Fresh-frozen tissue samples (pig adrenal gland and rat brain) were obtained frozen on dry ice from Pel-Freez Biologicals (Rogers, AR), and subsequently stored at −80°C until use.
Pretreatment of MALDI Plates with CHCA for Analysis of Underivatized AAs and NTs
In order to analyze AAs and NTs directly, we placed tissues onto gold-coated stainless-steel MALDI targets (Applied Biosystems, Foster City, CA or Hudson Surface Technology, Fort Lee, NJ) that were pre-coated with α-cyano-4-hydroxycinnamic acid (CHCA) MALDI matrix. We prepared the coating solution at 5 mg/mL CHCA in 60: 40 acetonitrile: water (with 0.1% TFA) and the matrix was coated at a density of ~ 0.36 mg/cm2 using automated spray deposition (TM Sprayer™, HTX Technologies, Carrboro, NC). The CHCA solution was sprayed at a flow rate of 150µL/min. We used nitrogen as the nebulization gas and it was set to 10 psi. The spray nozzle was housed inside a heated block maintained at 110 °C. The spray was deposited at a linear velocity of 370 mm/min with an offset line spacing of 3 mm. Eight passes were performed with each pass consisting of spray application to the entire plate. On alternating passes, the spray pattern was changed by a 90-degree angle. On passes 3, 4, 7 and 8 the spray pattern was offset 1.5-mm to achieve a more homogeneous coating coverage. The nozzle to MALDI target distance was approximately 34 mm.
Method Development with Derivatization Reagents for MALDI IMS Analysis (See Supplementary Information)
Pre-treatment of MALDI Plates with Derivatization Reagent/MALDI Matrix Using Automated Spray Deposition
In order to prepare the optimized precoated targets for derivatization of AAs and NTs, a mixture of 4-hydroxy-3-methoxycinnamaldehyde (CA) and its MALDI matrix acid equivalent trans-4-hydroxy-3-methoxycinnamic acid (trans-ferulic acid; FA) was applied to MALDI targets. A methanol solution, consisting of 23mg/mL CA and 8.5 mg/mL FA was sprayed using the TM Sprayer™ and conditions detailed above, except the nozzle temperature was maintained at 100 °C. This method gave a tacky yellow coating on MALDI targets with a density of approximately 1.7 mg/cm2. If targets were not utilized the same day, they were stored at −4 °C to −20 °C to slow the potential oxidation of CA to FA.
Tissue Preparation for MALDI IMS
We sectioned frozen pig adrenal gland and rat brain cerebellum at −20°C into 3 to 12-µm thick sections on a cryostat (Leica Microsystems Inc., Bannockburn, IL) and thaw-mounted the tissues onto gold-coated stainless-steel MALDI targets. The targets were either precoated with CHCA for “underivatized” analysis or precoated with a mixture of CA and FA for “derivatized” AA and NT analysis. If prepared tissues were not analyzed the same day, they were stored at −4 °C to −20 °C.
Tissue Preparation for HPLC-MS/MS Analysis
We removed the medulla region from pig adrenal gland (at −20°C) by manually dissecting away the outer cortex layer. We also detached the cerebellum from the rat brain but did not attempt to separate gray and white matter regions. Sections (50-µm thick; 20–50 mg total) of pig adrenal gland medulla and rat brain cerebellum were cut at −20°C on the Leica cryostat and each tissue type collected into separate microcentrifuge tubes. The CA derivatization and FA matrix precoating solution was used to extract/derivatize AAs and NTs. This methanolic solution (100 µL) was added to the tissue sections and the samples were vortexed for one minute, then centrifuged at 16,000 × g for 10 minutes at 4 °C. An aliquot (40 µL) of the supernatant was removed, concentrated under a stream of nitrogen, and then diluted to give 100 µL of extract in 90:10 water: methanol. An extraction blank was prepared in an identical fashion, except no tissue was added.
MALDI Imaging Mass Spectrometry by MS/MS
Mass spectra were acquired in positive-ion mode using a MALDI LTQ XL linear ion trap mass spectrometer (Thermo Scientific, San Jose, CA), equipped with a 337-nm nitrogen laser operating at 60 Hz and resulting in ~ 40 × 100 µm laser spot size. Dissociation of protonated molecules was achieved using an isolation window of 1 Da and optimized normalized CID energy of 30–45% for underivatized and derivatized AAs and NTs. Helium was used as the trapping/collision gas. Isolated precursor ions were activated for 30 ms using an RF frequency activation Q value of 0.25 and product ion mass spectra were acquired. For MS/MS imaging experiments, generally an average of 15–45 laser shots per scan was used to produce a mass spectrum at each pixel. Two dimensional ion density images were extracted from the raw data using ImageQuest version 1.0.1 (Thermo Scientific, San Jose, CA).
FTICR Imaging Mass Spectrometry
Data were collected using a MALDI Fourier transform ion cyclotron resonance (FTICR) mass spectrometer (9.4Tesla SolariX, Bruker Daltonics, Billerica, MA) equipped with an Apollo II dual ion source and a Smartbeam II 1 KHz Nd:YAG 355 nm solid-state laser. External calibration was done using a series of phosphorus clusters resulting in mass accuracies within 2 ppm. Images were acquired at a spatial resolution of 50 µm using the small laser focus setting (~40 µm diameter) and averaging 500 laser shots per pixel. Ion images were then generated using FlexImaging 3.0 (Bruker Daltonics, Billerica, MA).
HPLC-MS/MS Analysis
We injected 3 µL of each reconstituted derivatized extract from pig adrenal gland and rat brain cerebellum into a Waters Acquity UPLC system (Waters ; Milford, MA). Analytes were separated at ambient temperature at a flow rate of 0.25 mL/min on a Vydac 218MS 2.1 × 250 mm column packed with 5µm, C18 particles (Grace Davison Discovery Science, Deerfield, IL). Mobile phase solvent A consisted of 95:5 water: methanol with 0.1% formic acid and mobile phase B was 5:95 water: methanol with 0.1% formic acid (v/v/v). A linear gradient elution was used with the following program: 0% B for 2 min, 0 to 30% B over 18 min, 30 to 100% B over 2 min, 100 to 0% B over 2 min and hold for 9 min. The MS analysis was performed using a LTQ XL linear ion trap mass spectrometer (Thermo Scientific, San Jose, CA, USA) in electrospray positive-ionization mode while monitoring product ions of fragmented [M+H]+ precursors in full scan mode. The ionization voltage was 3.2 kV and the capillary temperature was set to 330°C. Nitrogen was used for the sheath and auxiliary gases and set to a flow rate of 42 and 22 (arbitrary units), respectively. The capillary and tube lens voltages were 10 V and 50 V, respectively. Helium was used as the trapping/collision gas and ions were fragmented at normalized collision-induced dissociation (CID) energy of 30%.
We derivatized AA and NT standards, along with isobaric analogs, and assayed each (2500 pmol in mobile phase A) for comparison to the tissue samples. Target analytes monitored were dopamine-CA (m/z 314→), norepinephrine-CA (m/z 330→312→), and epinephrine-CA (m/z 344→) for pig adrenal gland and GABA-CA (m/z 264→) for rat brain cerebellum.
RESULTS
Imaging MS/MS Analysis of Underivatized Amino Acids and Neurotransmitters
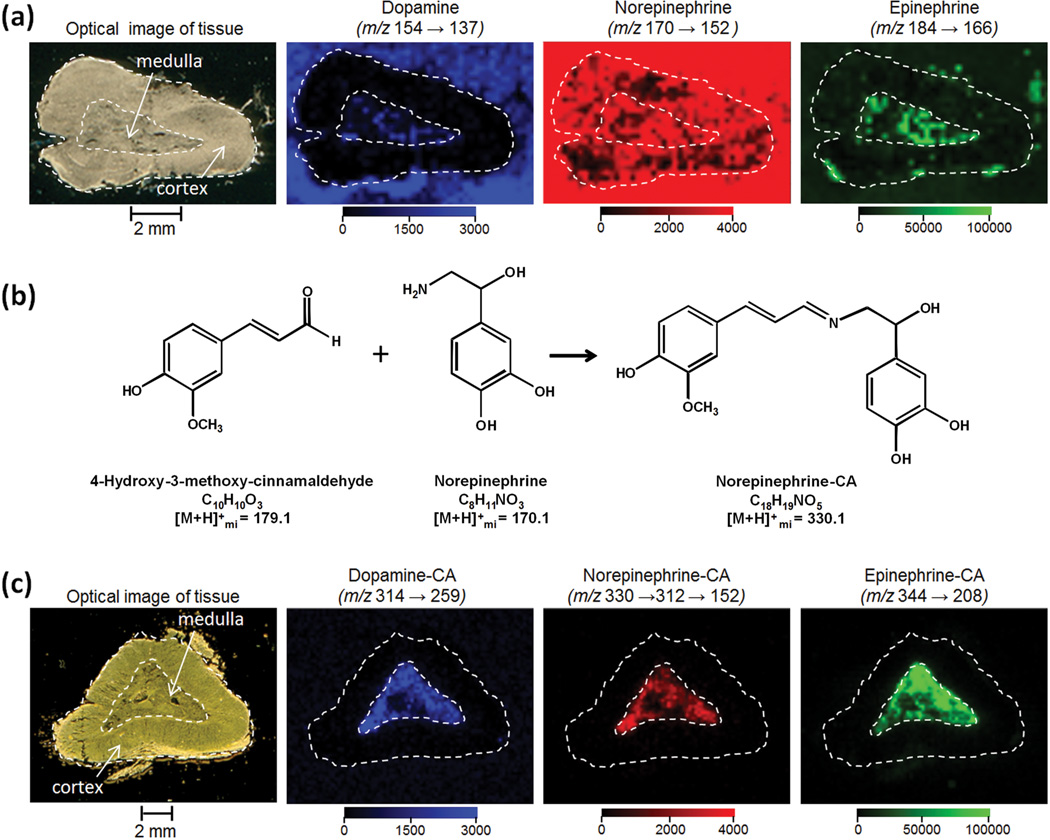
For preliminary tissue analysis, we focused on pig adrenal gland because the localizations of neurotransmitters/hormones, such as dopamine (DA), epinephrine (E), and norepinephrine (NE), have already been studied using immunohistochemical methods [26–27], radioisotopic techniques [28] and desorption electrospray ionization [19]. Epinephrine (a.k.a. adrenaline) and norepinephrine (a.k.a. noradrenaline) each localize to different chromaffin cell types, E cells and NE cells, respectively, that exhibit differential spatial localization within the central medulla region [26–27]. Peripheral areas in the medulla of the pig adrenal gland are occupied with E cells and central regions with NE cells [27]. The MALDI image of pig adrenal gland for underivatized DA, NE and E is shown in Figure 2a. We thaw-mounted a thin tissue section (3 µm) onto a target precoated with CHCA matrix. This matrix pre-coating method has the advantage of reducing analyte delocalization, as no solvent is applied to the tissue section. The optical image in Figure 2 is shown after matrix removal from the target using ethanol and acetone washes. Data were acquired on a linear ion trap instrument equipped with a nitrogen laser. MS/MS ion images were created for the most intense product ions of norepinephrine (m/z 170→152), epinephrine (m/z 184→166), and dopamine (m/z 154→137) from data collected at a spatial resolution of 250 µm. While signals at the MS/MS transitions for all 3 analytes appear in the medulla (Fig. 2a), there are signals of comparable intensity in the cortex region and/or off tissue on the MALDI plate. These signals are likely due to chemical background or MALDI matrix interferences as these images are not consistent with predominant medulla localization obtained by other methods [19, 26–27]. We also investigated the more traditional method of post coating the tissues with CHCA matrix, as well as pre- and post coating using a different matrix (trans-ferulic acid), however these methods did not improve the images. Definitive detection and identification of these 3 analytes as well as six other neurotransmitters and amino acids (tryptophan, tyrosine, glutamic acid, γ-aminobutyric acid, serotonin, and melatonin) in adrenal gland directly by MALDI MS/MS was not possible. Fragmentation of these compounds yields predominately non-specific fragments such as loss of water (m/z 18) or ammonia (m/z 17), with significant in-source fragmentation upon laser irradiation. As a result, overall MS/MS sensitivity is reduced and unequivocal identification of the exact chemical species, especially in order to distinguish isobaric compounds, is difficult.
Figure 2.
MALDI LTQ analysis of amino acids and neurotransmitters in pig adrenal gland. (a) Underivatized analysis: A 3-µm thick tissue section was thaw mounted on a MALDI target precoated with CHCA matrix. Shown are the optical image of tissue following analysis and solvent removal of matrix, and the MS/MS reconstructed ion images (250 µm spatial resolution) consistent with fragmentation of dopamine (m/z 154 → 137), norepinephrine (m/z 170 → 152), and epinephrine (m/z 184 → 166) (b) Derivatization Example: structures of Norepinephrine, CA and Norepinephrine -CA with monoisotopic molecular weights of protonated molecules (c) Derivatized analysis: A 12-µm thick section was thaw mounted on a MALDI target precoated with a mixture of 4-hydroxy-3-methoxycinnamaldehyde derivatization reagent and ferulic acid MALDI matrix. Shown are the optical image of tissue following placement on the precoated target, and reconstructed ion images (200 µm spatial resolution) consistent with fragmentation of dopamine-CA (m/z 314 → 259), norepinephrine-CA (m/z 330 → 312 → 152), epinephrine-CA (m/z 344 → 208). Localization of analytes is to the medulla region.
Similar methods were tried for the assay of AAs and NTs in rat brain. For example, GABA is known to be relatively concentrated in brain tissue (ca. 50 µg/g in striatum and amygdala) [29]. Direct analysis (m/z 104→87), showed no localization to the white or gray matter of the cerebellum and significant background signal was obtained on target (not shown). To improve specificity and sensitivity, chemical derivatization of the amino acids and neurotransmitters was investigated.
Chemical Derivatization of Amino Acids and Neurotransmitters
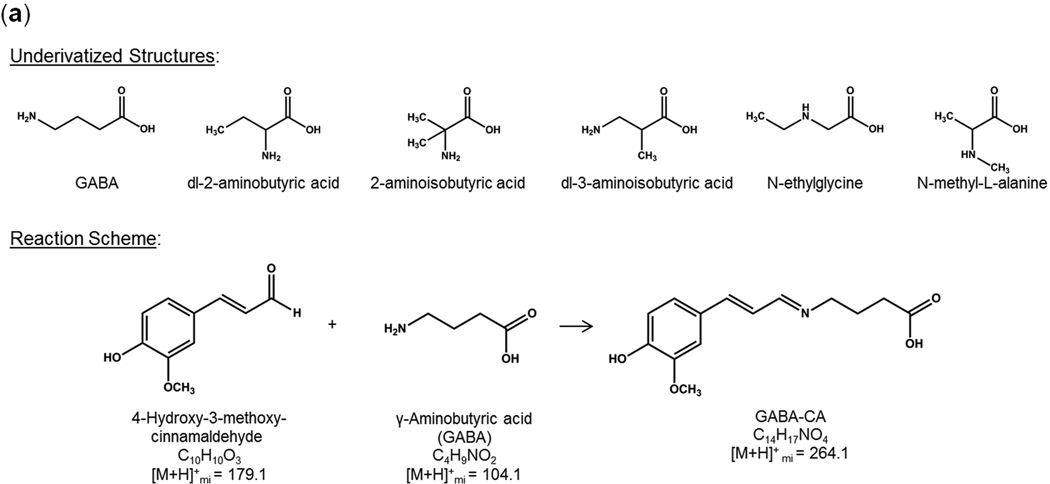
A number of derivatization reagents and conditions were evaluated with the same nine AAs and NTs as described above (results in Supplementary Information). The optimal reagent, 4-hydroxy-3-methoxycinnamaldehyde (CA), was applied to MALDI targets along with the structurally similar MALDI matrix acid, 4-hydroxy-3-methoxycinnamic acid (trans-ferulic acid), prior to tissue placement. This allows the tissues to be directly thaw-mounted, derivatized, and mixed with MALDI matrix, thus simplifying the sample preparation procedure. A representative derivatization reaction between norepinephrine and CA is shown in Figure 2b. The carbonyl of CA forms a stable Schiff base with the amine moiety. This mechanism for derivatization is common to all nine amino acids and neurotransmitters tested. The precursor masses and their major and distinguishing MS/MS fragments for the CA derivatives of the nine AAs and NTs, as well as some isobaric analogs, are given in Table 1.
Table 1.
MS/MS fragmentation of CA derivatized amino acids, neurotransmitters and isobaric analogs
| Compounda | [M + H]+ | MS/MS Fragments, m/zb | MS3 Fragment, m/z |
|---|---|---|---|
| Dopamine | 314 | 137, 178, 190, 259 | |
| 4-hydroxy-3-methoxybenzylamine | 137, 178 | ||
| Norepinephrine | 330 | 161, 188, 312 | 152c |
| 5-hydroxydopamine | 153, 178, 206, 275 | ||
| 6-hydroxydopamine | 151, 153, 178, 275 | ||
| Epinephrine | 344 | 137, 166, 190, 208 | |
| dl-normetanephrine | 175, 202, 326 | ||
| GABA | 264 | 161, 178, 191, 209 | 191d |
| dl-2-aminobutyric acid | 161, 218 | ||
| 2-aminoisobutyric acid | 161,218 | ||
| dl-3-aminoisobutyric acid | 161, 190, 209, 246 | 153d | |
| N-ethylglycine | 151, 161, 181 | ||
| N-methyl-L-alanine | 128, 137, 161 | ||
| Tryptophan | 365 | 178, 236, 272, 348 | |
| Tyrosine | 342 | 161, 165, 342182, 296 | |
| Glutamic acid | 308 | 148, 161, 262, 290 | |
| Serotonin | 337 | 160, 178, 228, 282, 294, 308, 320 | |
| Melatonin | 393 | 233, 243, 269, 334 |
Derivatized (with 4-hydroxy-3-methoxycinnamaldehyde) amino acid or neurotransmitter is underlined with its isobaric analog listed below
Target amino acid or neurotransmitter fragment mass highlighted in bold if used for ion image reconstruction
Transition used for norepinephrine-CA (m/z 330→312→152) ion image reconstruction
Fragment mass used for confirmation of identity (m/z 264→209→)
Analysis of Derivatized Amino Acids and Neurotransmitters in Pig Adrenal Gland and Rat Brain
Preliminary analysis of pig adrenal gland for the nine target compounds after CA derivatization indicated ions that are consistent with the presence of CA derivatives of glutamic acid, GABA, dopamine, norepinephrine, and epinephrine. The MS/MS transitions specified in Table 1 were used to construct ion images. Product ion spectra were consistent with those from derivatized standards (data not shown). Following a more targeted analysis, the ion images for transitions consistent with dopamine-CA (m/z 314→259), norepinephrine-CA (m/z 330→312→152), and epinephrine-CA (m/z 344→208) are shown in Figure 2c. MS3 was used for analysis of norepinephrine due to the non-specific loss of water upon MS/MS analysis. The derivatization protocol resulted in images where the analytes showed predominant signal localization to the medulla region, which is consistent with the biological function of each [26–28]. In addition, norepinephrine and epinephrine localize to different regions in the medulla consistent with previously published data [27].
To further validate the MS/MS data, we conducted high resolution/accurate mass measurements. FTICR MS analysis verified the presence of signals consistent with CA derivatives of dopamine, norepinephrine, and epinephrine in pig adrenal gland and GABA in rat brain cerebellum (≤ 2 ppm accuracy) with similar spatial localizations in tissues as the MALDI ion-trap images. FTICR MS revealed 14, 7, 0 and 5 nominally isobaric ions within ± 0.5 Da of dopamine-CA (DA-CA), norepinephrine-CA (NE-CA) and epinephrine-CA (E-CA) in pig adrenal gland and GABA-CA in rat brain cerebellum, respectively. These nominally isobaric species can interfere with the LTQ MS/MS analysis if the fragments produced are similar to those of the derivatized target analytes. However, the images produced by FTICR MS showed that no other significant (> 2% intensity) nominally isobaric spectral peaks had the same spatial localizations as the target analytes, therefore only exactly isobaric compounds could be responsible for the images.
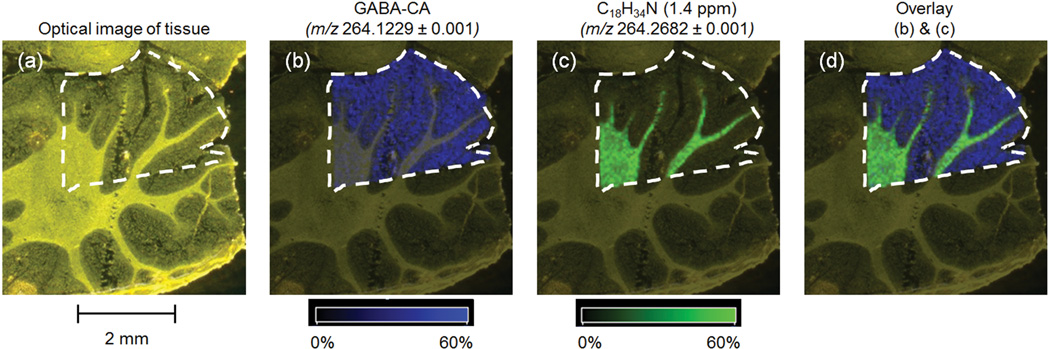
The analysis for GABA-CA in rat cerebellum by FTICR MS is shown at 50 µm spatial resolution in Figure 3. A 12-µm thick section was thaw mounted on a MALDI target precoated with a mixture of CA derivatization reagent and FA matrix. Figure 3 shows the (a) optical image of tissue following placement on the precoated target and (b) reconstructed ion image for m/z 264.1229 (within 0.4 ppm of the accurate mass of the [M+H]+ for GABA-CA at 264.1230). In Figure 3c a reconstructed ion image for a species at m/z 264.2682 (predicted to be C18H34N based on mass accuracy; 1.4 ppm) shows localization to the white matter. Although the molecule does not appear to be derivatized (molecular formula lacks oxygen), its decisive localization to the white matter indicates that delocalization of small molecules did not occur during sample preparation. Figure 3d overlays the ion images from (3b) and (3c) to provide spatial reference.
Figure 3.
MALDI FTICR IMS data for CA-derivatized GABA in rat brain cerebellum at 50 µm spatial resolution. A 12-µm thick section was thaw mounted on a MALDI target precoated with a mixture of 4-hydroxy-3-methoxycinnamaldehyde derivatization reagent and ferulic acid MALDI matrix. Shown are the (a) optical image of tissue following placement on the precoated target, (b) reconstructed ion image for m/z 264.1229 consistent within 0.4 ppm of the accurate mass of the [M+H]+ for GABA-CA (264.1230), (c) reconstructed ion image for a species at m/z 264.2682 (likely C18H34N based on mass accuracy;1.4 ppm) that shows localization to the white matter, and (d) overlay of ion images from (b) and (c).
Exact Isobaric Species and MSn Comparison
The FTICR MS high resolution analysis ruled out the possibility of nominal isobars contributing to the ion images. However, compounds with the same exact mass (e.g., structural isomers) cannot be distinguished by MS alone. Therefore, a comparison of the fragmentation patterns of derivatized target analytes to those of derivatized isomers, as well as underivatized compounds with the same exact mass, was necessary. The Metlin (http://masspec.scripps.edu/) and the Human Metabolome Database [30] were searched for compounds containing the same exact mass of the underivatized as well as the CA-derivatized target analytes. Underivatized isomers were further considered if they would likely be derivatized by CA (e.g., primary and secondary amines) and could conceivably be present in these tissues. Compounds with the same exact mass of a CA-derivatized target analyte were considered if biologically appropriate and suspected to fragment similarly. The precursor mass and major MS/MS fragments for the CA derivatives of the purchased isomeric analogs are given in Table 1. Target AA and NTs could be distinguished from these isomers using MSn and gave spectra consistent with those found from tissues, thus improving the confidence in analyte identity. These spectral comparisons on the MALDI LTQ are consistent with those obtained during the HPLC analysis below.
HPLC-MS/MS Analysis of Tissue Extracts
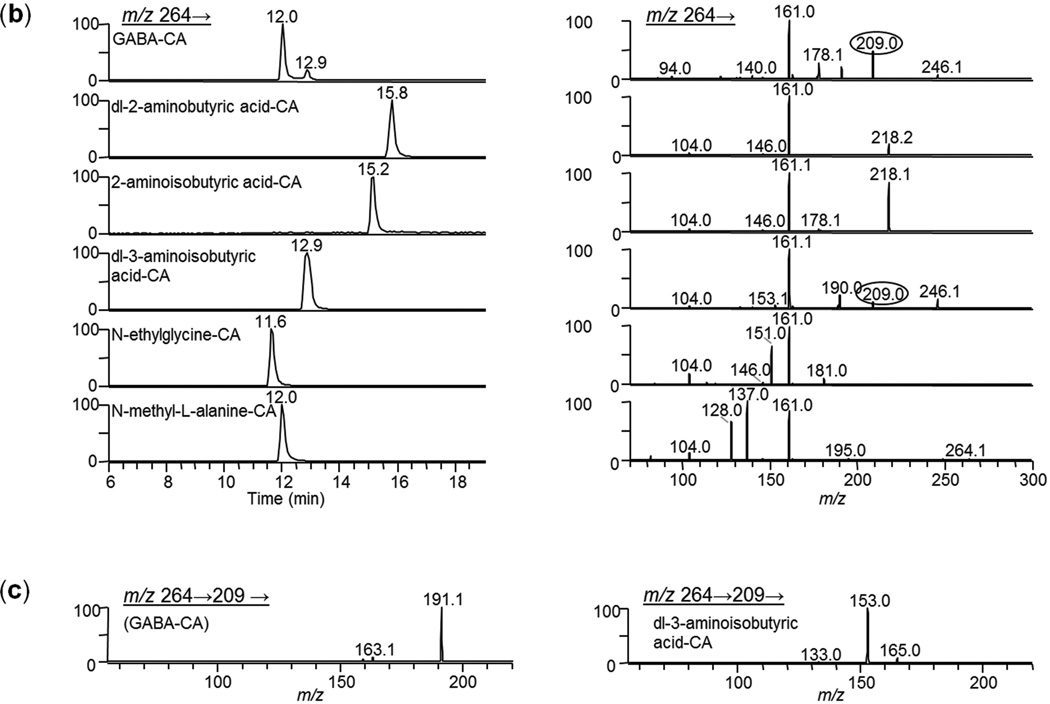
Even though accurate mass measurements and MS/MS spectra from MALDI analysis of tissues provided good indication that analytes could be distinguished from isobars and were properly identified, not all possible small molecule interfering compounds are available, or are even known. To gain additional confidence in the identifications, we investigated alternative orthogonal separation techniques. Matching the HPLC retention time and MS/MS or MSn spectrum from a chromatographic peak in a tissue extract to that of a derivatized standard gives compelling evidence of the proper identification. Molecules from the medulla region from pig adrenal gland and cerebellum from rat brain were each separately extracted and derivatized using the target pre-coating solution (CA/FA in methanol). Reconstituted tissue extracts were analyzed using HPLC- MSn. Retention times and mass spectra from the tissue extracts were compared to those of derivatized target standards and structurally similar isobaric compounds. An example for identification of GABA-CA in rat brain cerebellum is shown in Figure 4. The chemical structure for GABA along with the structures of five isomers and the CA-derivatization reaction scheme are shown in Figure 4a. The HPLC chromatograms for the fragmentation products of [M+H]+ = 264 are shown on the left-side panel of Figure 4b and the product ion spectra for the corresponding chromatographic peaks are on the right-side panel. We generally construct MALDI ion images for GABA-CA using m/z 264→209. These results show that some interference may occur if dl-3-aminoisobutyric acid-CA were also present in the tissue as it also produces m/z 209 upon fragmentation. A close examination of the spectra shows that no major fragment ion peak is unique to GABA compared to this set of isomers. However, the MSn capability of the ion trap is advantageous in that added confidence can be gained upon comparing consecutive spectra from each level of MSn for a tissue extract peak to that of a standard. For example, MS3 spectra (m/z 264→209→) for GABA-CA and dl-3-aminoisobutyric acid-CA are shown in Figure 4c. GABA-CA fragmentation produces predominately m/z 191 while dl-3-aminoisobutyric acid-CA produces m/z 153, therefore these two isomers can be easily distinguished via MS3.
Figure 4.
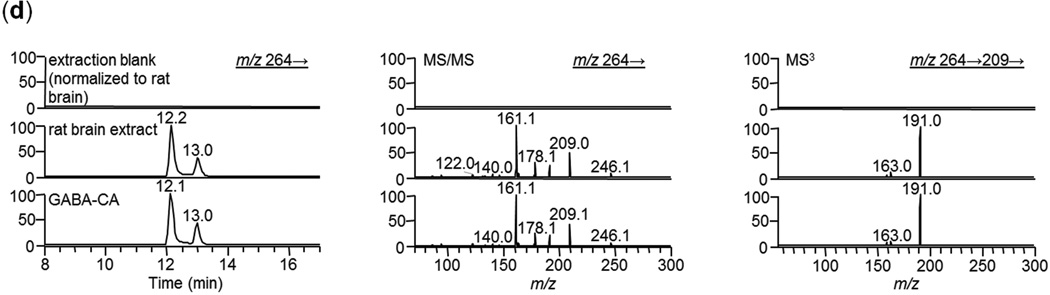
LC-MS/MS analysis of CA derivatization products of GABA, a representative subset of isobaric compounds and rat brain cerebellum extract. (a) Compound structures and reaction scheme, (b) HPLC extracted-ion chromatograms (of fragmentation product of m/z 264) for derivatized standards and MS/MS spectra (m/z 264→) of the chromatographic CA-derivative peaks. Note: peaks at 12 and 13min have ~ identical spectra (c) MS3 spectra of the chromatographed CA-derivatives of GABA and dl-3-aminoisobutyric acid (m/z 264→ 209→). (d) HPLC extracted-ion chromatograms (m/z 264) for derivatized extraction blank, derivatized extract of rat brain cerebellum and GABA-CA standard (left panel), MS/MS spectra (m/z 264→) of the left-side chromatographic peaks (middle panel), and (MS3 spectra (m/z 264→ 209→)of the chromatographic peaks (right panel).
The results for the rat brain cerebellum extract are shown in Figure 4d. Again fragmentation products of m/z 264 were monitored. No significant chromatographic peak was obtained for the extraction blank (top row; intensity is normalized to the rat brain cerebellum extract). The brain extract (middle row) showed chromatographic peaks at 12.2 and 13.0 minutes. These two peaks have nearly identical MS/MS spectra. The retention times and MS/MS spectra from rat cerebellum are virtually identical to that of standard GABA-CA (third row) which also contains two peaks (likely due to cis- and trans- isomer formation). MS3 spectra (m/z 264→209→) for the rat brain extract and GABA-CA (third column) are also very similar. Because no m/z 153 is present in the MS3 spectra, we can confirm that there is no interference from dl-3-aminoisobutyric acid-CA. The HPLC results along with the MALDI MS/MS and FTICR MS accurate mass data confirm the presence of GABA in rat brain cerebellum. In a similar fashion dopamine, norepinephrine, and epinephrine in pig adrenal gland were confirmed by HPLC analysis. The data for target standards, isobars, and tissue extracts are shown in Supplementary Figure 1.
DISCUSSION
MALDI imaging of underivatized endogenous amine metabolites such as amino acids and neurotransmitters in tissues suffers from poor sensitivity and specificity, likely due to problems associated with in-source fragmentation, analyte delocalization, and interferences from other small molecular weight compounds. However, derivatization of these compounds using 4-hydroxy-3-methoxycinnamaldehyde allowed us to obtain spatially localized images of the analytes. For proof of concept and validation purposes we chose to focus on dopamine, norepinephrine, and epinephrine in pig adrenal gland and GABA in rat brain, although, there was preliminary indication of (m/z signals consistent with) glutamic acid and GABA in pig adrenal gland and glutamic acid and dopamine in other brain areas examined. The focus of this Perspective is proper identification of metabolite species, thus qualitative in nature. Once interference-free identifications are made, compound spatial distributions which are more quantitative in nature will require further validation.
Pre-coating the target with derivatization agent along with MALDI matrix allows tissues to be directly thaw mounted and analyzed thus simplifying sample preparation while minimizing delocalization of analytes. Indeed, the process of thaw-mounting the moisture- containing frozen tissue onto the surface of the precoated target was sufficient for good analyte derivatization and subsequent mixing with the MALDI matrix. The MS/MS spectra of derivatized compounds yielded more structurally specific fragmentation product ions than did the underivatized analogs (which were generally losses of water or ammonia). Chemical derivatization along with MSn and accurate mass measurements were used for added confidence in analyte identification. Both the tandem mass spectrometry and accurate mass measurements were necessary. For example, the ion-trap instrument (LTQ) used in this study for MS/MS imaging, utilized an isolation width of 1 Da (±0.5 Da or ± approximately 1500 ppm at m/z 335). The FTICR MS allowed peaks within 5ppm to be differentiated; therefore by using the FTICR, the number of potential candidates giving rise to a measured signal is greatly reduced. This is demonstrated by a search of the Metlin database (http://masspec.scripps.edu/) for the exact mass ([M+H]+) of norepinephrine-CA (330.1336). The number of candidates is reduced from 93 at the measurement conditions of the LTQ (±0.5 Da) to 5 candidates at 5 ppm (FTICR). These 5 candidates (also identified at 0 ppm) are exactly isobaric and have the same chemical formula therefore cannot be distinguished by mass-to-charge alone. Therefore MSn must be used and compound fragmentation differences used to discern each species. Similar results for the other metabolites (either derivatized or underivatized) are shown in Supplementary Table 2.
To gain additional confidence in the FTICR and ion trap identifications, we investigated alternative orthogonal separation techniques including ion mobility and HPLC. MALDI ionization with ion-mobility separation can be used to differentiate isobaric species while maintaining the spatial localization of analytes within the tissue.[31–32] We attempted drift-time separation of GABA-CA and 5 of its isobaric analogs using a Waters Synapt G2 ion-mobility mass spectrometer (Waters, Milford, MA) with nitrogen as the buffer gas; however, baseline separation of the analytes could not be achieved. Recent literature reports have demonstrated that optimization of the buffer gas can enhance ion mobility separations[33–34]; therefore, it is possible that ion mobility separations could be used to obtain the requisite chemical specificity to make an unambiguous identification of certain metabolites. However in this case, we were not successful and we instead used chromatographic separations and MS/MS analysis of tissue extracts to obtain sufficient separation of isobaric species and mass spectral confirmation of analyte identity.
As chemical derivatization becomes increasingly used in Imaging Mass Spectrometry to improve the analysis of endogenous metabolites in tissues, several properties of the derivative must be considered. Derivatives that are pre-charged can greatly enhance signal and therefore are desirable if most of the following characteristics are met. These reagents should be inexpensive since a significant amount of reagent will be needed. Derivatives should be stable and easily formed without interfering side reactions. Ideally, the derivatization reaction should proceed quickly at room temperature and without the addition of specific buffers or other reagents. More importantly, in order to distinguish a target analyte from other exactly isobaric species present in the tissue, the derivative must fragment to produce ions related to the target analyte and not primarily to the derivatization reagent. In addition, the m/z chosen for image reconstruction must be specific to the target analyte as compared to other exactly isobaric species. For example, upon HPLC-MS/MS confirmation, all non-target chromatographic peaks must be evaluated to verify no ion signal is present in the spectrum at the same m/z used to create the ion image for the target species. In situations where these criteria are not met, investigators must acknowledge the potential presence of multiple species when reporting IMS results. See Supplementary Information-Results (HPLC-MS/MS Confirmation for Dopamine) and Supplementary Figure 2 for a specific example.
As the study of endogenous metabolites by MALDI IMS increases, inclusion of MALDI fragmentation data, retention time information, and validated spatial localization into the public metabolite databases such as Metlin (http://masspec.scripps.edu/), the Human Metabolome Database [30] and LIPID MAPS (http://www.lipidmaps.org/) would provide a valuable resource that would better enable the application of the technology to the study of important biological problems.
The experimental strategy described in this study combines a number of advanced instrumental methods to identify and confirm the accurate spatial localization of target AAs and NTs. Although many literature reports describe these approaches for the analysis of low molecular weight compounds from tissues, often only a single approach is used for identification. Our experience has been that for many analytes, the presence of numerous nominal or exact isobars are possible and only through a combination of tandem MS, exact mass measurement and confirmation by an orthogonal separation and analysis technique such as ion mobility or HPLC-MS/MS can unambiguous assignment of the image data to the target analyte be made. We believe that the approach described is a model for the development of IMS assays of metabolites and will minimize the reporting of non-specific analytical results.
Supplementary Material
Acknowledgments
This project was supported by grants from the National Center for Research Resources (GM103391-04) and by NIH/NIGMS grants 5R01 GM058008-15.
Footnotes
Supporting Information
Supporting information may be found in the online version of this article.
References
- 1.Zhao XE, Suo YR. Simultaneous determination of monoamine and amino acid neurotransmitters in rat endbrain tissues by pre-column derivatization with high-performance liquid chromatographic fluorescence detection and mass spectrometric identification. Talanta. 2008;76:690. doi: 10.1016/j.talanta.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 2.Yoshitake T, Kehr J, Todoroki K, Nohta H, Yamaguchi M. Derivatization chemistries for determination of serotonin, norepinephrine and dopamine in brain microdialysis samples by liquid chromatography with fluorescence detection. Biomedical Chromatography. 2006;20:267. doi: 10.1002/bmc.560. [DOI] [PubMed] [Google Scholar]
- 3.Burke WJ, Li SW, Schmitt CA, Xia P, Chung HD, Gillespie KN. Accumulation of 3,4-dihydroxyphenylglycolaldehyde, the neurotoxic monoamine oxidase A metabolite of norepinephrine, in locus ceruleus cell bodies in Alzheimer's disease: mechanism of neuron death. Brain Res. 1999;816:633. doi: 10.1016/s0006-8993(98)01211-6. [DOI] [PubMed] [Google Scholar]
- 4.Norris JL, Caprioli RM. Analysis of tissue specimens by matrix-assisted laser desorption/ionization imaging mass spectrometry in biological and clinical research. Chem Rev. 2013;113:2309. doi: 10.1021/cr3004295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hankin JA, Barkley RM, Murphy RC. Sublimation as a method of matrix application for mass spectrometric imaging. J Am Soc Mass Spectrom. 2007;18:1646. doi: 10.1016/j.jasms.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnum KE, Cornett DS, Puolitaival SM, Milne SB, Myers DS, Tranguch S, Brown HA, Dey SK, Caprioli RM. Spatial and temporal alterations of phospholipids determined by mass spectrometry during mouse embryo implantation. J Lipid Res. 2009;50:2290. doi: 10.1194/jlr.M900100-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrett TJ, Yost RA. Analysis of Intact Tissue by Intermediate-Pressure MALDI on a Linear Ion Trap Mass Spectrometer. Analytical Chemistry. 2006;78:2465. doi: 10.1021/ac0522761. [DOI] [PubMed] [Google Scholar]
- 8.Anderson DM, Mills D, Spraggins J, Lambert WS, Calkins DJ, Schey KL. High-resolution matrix-assisted laser desorption ionization-imaging mass spectrometry of lipids in rodent optic nerve tissue. Mol Vis. 2013;19:581. [PMC free article] [PubMed] [Google Scholar]
- 9.Troendle FJ, Reddick CD, Yost RA. Detection of pharmaceutical compounds in tissue by matrix-assisted laser desorption/ionization and laser desorption/chemical ionization tandem mass spectrometry with a quadrupole ion trap. Journal of the American Society for Mass Spectrometry. 1999;10:1315. [Google Scholar]
- 10.Reyzer ML, Hsieh Y, Ng K, Korfmacher WA, Caprioli RM. Direct analysis of drug candidates in tissue by matrix-assisted laser desorption/ionization mass spectrometry. J Mass Spectrom. 2003;38:1081. doi: 10.1002/jms.525. [DOI] [PubMed] [Google Scholar]
- 11.Khatib-Shahidi S, Andersson M, Herman JL, Gillespie TA, Caprioli RM. Direct Molecular Analysis of Whole-Body Animal Tissue Sections by Imaging MALDI Mass Spectrometry. Analytical Chemistry. 2006;78:6448. doi: 10.1021/ac060788p. [DOI] [PubMed] [Google Scholar]
- 12.Ye H, Wang J, Greer T, Strupat K, Li L. Visualizing Neurotransmitters and Metabolites in the Central Nervous System by High Resolution and High Accuracy Mass Spectrometric Imaging. ACS Chem Neurosci. 2013;4:1049. doi: 10.1021/cn400065k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burrell M, Earnshaw C, Clench M. Imaging Matrix Assisted Laser Desorption Ionization Mass Spectrometry: a technique to map plant metabolites within tissues at high spatial resolution. J Exp Bot. 2007;58:757. doi: 10.1093/jxb/erl139. [DOI] [PubMed] [Google Scholar]
- 14.Goto-Inoue N, Setou M, Zaima N. Visualization of spatial distribution of gamma-aminobutyric acid in eggplant (Solanum melongena) by matrix-assisted laser desorption/ionization imaging mass spectrometry. Anal Sci. 2010;26:821. doi: 10.2116/analsci.26.821. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Shrestha B, Vertes A. Atmospheric pressure infrared MALDI imaging mass spectrometry for plant metabolomics. Anal Chem. 2008;80:407. doi: 10.1021/ac701703f. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson L, Bradshaw R, Wolstenholme R, Clench M, Francese S. Two-step matrix application for the enhancement and imaging of latent fingermarks. Anal Chem. 2011;83:5585. doi: 10.1021/ac200619f. [DOI] [PubMed] [Google Scholar]
- 17.Sugiura Y, Zaima N, Setou M, Ito S, Yao I. Visualization of acetylcholine distribution in central nervous system tissue sections by tandem imaging mass spectrometry. Anal Bioanal Chem. 2012;403:1851. doi: 10.1007/s00216-012-5988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toue S, Sugiura Y, Kubo A, Ohmura M, Karakawa S, Mizukoshi T, Yoneda J, Miyano H, Noguchi Y, Kobayashi T, Kabe Y, Suematsu M. Microscopic imaging mass spectrometry assisted by on-tissue chemical derivatization for visualizing multiple amino acids in human colon cancer xenografts. PROTEOMICS. 2014;14:810. doi: 10.1002/pmic.201300041. [DOI] [PubMed] [Google Scholar]
- 19.Wu C, Ifa DR, Manicke NE, Cooks RG. Molecular imaging of adrenal gland by desorption electrospray ionization mass spectrometry. Analyst. 2010;135:28. doi: 10.1039/b919816d. [DOI] [PubMed] [Google Scholar]
- 20.Shanta SR, Kim TY, Hong JH, Lee JH, Shin CY, Kim KH, Kim YH, Kim SK, Kim KP. A new combination MALDI matrix for small molecule analysis: application to imaging mass spectrometry for drugs and metabolites. Analyst. 2012;137:5757. doi: 10.1039/c2an35782h. [DOI] [PubMed] [Google Scholar]
- 21.Wang H-YJ, Jackson SN, McEuen J, Woods AS. Localization and Analyses of Small Drug Molecules in Rat Brain Tissue Sections. Analytical Chemistry. 2005;77:6682. doi: 10.1021/ac050868d. [DOI] [PubMed] [Google Scholar]
- 22.Shariatgorji M, Nilsson A, Goodwin RJ, Svenningsson P, Schintu N, Banka Z, Kladni L, Hasko T, Szabo A, Andren PE. Deuterated matrix-assisted laser desorption ionization matrix uncovers masked mass spectrometry imaging signals of small molecules. Anal Chem. 2012;84:7152. doi: 10.1021/ac301498m. [DOI] [PubMed] [Google Scholar]
- 23.Manier ML, Reyzer ML, Goh A, Dartois V, Via LE, Barry CE, 3rd, Caprioli RM. Reagent precoated targets for rapid in-tissue derivatization of the anti-tuberculosis drug isoniazid followed by MALDI imaging mass spectrometry. J Am Soc Mass Spectrom. 2011;22:1409. doi: 10.1007/s13361-011-0150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chacon A, Zagol-Ikapitte I, Amarnath V, Reyzer ML, Oates JA, Caprioli RM, Boutaud O. On-tissue chemical derivatization of 3-methoxysalicylamine for MALDI-imaging mass spectrometry. J Mass Spectrom. 2011;46:840. doi: 10.1002/jms.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornett DS, Frappier SL, Caprioli RM. MALDI-FTICR imaging mass spectrometry of drugs and metabolites in tissue. Anal Chem. 2008;80:5648. doi: 10.1021/ac800617s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki T, Kachi T. Differences between adrenaline and noradrenaline cells in cellular association with supporting cells in the adrenal medulla of the pig: An immunohistochemical study. Neuroscience Letters. 1994;176:217. doi: 10.1016/0304-3940(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T, Kachi T. Similarities and differences in supporting and chromaffin cells in the mammalian adrenal medullae: An immunohistochemical study. The Anatomical Record. 1996;244:358. doi: 10.1002/(SICI)1097-0185(199603)244:3<358::AID-AR7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi S, Coupland RE. Morphological aspects of chromaffin tissue: the differential fixation of adrenaline and noradrenaline. J Anat. 1993;183(Pt 2):223. [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez RR, Fernandez RF, Vidal JLM, Frenich AG, Perez MLG. Development and validation of an ultra-high performance liquid chromatography-tandem mass-spectrometry (UHPLC-MS/MS) method for the simultaneous determination of neurotransmitters in rat brain samples. Journal of Neuroscience Methods. 2011;198:187. doi: 10.1016/j.jneumeth.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, Bouatra S, Sinelnikov I, Arndt D, Xia J, Liu P, Yallou F, Bjorndahl T, Perez-Pineiro R, Eisner R, Allen F, Neveu V, Greiner R, Scalbert A. HMDB 3.0--The Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41:D801. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanu AB, Dwivedi P, Tam M, Matz L, Hill HH. Ion mobility–mass spectrometry. J Mass Spectrom. 2008;43:1. doi: 10.1002/jms.1383. [DOI] [PubMed] [Google Scholar]
- 32.Lapthorn C, Pullen F, Chowdhry BZ. Ion mobility spectrometry-mass spectrometry (IMS-MS) of small molecules: Separating and assigning structures to ions. Mass Spectrometry Reviews. 2013;32:43. doi: 10.1002/mas.21349. [DOI] [PubMed] [Google Scholar]
- 33.Lalli PM, Corilo YE, Fasciotti M, Riccio MF, de Sa GF, Daroda RJ, Souza GHMF, McCullagh M, Bartberger MD, Eberlin MN, Campuzano IDG. Baseline resolution of isomers by traveling wave ion mobility mass spectrometry: investigating the effects of polarizable drift gases and ionic charge distribution. J Mass Spectrom. 2013;48:989. doi: 10.1002/jms.3245. [DOI] [PubMed] [Google Scholar]
- 34.Fasciotti M, Sanvido GB, Santos VG, Lalli PM, McCullagh M, de Sá GF, Daroda RJ, Peter MG, Eberlin MN. Separation of isomeric disaccharides by traveling wave ion mobility mass spectrometry using CO2 as drift gas. J Mass Spectrom. 2012;47:1643. doi: 10.1002/jms.3089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.