Abstract
Rats selectively bred for high- and low-capacity for running on a treadmill (HCR; LCR) also differ in wheel-running behavior, but whether wheel-running can be explained by intrinsic or adaptive brain mechanisms is not as yet understood. It is established that motivation of locomotory behavior is driven by dopaminergic transmission in mesolimbic and mesostriatal systems. However, whether voluntary wheel running is associated with enkephalinergic activity in the ventral striatum is not known.
Materials & Methods
40 male (20 HCR and 20 LCR) and 40 female (20 HCR and 20 LCR) rats were randomly assigned to 3 weeks of activity wheel exposure or sedentary conditions without wheel access. After 3 weeks of activity-wheel running, rats were decapitated and brains were extracted. Coronal sections were analyzed utilizing in situ hybridization histochemistry for enkephalin (ENK) mRNA in the ventral striatum.
Results
HCR rats expressed less ENK than LCR rats in the nucleus accumbens among females (p <.01) and in the olfactory tubercle among both females (p <.05) and males (p<.05). There was no effect of wheel running on ENK mRNA expression.
Conclusion
Line differences in ENK expression in the olfactory tubercle, and possibly the nucleus accumbens, partly explain divergent wheel-running behavior. The lower striatal ENK in the HCR line is consistent with enhanced dopaminergic tone, which may explain the increased motivation for wheel running observed in the HCR line.
Keywords: Enkephalin, Nucleus Accumbens, Olfactory tubercle, Activity wheel, in situ hybridization
1. Introduction
Family and twin studies indicate that variation in human physical activity levels is heritable (Eriksson et al., 2006; Simonen et al., 2002; Stubbe et al., 2006), but the genetic determinants of physical activity are poorly understood (Dishman, 2008). Voluntary wheel running by rodents also has a genetic component (Knab and Lightfoot, 2010; Lightfoot et al., 2004; Lightfoot et al., 2008; Roberts et al., 2013; Swallow et al., 1998; Waters et al., 2013). Rats selectively bred at the University of Michigan for high-capacity running (HCR) or low-capacity running (LCR) (Koch and Britton, 2001) demonstrate substantial divergence in treadmill performance, including running speed and distance (Høydal et al., 2007; Koch and Britton, 2008) and also daily wheel-running (Groves-Chapman et al., 2011; Waters et al., 2008), an activity that appears to represent a preferred and evolutionarily salient behavior in rodents (Belke and Wagner 2005; Brené et al., 2007; Iversen, 1993; Lett, 2000; Sherwin, 1998).
The HCR line is associated with several traits subordinate to exercise performance, including a greater capacity to deliver and utilize O2 in skeletal muscle (Howlett et al., 2009; Gonzalez et al., 2006), but these differences do not fully account for the large differences in running behavior between lines. Instead, these variations may reflect traits that mediate the relationship between a central drive to engage in motor behavior and observed locomotion (Jónás et al., 2010; Novak et al., 2010). The HCR and LCR rats provide a model from which the brain pathways underlying heritable running behavior and gene-environment interaction can be investigated (Koch and Britton, 2008).
Although the neurobiology of motivated wheel running is as yet unknown, there is substantial evidence for a mechanism involving the mesolimbic-motor interface (Burgess, 2010; Knab et al., 2009; Scheurink et al., 2010).The cumulative evidence suggests this junction exists at the basal ganglia (Garcia-Rill, 1986; Mogenson, 1987; Parent and Hazrati, 1995; Smith et al., 1988; Takakusaki et al., 2004), particularly in striatal GABA/opioidergic neurons located in distinct areas of the striatum that receive dopaminergic projections from the ventral tegmental area (Cardinal et al., 2002; Depue and Collins, 1999; Horvitz, 2002).
Striatal GABAergic medium spiny neurons express D2-like dopamine receptors and enkephalin or D1-like receptors and dynorphin in the direct (striatonigral) pathway and indirect (striatopallidal) pathway, respectively (Gerfen and Young, 1988; Surmeier et al., 1996). Midbrain dopaminergic transmission sensitizes the striatum to rewarding stimuli, mediates the incentive salience associated with these stimuli (Berridge and Robinson, 1998; Ikemoto, 2007; Morales-Mulia, 2013), increases in response to acute (Hattori et al., 1994) and chronic treadmill training (Gilliam et al., 1984), and is up-regulated in the striatum of mice selectively bred for high levels of activity-wheel running (Mathes et al., 2010). The motivational drive to run is plausibly mediated by striatal enkephalinergic neurotransmission in the nucleus accumbens septi (NAS) and olfactory tubercle (OT) or through the efferent targets of these neurons in the ventral pallidum (LeMoine et al., 1990; Young et al., 1986). The striatal enkephalin-dopamine environment may be important for understanding voluntary locomotory behavior (Dishman & Holmes, 2012; Kalivas et al., 1983). Enkephalin (ENK) is a peptide neuromodulator of GABAergic projections to the ventral pallidum (the limbic structure contiguous with motor pathways) that appears to suppress motor activity and motivated behavior (Durieux et al., 2009; Ena et al., 2011; Kravitz et al., 2013). Wheel-running behavior in rats may be directly attributable to differences in ENK expression (Werme et al., 2002), and divergent running performance observed between HCR and LCR rats may be explained by differences in striatal ENK expression. We hypothesized that HCR rats would have less ventral striatal ENK expression than LCR rats and that three weeks of access to wheel-running would down-regulate ENK expression in the ventral striatum compared to a sedentary housing condition.
2. Results
2.1 Running Distance and Body Weight
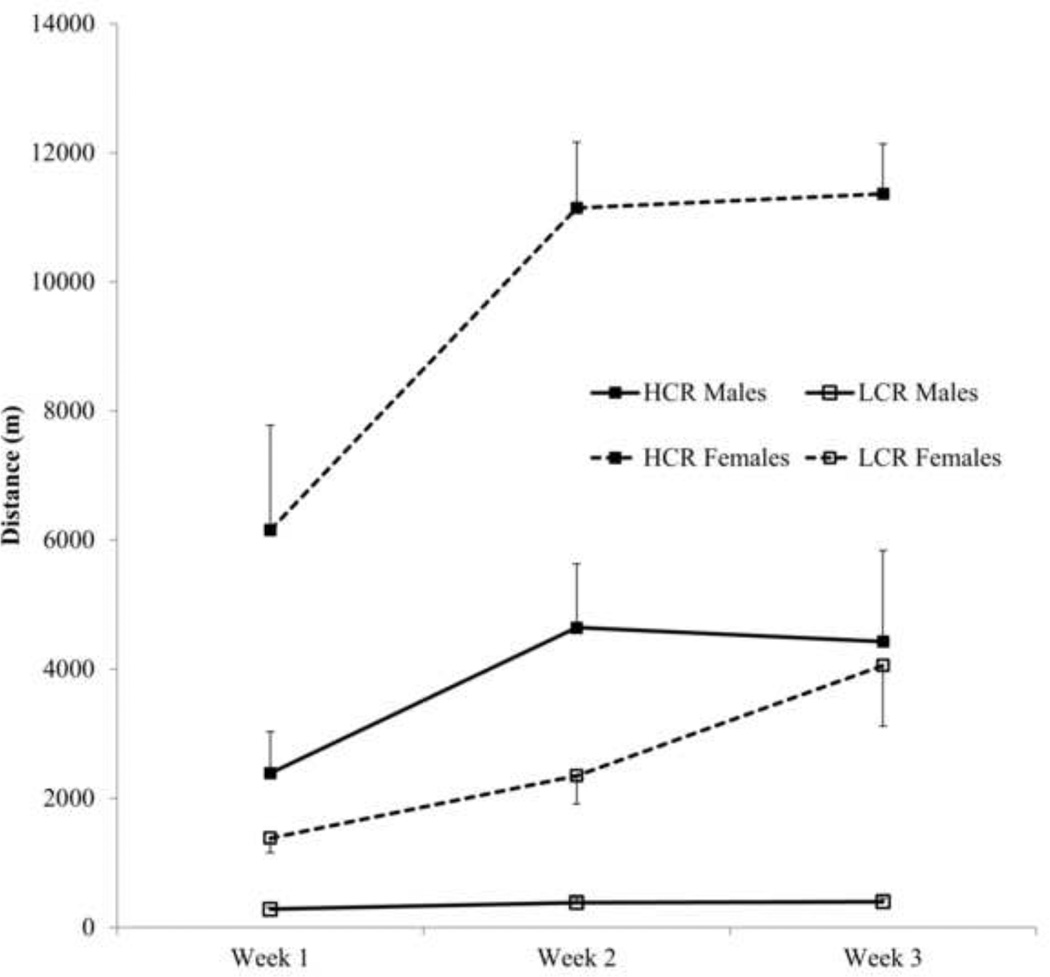
Weekly running was reliable across the three weeks in females, intraclass correlation (ICC) (2,3) =.875 and in males, ICC (2,3) = .900. Running increased over time in females, F(2,36)=14.486, ε=.846, η2 = .45, p<.001, and males, F(2,36)=4.45, ε=.980, η2 = .20, p<.05. There was an effect of line in females, F(1, 18)=47.289, η2 = .72, p<.001, and in males, F(1, 18)=13.766, η2 = .43, p<.01. HCR rats ran more on average than LCR rats, but there was also a line × quadratic trend across time in females, F(1,18) = 10.192, η2 = .36, p=.005. There was a quadratic effect of time independent of line in males, F(1,18) = 4.927, η2 = .22, p=.04. Among females, weekly running distance increased linearly in LCR, F(1,9) = 12.212, ε=.564, η2 = .58, p=.007, but it reached a plateau after week 2 in HCR, F(1,9) = 8.168, ε=.908, η2 = .48, p=.017. Among males, running distance increased linearly in LCR, F(1,9) = 8.805, ε=.908, η2 = .50, p=.016 (Fig. 1).
Fig. 1.
Mean daily running distances (± SEM) on the activity-wheel over 3 weeks. High-capacity running (HCR) rats ran more on average than low-capacity running (LCR) rats. There was an interaction effect between lines over 3 weeks in females; the effect was independent of line in males.
Body weight was reliable across the five weeks in females, ICC (2,3) =.978, and in males, ICC (2,3) = .986. Body weight increased linearly over time in females, F(2,72)=653.246, ε= 1.0, η2 = .95, p<.001, and in males, F(2,72)=954.299, ε= 1.0, η2 = .964, p<.001. There was an effect of line in females, F(1, 36)=126.081, η2 = .78, p<.001, and in males, F(1, 36)=110.919, η2 = .76, p<.001. HCR rats weighed less on average (initial mean ± SD, pre-decapitation mean ± SD) (females: 163 ± 11 g, 204 ± 17 g; males: 230 ± 18 g, 318 ± 35 g) than LCR rats (females: 209 ± 15 g, 259 ± 16 g; males: 330 ± 38 g, 438 ± 48 g), but line and line × condition effects were not significant between wheel running rats (females: 184 ± 27 g, 223 ± 33 g; males: 277 ± 57 g, 366 ± 66 g), and SED rats (females: 187 ± 28 g, 239 ± 31 g; males: 283 ± 61 g, 391 ± 81 g) (females: F-values < 3.2, η2 < .08, p-values >.08; males: F-values < 2.4, η2 < .07, p-values >.13).
2.2 ENK mRNA in the Striatum
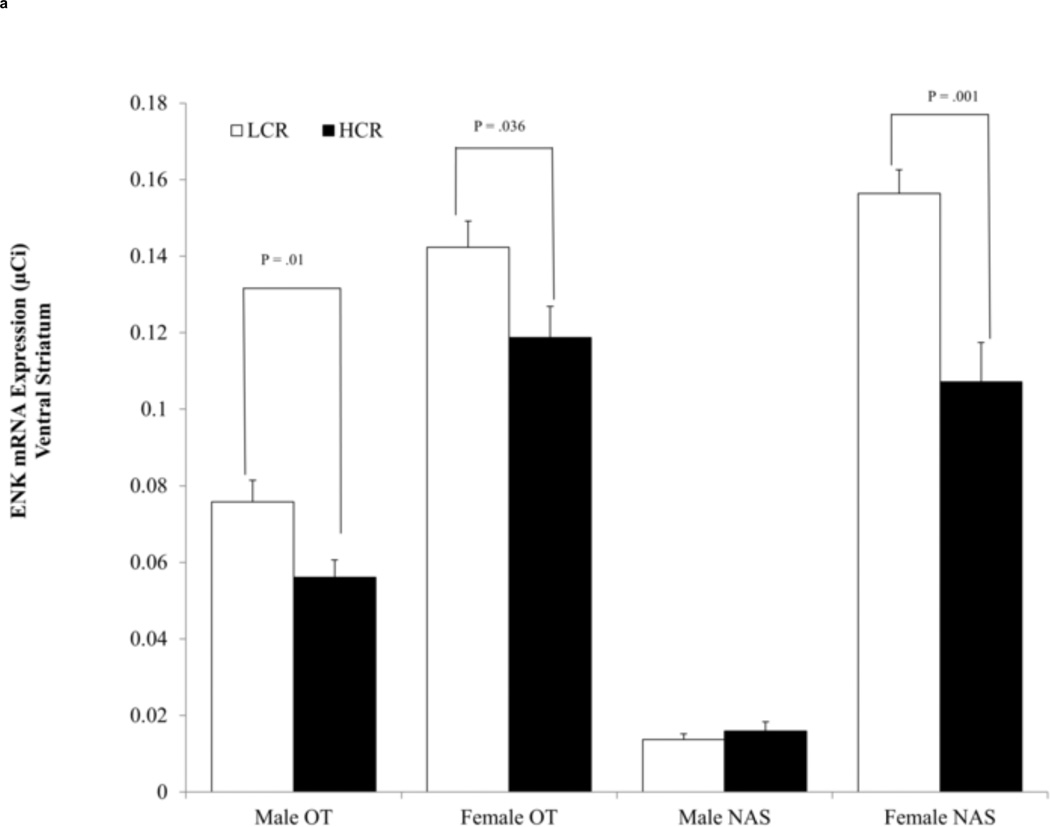
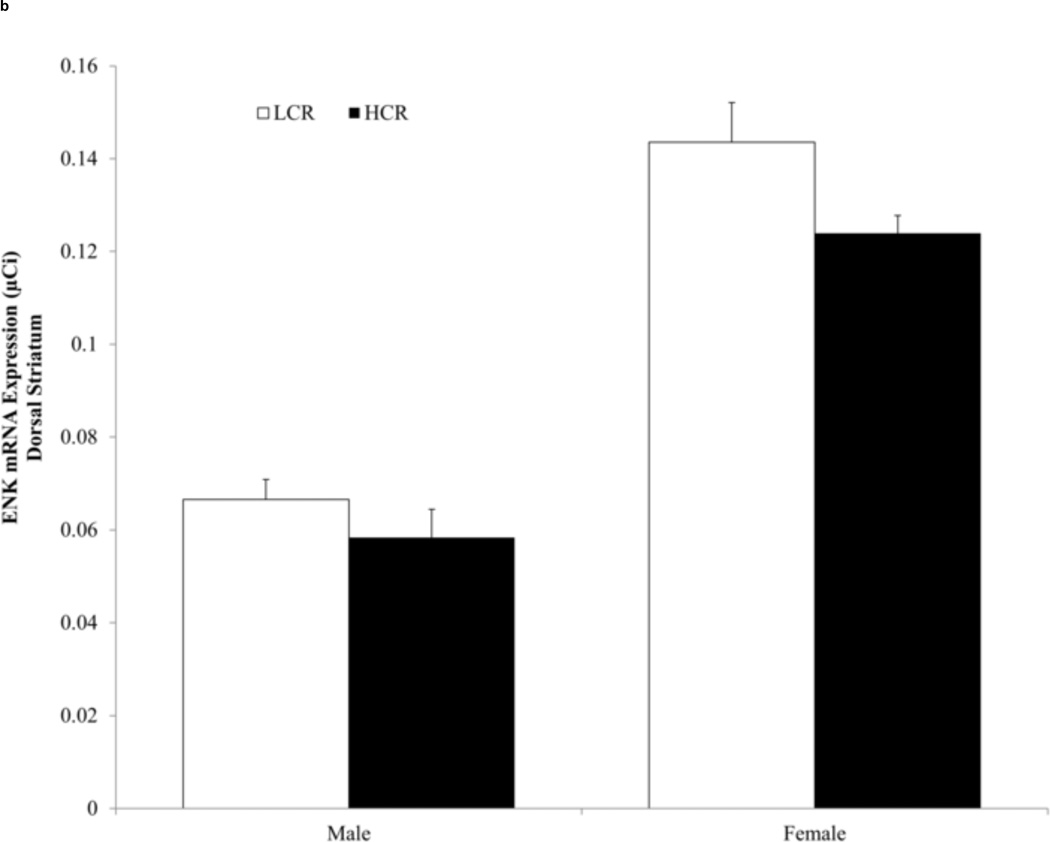
There was a line effect in the NAS among females, F (1,25)=13.038, η2 = .343, p =.001, and the OT among males, F(1,30) =7.510, η2 = .20, p =.01 and females, F(1,25) =4.924, η2 = .165, p =.036. HCR expressed less ENK mRNA compared to LCR in the ventral striatum (Fig. 2a). A similar effect in the dorsal striatum did not reach statistical significance for females, η2 = .13, p=.067, or males, η2 = .03, p=.29 (Fig. 2b). There were no condition, condition × line, or condition × line × sex effects of wheel running on ENK mRNA (F-values ≤ 1.815, η2 ≤ .032, p ≥.183).
Fig. 2.
(a) Average enkephalin (ENK) mRNA expression (± SEM) in the nucleus accumbens septi (NAS). High capacity running (HCR) rats expressed less ENK than low capacity running (LCR) rats; OT=olfactory tubercle, NAS= nucleus accumbens septi. (b) Average enkephalin (ENK) mRNA expression (± SEM) in the dorsal striatum. There were no significant line differences in ENK expression.
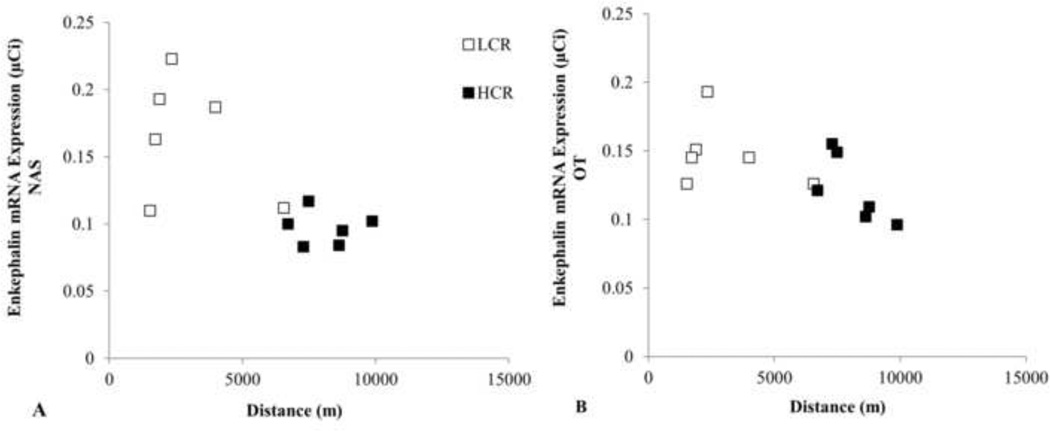
Among females, average running distance was correlated with ENK mRNA in the NAS, r = −.740, t =3.48, p = .006, and the OT, r = −.631, t = 2.57, p=.028 (Fig 3). Among males, ENK mRNA was uncorrelated with running distance in the OT (r = .069, t =.26, p = .80). Dorsal striatal ENK mRNA was uncorrelated with running distance in females, r = −.479, t =1.724, p = .115, or males, r = .109, t =.453, p = .656.
Fig. 3.
Mean daily running distance and ENK mRNA expression among female rats in the nucleus accumbens septi (NAS; panel A) and the olfactory tubercle (OT; panel B). Correlations between running distance and ENK mRNA in the NAS and the OT were mainly explained by lower mean mRNA and higher mean running distance in HCR females compared to LCR females.
3. Discussion
We report that HCR rats expressed less ENK than LCR rats in the NAS among females and in the OT among both females and males. Sex differences seen here in striatal ENK expression among rats selectively bred for divergent treadmill performance are consistent with those described in non-selectively bred rats (Tang and Man, 1991). High intrinsic drive to run, evidenced here by four- to six-fold greater distances run each day by HCR rats, was marked by low ENK mRNA expression in the ventral striatum, suggesting that a central opioidergic mechanism underlies voluntary running behavior. Multiple lines of evidence conclude that ENK projections from the ventral striatum directly modulate neural activity in the ventral pallidum to inhibit motivated locomotory behavior (Haber, 2011) and that low ENK gene expression may reflect increased dopaminergic tone (Carta et al., 2001; Gerfen et al., 1991; Li et al., 1990; Nikoshkov et al., 2008). Reduced ENK may elevate striatal opioidergic receptor expression, sensitizing the organism to natural reward and promoting wheel-running behavior in HCR rats (Greenwood et al., 2011). Improved animal models and epigenetic human studies are necessary to further elucidate the effects of motivational and hedonic processing on exercise (Dishman and Holmes, 2012).
Three weeks of exposure to wheel running had no effect on ventral or dorsal striatal ENK transcription compared to sedentary housing, consistent with a prior report on female rats (Bjornebekk et al., 2005). It has been demonstrated that thirty days of wheel access reduces ΔFosB expression in enkephalinergic cells of the NAS (Werme et al., 2002) and that eight weeks of treadmill training blunts ENK release in the basal ganglia after a fatiguing bout of treadmill running (Blake et al., 1984). Although nine days of wheel access increased hippocampal ENK in spontaneously hypertensive rats (Persson et al., 2004), it appears that three weeks of wheel running is not sufficient to induce ENK changes in the striatopallidal pathway. Here, intrinsic transcription of ENK was negatively correlated with average running distances in females, mainly because of lower ENK mRNA and higher running distances characteristic of HCR.
Striatal enkephalinergic differences between HCR and LCR rats support two other putative mechanisms of motivated behavior in rodents. First, differential ENK expression is consistent with previous evidence that dopaminergic transmission to the striatum contributes to differences in locomotory behavior (Mathes et al., 2010; Wise, 2004), possibly via opioidergic regulation (Roberts et al., 2013). It is plausible that greater dopaminergic tone suppresses transcription of ENK in HCR (Foley et al., 2006; Li et al., 1990; Young et al., 1986) promoting wheel-running and other appetitive behaviors related to energy balance (Borer, 2010; Smith et al., 2011). Second, it has been theorized that ENK innervation from the dorsal striatum drives motivation specific to food seeking (Salamone et al., 2003) and reward (Hayward and Low, 2007). The tendency toward less dorsal striatal ENK expression observed here in HCR compared to LCR suggests a novel, central mechanism underlying reduced sensitivity of HCR to an obesogenic environment compared to LCR rats (Koch et al., 2012; Novak et al., 2010).
Our findings encourage investigation of whether decreased transmission (lower ENK) or increased transmission (upregulated opioid receptors) is associated with wheel running. The complex interactions between opioidergic and dopaminergic pathways through the basal ganglia require further study to identify an integrated neural mechanism underlying voluntary locomotory behavior.
4. Experimental Procedures
4.1. Animals and Experimental Design
40 adult Female rats (n=20 HCR, n=20 LCR) and 40 adult Male rats (n=20 HCR, n=20 LCR) were blocked by sex and line before random allocation (www.randomizer.org) to activity wheel (n=40) or sedentary (n=40) conditions. Animals were housed individually in polycarbonate cages in a temperature and humidity-controlled environment on a 12-hour light/dark schedule. Food and water were available ad libitum and animals were weighed upon housing assignment and prior to decapitation. Selectively bred HCR and LCR rats were obtained from the University of Michigan where the running capacities were estimated by treadmill tests performed at 11 weeks of age (Table 1). All procedures were approved by the institutional animal use committees at the University of Georgia and the University of Michigan and conducted in accordance with NIH Guide for Care and Use of Laboratory Animals.
4.2 Exercise Protocol
Activity wheels with a circumference of 105 cm were placed in polycarbonate shoebox cages and attached to magnetic revolution counters (MiniMitter; Bend, Oregon). Home cages of sedentary rats did not contain an activity wheel. Activity-wheel running rats were given unlimited access to activity wheels for 21 days. Wheel revolutions were recorded and daily distances were determined by multiplying the circumference (105 cm) of the activity wheel by the number of revolutions.
4.3 In Situ Hybridization Histochemistry
Animals were killed by rapid decapitation upon termination of the 21 day exercise or control exposure at the end of a full light cycle. Brains were extracted and stored at −80°C. Brains were sliced into 12 µm coronal sections at the level of the nucleus accumbens and thaw-mounted to gelatin coated microscope slides. Anatomical location was verified using a 0.1% thionin stain. In situ hybridization methods used are reported in detail elsewhere (Van Hoomissen et al., 2003). Briefly, sections were fixed in 4% (v/v) formaldehyde in 0.12 M sodium phosphate-buffered saline (PBS) solution, rinsed in PBS, and soaked in 0.25% (v/v) acetic anhydride in 0.1 M triethanolamine HCl-0.9% (v/v) NaCl. Sections were then dehydrated through a series of ethanol washes, delipidated in chloroform, rinsed again in ethanol, and allowed to dry.
An oligonucleotide probe complementary to prepro-ENK mRNA was obtained from Oligos, Etc. (Wilsonville, OR) and labeled at the 3’ end with [35S]-dATP (New England Nuclear, Boston, Massachusetts), terminal deoxynucleotidyl transferase (TdT, 25 units/ml; Roche, Indianapolis, Indiana), and tailing buffer. Agilent (Santa Clara, CA) NucTrap size exclusionary columns were used to separate unincorporated nucleotides from labeled ENK. Sections were hybridized with the radiolabeled ENK in solutions containing 25% (v/v) formamide, 72 mM NaCl, 3.2 mM Tris-HCl, .0032 mM EDTA, 0.001% (v/v) sodium pyrophosphate, 0.004% (v/v) sodium dodecyl sulfate, 0.002 mg/ml heparin sulfate, and 2% (v/v) dextran sulfate. Hybridized sections were incubated overnight at 37°C, followed by a series of washes in SSC and SSC-50% formamide, water and ethanol to reduce nonspecific binding, and then were dried. Hybridized brain sections were exposed, with a radioactive standard, to autoradiographic film for four weeks.
Developed autoradiographic film was photographed (Nikon D5000) and analysis was conducted, blind to assignment and line, using NIH ImageJ (version 1.44). Gray-scale was converted to µCi values using the radioactive standard and calibration was performed for each film. Measurements were taken at points in the left and right ventral (NAS and OT) and dorsal striatum and compared to background recorded for each slide. Outliers were omitted within each site across all slides using Grubbs Criteria (Grubbs and Beck, 1972), and as a result one HCR female was removed from analysis of ENK expression in the OT. There was moderate-to-high agreement (ICC (2, 4)) among females (Cronbach α = .833, .886, and .819) and males (Cronbach α = .774, .658, and .849) for ENK in the OT, NAS, and dorsal striatum respectively.
4.4 Data Analysis
Data were analyzed using SPSS Windows version 21.0 (IBM Corporation, USA). All significance levels were set at p < 0.05. For descriptive purposes, distance run was analyzed by a 2 (line: HCR vs. LCR) × 3 (time: weeks 1–3) mixed-model ANOVA with time repeated. Body weight was analyzed using a two-way (condition × time) mixed model ANOVA with time repeated. Huynh-Feldt (ε) was used to adjust degrees of freedom (df) for sphericity violation of the repeated measure. For hypothesis testing, levels (µCi) of ENK mRNA in each brain region were compared using a 2 group (HCR vs. LCR) × 2 condition (activity wheel vs. sedentary) ANOVA. Bonferroni-adjusted post-hoc tests were used. Effect sizes were estimated by η2. Relations between ENK mRNA and running distance were estimated by linear regression analysis. The sample size was sufficiently powered to detect moderately large effect sizes (η2 ≥ .15) at a statistical power >.80, and p< .05.
Research Highlights.
ENK mRNA in ventral striatum was lower in a divergent line of high running rats.
Exposure to wheel running did not alter striatal ENK.
Motivational drive to run is plausibly mediated by intrinsic striatal ENK.
Acknowledgments
The LCR-HCR rat model system was funded by the National Center for Research Resources grant R24RR017718 and is currently supported by the Office of Research Infrastructure Programs/OD grant R24OD010950 (to LGK. and SLB) from the National Institutes of Health. SLB was also supported by National Institutes of Health grants R01 DK077200 and R01GM104194. We acknowledge the expert care of the rat colony provided by Molly Kalahar and Lori Heckenkamp. Contact LGK (lgkoch@umich.edu) or SLB (brittons@umich.edu) for information on the LCR and HCR rats: these rat models are maintained as an international resource with support from the Department of Anesthesiology at the University of Michigan, Ann Arbor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Derek C. Monroe, Email: dmon@uga.edu.
Philip V. Holmes, Email: pvholmes@uga.edu.
Lauren G. Koch, Email: lgkoch@med.umich.edu.
Steven L. Britton, Email: brittons@med.umich.edu.
References
- Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav. Process. 2005;68(2):165–172. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Rev. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bjornebekk A, Mathé AA, Brené S. The antidepressant effect of running is associated with increased hippocampal cell proliferation. Int. J. Neuropsychopharmacol. 2005;8(3):357–368. doi: 10.1017/S1461145705005122. [DOI] [PubMed] [Google Scholar]
- Blake MJ, Stein EA, Vomachka AJ. Effects of exercise training on brain opioid peptides and serum LH in female rats. Pept. 1984;5(5):953–958. doi: 10.1016/0196-9781(84)90122-0. [DOI] [PubMed] [Google Scholar]
- Borer KT. Nonhomeostatic Control of Human Appetite and Physical Activity in Regulation of Energy Balance. Exer. Sport Sci. Rev. 2010;38(3):114–121. doi: 10.1097/JES.0b013e3181e3728f. 110.1097/JES.1090b1013e3181e3728f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brené S, Bjørnebekk A, Åberg E, Mathé AA, Olson L, Werme M. Running is rewarding and antidepressive. Physiol. & Behav. 2007;92(1–2):136–140. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess CR. Histamine and Orexin in the Control of Arousal, Locomotion, and Motivation. The J. of Neurosci. 2010;30(8):2810–2811. doi: 10.1523/JNEUROSCI.0045-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002;26(3):321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carta A, Fenu S, Morelli M. Alterations in GAD67, dynorphin and enkephalin mRNA in striatal output neurons following priming in the 6-OHDA model of Parkinson's disease. Neurol. Sci. 2001;22(1):59–60. doi: 10.1007/s100720170046. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behav. Brain Sci. 1999;22(3):491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Dishman RK. Gene–physical activity interactions in the etiology of obesity: behavioral considerations. Obesity. 2008;16(S3):S60–S65. doi: 10.1038/oby.2008.520. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Holmes PV. Opioids and Exercise: Animal Models Functional. In: Boecker H, Hillman CH, Scheef L, Strüder HK, editors. Neuroimaging Exerc. Sport Sci. New York, NY: Springer; 2012. pp. 45–58. [Google Scholar]
- Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M, de Kerchove d'Exaerde A. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat. Neurosci. 2009;12(4):393–395. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Rasmussen F, Tynelius P. Genetic factors in physical activity and the equal environment assumption–the Swedish young male twins study. Behav. Genet. 2006;36(2):238–247. doi: 10.1007/s10519-005-9018-7. [DOI] [PubMed] [Google Scholar]
- Ena S, de Kerchove d'Exaerde A, Schiffmann SN. Unraveling the Differential Functions and Regulation of Striatal Neuron Sub-Populations in Motor Control, Reward, and Motivational Processes. Front. Behav. Neurosci. 2011;5:47. doi: 10.3389/fnbeh.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley TE, Greenwood BN, Day HE, Koch LG, Britton SL, Fleshner M. Elevated central monoamine receptor mRNA in rats bred for high endurance capacity: implications for central fatigue. Behav. Brain Res. 2006;174(1):132–142. doi: 10.1016/j.bbr.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E. The basal ganglia and the locomotor regions. Brain Res. Rev. 1986;11(1):47–63. [PubMed] [Google Scholar]
- Gerfen CR, McGinty JF, Young WS. Dopamine differentially regulates dynorphin, substance P, and enkephalin expression in striatal neurons: in situ hybridization histochemical analysis. J. of Neurosci. 1991;11(4):1016–1031. doi: 10.1523/JNEUROSCI.11-04-01016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Young WS. Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460(1):161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- Gilliam PE, Spirduso WW, Martin TP, Walters TJ, Wilcox RE, Farrar RP. The effects of exercise training on [3H]-spiperone binding in rat striatum. Pharmacol. Biochem. Behav. 1984;20(6):863–867. doi: 10.1016/0091-3057(84)90008-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez NC, Howlett RA, Henderson KK, Koch LG, Britton SL, Wagner HE, Wagner PD. Systemic oxygen transport in rats artificially selected for running endurance. Respir. Physiol. & Neurobiol. 2006;151(2):141–150. doi: 10.1016/j.resp.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HEW, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav. Brain Res. 2011;217(2):354–362. doi: 10.1016/j.bbr.2010.11.005. doi: http://dx.doi.org/10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs FE, Beck G. Extension of sample sizes and percentage points for significance tests of outlying observations. Technometrics. 1972;14(4):847–854. [Google Scholar]
- Groves-Chapman JL, Murray PS, Stevens KL, Monroe DC, Koch LG, Britton SL, Dishman RK. Changes in mRNA levels for brain-derived neurotrophic factor after wheel running in rats selectively bred for high- and low-aerobic capacity. Brain Res. 2011;1425(0):90–97. doi: 10.1016/j.brainres.2011.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. Neuroanatomy of Reward: A View from the Ventral Striatum. In: Gottfried JA, editor. Neurobiol. of Sensat. and Reward. Boca Raton, FL: CRC Press; 2011. [PubMed] [Google Scholar]
- Hattori S, Naoi M, Nishino H. Striatal dopamine turnover during treadmill running in the rat: Relation to the speed of running. Brain Res. Bull. 1994;35(1):41–49. doi: 10.1016/0361-9230(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Hayward M, Low M. The contribution of endogenous opioids to food reward is dependent on sex and background strain. Neurosci. 2007;144(1):17–25. doi: 10.1016/j.neuroscience.2006.08.067. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Dopamine gating of glutamatergic sensorimotor and incentive motivational input signals to the striatum. Behav. Brain Res. 2002;137(1–2):65–74. doi: 10.1016/s0166-4328(02)00285-1. [DOI] [PubMed] [Google Scholar]
- Howlett RA, Kirkton SD, Gonzalez NC, Wagner HE, Britton SL, Koch LG, Wagner PD. Peripheral oxygen transport and utilization in rats following continued selective breeding for endurance running capacity. J. of Appl. Physiol. 2009;106(6):1819–1825. doi: 10.1152/japplphysiol.00914.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res. Rev. 2007;56(1):27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen IH. Techniques for establishing schedules with wheel running as reinforcement in rats. J. Exp. Anal. Behav. 1993;60(1):219–238. doi: 10.1901/jeab.1993.60-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Widerlöv E, Stanley D, Breese G, Prange A. Enkephalin action on the mesolimbic system: a dopamine-dependent and a dopamine-independent increase in locomotor activity. J. Pharmacol. Exp. Ther. 1983;227(1):229–237. [PubMed] [Google Scholar]
- Knab AM, Bowen RS, Hamilton AT, Gulledge AA, Lightfoot JT. Altered dopaminergic profiles: Implications for the regulation of voluntary physical activity. Behav. Brain Res. 2009;204(1):147–152. doi: 10.1016/j.bbr.2009.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knab AM, Lightfoot JT. Does the difference between physically active and couch potato lie in the dopamine system? Int J. Biol. Sci. 2010;6(2):133. doi: 10.7150/ijbs.6.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol. Genomics. 2001;5(1):45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- Koch LG, Britton SL. Development of animal models to test the fundamental basis of gene–environment interactions. Obesity. 2008;16(S3):S28–S32. doi: 10.1038/oby.2008.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch LG, Britton SL, Wisløff U. A rat model system to study complex disease risks, fitness, aging, and longevity. Trends Cardiovasc. Med. 2012;22(2):29–34. doi: 10.1016/j.tcm.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466(7306):622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moine C, Normand E, Guitteny A, Fouque B, Teoule R, Bloch B. Dopamine receptor gene expression by enkephalin neurons in rat forebrain. Proc. Natl. Acad. Sci. 1990;87(1):230–234. doi: 10.1073/pnas.87.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett B, Grant V, Byrne M, Koh M. Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite. 2000;34(1):87–94. doi: 10.1006/appe.1999.0274. [DOI] [PubMed] [Google Scholar]
- Li S, Jiang H, Stachowiak M, Hudson P, Owyang V, Nanry K, Hong J. Influence of nigrostriatal dopaminergic tone on the biosynthesis of dynorphin and enkephalin in rat striatum. Mol. Brain Res. 1990;8(3):219–225. doi: 10.1016/0169-328x(90)90020-e. [DOI] [PubMed] [Google Scholar]
- Lightfoot JT, Turner MJ, Daves M, Vordermark A, Kleeberger SR. Genetic influence on daily wheel running activity level. Physiol. Genomics. 2004;19(3):270–276. doi: 10.1152/physiolgenomics.00125.2004. [DOI] [PubMed] [Google Scholar]
- Lightfoot JT, Turner MJ, Pomp D, Kleeberger SR, Leamy LJ. Quantitative trait loci for physical activity traits in mice. Physiol. Genomics. 2008;32(3):401–408. doi: 10.1152/physiolgenomics.00241.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathes WF, Nehrenberg DL, Gordon R, Hua K, Garland T, Jr, Pomp D. Dopaminergic dysregulation in mice selectively bred for excessive exercise or obesity. Behav. Brain Res. 2010;210(2):155–163. doi: 10.1016/j.bbr.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson G. Limbic-motor integration. Prog. Psychobiol. Physiol. Psychol. 1987;12:117–170. [Google Scholar]
- Morales-Mulia M, de Gortari P, Amaya M-I, Méndez M. Acute Ethanol Administration Differentially Alters Enkephalinase and Aminopeptidase N Activity and mRNA Levels in Regions of the Nigrostriatal Pathway. J. Mol. Neurosci. 2013;49(2):289–300. doi: 10.1007/s12031-012-9823-4. [DOI] [PubMed] [Google Scholar]
- Nikoshkov A, Drakenberg K, Wang X, Horvath M, Keller E, Hurd Y. Opioid neuropeptide genotypes in relation to heroin abuse: dopamine tone contributes to reversed mesolimbic proenkephalin expression. Proc. Natl. Acad. Sci. U. S. A. 2008;105(2):786–791. doi: 10.1073/pnas.0710902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak CM, Escande C, Burghardt PR, Zhang M, Barbosa MT, Chini EN, Levine JA. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm. Behav. 2010;58(3):355–367. doi: 10.1016/j.yhbeh.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Hazrati L-N. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Rev. 1995;20(1):91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Persson AI, Naylor AS, Jonsdottir IH, Nyberg F, Eriksson PS, Thorlin T. Differential regulation of hippocampal progenitor proliferation by opioid receptor antagonists in running and non-running spontaneously hypertensive rats. Eur. J. Neurosci. 2004;19(7):1847–1855. doi: 10.1111/j.1460-9568.2004.03268.x. [DOI] [PubMed] [Google Scholar]
- Roberts MD, Brown JD, Oberle LP, Heese AJ, Toedebusch RG, Wells KD, Childs TE. Phenotypic and molecular differences between rats selectively bred to voluntarily run high vs. low nightly distances. Am. J. Physiol.-Regul., Integr. Comparat. Physiol. 2013;304(11):R1024–R1035. doi: 10.1152/ajpregu.00581.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J. Pharm. Exp. Therap. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Scheurink AJW, Boersma GJ, Nergårdh R, Södersten P. Neurobiology of hyperactivity and reward: Agreeable restlessness in Anorexia Nervosa. Physiol. Behav. 2010;100(5):490–495. doi: 10.1016/j.physbeh.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Sherwin CM. Voluntary wheel running: a review and novel interpretation. Anim. Behav. 1998;56(1):11–27. doi: 10.1006/anbe.1998.0836. [DOI] [PubMed] [Google Scholar]
- Simonen RL, Perusse L, Rankinen T, Rice T, Rao DC, Bouchard C. Familial aggregation of physical activity levels in the Quebec family study. Med. Sci. Sports Exerc. 2002;34(7):1137–1142. doi: 10.1097/00005768-200207000-00014. [DOI] [PubMed] [Google Scholar]
- Smith J, Feldman J, Schmidt B. Neural mechanisms generating locomotion studied in mammalian brain stem-spinal cord in vitro. FASEB J. 1988;2(7):2283–2288. doi: 10.1096/fasebj.2.7.2450802. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc. Natl. Acad. Sci. 2011 doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbe JH, Boomsma DI, Vink JM, Cornes BK, Martin NG, Skytthe A, Kaprio J. Genetic influences on exercise participation in 37.051 twin pairs from seven countries. PloS One. 2006;1(1):e22. doi: 10.1371/journal.pone.0000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Song W-J, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J. Neurosci. 1996;16(20):6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow JG, Carter PA, Garland T., Jr Artificial selection for increased wheel-running behavior in house mice. Behav. Genet. 1998;28(3):227–237. doi: 10.1023/a:1021479331779. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Saitoh K, Harada H, Kashiwayanagi M. Role of basal ganglia–brainstem pathways in the control of motor behaviors. Neurosci. Res. 2004;50(2):137–151. doi: 10.1016/j.neures.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Tang F, Man W. The regional distribution of thyrotropin releasing hormone, leu-enkephalin, met-enkephalin, substance P, somatostatin and cholecystokinin in the rat brain and pituitary. Neuropeptides. 1991;19(4):287–292. doi: 10.1016/0143-4179(91)90096-2. [DOI] [PubMed] [Google Scholar]
- Van Hoomissen JD, Chambliss HO, Holmes PV, Dishman RK. Effects of chronic exercise and imipramine on mRNA for BDNF after olfactory bulbectomy in rat. Brain Res. 2003;974(1–2):228–235. doi: 10.1016/s0006-8993(03)02584-8. [DOI] [PubMed] [Google Scholar]
- Waters RP, Pringle RB, Forster GL, Renner KJ, Malisch JL, Garland T, Jr, Swallow JG. Selection for increased voluntary wheel-running affects behavior and brain monoamines in mice. Brain Res. 2013;1508:9–22. doi: 10.1016/j.brainres.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters RP, Renner K, Pringle R, Summers CH, Britton S, Koch L, Swallow J. Selection for aerobic capacity affects corticosterone, monoamines and wheel-running activity. Physiol. Behav. 2008;93(4):1044–1054. doi: 10.1016/j.physbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werme M, Messer C, Olson L, Gilden L, Thoren P, Nestler EJ, Brene S. Delta FosB regulates wheel running. J. Neurosci. 2002;22(18):8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Young WS, Bonner TI, Brann MR. Mesencephalic dopamine neurons regulate the expression of neuropeptide mRNAs in the rat forebrain. Proc. Natl. Acad. Sci. 1986;83(24):9827–9831. doi: 10.1073/pnas.83.24.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]