Abstract
Gamma oscillations (∼30 to 100 Hz) provide a fundamental mechanism of information processing during sensory perception, motor behavior, and memory formation by coordination of neuronal activity in networks of the hippocampus and neocortex. We review the cellular mechanisms of gamma oscillations about the underlying neuroenergetics, i.e., high oxygen consumption rate and exquisite sensitivity to metabolic stress during hypoxia or poisoning of mitochondrial oxidative phosphorylation. Gamma oscillations emerge from the precise synaptic interactions of excitatory pyramidal cells and inhibitory GABAergic interneurons. In particular, specialized interneurons such as parvalbumin-positive basket cells generate action potentials at high frequency (‘fast-spiking') and synchronize the activity of numerous pyramidal cells by rhythmic inhibition (‘clockwork'). As prerequisites, fast-spiking interneurons have unique electrophysiological properties and particularly high energy utilization, which is reflected in the ultrastructure by enrichment with mitochondria and cytochrome c oxidase, most likely needed for extensive membrane ion transport and γ-aminobutyric acid metabolism. This supports the hypothesis that highly energized fast-spiking interneurons are a central element for cortical information processing and may be critical for cognitive decline when energy supply becomes limited (‘interneuron energy hypothesis'). As a clinical perspective, we discuss the functional consequences of metabolic and oxidative stress in fast-spiking interneurons in aging, ischemia, Alzheimer's disease, and schizophrenia.
Keywords: brain slice, electrophysiology, energy metabolism, γ-aminobutyric acid, inhibitory interneuron, mitochondria
Introduction
Research on brain energy metabolism in the neocortex and the hippocampus has made major progress with regard to oxidative metabolism, energy utilization, and survival of neurons.1, 2, 3, 4, 5, 6 Notably, most of this research has focused on excitatory neurons such as glutamatergic pyramidal cells that release neurotransmitter, glutamate. These principal projection neurons are generally thought to compute, transfer, store, and retrieve information, which underlies higher brain functions such as sensory perception, motor behavior, and memory formation.7, 8, 9
Neuronal information processing, however, requires precise spatial and temporal coordination of pyramidal cell activities in cortical networks.9, 10, 11 Different classes of network oscillations provide such a fundamental mechanism by enabling coordinated synchronous activity during normal brain function.9, 12, 13 There are multiple different classes of network oscillations, which span a wide range of frequencies from ∼0.05 to almost 1,000 Hz and which define different cognitive and behavioral states.12 Dominant patterns in cortical networks comprise theta- (4 to 12 Hz), beta- (13 to 30 Hz) and gamma- (∼30 to 100 Hz) frequency oscillations, which synchronize the action potential generation (‘spiking') of principal cells with great precision, sometimes over large distances of corticocortical connections.10, 13, 14, 15
Importantly, inhibitory interneurons have a key role in organizing these highly coordinated patterns of activity. In cortical networks, inhibition is mainly mediated by neurotransmitter, γ-aminobutyric acid (GABA), and research during the past decade has revealed an astonishing heterogeneity of GABAergic interneurons at the molecular, morphologic and functional level.16, 17 This raises the fundamental question of electrophysiological and neuroenergetical properties of GABAergic interneurons, including the impact on cortical information processing under physiologic conditions as well as pathologic conditions such as ischemia. However, GABAergic interneurons have attracted little attention about brain energy metabolism so far.18
Here, we summarize and discuss studies on the cellular mechanisms and neuroenergetics of hippocampal gamma oscillations (∼30 to 100 Hz). Gamma oscillations have a strong relationship to higher brain functions.7, 11, 13 Despite occurring in many cortical regions, gamma oscillations have been particularly well studied in the hippocampus that has become an important model system in cognitive neuroscience for several reasons.10, 16, 19, 20, 21 First, hippocampal gamma oscillations are evoked during specific behavioral states such as exploration. Second, as the hippocampus has a central role for spatial navigation and episodic memory, the relevance of network oscillations for encoding, storage, and retrieval of information can be studied. Third, extracellularly recorded hippocampal gamma oscillations show larger amplitudes because of the more simple laminated architecture of the hippocampus compared with the neocortex. We will focus on hippocampal gamma oscillations in the present review, combining mechanistic results from in vitro and in vivo studies in rodents. Where available, we will include data from the neocortex and from humans.
Collectively, the available evidence indicates that information processing in the cortex critically depends on the activity of a specific type of inhibitory interneurons, so-called ‘fast-spiking' interneurons that generate action potentials at high frequencies. At the same time, these interneurons feature particularly high energy utilization, which puts them into a critical position for the maintenance or failure of cognitive functions when metabolic stress occurs (‘interneuron energy hypothesis').
Gamma oscillations and cortical information processing
Gamma oscillations have been found in many mammalian brain regions, including the hippocampus and the neocortex.9, 13, 20 They are characterized by rhythmic and synchronous fluctuations of the membrane potential of most or all neurons in a neuronal network, with a characteristic frequency domain of ∼30 to 100 Hz.11, 22 These subthreshold fluctuations are generated by a complex interplay between excitatory pyramidal cells and inhibitory GABAergic interneurons.16, 19, 23, 24 Functionally, gamma oscillations in neuronal networks bind neurons into a common temporal regime during higher brain functions like motor behavior, sensory perception, or memory formation.7, 25, 26, 27, 28 The synchronizing effect of gamma oscillations permits the coordinated activation of defined sets of neurons, which constitute functional ensembles—the putative information-carrying multicellular subsets of neuronal networks.9, 11, 29 Moreover, the precise timing of action potentials is central for use-dependent synaptic plasticity and, thus, supports learning and memory formation.13, 20, 30, 31
In neuronal networks in vivo, gamma oscillations occur transiently on the hundred millisecond time scale upon sensory input or during specific cognitive tasks. In the human brain and dependent on the task, however, they can last for prolonged times in the range of minutes.28, 32 This aspect is important for the validation of in vitro models, most of which feature persistent gamma oscillations.8, 9 Another characteristic of gamma oscillations is the synchronization of remote cortical networks. This peculiar phenomenon has been proposed to underlie binding of the distributed neuronal coding, and may thus form a crucial prerequisite for the unity of perception, attention and, perhaps, consciousness.10, 13, 33 Of course, the fact that research on neuronal network phenomena has focused on gamma oscillations does not withstand the importance of slower network oscillations for higher brain functions.10, 12 Interestingly, high-frequency network oscillations are consistently present during cognitive tasks that require attention or consciousness, and they are often correlated with adaptations in local cerebral blood flow.13, 34
Cellular mechanisms of gamma oscillations: insight from in vitro models
In vivo studies have yielded valuable insights into the relationships between cortical gamma oscillations and behavior, including the identification of the involved neuronal subpopulations.13, 22, 24, 35, 36 However, detailed experimental analyses of the cellular and subcellular mechanisms underlying gamma oscillations have been widely performed in vitro, particularly in neuronal networks of the hippocampus.16, 17, 19, 37 Rodent hippocampal slices express various patterns of naturally occurring network oscillations and permit high-resolution recordings with electrophysiological methods, live-cell imaging techniques and oxygen sensor microelectrodes under well-defined recording conditions.38, 39, 40, 41 This in vitro preparation also provides a reasonable model for examining energy metabolism during different forms of neuronal network activation.42, 43, 44, 45
In the hippocampal slices, persistent gamma oscillations can be reliably induced by bath application of cholinergic or glutamatergic receptor agonists at nanomolar to low micromolar concentrations (Figures 1A and 1B). The most widespread models use acetylcholine or carbachol for activation of metabotropic cholinergic receptors, or kainate (kainic acid) for activation of ionotropic glutamatergic receptors.40, 41, 46, 47 Pharmacologically induced hippocampal gamma oscillations in vitro are most prominent in the neuronal network of subfield CA3, weaker in subfield CA1, and less prominent or absent in the dentate gyrus.16, 41 They can occur in the presence or in the absence of theta oscillations,40, 41 similar to the pattern of gamma oscillations in vivo.8, 30, 48 Alternative models are based on the induction of transient phases of gamma oscillations: local tetanic electrical stimulation, topic application of potassium-rich solution, or application of metabotropic glutamate receptor agonists.16, 38, 49 However, electrical stimulation of neuronal tissue can only partially mimic the features of naturally occurring network oscillations.9 Nonetheless, electrical stimulation of neuronal tissue at different frequencies and intensities is a very useful tool for well-defined, transient neuronal activation in experimental studies on ion homeostasis and energy metabolism.42, 43, 45 All in vitro models of hippocampal gamma oscillations share a critical role for rhythmic, transient activation of GABAA receptors because the oscillations are all blocked by GABAergic receptor antagonists such as bicuculline. They do, however, differ in the relative contribution of glutamatergic synaptic transmission: while gamma oscillations that are induced by metabotropic glutamate receptor agonists or kainate persist in the presence of AMPA receptor antagonists, carbachol-induced gamma oscillations depend on the function of AMPA receptor-mediated excitatory postsynaptic potentials.8, 16, 19 Studies using transgenic mice with cell-specific deletion of glutamate receptor subunits show that synaptic excitation of fast-spiking interneurons is required for generation of normal gamma oscillations in vivo.50, 51
Figure 1.
Gamma oscillations are exemplified from the CA3 network of the hippocampus. (A) The hippocampal formation consists of the dentate gyrus (DG) with excitatory granule cells (white ovals) and subfields, CA3 and CA1 with excitatory pyramidal cells (orange triangles). Parvalbumin-positive (PV+) interneurons are also illustrated (intermittent blue spheres). Red arrows indicate main routes of information processing in the hippocampal formation. (B) Example trace of persistent gamma oscillations that occur in local field potential (LFP) recordings in the presence of cholinergic or glutamatergic receptor agonists. The power spectrum (PS) was calculated from the same trace. (C) and (D) Immunohistochemical stainings of PV+ interneurons, visualized with 3,3'-diaminobenzidine (DAB) (C) or fluorescent secondary antibody (D). The images show parts of the CA3 subfield. Note both location of somata (C, black dots) and numerous synaptic contacts of PV+ interneurons to the perisomatic region of pyramidal cells (D, turquoise meshwork). Nuclei of neurons and glial cells are counterstained with 4',6-diamidino-2-phenylindole (DAPI) (D, grey dots). (E) A single PV+ interneuron contacts numerous pyramidal cells (PC) via GABAergic synapses. Excitatory synapses from pyramidal cells onto the interneuron are not shown. (F) Rhythmic action potentials (blue vertical bars) of PV+, fast-spiking interneurons (first line) evoke synchronous inhibitory postsynaptic potentials in pyramidal cells that are reflected by oscillations in the local field potential (LFP) (second line). This rhythmic perisomatic inhibition provides a temporal matrix for individual pyramidal cells (orange triangles) to generate action potentials (red vertical bars) during brief time windows, resulting in a well-defined aggregate sequence of action potentials (black vertical bars) from a given pyramidal cell group and thus precise information processing in neuronal networks (bottom line). Scale bar, 100 μm (C and D).
The indispensable role of phasic GABAergic inhibition for generation of gamma oscillations is underlined by the fact that local field potentials in the gamma-frequency band are dominated by inhibitory postsynaptic potentials (IPSPs), rather than action potentials or excitatory postsynaptic potentials.17, 52, 53 It is also in line with the models of gamma oscillations in neuronal networks that emphasize the importance of rhythmic and synchronous inhibition of both pyramidal cells and interneurons, which finally generates alternating phases of enhanced or decreased probability of spiking.9, 54 Initial models argued that the cycle of gamma oscillations (∼25 ms for 40 Hz) is dominated by the duration of slow IPSPs. Subsequent direct electrophysiological measurements revealed faster kinetics of IPSPs than compatible with the gamma cycle.16 This has led to the proposals of modified models that include conduction delays in axons of interneurons but have not changed the basic concept of rhythmic inhibition as a key factor for gamma oscillations.16 Indeed, synchronized inhibition of multiple interneurons and pyramidal cells, followed by feedback excitation of interneurons is the common core concept of the existing network models on gamma oscillations.9, 55
High energy utilization during hippocampal gamma oscillations
Recent studies have started to directly address the energy utilization of different activity states in neuronal networks using slice preparations from mouse and rat. Recordings of local field potential and interstitial partial oxygen pressure (pO2) with high spatiotemporal resolution revealed a positive correlation between the power of gamma oscillations and oxygen consumption.41 The power spectrum reveals the strength of potential variations (energy) as a function of frequency and, thus, the contribution of different frequencies to a complex oscillation40, 41, 56 (Figure 1B). Surprisingly, oxygen consumption during gamma oscillations reached similar levels as observed during pathologic hyperactive states, i.e., seizure-like events. High-resolution depth profiles of local pO2 and mathematical modeling of convective transport, diffusion- and activity-dependent consumption of oxygen suggest a 2.2-fold increase in the oxygen consumption rate during gamma oscillations compared with asynchronous neuronal network activity (‘baseline' activity) in vitro, indicating that gamma oscillations are associated with high energy utilization.57 This value is higher than observed in vivo where local cerebral metabolic rate of oxygen consumption rarely increases more than 30% during functional activation of cortical regions.58, 59, 60, 61 This discrepancy might primarily reflect much lower levels of ‘baseline' activity and thus ‘resting' metabolic rates in slice preparations compared with ‘baseline' activity in vivo. In addition, methodological aspects need to be considered (see below). Support for the notion of high energy utilization during gamma oscillations comes from studies in humans based on positron emission tomography with 2-deoxy-2-[18 F] fluoro-D-glucose. These data show stimulus-dependent increases in glucose metabolic rate in primary and associative visual cortices of ∼40% and 60%, respectively62 as well as the positive correlation between spectral amplitudes of gamma oscillations and regional glucose uptake measured during seizure-free intervals, i.e., interictally, in patients with nonlesional focal epilepsy.63 Notably, correlations between glucose uptake and oscillations in other human frequency bands such as theta (4 to 7 Hz), alpha (8 to 12 Hz), and beta (16 to 32 Hz) were either marginal or even absent. The tight correlation between gamma oscillations and energy utilization was further confirmed by several recent studies using functional magnetic resonance imaging as a measure of neurovascular coupling. The power of gamma oscillations is positively correlated with the hemodynamic functional magnetic resonance imaging response in the cat visual cortex.64 There is also evidence for positive correlations between functional magnetic resonance imaging signals and gamma oscillations, for nearly the entire cerebral cortex of the monkey65 and for the human cortex during specific tasks.66, 67 Other authors, however, observed considerably smaller increases in local energy metabolism, amounting to 5% to 15% of ‘baseline versus task'-related differences in glucose consumption and blood flow.18, 60, 68 In any case, there is a large body of evidence for a correlation between enhanced energy metabolism and gamma oscillations in neuronal networks,34, 53 and for a correlation between this pattern of activity and higher brain functions, including consciousness.13, 33, 69
Several methodological aspects need to be considered when comparing the above-cited results from experiments in vitro and in vivo. First, the 2.2-fold increase in oxygen consumption rate in vitro was determined during persistent gamma oscillations in the pyramidal cell layer (perisomatic region) of the CA3 network in the hippocampal slices of the rat. Thus, ‘local' in the pO2 depth profile refers to a distance of ∼0.2 mm57 while the resolution of functional magnetic resonance imaging technologies is in the order of 1 mm3 and more. Second, the CA3 network is known to be an intrahippocampal generator of high-power gamma oscillations,9, 10 and the electrophysiological and neuroenergetical features of this network may not entirely apply to other cortical regions that express gamma oscillations. Third, ‘baseline' activity without detectable episodes of gamma oscillations might be higher in vivo than in partly deafferentiated slice preparations. For a full understanding of the state-, task-, region-, and lamina-specific energy metabolism, we will need further experimental studies in vitro and in vivo, including electrode-based and optical methods with improved spatiotemporal resolution.18, 60
The high energy utilization of neurons during gamma oscillations is most likely caused by increased rates of action potentials and synaptic interactions. In particular, the significant increase in the rates of excitatory postsynaptic potentials and IPSPs during gamma oscillations elicits strong ion fluxes across the neuronal membrane of pyramidal cells and inhibitory interneurons.8, 19, 70 These ion fluxes tend to dissipate the gradients of sodium, calcium, potassium, and chloride ions, thus utilizing potential energy. In order to maintain physiologic levels of excitability, these ionic gradients have to be continuously restored by ion pumps such as Na+/K+-ATPase and Ca2+-ATPase as well as transporters such as Na+/Ca2+-exchanger, Na+/H+-exchanger, K+/Cl−-cotransporter, and Na+/K+/Cl−-cotransporter. These transport processes are directed against equilibrium and finally fueled by adenosine-5'-triphosphate (ATP) that is generated mainly by oxidative phosphorylation in the neuronal mitochondria.2, 3, 70 This was recently supported by fluorescence imaging of nicotinamide adenine dinucleotide (phosphate) [NAD(P)H] and flavin adenine dinucleotide, i.e., by monitoring the cellular and mitochondrial redox state. Hippocampal gamma oscillations in vitro were associated with near-limit utilization of mitochondrial oxidative capacity, in line with oxygen consumption data.41, 57 Interestingly, subunits of mitochondrial complex I are strongly expressed in CA3, the hippocampal network with highest gamma oscillation power and oxygen consumption.41, 71 Complex I (NADH:ubiquinone oxidoreductase) is a large protein composed of up to 45 individual subunits that is part of the mitochondrial respiratory chain.72 It seems to have a key role for oxidative phosphorylation under normal conditions as well as in neurodegenerative diseases.73 The peculiar pattern of complex I subunit expression in the hippocampus likely reflects special enzymatic properties of neuronal mitochondria in the CA3 network, in agreement with the high cytochrome c oxidase activities in this region.70, 74 These data also support the notion that mitochondrial DNA genotype and nuclear–mitochondrial interactions are important determinants of higher cognitive tasks.75
It is currently hard to quantify the relative contribution of excitatory and inhibitory neurons to energy utilization during gamma oscillations. Owing to the lack of cell-specific data and in the interest of simplicity, global energy budgets for gray matter have been based on glutamatergic neurons that provide 85% to 90% of the neuronal population in the cortical circuits.3, 18 Based on the assumption of an average spiking rate of 4 Hz and a ‘sodium entry ratio' of 1.24 during the action potential, the updated calculations predict energy costs in the cerebral cortex of ∼25% for housekeeping, 15% for resting potentials, 16% for action potentials, and 44% for synaptic processes.3, 76 In primates, the synaptic costs might be even larger because of the higher numbers of synapses per neuron.76 Other studies also suggested that the major neuronal energy utilization is for excitatory synaptic signaling (neuronal input) rather than action potentials of the axon (neuronal output).60, 77, 78, 79 This might be reflected by the strong linear correlation between synaptic activities, oxygen metabolism, and cerebral blood flow in vivo, at least for some experimental stimulation paradigms.61, 80 In contrast to the excitatory projection cells, estimates about energy costs of inhibitory interneurons are widely lacking because of insufficient experimental data and cell-specific neuroenergetic knowledge.18, 76 Few studies from hippocampus and neocortex provide first evidence that (i) glucose metabolism is increased during long-term recurrent inhibition of hippocampal pyramidal cells,81 (ii) the contribution of GABA to the glutamate/GABA–glutamine cycle might account for 10% to 15% of the total oxidative metabolism82 and (iii) glucose metabolism might be significantly stronger in GABAergic neurons than in glutamatergic neurons as revealed by combining high-resolution 2-deoxyglucose and immunohistochemistry.83 For the latter technically advanced study with single-cell resolution, the methodological obstacles such as adequate label retention during immunohistochemical processing that may limit interpretation have been discussed.83, 84 For the remainder of this review, we will summarize current knowledge on the role of interneurons in cortical high-frequency oscillations, in particular, gamma oscillations, and on the possible neuroenergetical and pathophysiological implications of high energy utilization in a specific subset of these GABAergic cells, i.e., fast-spiking, parvalbumin-positive (PV+) interneurons.
Hippocampal gamma oscillations and fast-spiking inhibitory interneurons
Inhibitory interneurons represent highly diverse neuronal subpopulations in both the hippocampus and neocortex. In the rodent hippocampus, GABAergic interneurons comprise about 10% to 15% of the entire neuronal population, and at least 16 distinct types have been identified in the hippocampal CA1 region.23, 85 However, from the functional point of view, inhibitory interneurons can be grossly classified into (i) dendritic-targeting interneurons that control synaptic input to principal cells, (ii) perisomatic interneurons that control principal cell spiking, and (iii) interneurons that innervate and control other interneurons.8
Some dendritic-targeting interneurons such as bistratified and trilaminar GABAergic interneurons were reported to show fast-spiking behavior during hippocampal gamma oscillations.23, 86 However, the class of perisomatic inhibitory interneurons has gained much more attention.8, 16, 19, 87 This class comprises two types of basket cells as well as axo-axonic (chandelier) cells.87 Basket cells that contain cholecystokinin show regular spiking behavior and have been proposed to modulate synchronous neuronal activities as a function of subcortical inputs carrying information about motivation and emotions;52, 87 these will not be further discussed in this review. By contrast, basket cells and axo-axonic cells that contain Ca2+-binding protein, parvalbumin (PV+) show fast-spiking behavior8, 16, 19, 88 (Figures 1C–1F). PV+ basket and axo-axonic cells are abundant; they account for about 20% of the GABAergic interneuron population in the hippocampus. Within or adjacent to the principal cell layers, this value even rises to ∼50%.85, 89 Notably, many of the studies discussed below focused on fast-spiking behavior and parvalbumin content (PV+) for identification. Thus, unless detailed morphologic post hoc analysis was included, the data may originate from basket cells and/or axo-axonic cells.23
A single PV+ axo-axonic cell provides GABAergic synapses exclusively to the axon initial segments of up to 1,200 pyramidal cells.23 The characteristics of axo-axonic cells have been less explored but recent evidence suggests that these cells exert highly specialized functions during information processing in cortical networks.52, 87, 90 For the hippocampus, it was demonstrated in vitro that axo-axonic cells generate action potentials at much higher rate (∼100 Hz) compared with the frequency of gamma oscillations (∼35 Hz) in the CA3 network, and that they can control the antidromic propagation of ectopically generated spikes from axons into the soma of pyramidal cells during gamma oscillations.88 Moreover, axo-axonic cells in the CA3 network show significant phase coupling to hippocampal gamma oscillations in vivo, comparable in strength and phase to those of pyramidal cells and PV+ basket cells.91 In the rat somatosensory cortex, axo-axonic cells have been reported to depolarize pyramidal cells and thus initiate stereotyped series of synaptic events in neuronal networks because of a depolarized reversal potential for axonal relative to perisomatic GABAergic inputs.92 Further studies are required to clarify the crucial role of axo-axonic cells as suggested by their morphology and strategical positioning.
For the generation of hippocampal gamma oscillations, PV+ basket cells are of special importance (Table 1). These interneurons show extensive mutual connections with strong inhibitory synapses.23, 87 Moreover, they are coupled via electrical synapses, thus forming functional interneuron networks.49, 93, 94, 95, 96, 97, 98 This special feature is well suited to synchronize basket cell spiking with high temporal fidelity in the hippocampus as well as in the neocortex.8, 16, 24, 99 In brief, an action potential exerts a short-lasting depolarization of electrically coupled basket cells, which is immediately curtailed by the subsequent strong IPSP.16, 100, 101 This mechanism defines a short time window for mutual excitation of PV+ basket cells, thereby generating near-synchronous inhibition of multiple excitatory and inhibitory neurons in the network. These models are also in line with the observed short phase delay (<90°) of interneuron discharges behind pyramidal cells during hippocampal gamma oscillations in vitro.8, 9 It has been suggested that a single PV+ basket cell receives excitatory synaptic input from about 2,000 pyramidal cells.85 However, the highly divergent axonal plexus of basket cells has a mediolateral extent of >700 μm within the hippocampus where it contacts somata and proximal dendrites of pyramidal cells.18, 37, 86, 102 This connectivity feature allows synchronous inhibition of a multitude of pyramidal cells; the estimated numbers range from several hundred up to 2,500 cells.85, 103, 104 Together with the dendritic-targeting interneurons, the pattern of convergence and divergence in hippocampal network connectivity thus reflects the intense feed-forward and feedback control of principal cells by GABAergic inhibition.17, 37, 101, 105, 106
Table 1. Features of fast-spiking inhibitory interneurons and gamma oscillations.
| Unique feature | References |
|---|---|
| A | |
| Basket cell network | 93, 94, 95, 96, 97 |
| Perisomatic control of pyramidal cells | 56, 86, 89, 103, 104 |
| Fast-spiking (up to >100 Hz) | 48, 56, 86, 88, 108 |
| Rapid AP kinetics and high Na+ entry ratio | 134, 135, 138, 139, 141 |
| High numbers of mitochondria | 74, 118, 119, 121, 123 |
| Generation of gamma oscillations | 22, 36, 49, 51, 52 |
| B | |
| Strong relationship to higher brain functions | 25, 26, 28, 30, 31, 33 |
| High energy utilization | 41, 57, 63, 64, 67 |
| Exquisite sensitivity to metabolic stress | 41, 44, 47, 163, 164 |
AP, action potential.
Some unique features of (A) fast-spiking, parvalbumin-positive interneurons and (B) gamma oscillations (30 to 100 Hz) are summarized, including a selection of key references. Note that mitochondria in parvalbumin-positive interneurons also contain higher levels of cytochrome c oxidase (complex IV) and cytochrome c.
Importantly, PV+ basket cells and axo-axonic cells strongly increase their spiking rate during specific oscillating network states of the hippocampus, especially during theta-, gamma-, and ripple oscillations.22, 35, 47, 91, 107 These interneurons are able to generate fast series of action potentials up to several hundred Hertz (hence: ‘fast-spiking'), and they are active in almost every cycle of gamma oscillations (∼30 to 100 Hz) in vitro and in vivo.35, 48, 52, 56, 86, 88, 108 The resulting fast periodic release of GABA induces strong rhythmic perisomatic inhibition of almost all local principal cells,9, 10, 23 which is reflected by the typical gamma oscillations in local field potential recordings.8, 48, 52 Thus, fast-spiking interneurons are ideally suited to control both pattern and timing of the action potential generation in principal cells (‘clockwork'), which has been demonstrated by experimental data and mathematical modeling.29, 37, 38, 102, 107 Based on these properties, fast-spiking, PV+ interneurons establish a temporal matrix for information processing in the cortex (Figures 1F and 2A). This notion has been underlined by elegant in vivo studies in mice showing that selective genetic modification of glutamate receptor subtypes in fast-spiking, PV+ interneurons strongly affects power and synchrony of gamma oscillations, together with impaired hippocampus-dependent cognitive functions.50, 51 Similar to the hippocampus, fast-spiking interneurons control pyramidal cell activity during gamma oscillations in the neocortex and thus promote neocortical information processing in vitro and in vivo.36, 99, 109, 110, 111 Pyramidal cells, in contrast, show strong action potential frequency accommodation and have typical spiking rates around 1 to 3 Hz during hippocampal gamma oscillations in vitro and in vivo.56, 86, 98, 112 This finding underlines the general principle of sparse coding by pyramidal cells, which has been suggested to provide an optimal trade-off between information processing and energy efficacy.113, 114 However, pyramidal cells comprise ∼85% of all neurons in the hippocampus proper, and even with sparse coding they are able to encode multiple representations of spatial and episodic memories.18, 20, 23, 85 By propagating specific activity patterns into downstream cortical and subcortical regions, pyramidal cells also impact on network activity and information processing in multiple other brain regions.9, 10, 21 From a neuronal network perspective, it can be said that gamma oscillations are characterized by high action potential frequencies, strongly divergent local connectivity and high synaptic efficacy of fast-spiking, PV+ interneurons. This contrasts with the far higher number but lower spiking frequency and efficacy of principal cells, such that excitation and inhibition are in balance.16, 18 This balance is dynamically regulated and maintained by the feedback connectivity between pyramidal cells and PV+ basket cells.17, 115, 116
In summary, convergent evidence from electrophysiological recordings, genetic interventions, quantitative neuroanatomical analysis, and mathematical modeling in both the hippocampus and neocortex reveals that fast-spiking, PV+ inhibitory interneurons are a central element for the generation of gamma oscillations and, therefore, information processing in the cortex.
Mitochondria in fast-spiking inhibitory interneurons
While this key role of fast-spiking, PV+ interneurons for fast rhythmic network activity has been consistently recognized, the underlying neuroenergetics have gained much less attention. It is likely, however, that patterns of neuronal network activity represent a compromise between maximal information processing and minimal energy utilization.113, 114 It is therefore important to understand the activity-dependent neuroenergetic demands and constraints of this highly active class of neurons. Indeed, several findings indicate that fast-spiking interneurons use much more energy than other cells in the central nervous system, which might render them particularly vulnerable to conditions of energy deficiency. Energy utilization is counterbalanced by generation of cellular energy carrier, ATP via cytosolic glycolysis and oxidative phosphorylation in mitochondria, the latter of which requiring oxygen as a final electron acceptor.2, 72 Indeed, most basket cells and axo-axonic cells contain considerably higher numbers of mitochondria in dendrites, somata and presynaptic terminals as compared with other interneuron subpopulations and pyramidal cells.74, 117, 118, 119, 120, 121, 122, 123 This property is highly conserved in various species of mammals.74, 117, 124 Moreover, mitochondria of fast-spiking, PV+ interneurons are enriched with proteins such as cytochrome c oxidase (complex IV) and cytochrome c that are crucial for electron transport in the mitochondrial respiratory chain.74, 121 Similar observations have been made in interneuron subpopulations of the visual cortex.124, 125 We note that these findings indicate relative metabolic capacity rather than actual activity of fast-spiking interneurons in vivo. However, as there is evidence for a positive correlation between the capacity for oxidative metabolism and the rates of metabolic activity,74, 126, 127, 128 the findings suggest that fast-spiking interneurons do indeed use high amounts of energy, which they gain from oxidative phosphorylation in mitochondria. Interestingly, several studies indicate that GABAergic interneurons, including PV+ interneurons participate in regulating hemodynamic responses.129, 130, 131 Moreover, the oxygen overshoot during the activity-dependent hemodynamic response might serve the upkeep of sufficient oxygen levels in mitochondria of fast-spiking interneurons, particularly when located in some distance from the vasculature.132, 133
Why would fast-spiking interneurons need higher amounts of energy than more scarcely spiking principal cells or other types of interneurons? Two distinguishing features of fast-spiking interneurons have been suggested as activity-dependent mechanisms of high energy utilization: (i) high frequency and fast kinetics of the action potential and (ii) synaptic inhibition per se.18, 134, 135 While there is no final consensus on the reasons for the specific neuroenergetic profile of fast-spiking interneurons, several arguments indicate that both distinguishing features contribute to the high energy utilization and thus oxygen consumption.
Energy utilization of fast-spiking behavior and synaptic inhibition
As indicated by their name, fast-spiking interneurons can generate action potentials at high frequencies (>100 Hz) for prolonged periods of time, with weak or no accommodation.37, 85 Several properties of these neurons support this high level of activity: (i) the ‘resting' membrane potential is ∼10 to 15 mV closer to spike threshold than in pyramidal cells; (ii) intrinsic membrane properties include resonance at frequencies within the gamma-frequency band; (iii) action potentials follow a particularly rapid time course and propagate with high reliability along the largely unmyelinated axon.37, 48, 102, 134, 136, 137, 138 Notably, the conduction velocity of fast-spiking interneurons is higher than that of unmyelinated axons of glutamatergic principal cells.138 These features of the action potential, however, come at a price: fast-spiking interneurons show (i) a ‘supercritical' density of voltage-gated Na+-channels all over the unmyelinated axon that results in high Na+-conductance density and (ii) a larger overlap of depolarizing Na+-entry and repolarizing K+-efflux (‘sodium entry ratio' of ∼1.98), which differs markedly from cortical principal cells.135, 138, 139 The ‘supercritical' density of Na+-channels is thought to compensate for the morphologic axonal properties of fast-spiking interneurons, i.e., small segmental diameter, extensive branching and high bouton density and to permit fast propagation of the action potential.138 Thus, high-frequency repetitive firing in fast-spiking interneurons likely causes a much larger dissipation of ion gradients than in neurons with energetically optimized action potential waveforms.138, 139 This would enhance the need for ATP to fuel Na+/K+-ATPase and Ca2+-ATPase to restore the original, non-equilibrium ion distributions. However, energy-saving mechanisms that may partially compensate for the high ATP demand have been discussed for fast-spiking interneurons, i.e., fewer excitatory input sites, partially higher input resistance as well as expression of voltage-gated K+-channels of the Kv3 subtype and rapidly inactivating Na+-channels.18, 134, 138, 140, 141, 142, 143 Examples from the basal ganglia also suggest that high spiking rates of GABAergic neurons do not necessarily correlate with their energetic needs.18 As long as we are lacking direct empirical evidence for the activity-dependent energy utilization of these interneurons, it will be difficult to decide how much energy is required to permit their fast-spiking behavior. Systematic recordings from dendrites and axons under physiologic and pathophysiological conditions are required to precisely assess the energy costs of fast-spiking interneurons.
As an alternative of complementary explanation for the high energy utilization the synaptic functions of fast-spiking GABAergic interneurons need to be considered. During fast network oscillations, almost all local neurons are phasically inhibited on every oscillation cycle, and most of this inhibition is perisomatic, i.e., at the soma, the proximal dendrites and the axon initial segment.35, 52, 87, 107 In neuronal networks of the hippocampus proper, fast-spiking PV+ interneurons form numerous GABAergic synaptic contacts to pyramidal cells in the perisomatic region23, 87 (Figures 1C–E). As outlined above, these synapses are strongly and rhythmically activated during fast network oscillations, and multiple lines of evidence indicate that this phasic inhibition is a key factor in coordinating neuronal activity during network states such as gamma oscillations or sharp wave–ripple complexes.9, 10 It would, therefore, be desirable to precisely know the energy utilization as well as the frequency of unitary GABAergic events during resting states and gamma oscillations. This would allow for a solid quantification of the inhibition-related energy utilization of fast-spiking interneurons, which includes the presynaptic GABA release as well as the postsynaptic GABA effects in both principal cells and interneurons. However, this turns out to be a difficult task.18 Fast phasic GABAergic inhibition acts via activation of postsynaptic anion channels, i.e., GABAA receptors.16, 37 These channels are selectively permeable to chloride and, to a lower extent, bicarbonate (HCO3−), thereby shifting the postsynaptic membrane potential more closely the reversal potential of these ions.144, 145 These processes contain several energy-utilizing steps for release and metabolism of GABA (see below) and, most importantly, the restoration of membrane ion gradients. Transmitter release is a very fast and highly specialized process that involves the presynaptic vesicular cycle, Ca2+-dependent membrane fusion and subsequent buffering or extrusion of calcium from the presynaptic terminal.146, 147 In principle, the energy costs of these processes should not differ between excitatory and inhibitory synapses, but the frequency of release is likely much higher in terminals of fast-spiking interneurons as compared with other cell types. Fast-spiking interneurons have by far the highest frequency of action potentials during gamma oscillations.23, 52, 85, 88 At the same time, the release of GABAergic synaptic vesicles from fast-spiking interneurons shows large quantal size, high release probability and, presumably, less conduction failures as compared with typical excitatory neurons.4, 37, 100, 148, 149 Moreover, an activity-dependent gating mechanism that reduces release probability appears to preserve the releasable pool of synaptic vesicles during high-frequency repetitive firing.100 While a quantification of energy costs is difficult in the absence of direct experimental data, a recent study in the hippocampal slices does indeed suggest that presynaptic Ca2+-removal, vesicle turnover, and neurotransmitter transport use more energy than generally assumed.150 Thus, it is well feasible that the sustained and fast cycling of vesicles as well as the very frequent Ca2+-entry in presynaptic terminals of fast-spiking interneurons require significant amounts of ATP, in line with the high numbers of presynaptic mitochondria.74, 117, 121, 123 A second energy-utilizing process in synaptic transmission is the restoration of ion gradients across neuronal membranes. This process primarily affects the postsynaptic target neurons of inhibitory synapses. Indeed, the perisomatic region of pyramidal cells in CA3, which is the hippocampal subfield with most prominent gamma oscillations and oxygen consumption, shows alternating current sinks and sources during gamma oscillations in vitro and in vivo.22, 56 Moreover, this perisomatic region contains numerous mitochondria, which is not a general feature of cortical principal cells.70, 74, 151 In case of inhibitory synapses with dominant Cl−-conductance, restoration of ion gradients is served by secondarily active transporters, especially the K+/Cl−-cotransporter, KCC2.16, 17, 18, 144 When chloride enters the cell during synaptic inhibition, subsequent Cl−-transport will be directed outwardly, against the equilibrium potential of chloride. The required driving force for the outward transport is provided by the electrochemical potential of potassium being established by the Na+/K+-ATPase. However, it is currently not clear how much Cl−-flux underlies synaptic inhibition during different activity states in cortical networks.18 Consider the case where resting membrane potential and equilibrium potential of chloride are identical: in this situation, there is no net flux of chloride, despite an increased membrane conductance because of the open GABAA receptors. Such ‘shunting inhibition' can still be highly efficient to reduce the effect of glutamatergic inputs,16, 17, 145 without changing the distribution of chloride. In addition to hyperpolarizing inhibition, shunting inhibition seems to be indeed a typical case rather than an exception in cortical principal cells.16, 152, 153 As a general rule, resting membrane potential of pyramidal cells and equilibrium potential of chloride are not very far from each other, which perhaps constitutes an energy-saving mechanism in networks with intense synaptic inhibition.18 Fast-spiking interneurons, in contrast, have ∼10 to 15 mV more depolarized membrane potentials than pyramidal cells37, 102 and may thus have considerably higher energy costs for Cl−-extrusion associated with mutual GABAergic inhibition. There might be yet another challenge to ion homeostasis even when the equilibrium potential of chloride is not far from the resting membrane potential: excitatory and inhibitory synaptic inputs do usually form a dynamic equilibrium, i.e., an increase of inhibition goes along with an increase of excitation and vice versa.154 This increase in overall synaptic activity keeps excitability within a narrow functional range; but in situations of high membrane conductance, it causes increased ion fluxes for excitatory transmission, even when inhibition is purely shunting.18 Thus, ion homeostasis during network activity states with strong synaptic inhibition may create a considerable energy demand, in both inhibitory interneurons and excitatory pyramidal cells.
A related important aspect is neurotransmitter metabolism, i.e., biosynthesis, vesicular storage, and release of GABA in inhibitory interneurons. GABA is derived from glutamate by glutamic acid decarboxylase, which occurs in two isoforms, GAD65 and GAD67. While GAD67 is expressed throughout the cytosol, GAD65 is the dominant presynaptic isoform, which is directly associated to presynaptic vesicles and therefore seems to be important for their filling.106, 155, 156 This filling process, however, requires activity of a vacuolar H+-ATPase that provides the electrical (ΔΨ) and chemical (ΔpH) components of the driving force for the vesicular inhibitory amino-acid transporter.147, 157 After release to the synaptic cleft, re-uptake of GABA occurs via Na+-/Cl−-dependent GABA transporter 1 in the presynaptic terminal or via GAT-3 in processes of astrocytes that enwrap the synapse. Again, these processes are secondarily active, thus utilizing potential energy that has been previously provided by the Na+/K+-ATPase and other transport systems.147 However, for mouse astrocytes in primary culture, it was described that GABA uptake resulted in increases of cytosolic Na+-concentration but not of glucose utilization.158 Systematic studies in complex brain preparations during naturally occurring network states are required to further elucidate the energy costs of astrocytic GABA uptake. In astrocytes, GABA is subsequently converted to glutamine by several enzymatic steps. Glutamine is finally shuttled from astrocytes to neurons by secondarily active transporters (glutamate/GABA–glutamine cycle), before being converted to glutamate via phosphate-activated glutaminase.106, 159 It is important to note that all involved membrane transport processes depend directly or indirectly on ATP. They may, therefore, significantly contribute to the energetic needs of enhanced synaptic inhibition. Mitochondria of interneurons are also involved in the conversion of α-ketoglutarate or glutamine to glutamate while conversion of GABA to glutamate takes place in astrocytic mitochondria.106, 159 Notably, several studies suggest that the mitochondrial tricarboxylic acid cycle is central for the biosynthesis of GABA from glutamine or lactate, an alternative source of the transmitter.155, 159, 160, 161, 162
In summary, electrophysiological recordings, quantitative neuroanatomical and biochemical analyses in different cortical preparations suggest that fast-spiking interneurons have unique electrophysiological and neuroenergetical properties that ultimately require sufficient substrate and oxygen supply as well as proper mitochondrial performance for generation of ATP and allocation of GABA.
Perspective: metabolic and oxidative stress in fast-spiking interneurons
The rapid abolishment in the power of gamma oscillations has been described under various experimental conditions in slice preparations: (i) when the pO2 of the ambient atmosphere was lowered to the normoxic range under semi-interface recording condition,44 (ii) when the flow rate of oxygenated recording solution was below a critical limit under submerged recording condition,47 and (iii) when hypoxic events were induced.46 In line with these findings, unilateral hippocampal ischemia in vivo caused a transient of pathologic high-frequency discharges followed by a dramatic and long-lasting selective decrease in the power of the gamma oscillations.163 Such decreases in gamma oscillation power were also observed in vitro during pharmacological interference with mitochondrial function (‘poisoning'), namely inhibition of the mitochondrial respiratory chain by rotenone (acting on complex I) or potassium cyanide (acting on cytochrome c oxidase in complex IV), or after mitochondrial uncoupling by protonophores.41, 164, 165 Intriguingly, other forms of activity such as electrical stimulus-evoked neuronal activation, low-frequency oscillations, and seizure-like events were much more resistant to low pO2 or pharmacological inhibition of the mitochondrial respiratory chain in vitro and in vivo,41, 44, 163 suggesting that fast-spiking interneurons that are crucial for the gamma oscillations were primarily affected.
The studies summarized above show that fast-spiking interneurons are capable to maintain cellular energy homeostasis during gamma oscillations under physiologic conditions. By contrast, fast-spiking interneurons may dramatically lower the threshold for neuronal network dysfunction during metabolic stress that occurs during hypoxia, ischemia, or dysfunction of mitochondria. This notion is indirectly supported by unique morphologic, electrophysiological, and neuroenergetical properties of fast-spiking interneurons as well as by their central role in the generation of network oscillations (see above). A recent study provided the first direct experimental insight into the cellular mechanisms underlying the rapid decrease of gamma oscillations during metabolic stress.164 The authors found no significant changes in resting membrane potential of pyramidal cells during application of potassium cyanide (acting on cytochrome c oxidase in complex IV), indicating maintenance of cellular excitability. By contrast, fast-spiking basket cells showed a sustained membrane depolarization, resulting in almost complete cessation of spiking and thus strongly reduced rates of IPSPs impinging on pyramidal cells. This rapid dysfunction does not necessarily imply a lower threshold of PV+ interneurons to ischemia-induced neuronal death.166 Similar findings were obtained for fast-spiking interneurons during gamma oscillations when oxygen supply was lowered.47 This first experimental evidence suggests that even moderate hypoxia or poisoning of mitochondrial oxidative phosphorylation selectively results in rapid decrease in ATP levels and GABA release in fast-spiking interneurons.41, 167 This might be of particular importance for those fast-spiking interneurons that are located in the periphery of cortical vascular supply regions, with limited oxygen availability.133 Moderate metabolic stress may thus disrupt fast phasic inhibition of fast-spiking interneurons more effectively than other types of inhibition or pyramidal cell excitation in neuronal networks87, 116 (Figure 2B). The disruption of rhythmic inhibition would cause rapid loss of gamma oscillations, with devastating consequences on information processing in local and, perhaps, remote cortical networks34, 41, 44, 164 (Figure 3). This pathophysiological mechanism might help to explain the exceptional vulnerability of higher brain functions during acute metabolic stress, which has long been known from clinical medicine and experimental neurology.53 In particular, symptoms such as visual field narrowing and unconsciousness being accompanied with slow-wave activity in the electroencephalogram occur very rapidly during ischemia in both humans and animals, whereas evoked responses and ion distributions are more resistant.168, 169, 170, 171
Figure 2.
Highly energized inhibitory interneurons are a central element for information processing in cortical networks (interneuron energy hypothesis). (A) Synchronized rhythmic activity of pyramidal cells (PC, orange cell population on the left) highly depends on mutual interactions with inhibitory GABAergic interneurons (IN, heterogeneous cell population on the right) via excitatory (EPSPs, arrow-headed connections) and inhibitory postsynaptic potentials (IPSPs, line-headed connections). GABAergic interneurons exert different types of inhibition in pyramidal cells (three solid spheres with diverse blue colors), including fast phasic inhibition (intermittent blue spheres). In particular, fast-spiking interneurons such as parvalbumin-positive basket cells are central for fast rhythmic synchronization of pyramidal cell activity (‘clockwork'). Fast-spiking interneurons are characterized by unique electrophysiological and neuroenergetical properties such as high numbers of mitochondria. Note that gamma oscillations in local field potential recordings (LFP) primarily reflect synchronized IPSPs. (B) As a perspective, the functional consequences of metabolic stress in fast-spiking interneurons are illustrated. Even moderate metabolic stress, which occurs either acutely (e.g., hypoxia/ischemia and mitochondrial poisoning) or chronically (e.g., arteriosclerosis or mitochondrial disorders), might cause dramatic decreases in ATP levels and GABA release in fast-spiking interneurons (switch from intermittent to solid blue spheres). This is associated with a decrease of action potentials in fast-spiking interneurons, the loss of gamma oscillations in the local field potential (LFP) (‘blurred clockwork') and the precise temporal sequence of information processing in the neuronal network, and, consequently, higher brain functions (compare three lines at the bottom in A and B). This pathophysiological mechanism might be worsened by acute or chronic oxidative stress in fast-spiking interneurons. By contrast, other types of inhibition (three solid spheres with diverse blue colors) as well as pyramidal cell excitation might be less affected because of higher cellular thresholds to metabolic stress. The scheme is modified from Bartos et al.16
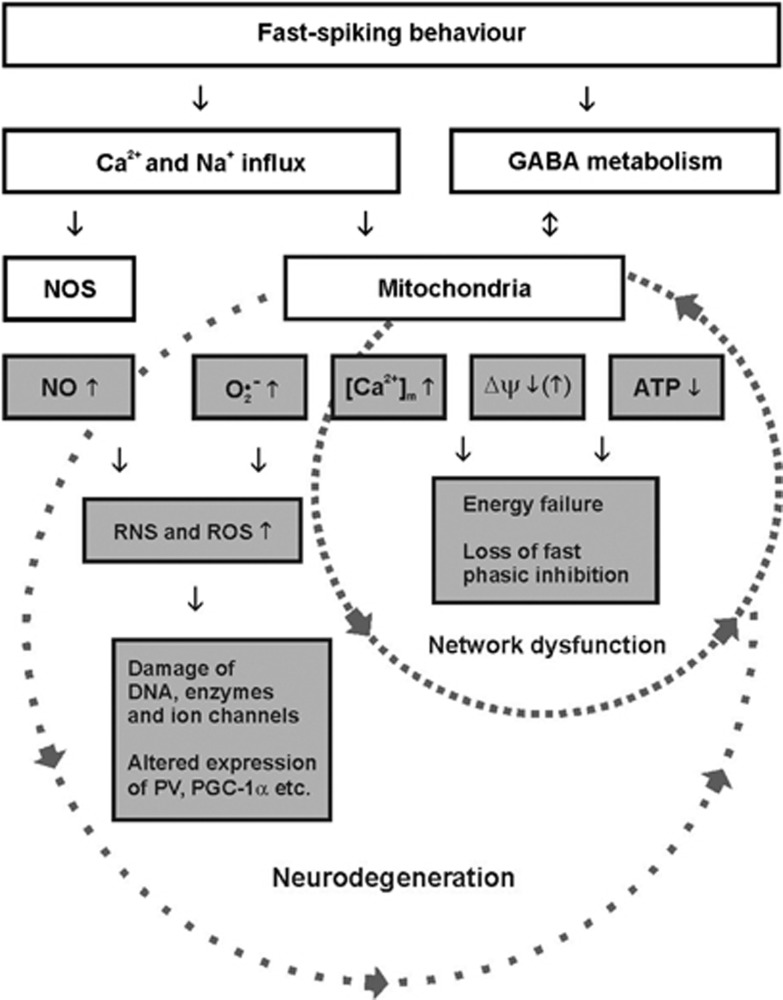
Figure 3.
The simplified scheme illustrates the perspective on metabolic and oxidative stress in fast-spiking, parvalbumin-positive interneurons. The small circle reflects acute effects of hypoxia/ischemia or mitochondrial poisoning that rapidly result in neuronal network dysfunction. The large circle reflects chronic effects of mitochondrial dysfunction and/or cerebral hypoperfusion that finally result in neurodegeneration. Note that the pathophysiological mechanisms illustrated in both circles may partially interact, depending on the course of the disease and pathogenic factors such as alterations in expression of ion channels, parvalbumin or PGC-1α that may result in imbalances of oxidative and anti-oxidative processes. [Ca2+]m, mitochondrial Ca2+-concentration; GABA, γ-aminobutyric acid; NO, nitric oxide; NOS, nitric oxide synthase; O2•−, superoxide anion; ΔΨ, mitochondrial membrane potential; RNS, reactive nitrogen species; ROS, reactive oxygen species; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PV, parvalbumin.
Fast-spiking interneurons might also experience higher levels of oxidative stress under both physiologic and pathophysiological conditions. At the mitochondrial respiratory chain, the small amount of electron leakage (0.1% to 4%) induces one-electron reduction of oxygen, which results in relatively stable superoxide anion—a free radical with biologic toxicity.172 Superoxide anion is generated at several mitochondrial sites such as complexes I and III, and this occurs readily during mitochondrial respiration at physiologic levels of neuronal activity. Specific genome mutations, for example, alter the electron transfer properties of respiratory chain complexes and result in abnormal high levels of superoxide anion.173 Such abnormal levels can favor the accumulation of hydrogen peroxide and the appearance of other molecular oxygen-derived free radicals and precursors, collectively known as reactive oxygen species (ROS).172, 173 Another gaseous molecule is nitric oxide (NO) that is generated through enzymatic conversion of L-arginine to L-citrulline by constitutive neuronal nitric oxide synthase. Nitric oxide is more labile and acts as both neurotransmitter and regulator of cerebral blood flow. It is released by many neuronal subtypes, including fast-spiking interneurons.174 ROS react with nitric oxide, and may finally generate more harmful reactive nitrogen species.173 Therefore, the maintenance of ROS at low levels is critical for normal cell function. Conversely, prolonged performance of mitochondria carries an inherent risk of increasing ROS levels. Fast-spiking interneurons might even generate higher ROS levels because the unique electrophysiological and neuroenergetical properties (see above) may frequently result in mismatching changes of metabolic state, Ca2+-load and pO2 in the mitochondria. Such mismatches have been considered to promote superoxide anion generation.173, 175 This might be the reason for the strong expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha in PV+ interneurons, at least during certain stages of brain development.176, 177 Peroxisome proliferator-activated receptor gamma coactivator 1-alpha has the capability to induce the expression of enzymes involved in glutathione biosynthesis as well as of ROS-detoxifying enzymes such as superoxide dismutase and glutathione peroxidase. Under pathologic conditions, the activation of ionotropic glutamate receptors with high Ca2+-permeability and/or alterations in parvalbumin expression in fast-spiking interneurons might result in cytosolic and mitochondrial Ca2+-overload that would also enhance the generation of ROS and nitric oxide.173, 178, 179, 180 Enhanced ROS generation combined with alterations of anti-oxidative mechanisms may finally cause higher levels of oxidative stress in fast-spiking interneurons as compared with other neuron subtypes, with devastating consequences on nuclear and mitochondrial DNA and/or functional proteins such as enzymes and ion channels.173, 181, 182, 183, 184
These putative pathophysiological mechanisms in fast-spiking interneurons, i.e., higher susceptibility to metabolic and oxidative stress, might apply to several neurologic and psychiatric disorders that go along with chronic mitochondrial dysfunction and/or chronic cerebral hypoperfusion (Figure 3). Clinical examples are mitochondrial disorders (mitochondriopathies), arteriosclerosis, ageing, Alzheimer's disease, Parkinson's disease, and schizophrenia.5, 13, 72, 167, 172, 185 Indeed, experimental evidence suggests that fast-spiking interneurons have a key role in the (patho)physiologic processes underlying ageing, Alzheimer's disease, and schizophrenia.165, 183, 186, 187, 188 While recent studies yielded considerable insight into mitochondria and neuroenergetics during gamma oscillations, important features of interneurons, in particular, of fast-spiking interneurons, have been less defined. This includes detailed information about electrophysiological properties, bioenergetics, energy efficacy, GABA metabolism, free radicals, microdomains with densely packed respiring mitochondria, as well as supply of oxygen and energy substrates distant from the vasculature. These aspects need to be studied under physiologic and pathophysiological conditions in adult and aged animals. Therefore, further understanding of neuronal network dysfunction as related to metabolic and oxidative stress requires combined efforts from molecular genetics, biochemistry, electrophysiology, and live-cell imaging techniques with high spatial and temporal resolution, including mathematical modeling.
Acknowledgments
The authors thank Dr Nikolaus Berndt for critical reading of the manuscript and Andrea Lewen for text editing assistance.
The authors declare no conflict of interest.
Footnotes
This work was supported by the German Ministry of Education and Science (BMBF 01GQ1003A, BCCN Heidelberg/Mannheim, B3).
References
- Siesjö BK.(ed). Brain Energy Metabolism John Wiley & Sons: New York, USA; 1978 [Google Scholar]
- Erecińska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol. 2001;128:263–276. doi: 10.1016/s0034-5687(01)00306-1. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol Rev. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- Lisman J, Buzsáki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr Bull. 2008;34:974–980. doi: 10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Paulsen O. Network mechanisms of gamma oscillations in the CA3 region of the hippocampus. Neural Netw. 2009;22:1113–1119. doi: 10.1016/j.neunet.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA.(eds). Cortical Oscillations in Health and Disease Oxford University Press Inc.: New York, USA; 2010 [Google Scholar]
- Buzsáki G.(ed). Rhythms of the Brain Oxford University Press Inc.: New York, USA; 2006 [Google Scholar]
- Fries P, Nikolić D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Stanford IM, Jefferys JGR. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature. 1996;383:621–624. doi: 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- Melzer S, Michael M, Caputi A, Eliava M, Fuchs EC, Whittington MA, et al. Long-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science. 2012;335:1506–1510. doi: 10.1126/science.1217139. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 2007;30:343–349. doi: 10.1016/j.tins.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Kaila K, Raichle M. Inhibition and brain work. Neuron. 2007;56:771–783. doi: 10.1016/j.neuron.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Paulsen O, Moser EI. A model of hippocampal memory encoding and retrieval: GABAergic control of synaptic plasticity. Trends Neurosci. 1998;21:273–278. doi: 10.1016/s0166-2236(97)01205-8. [DOI] [PubMed] [Google Scholar]
- de Almeida L, Idiart M, Lisman JE. Memory retrieval time and memory capacity of the CA3 network: role of gamma frequency oscillations. Learn Mem. 2007;14:795–806. doi: 10.1101/lm.730207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsáki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamás G, Buhl EH, Lörincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- Gray CM, König P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Hu L, Hung YS, Mouraux A, Iannetti GD. Gamma-band oscillations in the primary somatosensory cortex—a direct and obligatory correlate of subjective pain intensity. J Neurosci. 2012;32:7429–7438. doi: 10.1523/JNEUROSCI.5877-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa D, Spolidoro M, Proville RD, Guyon N, Belliveau L, Léna C. Functional role of the cerebellum in gamma-band synchronization of the sensory and motor cortices. J Neurosci. 2013;33:6552–6556. doi: 10.1523/JNEUROSCI.5521-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Greischar LL, Rawlings NB, Ricard M, Davidson RJ. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proc Natl Acad Sci USA. 2004;101:16369–16373. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Montgomery SM, Buzsáki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci USA. 2007;104:14495–14500. doi: 10.1073/pnas.0701826104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vugt MK, Schulze-Bonhage A, Litt B, Brandt A, Kahana MJ. Hippocampal gamma oscillations increase with memory load. J Neurosci. 2010;30:2694–2699. doi: 10.1523/JNEUROSCI.0567-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann D, Faber PL, Achermann P, Jeanmonod D, Gianotti LRR, Pizzagalli D. Brain sources of EEG gamma frequency during volitionally meditation-induced, altered states of consciousness, and experience of the self. Psychiatry Res. 2001;108:111–121. doi: 10.1016/s0925-4927(01)00116-0. [DOI] [PubMed] [Google Scholar]
- Melloni L, Molina C, Pena M, Torres D, Singer W, Rodriguez E. Synchronization of neural activity across cortical areas correlates with conscious perception. J Neurosci. 2007;27:2858–2865. doi: 10.1523/JNEUROSCI.4623-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord LD, Expert P, Huckins JF, Turkheimer FE. Cerebral energy metabolism and the brain's functional network architecture: an integrative review. J Cereb Blood Flow Metab. 2013;33:1347–1354. doi: 10.1038/jcbfm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Márton LF, Roberts JDB, Cobden PM, Buzsáki G, et al. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Fricker D, Miles R. Interneuron diversity series: fast in, fast out—temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci. 2004;27:30–40. doi: 10.1016/j.tins.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Colling SB, Buzsáki G, Jefferys JGR. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol. 1996;493:471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draguhn A, Traub RD, Schmitz D, Jefferys JGR. Electrical coupling underlies high-frequency oscillations in the hippocampus in vitro. Nature. 1998;394:189–192. doi: 10.1038/28184. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Kann O, Huchzermeyer C, Kovács R, Wirtz S, Schuelke M. Gamma oscillations in the hippocampus require high complex I gene expression and strong functional performance of mitochondria. Brain. 2011;134:345–358. doi: 10.1093/brain/awq333. [DOI] [PubMed] [Google Scholar]
- Kann O, Kovács R, Njunting M, Behrens CJ, Otáhal J, Lehmann T-N, et al. Metabolic dysfunction during neuronal activation in the ex vivo hippocampus from chronic epileptic rats and humans. Brain. 2005;128:2396–2407. doi: 10.1093/brain/awh568. [DOI] [PubMed] [Google Scholar]
- Brennan AM, Connor JA, Shuttleworth CW. NAD(P)H fluorescence transients after synaptic activity in brain slices: predominant role of mitochondrial function. J Cereb Blood Flow Metab. 2006;26:1389–1406. doi: 10.1038/sj.jcbfm.9600292. [DOI] [PubMed] [Google Scholar]
- Huchzermeyer C, Albus K, Gabriel H-J, Otáhal J, Taubenberger N, Heinemann U, et al. Gamma oscillations and spontaneous network activity in the hippocampus are highly sensitive to decreases in pO2 and concomitant changes in mitochondrial redox state. J Neurosci. 2008;28:1153–1162. doi: 10.1523/JNEUROSCI.4105-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CN, Klein-Flügge MC, Howarth C, Attwell D. Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci. 2012;32:8940–8951. doi: 10.1523/JNEUROSCI.0026-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fano S, Behrens CJ, Heinemann U. Hypoxia suppresses kainate-induced γ-oscillations in rat hippocampal slices. NeuroReport. 2007;18:1827–1831. doi: 10.1097/WNR.0b013e3282f13e4f. [DOI] [PubMed] [Google Scholar]
- Hájos N, Ellender TJ, Zemankovics R, Mann EO, Exley R, Cragg SJ, et al. Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur J Neurosci. 2009;29:319–327. doi: 10.1111/j.1460-9568.2008.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttonen M, Kamondi A, Acsády L, Buzsáki G. Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. Eur J Neurosci. 1998;10:718–728. doi: 10.1046/j.1460-9568.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JGR. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, LeBeau FEN, Bannerman DM, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Szabó GG, Ulbert I, Holderith N, Monyer H, Erdélyi F, et al. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci. 2010;30:15134–15145. doi: 10.1523/JNEUROSCI.4104-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann O. The energy demand of fast neuronal network oscillations: insights from brain slice preparations. Front Pharmacol. 2011;2:90. doi: 10.3389/fphar.2011.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Wang X-J. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Radcliffe CA, Paulsen O. Hippocampal gamma-frequency oscillations: from interneurones to pyramidal cells, and back. J Physiol. 2005;562:55–63. doi: 10.1113/jphysiol.2004.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Pálhalmi J, Mann EO, Németh B, Paulsen O, Freund TF. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci. 2004;24:9127–9137. doi: 10.1523/JNEUROSCI.2113-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huchzermeyer C, Berndt N, Holzhütter H-G, Kann O. Oxygen consumption rates during three different neuronal activity states in the hippocampal CA3 network. J Cereb Blood Flow Metab. 2013;33:263–271. doi: 10.1038/jcbfm.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafaee MS, Gjedde A. Spatially dissociated flow-metabolism coupling in brain activation. NeuroImage. 2004;21:507–515. doi: 10.1016/j.neuroimage.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Lactate efflux and the neuroenergetic basis of brain function. NMR Biomed. 2001;14:389–396. doi: 10.1002/nbm.741. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Mathiesen C, Schaefer K, Thomsen KJ. Neuronal inhibition and excitation, and the dichotomic control of brain hemodynamic and oxygen responses. NeuroImage. 2012;62:1040–1050. doi: 10.1016/j.neuroimage.2012.01.040. [DOI] [PubMed] [Google Scholar]
- Phelps ME, Kuhl DE, Mazziota JC. Metabolic mapping of the brain's response to visual stimulation: studies in humans. Science. 1981;211:1445–1448. doi: 10.1126/science.6970412. [DOI] [PubMed] [Google Scholar]
- Nishida M, Juhász C, Sood S, Chugani HT, Asano E. Cortical glucose metabolism positively correlates with gamma-oscillations in nonlesional focal epilepsy. NeuroImage. 2008;42:1275–1284. doi: 10.1016/j.neuroimage.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RAW. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Schölvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci USA. 2010;107:10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux J-P, Fonlupt P, Kahane P, Minotti L, Hoffmann D, Bertrand O, et al. Relationship between task-related gamma oscillations and BOLD signal: new insights from combined fMRI and intracranial EEG. Hum Brain Mapp. 2007;28:1368–1375. doi: 10.1002/hbm.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa R, Fries P, Petersson K-M, Oostenveld R, Grothe I, Norris DG, et al. Neuronal dynamics underlying high- and low-frequency EEG oscillations contribute independently to the human BOLD signal. Neuron. 2011;69:572–583. doi: 10.1016/j.neuron.2010.11.044. [DOI] [PubMed] [Google Scholar]
- Lin A-L, Fox PT, Hardies J, Duong TQ, Gao J-H. Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proc Natl Acad Sci USA. 2010;107:8446–8451. doi: 10.1073/pnas.0909711107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Schmitz DP, Wagner T, Elger CE, Fell J. Interactions between medial temporal lobe, prefrontal cortex, and inferior temporal regions during visual working memory: a combined intracranial EEG and functional magnetic resonance imaging study. J Neurosci. 2008;28:7304–7312. doi: 10.1523/JNEUROSCI.1778-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MTT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989;12:94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- Wirtz S, Schuelke M. Region-specific expression of mitochondrial complex I genes during murine brain development. PLoS One. 2011;6:e18897. doi: 10.1371/journal.pone.0018897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelmaier F, Koopman WJH, van den Heuvel LP, Rodenburg RJ, Mayatepek E, Willems PHGM, et al. Mitochondrial complex I deficiency: from organelle dysfunction to clinical disease. Brain. 2009;132:833–842. doi: 10.1093/brain/awp058. [DOI] [PubMed] [Google Scholar]
- Pathak RU, Davey GP. Complex I and energy thresholds in the brain. Biochim Biophys Acta. 2008;1777:777–782. doi: 10.1016/j.bbabio.2008.05.443. [DOI] [PubMed] [Google Scholar]
- Kageyama GH, Wong-Riley MTT. Histochemical localization of cytochrome oxidase in the hippocampus: correlation with specific neuronal types and afferent pathways. Neuroscience. 1982;7:2337–2361. doi: 10.1016/0306-4522(82)90199-3. [DOI] [PubMed] [Google Scholar]
- Roubertoux PL, Sluyter F, Carlier M, Marcet B, Maarouf-Veray F, Chérif C, et al. Mitochondrial DNA modifies cognition in interaction with the nuclear genome and age in mice. Nat Genet. 2003;35:65–69. doi: 10.1038/ng1230. [DOI] [PubMed] [Google Scholar]
- Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab. 2012;32:1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen M. Relationship of spikes, synaptic activity, and local changes of cerebral blood flow. J Cereb Blood Flow Metab. 2001;21:1367–1383. doi: 10.1097/00004647-200112000-00001. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Viswanathan A, Freeman RD. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat Neurosci. 2007;10:1308–1312. doi: 10.1038/nn1977. [DOI] [PubMed] [Google Scholar]
- Mathiesen C, Caesar K, Lauritzen M. Temporal coupling between neuronal activity and blood flow in rat cerebellar cortex as indicated by field potential analysis. J Physiol. 2000;523:235–246. doi: 10.1111/j.1469-7793.2000.t01-1-00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann RF, Finch DM, Babb TL, Engel JJr. Increased glucose metabolism during long-duration recurrent inhibition of hippocampal pyramidal cells. J Neurosci. 1984;4:251–264. doi: 10.1523/JNEUROSCI.04-01-00251.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci USA. 2005;102:5588–5593. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCasland JS, Hibbard LS. GABAergic neurons in barrel cortex show strong, whisker-dependent metabolic activation during normal behavior. J Neurosci. 1997;17:5509–5527. doi: 10.1523/JNEUROSCI.17-14-05509.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCasland JS. Metabolic activity in antigenically identified neurons: a double labeling method for high-resolution 2-deoxyglucose and immunohistochemistry. J Neurosci Methods. 1996;68:113–123. doi: 10.1016/0165-0270(96)00063-5. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gloveli T, Dugladze T, Saha S, Monyer H, Heinemann U, Traub RD, et al. Differential involvement of oriens/pyramidale interneurones in hippocampal network oscillations in vitro. J Physiol. 2005;562:131–147. doi: 10.1113/jphysiol.2004.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Dugladze T, Schmitz D, Whittington MA, Vida I, Gloveli T. Segregation of axonal and somatic activity during fast network oscillations. Science. 2012;336:1458–1461. doi: 10.1126/science.1222017. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Katsumaru H, Hama K, Wu J-Y, Heizmann CW. GABAergic neurons containing the Ca2+-binding protein parvalbumin in the rat hippocampus and dentate gyrus. Brain Res. 1987;419:119–130. doi: 10.1016/0006-8993(87)90575-0. [DOI] [PubMed] [Google Scholar]
- Howard A, Tamas G, Soltesz I. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci. 2005;28:310–316. doi: 10.1016/j.tins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Viney TJ, Lasztoczi B, Katona L, Crump MG, Tukker JJ, Klausberger T, et al. Network state-dependent inhibition of identified hippocampal CA3 axo-axonic cells in vivo. Nat Neurosci. 2013;16:1802–1811. doi: 10.1038/nn.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnár G, Oláh S, Barzó P, Tamás G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A, Buzsáki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci. 1995;15:6651–6665. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormuzdi SG, Pais I, LeBeau FEN, Towers SK, Rozov A, Buhl EH, et al. Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron. 2001;31:487–495. doi: 10.1016/s0896-6273(01)00387-7. [DOI] [PubMed] [Google Scholar]
- Traub RD, Kopell N, Bibbig A, Buhl EH, LeBeau FEN, Whittington MA. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. J Neurosci. 2001;21:9478–9486. doi: 10.1523/JNEUROSCI.21-23-09478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumaru H, Kosaka T, Heizmann CW, Hama K. Gap junctions on GABAergic neurons containing the calcium-binding protein parvalbumin in the rat hippocampus (CA1 region) Exp Brain Res. 1988;72:363–370. doi: 10.1007/BF00250257. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Seress L, Leranth C. Electron microscopic immunocytochemical study of the distribution of parvalbumin-containing neurons and axon terminals in the primate dentate gyrus and Ammon's horn. J Comp Neurol. 1993;327:298–321. doi: 10.1002/cne.903270211. [DOI] [PubMed] [Google Scholar]
- Traub RD, Bibbig A, Fisahn A, LeBeau FEN, Whittington MA, Buhl EH. A model of gamma-frequency network oscillations induced in the rat CA3 region by carbachol in vitro. Eur J Neurosci. 2000;12:4093–4106. doi: 10.1046/j.1460-9568.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- Kraushaar U, Jonas P. Efficacy and stability of quantal GABA release at a hippocampal interneuron-principal neuron synapse. J Neurosci. 2000;20:5594–5607. doi: 10.1523/JNEUROSCI.20-15-05594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R. Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea-pig in vitro. J Physiol. 1990;428:61–77. doi: 10.1113/jphysiol.1990.sp018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, McBain CJ.Structural and functional properties of hippocampal neuronsIn: Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J (eds). The Hippocampus Book Oxford University Press: New York, USA; 2007133–201. [Google Scholar]
- Halasy K, Buhl EH, Lörinczi Z, Tamás G, Somogyi P. Synaptic target selectivity and input of GABAergic basket and bistratified interneurons in the CA1 area of the rat hippocampus. Hippocampus. 1996;6:306–329. doi: 10.1002/(SICI)1098-1063(1996)6:3<306::AID-HIPO8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Mercer A, Eastlake K, Trigg HL, Thomson AM. Local circuitry involving parvalbumin-positive basket cells in the CA2 region of the hippocampus. Hippocampus. 2012;22:43–56. doi: 10.1002/hipo.20841. [DOI] [PubMed] [Google Scholar]
- Ferrante M, Migliore M, Ascoli GA. Feed-forward inhibition as a buffer of the neuronal input-output relation. Proc Natl Acad Sci USA. 2009;106:18004–18009. doi: 10.1073/pnas.0904784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth FC, Draguhn A. GABA metabolism and transport: effects on synaptic efficacy. Neural Plast. 2012;2012:805830. doi: 10.1155/2012/805830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähner F, Weiss EK, Birke G, Maier N, Schmitz D, Rudolph U, et al. Cellular correlate of assembly formation in oscillating hippocampal networks in vitro. Proc Natl Acad Sci USA. 2011;108:E607–E616. doi: 10.1073/pnas.1103546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukker JJ, Lasztóczi B, Katona L, Roberts JD, Pissadaki EK, Dalezios Y, et al. Distinct dendritic arborization and in vivo firing patterns of parvalbumin-expressing basket cells in the hippocampal area CA3. J Neurosci. 2013;33:6809–6825. doi: 10.1523/JNEUROSCI.5052-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MO, Whittington MA, Bibbig A, Roopun A, LeBeau FEN, Vogt A, et al. A role for fast rhythmic bursting neurons in cortical gamma oscillations in vitro. Proc Natl Acad Sci USA. 2004;101:7152–7157. doi: 10.1073/pnas.0402060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah BV, Bruns W, Carandini M, Scanziani M. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron. 2012;73:159–170. doi: 10.1016/j.neuron.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurkó A, Mamiya A, Buzsáki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy WB, Baxter RA. Energy efficient neural codes. Neural Comput. 1996;8:531–543. doi: 10.1162/neco.1996.8.3.531. [DOI] [PubMed] [Google Scholar]
- Sengupta B, Stemmler MB, Friston KJ. Information and efficiency in the nervous system—a synthesis. PLoS Comput Biol. 2013;9:e1003157. doi: 10.1371/journal.pcbi.1003157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhler H. Molecular regulation of cognitive functions and developmental plasticity: impact of GABAA receptors. J Neurochem. 2007;102:1–12. doi: 10.1111/j.1471-4159.2007.04454.x. [DOI] [PubMed] [Google Scholar]
- Campanac E, Gasselin C, Baude A, Rama S, Ankri N, Debanne D. Enhanced intrinsic excitability in basket cells maintains excitatory-inhibitory balance in hippocampal circuits. Neuron. 2013;77:712–722. doi: 10.1016/j.neuron.2012.12.020. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Anderson L. Ultrastructure of the pyramidal basket cells in the dentate gyrus of the rat. J Comp Neurol. 1980;192:903–916. doi: 10.1002/cne.901920416. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS, Leranth C. The synaptology of parvalbumin-immunoreactive neurons in the primate prefrontal cortex. J Comp Neurol. 1992;320:353–369. doi: 10.1002/cne.903200307. [DOI] [PubMed] [Google Scholar]
- Halasy K, Somogyi P. Subdivisions in the multiple GABAergic innervation of granule cells in the dentate gyrus of the rat hippocampus. Eur J Neurosci. 1993;5:411–429. doi: 10.1111/j.1460-9568.1993.tb00508.x. [DOI] [PubMed] [Google Scholar]
- Shepherd GMG, Harris KM. Three-dimensional structure and composition of CA3→CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás AI, Buzsáki G, Freund TF, Hirase H. Populations of hippocampal inhibitory neurons express different levels of cytochrome c. Eur J Neurosci. 2006;23:2581–2594. doi: 10.1111/j.1460-9568.2006.04814.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald ML, Chan J, Mackie K, Lupica CR, Pickel VM. Altered dendritic distribution of dopamine D2 receptors and reduction in mitochondrial number in parvalbumin-containing interneurons in the medial prefrontal cortex of cannabinoid-1 (CB1) receptor knockout mice. J Comp Neurol. 2012;520:4013–4031. doi: 10.1002/cne.23141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takács VT, Szönyi A, Freund TF, Nyiri G, Gulyás AI.Quantitative ultrastructural analysis of basket and axo-axonic cell terminals in the mouse hippocampus Brain Struct Funct 2014. doi: 10.1007/s00429-013-0692-6(e-pub ahead of print). [DOI] [PubMed]
- Nie F, Wong-Riley MTT. Double labeling of GABA and cytochrome oxidase in the macaque visual cortex: quantitative EM analysis. J Comp Neurol. 1995;356:115–131. doi: 10.1002/cne.903560108. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT, Carroll EW. Quantitative light and electron microscopic analysis of cytochrome oxidase-rich zones in V II prestriate cortex of the squirrel monkey. J Comp Neurol. 1984;222:18–37. doi: 10.1002/cne.902220103. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Sharp FR. Autoradiographic maps of regional brain glucose consumption in resting, awake rats using (14C) 2-deoxyglucose. J Comp Neurol. 1978;177:335–359. doi: 10.1002/cne.901770210. [DOI] [PubMed] [Google Scholar]
- Borowsky IW, Collins RC. Metabolic anatomy of brain: a comparison of regional capillary density, glucose metabolism, and enzyme activities. J Comp Neurol. 1989;288:401–413. doi: 10.1002/cne.902880304. [DOI] [PubMed] [Google Scholar]
- Borowsky IW, Collins RC. Histochemical changes in enzymes of energy metabolism in the dentate gyrus accompany deafferentation and synaptic reorganization. Neuroscience. 1989;33:253–262. doi: 10.1016/0306-4522(89)90204-2. [DOI] [PubMed] [Google Scholar]
- Fergus A, Lee KS. GABAergic regulation of cerebral microvascular tone in the rat. J Cereb Blood Flow Metab. 1997;17:992–1003. doi: 10.1097/00004647-199709000-00009. [DOI] [PubMed] [Google Scholar]
- Cauli B, Tong X-K, Rancillac A, Serluca N, Lambolez B, Rossier J, et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enager P, Piilgaard H, Offenhauser N, Kocharyan A, Fernandes P, Hamel E, et al. Pathway-specific variations in neurovascular and neurometabolic coupling in rat primary somatosensory cortex. J Cereb Blood Flow Metab. 2009;29:976–986. doi: 10.1038/jcbfm.2009.23. [DOI] [PubMed] [Google Scholar]
- Devor A, Sakadzic S, Saisan PA, Yaseen MA, Roussakis E, Srinivasan VJ, et al. ‘Overshoot' of O2 is required to maintain baseline tissue oxygenation at locations distal to blood vessels. J Neurosci. 2011;31:13676–13681. doi: 10.1523/JNEUROSCI.1968-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasischke KA, Lambert EM, Panepento B, Sun A, Gelbard HA, Burgess RW, et al. Two-photon NADH imaging exposes boundaries of oxygen diffusion in cortical vascular supply regions. J Cereb Blood Flow Metab. 2011;31:68–81. doi: 10.1038/jcbfm.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Schultz JH, Ehmke H, Monyer H, Jonas P. Functional and molecular differences between voltage-gated K+ channels of fast-spiking interneurons and pyramidal neurons of rat hippocampus. J Neurosci. 1998;18:8111–8125. doi: 10.1523/JNEUROSCI.18-20-08111.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BC, Bean BP. Sodium entry during action potentials of mammalian neurons: incomplete inactivation and reduced metabolic efficiency in fast-spiking neurons. Neuron. 2009;64:898–909. doi: 10.1016/j.neuron.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike FG, Goddard RS, Suckling JM, Ganter P, Kasthuri N, Paulsen O. Distinct frequency preferences of different types of rat hippocampal neurones in response to oscillatory input currents. J Physiol. 2000;529:205–213. doi: 10.1111/j.1469-7793.2000.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, et al. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- Hu H, Jonas P. A supercritical density of Na+ channels ensures fast signaling in GABAergic interneuron axons. Nat Neurosci. 2014;17:686–693. doi: 10.1038/nn.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alle H, Roth A, Geiger JRP. Energy-efficient action potentials in hippocampal mossy fibers. Science. 2009;325:1405–1408. doi: 10.1126/science.1174331. [DOI] [PubMed] [Google Scholar]
- Hasenstaub A, Otte S, Callaway E, Sejnowski TJ. Metabolic cost as a unifying principle governing neuronal biophysics. Proc Natl Acad Sci USA. 2010;107:12329–12334. doi: 10.1073/pnas.0914886107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nörenberg A, Hu H, Vida I, Bartos M, Jonas P. Distinct nonuniform cable properties optimize rapid and efficient activation of fast-spiking GABAergic interneurons. Proc Natl Acad Sci USA. 2010;107:894–899. doi: 10.1073/pnas.0910716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- Okaty BW, Miller MN, Sugino K, Hempel CM, Nelson SB. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J Neurosci. 2009;29:7040–7052. doi: 10.1523/JNEUROSCI.0105-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Prog Neurobiol. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Birke G, Draguhn A. No simple brake—the complex functions of inhibitory synapses. Pharmacopsychiatry. 2010;43 (Suppl 1:S21–S31. doi: 10.1055/s-0030-1253376. [DOI] [PubMed] [Google Scholar]
- Südhof TC. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013;80:675–690. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RH. The neurotransmitter cycle and quantal size. Neuron. 2007;55:835–858. doi: 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P. Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron. 2008;57:536–545. doi: 10.1016/j.neuron.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Biró AA, Holderith NB, Nusser Z. Quantal size is independent of the release probability at hippocampal excitatory synapses. J Neurosci. 2005;25:223–232. doi: 10.1523/JNEUROSCI.3688-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta A, Rösner J, Huchzermeyer C, Wojtowicz A, Kann O, Schmitz D, et al. Energy demand of synaptic transmission at the hippocampal Schaffer-collateral synapse. J Cereb Blood Flow Metab. 2012;32:2076–2083. doi: 10.1038/jcbfm.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann O, Taubenberger N, Huchzermeyer C, Papageorgiou IE, Benninger F, Heinemann U, et al. Muscarinic receptor activation determines the effects of store-operated Ca2+-entry on excitability and energy metabolism in pyramidal neurons. Cell Calcium. 2012;51:40–50. doi: 10.1016/j.ceca.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Andersen P, Dingledine R, Gjerstad L, Langmoen IA, Mosfeldt Laursen A. Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J Physiol. 1980;305:279–296. doi: 10.1113/jphysiol.1980.sp013363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Royer S, Paré D. Cell-type-specific GABA responses and chloride homeostasis in the cortex and amygdala. J Neurophysiol. 2001;86:2887–2895. doi: 10.1152/jn.2001.86.6.2887. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Turrigiano GG. Strength through diversity. Neuron. 2008;60:477–482. doi: 10.1016/j.neuron.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waagepetersen HS, Sonnewald U, Gegelashvili G, Larsson OM, Schousboe A. Metabolic distinction between vesicular and cytosolic GABA in cultured GABAergic neurons using 13C magnetic resonance spectroscopy. J Neurosci Res. 2001;63:347–355. doi: 10.1002/1097-4547(20010215)63:4<347::AID-JNR1029>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Walls AB, Eyjolfsson EM, Smeland OB, Nilsen LH, Schousboe I, Schousboe A, et al. Knockout of GAD65 has major impact on synaptic GABA synthesized from astrocyte-derived glutamine. J Cereb Blood Flow Metab. 2011;31:494–503. doi: 10.1038/jcbfm.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, Katsurabayashi S, Guillemin I, Friauf E, Rosenmund C, Brose N, et al. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron. 2006;50:575–587. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Chatton J-Y, Pellerin L, Magistretti PJ. GABA uptake into astrocytes is not associated with significant metabolic cost: implications for brain imaging of inhibitory transmission. Proc Natl Acad Sci USA. 2003;100:12456–12461. doi: 10.1073/pnas.2132096100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- Westergaard N, Sonnewald U, Petersen SB, Schousboe A. Glutamate and glutamine metabolism in cultured GABAergic neurons studied by 13C NMR spectroscopy may indicate compartmentation and mitochondrial heterogeneity. Neurosci Lett. 1995;185:24–28. doi: 10.1016/0304-3940(94)11216-6. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Bakken IJ, Larsson OM, Sonnewald U, Schousboe A. Metabolism of lactate in cultured GABAergic neurons studied by 13C nuclear magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 1998;18:109–117. doi: 10.1097/00004647-199801000-00011. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Wang RY, Cruz NF. Generalized sensory stimulation of conscious rats increases labeling of oxidative pathways of glucose metabolism when the brain glucose-oxygen uptake ratio rises. J Cereb Blood Flow Metab. 2002;22:1490–1502. doi: 10.1097/01.WCB.0000034363.37277.89. [DOI] [PubMed] [Google Scholar]
- Barth AMI, Mody I. Changes in hippocampal neuronal activity during and after unilateral selective hippocampal ischemia in vivo. J Neurosci. 2011;31:851–860. doi: 10.1523/JNEUROSCI.5080-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker RG, Turnbull DM, Whittington MA, Cunningham MO. Impaired mitochondrial function abolishes gamma oscillations in the hippocampus through an effect on fast-spiking interneurons. Brain. 2011;134:e180. doi: 10.1093/brain/awr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CB, Vreugdenhil M, Toescu EC. The effect of aging-associated impaired mitochondrial status on kainate-evoked hippocampal gamma oscillations. Neurobiol Aging. 2012;33:2692–2703. doi: 10.1016/j.neurobiolaging.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch C, Scotti A, Sommacal A, Kalt G. GABAergic hippocampal neurons resistant to ischemia-induced neuronal death contain the Ca2+-binding protein parvalbumin. Neurosci Lett. 1989;105:263–268. doi: 10.1016/0304-3940(89)90631-9. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Oxidative stress and energy crises in neuronal dysfunction. Ann N Y Acad Sci. 2008;1147:53–60. doi: 10.1196/annals.1427.002. [DOI] [PubMed] [Google Scholar]
- Dennis C, Kabat H. Behavior of dogs after complete temporary arrest of the cephalic circulation. Exp Biol Med. 1939;40:559–561. [Google Scholar]
- Rossen R, Kabat H, Anderson JP. Acute arrest of cerebral circulation in man. Arch Neurol Psychiatry. 1943;50:510–528. [Google Scholar]
- Colin F, Manil J, Bourgain RH. The effect of acute anoxia on the cortical somatosensory evoked potential in the rabbit. Neurol Res. 1981;3:393–407. doi: 10.1080/01616412.1981.11739612. [DOI] [PubMed] [Google Scholar]
- Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985;65:101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- Morán M, Moreno-Lastres D, Marín-Buera L, Arenas J, Martín MA, Ugalde C. Mitochondrial respiratory chain dysfunction: implications in neurodegeneration. Free Radic Biol Med. 2012;53:595–609. doi: 10.1016/j.freeradbiomed.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Kann O, Kovács R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007;292:C641–C657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- Duchemin S, Boily M, Sadekova N, Girouard H. The complex contribution of NOS interneurons in the physiology of cerebrovascular regulation. Front Neural Circuits. 2012;6:51. doi: 10.3389/fncir.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam-Vizi V, Starkov AA. Calcium and mitochondrial reactive oxygen species generation: how to read the facts. J Alzheimers Dis. 2010;20 (Suppl 2:S413–S426. doi: 10.3233/JAD-2010-100465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Lucas EK, Markwardt SJ, Gupta S, Meador-Woodruff JH, Lin JD, Overstreet-Wadiche L, et al. Parvalbumin deficiency and GABAergic dysfunction in mice lacking PGC-1α. J Neurosci. 2010;30:7227–7235. doi: 10.1523/JNEUROSCI.0698-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga D, Hof PR, Vissavajjhala P, Moran TM, Morrison JH. Parvalbumin-containing interneurons in rat hippocampus have an AMPA receptor profile suggestive of vulnerability to excitotoxicity. J Chem Neuroanat. 2002;23:249–253. doi: 10.1016/s0891-0618(02)00012-1. [DOI] [PubMed] [Google Scholar]
- Geiger JRP, Melcher T, Koh D-S, Sakmann B, Seeburg PH, Jonas P, et al. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Eyles DW, McGrath JJ, Reynolds GP. Neuronal calcium-binding proteins and schizophrenia. Schizophr Res. 2002;57:27–34. doi: 10.1016/s0920-9964(01)00299-7. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Yang SF, Xia Y, Johnson KM. Postnatal phencyclidine administration selectively reduces adult cortical parvalbumin-containing interneurons. Neuropsychopharmacology. 2008;33:2442–2455. doi: 10.1038/sj.npp.1301647. [DOI] [PubMed] [Google Scholar]
- Steullet P, Cabungcal J-H, Kulak A, Kraftsik R, Chen Y, Dalton TP, et al. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci. 2010;30:2547–2558. doi: 10.1523/JNEUROSCI.3857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Peters A, Barbas H. Mitochondrial degeneration in dystrophic neurites of senile plaques may lead to extracellular deposition of fine filaments. Brain Struct Funct. 2007;212:195–207. doi: 10.1007/s00429-007-0153-1. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Critically attained threshold of cerebral hypoperfusion: the CATCH hypothesis of Alzheimer's pathogenesis. Neurobiol Aging. 2000;21:331–342. doi: 10.1016/s0197-4580(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Kittelberger K, Hur EE, Sazegar S, Keshavan V, Kocsis B. Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizophrenia. Brain Struct Funct. 2012;217:395–409. doi: 10.1007/s00429-011-0351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AMI, Cobos I, Ho K, et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149:708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Rompala GR, Zhang S, Cowell RM, Nakazawa K. Social isolation exacerbates schizophrenia-like phenotypes via oxidative stress in cortical interneurons. Biol Psychiatry. 2013;73:1024–1034. doi: 10.1016/j.biopsych.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]