Abstract
Surgical flow augmentation for treatment of cerebral hemodynamic impairment remains controversial. Here, we investigated the benefit of endothelial progenitor cell (EPC) treatment in a rat model of chronic cerebral hypoperfusion. At repeated time points after 3-vessel occlusion (3-VO), animals were treated with 1 × 106 DiI-labeled (a) ex vivo-expanded embryonic-EPC (e-EPC), (b) cyclic AMP-differentiated embryonic-endothelial progenitor-derived cells (e-EPDC as biologic control) or, (c) saline. The cerebrovascular reserve capacity (CVRC) was assessed immediately before and on days 7 and 21 after 3-VO. Structural effects were assessed by latex perfusion, immunohistochemistry, and intravital fluorescence video microscopy on day 21. Three-vessel occlusion resulted in a significant impairment of the CVRC with better functional recovery after treatment with e-EPC (16.4±8%) compared with e-EPDC (3.7±8%) or saline (6.4±9%) by day 21 (P<0.05), which was paralleled by a significant increase in the vessel diameters of the anterior Circle of Willis, a significantly higher number of leptomeningeal anastomoses and higher parenchymal capillary density in e-EPC-treated animals. Interestingly, despite in vivo interaction of e-EPC with the cerebral endothelium, e-EPC incorporation into the cerebral vasculature was not observed. Our results suggest that EPC may serve as a novel therapeutic agent in clinical trials for nonsurgical treatment of chronic cerebral hemodynamic impairment.
Keywords: arteriogenesis, cerebral blood flow, cerebrovascular disease, collateral circulation, endothelial progenitor cells
Introduction
Chronic impairment of blood supply to the brain due to arterial steno-occlusive disease contributes to ischemic stroke in ∼10% of all cases.1 Owing to the slow disease progression, patients typically present with transient ischemic symptoms before the manifestation of brain infarction. Here, the outgrowth of preformed collateral vessels (arteriogenesis) is the most important endogenous rescue system to cope with the chronic cerebral hypoperfusion. However, the natural collateral network is often not efficient enough to compensate the progressive disease.2
Arteriogenesis is regulated by molecular and cellular mechanisms, which can be manipulated to induce and accelerate the outgrowth of preexisting collaterals.3 This concept of therapeutic stimulation of collateral vessel growth has recently been adapted to the cerebrovascular system.4, 5 In contrast, postnatal vasculogenesis describes the de novo formation of blood vessels through migration and differentiation of circulating endothelial progenitor cells (EPC).
Against this background, several experimental and clinical studies have reported beneficial implications of EPC to stimulate postnatal neovascularization in peripheral6, 7, 8 and coronary artery disease.9, 10 However, little is known about the contribution of vasculogenesis and its main effectors—EPC—to collateral vessel formation in the cerebrovascular system.
In this study, we investigated whether ex vivo-expanded embryonic EPC (e-EPC), which were isolated at E7.5 of mouse development at the onset of vasculogenesis, (I) restore hampered cerebrovascular reserve capacity (CVRC), (II) contribute to collateral vessel formation and (III), interact with the cerebral vasculature in a xenograft rat model of chronic cerebral hypoperfusion.
Materials and Methods
Ethics Statement
Experiments were permitted by the local ethics committees on animal research (Regierungspräsidium Karlsruhe, AZ: 35-9185.81/G-105/04, Karlsruhe, Germany and LaGeSo No. G 0262/07, Berlin, Germany) and in conformity with the German Law for Animal Protection and the National Institute of Health Guidelines for Care and Use of Laboratory Animals.
Cell Culture Experiments
In terms of cell therapy aimed at revascularization, one advantage of e-EPC over differentiated endothelial cells is the higher plasticity of e-EPC. Thus, it is important to provide evidence that the e-EPC used in this study acquire endothelial cell (human umbilical vein endothelial cells; HUVEC) characteristics after differentiation to e-EPDC and that e-EPDC have the same characteristics as differentiated endothelial cells (HUVEC). Before our animal experiments, we therefore studied e-EPDC and e-EPC in tube formation assays on Matrigel (BD Biosciences, Heidelberg, Germany) with and without coculture of HUVEC.
Isolation and Labeling of Human Umbilical Vein Endothelial Cells, Embryonic-Endothelial Progenitor Cells and Embryonic-Endothelial Progenitor-Derived Cells
Human umbilical vein endothelial cells were used for in vitro studies. Umbilical cords were obtained from normal pregnancies immediately after delivery. Human umbilical vein endothelial cells were isolated by trypsin treatment of umbilical cord veins and used at passages 1–6. Cells were grown in culture flasks precoated with 0.5% gelatin (Sigma-Aldrich, Steinheim, Germany) at 37 °C in 5% CO2 and cultured in endothelial cell growth medium (Promocell, Heidelberg, Germany).
The isolation and ex vivo expansion of e-EPC from E7.5 mouse embryos as well as their differentiation with cyclic AMP and retinoic acid to e-EPDC (biologic control to e-EPC) was performed as described previously.11, 12 The isolated cells were labeled with the fluorescent lipid soluble dye DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; Life Technologies, Invitrogen, Molecular Probes Darmstadt, Germany).13 Directly before infusion, the cells were washed in isotonic saline to reduce toxic effects of the culture medium.
In vitro Tube Formation Assays
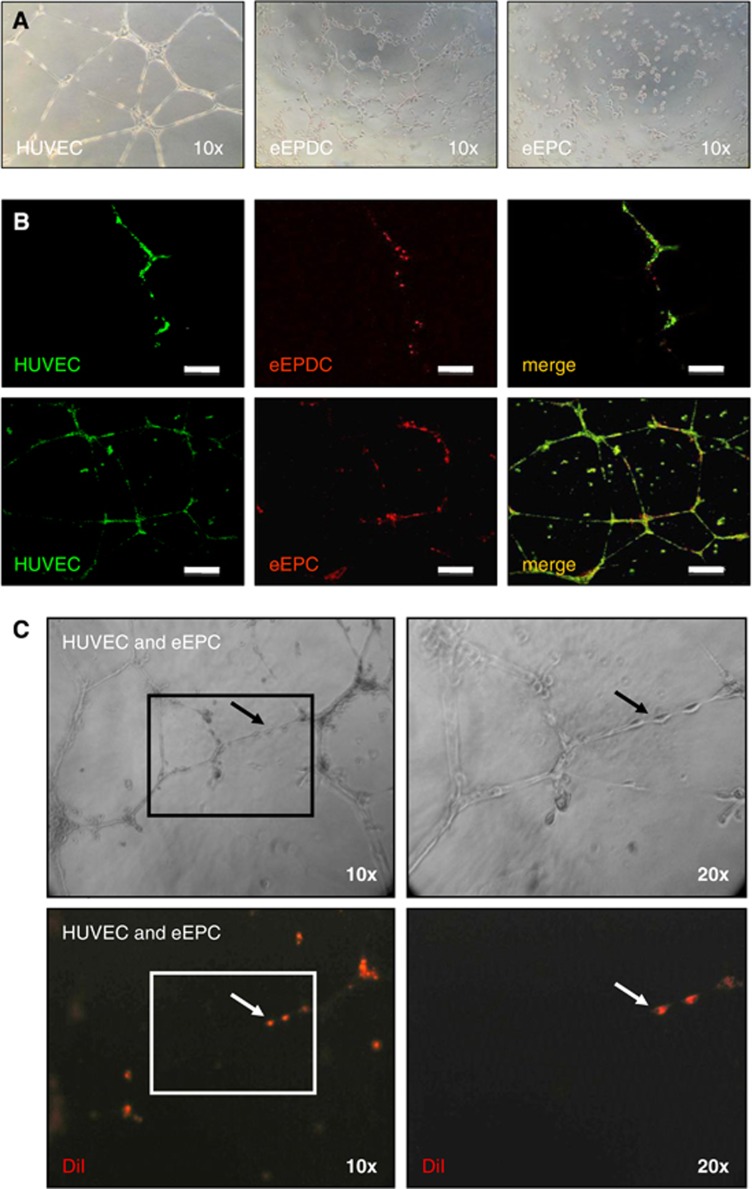
Matrigel was thawed on ice at 2°C to 8°C and kept on ice during preparation. Matrigel was then added to the wells of a 96-well plate in a volume of 50 μL and allowed to solidify at 37°C for 30 minutes. Embryonic-endothelial progenitor cells (10 × 104 cells/mL; n=4), e-EPDC (10 × 104 cells/mL; n=4), or HUVEC (17 × 104/mL; n=4) were added to each well in 100 μL of endothelial cell growth medium and incubated at 37°C in 5% CO2. After 24 hours, clear field microscopic images were obtained (Figure 1A).
Figure 1.
In vitro characterization of embryonic-endothelial progenitor-derived cells (e-EPDC) and embryonic-endothelial progenitor cells (e-EPC). The images show representative examples of four independent series of experiments for each cell type. (A) Human umbilical vein endothelial cells (HUVEC) (used as positive control for differentiated endothelial cells), e-EPDC and e-EPC were plated on Matrigel. The distinct appearance of e-EPC in contrast to the spontaneous tube formation of e-EPDC similar to HUVEC can be noted. (B) The integration of e-EPDC (upper panels) and e-EPC (lower panels) into a developing HUVEC network shows the plasticity of e-EPC and that both cell types acquire endothelial cell characteristics after differentiation. Bar=200 μm. (C) Clear field (upper panels) and fluorescence (lower panels) images highlight the spontaneous incorporation of DiI prelabeled e-EPC into a preformed HUVEC network. The right panels show the indicated area of detail enlargement.
For coculture experiments (n=4 per cell type), HUVEC were prelabeled with 3,3′-dioctadecyloxacarbocyanine perchlorate for 3 hours at 37°C in 5% CO2. Embryonic-endothelial progenitor cells and embryonic-endothelial progenitor-derived cells were labeled with DiI under the same conditions. Cells were harvested and cell suspensions were prepared using HUVEC culture medium. Next, e-EPC and e-EPDC were plated in a ratio of 1:50 to HUVEC. Twenty-four hours later, the formation of cord-like structures was determined through confocal imaging (Figure 1B).
In an additional set of experiments (n=4 per cell type), HUVEC were first plated on Matrigel before DiI prelabeled e-EPC (1/50 of the total cell number) were added 5 hours later. After 24 hours, the formation of cord-like structures and the incorporation of e-EPC into the preexisting vascular HUVEC network were assessed. Contrast-phase and fluorescent images were obtained with an inverted microscope (Figure 1C).
Animals and Experimental Design
Forty-eight adult, male Sprague–Dawley rats (body weight 296 to 332 g) were randomized to the following groups:
Embryonic-endothelial progenitor cells (n=18): 3-VO and treatment with intravenously applied mouse e-EPC (1 × 106 cells in 1 mL saline).
Embryonic-endothelial progenitor-derived cells (n=16): 3-VO and treatment with intravenously applied cAMP-differentiated mouse e-EPDC as biologic control (1 × 106 cells in 1 mL saline).
Saline (n=14): 3-VO and treatment with 1 mL saline.
Intravenous injections via the tail vein were performed immediately after and on days 7 and 14 after induction of chronic cerebral hypoperfusion. Each procedure was performed under general anesthesia with intraperitoneally applied chloral hydrate (0.4 g/kg bw). Postoperatively, animals received a subcutaneous injection of 30 μg/kg bw buprenorphine (Temgesic, Essex Pharma, Munich, Germany) for postoperative analgesia. During surgery, body temperature was maintained at 37°C using a temperature-controlled heating pad. All data regarding CVRC assessment, latex perfusion, and histology were obtained and analyzed in a masked fashion.
3-Vessel Occlusion
Rats were immobilized in the prone position with a stereotactic head fixation device. First, the cervical spine and the atlanto-occipital junction were exposed via a paravertebral access and the vertebral arteries were bilaterally cauterized according to Pulsinelli and Brierley.14 Next, the animals were turned to supine position and the right common carotid artery was exposed through a midline neck incision. The common carotid artery was carefully dissected from the vagal nerve and permanently ligated with an 8-0 silk suture. The skin was sutured with 6-0 nylon.
Measurement of the Cerebrovascular Reserve Capacity
Measurements of the CVRC were performed immediately before (day 0) and on days 7 and 21 after 3-VO (saline: n=8; e-EPDC: n=10; e-EPC: n=10). Rats were immobilized in the prone position with a stereotactic head fixation device. The skull was exposed through a midline scalp incision and two burr holes were drilled 3 mm lateral of the bregma on each side leaving the internal lamina of the scull intact. Two LDF probes (1 mm diameter, Moor Instruments, Devon, England) were positioned in each burr hole and connected to an LDF monitor (DRT4, Moor Instruments). Baseline perfusion was recorded in the arbitrary perfusion unit CBF-Flux for 120 seconds. Thereafter, 20 mg/kg acetazolamide (Diamox, Goldshield Pharmaceuticals, Surrey, England) was injected intraperitoneally and CBF-Flux was continuously recorded for 18 minutes. The acetazolamide-specific CVRC was calculated as the percent change between a 60-second baseline perfusion plateau and a 120-second perfusion plateau after the maximum change in CBF-Flux. After imaging, the scalp was sutured with 6-0 nylon.
Assessment of Parenchymal Capillary Density and the Localization of DiI-Positive Cells
For histologic assessment of the parenchymal capillary density and the localization of DiI-positive cells on day 21, six animals of each group received 1.5 mL of Evans Blue (Sigma-Aldrich, 2% dissolved in saline) via the tail vein. After a 90-second perfusion period, the rats were killed and the brains were removed and frozen. From each brain, 10 μm coronal cryosections were obtained from bregma level −0.4 mm to −6.4 mm in 2 mm intervals. The sections were mounted and observed under a fluorescence-enhanced microscope (Axioplan 2, Zeiss, Oberkochem, Germany). For each section level, specific cortical and subcortical brain areas were analyzed (−0.4 mm: caudate putamen; −2.4 mm: primary sensory cortex (S1); −4.4 mm, hippocampus (Ca1); −6.4 mm, primary auditory cortex (Au1)). Capillaries were defined as a round shape or transverse line with a maximum diameter of 8 μm and capillary vessel density was assessed as number of vessels per field of view. Embryonic-endothelial progenitor cells and e-EPDC were identified according to DiI-positive fluorescence.
Assessment of the Cerebral Collateral Vessel Growth by Latex/Carbon Black Perfusion
In a separate set of animals (n=6 per group), the brain angioarchitecture was visualized on day 21 with the latex/carbon black perfusion technique as described previously.5 Under deep anesthesia, an aortal catheter (internal diameter 0.76 mm, Vasofix Braunüle, Braun, Melsungen, Germany) was inserted and arterial blood gas samples were drawn for blood gas analysis and mean arterial pressure was determined before perfusion (Table 1). A sublethal dose of 50 mg/kg papaverine hydrochloride was injected to achieve maximal vasodilation. After 60 seconds, the vena cava was incised to allow venous outflow. Immediately after, a white liquid latex compound (Chicago Latex Product no. 563, Chicago Latex Products, Crystal Lake, USA) mixed with 20 μL/mL carbon black (Derussol N25/L, Degussa, Frankfurt, Germany) was infused into the aorta at 150 mm Hg for 5 minutes. The animals were placed in ice-cold water for 20 minutes to ensure hardening of the latex. The brains were carefully removed and the basal and leptomeningeal vasculature were photographed at 16-fold magnification.
Table 1. Body weight, MAP, and arterial blood gases on day 21.
| Saline | e-EPDC | e-EPC | |
|---|---|---|---|
| Body weight (g) | 380±15 | 354±21 | 368±21 |
| MAP (mm Hg) | 80±8 | 83±9 | 81±12 |
| pH (mm Hg) | 7.30±0.03 | 7.32±0.03 | 7.32±0.02 |
| PaCO2 (mm Hg) | 59±4 | 58±3 | 56±4 |
| PaO2 (mm Hg) | 104±5 | 97±6 | 107±13 |
| HCO3 (mm Hg) | 28±3 | 29±2 | 29±2 |
HCO3, hydrogen carbonate; MAP, mean arterial blood pressure; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; pH, potential of hydrogen.
Values are expressed as mean±s.d.
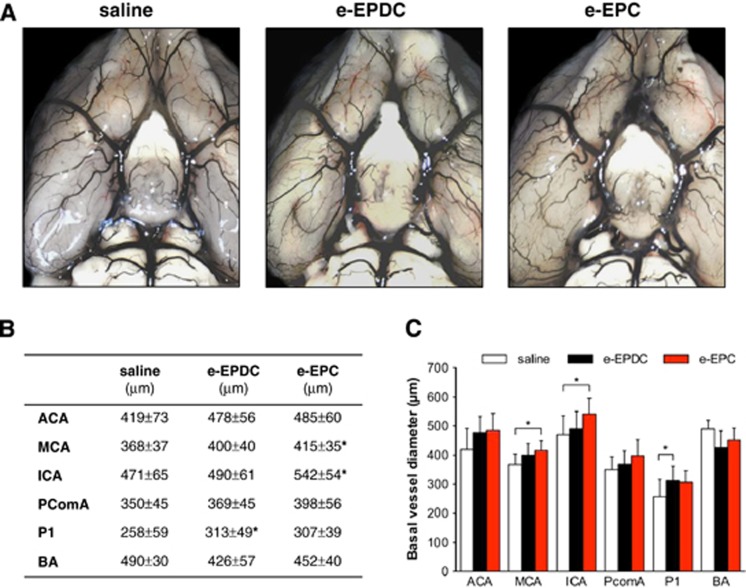
Measurements of the external diameters of the basal vasculature (anterior cerebral artery (ACA), middle cerebral artery (MCA), internal carotid artery (ICA), posterior cerebral artery (P1 segment), posterior communicating artery and basilar artery) and the diameter and number of leptomeningeal anastomoses of both hemispheres were performed with freeware image analysis software (ImageJ64, http://rsbweb.nih.gov/ij/) by a masked observer: The diameters of the ACA, MCA, posterior communicating artery and P1 segment were determined in the middle third of the vessel between its origin and next branching point or confluence (i.e., the ACA diameter was determined between its origin from the ICA and the just proximal point where ACA diverges the olfactory artery). The ICA diameter was determined just proximal to the ICA bifurcation. The basilar artery diameter was determined just proximal to the origin of the superior cerebellar arteries. In each vessel, three measurements were performed and the mean of these three values per vessel was compared between all groups. Leptomeningeal anastomoses were defined and counted at their point of confluence between the distal MCA and the distal ACA or between the distal MCA and the posterior cerebral artery. The mean of the total number of anastomoses was compared between all groups. The diameter of each anastomosis was obtained as the average of three measurements along the middle third of the collateral. The mean of these three values per vessel was used to compile relative frequency histograms of vessel diameters for each group.
Intravital Fluorescence Video Microscopy
In a separate set of animals (n=2), a cranial window was established 2 days before 3-VO and injection of 1 × 106 DiI-labeled e-EPC according to our experimental protocol for CVRC assessment and latex perfusion. For cranial window preparation, animals were immobilized in the prone position with a stereotactic head fixation device. The scalp was incised and a 6 × 9 mm craniectomy was performed using a microdrill. The dura was carefully incised and removed over both hemispheres. A transparent cover slip was placed above the exposed cortical surface and attached to the surrounding bone with acrylic cement. The skin was sutured with 6-0 nylon and the animals were returned to their cages with free access to food and water. Intravital video microscopy was performed as described previously.15 To enable colocalization of the e-EPC with respect to the blood vessel lumina, the vasculature was visualized through intravenous injection of fluorescein isothiocyanate-conjugated dextran before injection of DiI-labeled e-EPC (Rhodamine excitation).
Statistical Analysis
Data are presented as mean±s.d. For comparison of CVRC after 3-VO, a two-way analysis of variance procedure for repeated measures with subsequent pair-wise comparison of means by Fisher's least projected difference test was performed. Differences in physiologic parameters, basal vessel diameters, the total number of leptomeningeal anastomoses, and the parenchymal capillary density were tested by a one-way analysis of variance and subsequent Bonferroni correction (two-tailed) for multiple comparisons. The relative frequency of leptomeningeal vessel diameters was compared by a two-way analysis of variance and subsequent Bonferroni correction. All statistics were generated with GraphPad Prism for Mac (Version 5.0d, GraphPad Software, San Diego, CA, USA). Statistical significance was set at P<0.05.
Results
Embryonic-Endothelial Progenitor Cells Do not Form Cord-Like Structures In Vitro but Incorporate into a Vascular Network
Embryonic-endothelial progenitor-derived cells (e-EPC stimulated with cAMP and retinoic acid) spontaneously formed cord-like structures on Matrigel, similar to HUVEC (Figure 1A). Embryonic-endothelial progenitor-derived cells incorporation into a developing HUVEC network (Figure 1B, upper panels) further confirmed that e-EPDC behave as differentiated endothelial cells and may serve as a biologic control. In contrast, e-EPC cultured alone failed to form tubes (Figure 1A). However, when cocultured, immediately or 5 hours after HUVEC were taken into culture, e-EPC elongated and became an integral part of the developing (Figure 1B, lower panels) or preformed (Figure 1C) HUVEC network as a sign of high plasticity and that e-EPC acquire endothelial cell characteristics after differentiation.
Improved Functional Recovery of the Cerebrovascular Reactivity After Treatment with embryonic-endothelial progenitor cells
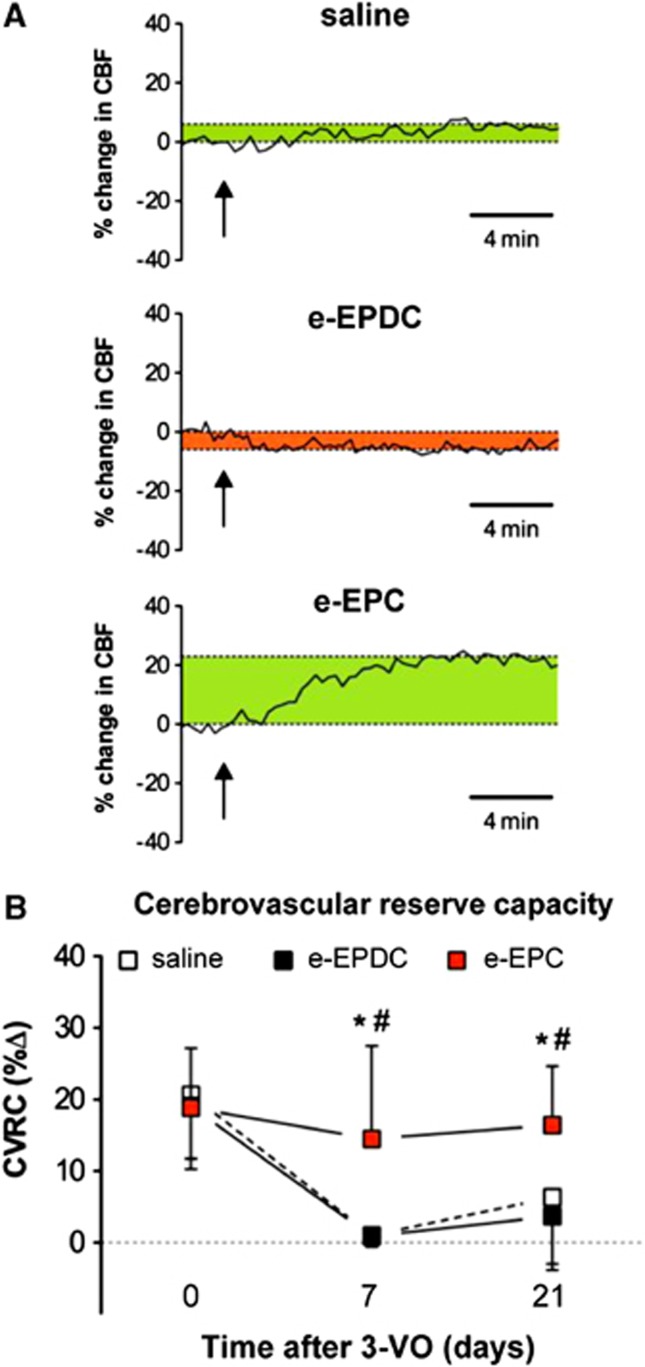
Graph traces of cerebral blood flow responses to acetazolamide stimulation on day 21 are shown in Figure 2A. On days 7 and 21 after 3-VO, CVRC was significantly higher after treatment with e-EPC compared with saline- and e-EPDC-treated animals (day 7: *P<0.01 for e-EPC versus saline and #P<0.01 for e-EPC versus e-EPDC; day 21: *P<0.05 for e-EPC versus saline and #P<0.01 for e-EPC versus e-EPDC; Figure 2B).
Figure 2.
Functional recovery of the cerebrovascular reserve capacity (CVRC). (A) Cerebral blood flow (CBF) response to acetazolamide on day 21 after 3-vessel occlusion (3-VO) in animals treated with saline (n=8), embryonic-endothelial progenitor-derived cells (e-EPDC) (n=10) and embryonic-endothelial progenitor cells (e-EPC) (n=10). The graph of the e-EPDC-treated animal illustrates a perfusion drop after acetazolamide as a sign of hemodynamic steal owing to an abolished CVRC (perfusion drop=red; perfusion increase=green). The arrows indicate the time point of acetazolamide administration. (B) Line graphs of the mean CVRC in animals treated with saline, e-EPDC or e-EPC over the course of the entire monitoring period illustrate the better CVRC recovery in animals treated with e-EPC compared with e-EPDC or saline (*P<0.05 for e-EPC versus saline and #P<0.01 for e-EPC versus e-EPDC).
Before 3-VO (day 0), CVRC did not differ between groups (saline: 20.7±9% e-EPDC: 19.1±9% e-EPC 18.9±8%). Seven days after 3-VO, CVRC was significantly reduced in animals treated with saline (1.1±2%, P<0.001 versus day 0) or e-EPDC (0.9±1%, P<0.001 versus day 0) but not in animals that received e-EPC (14.5±13). At day 21, CVRC remained exhausted in animals that received saline (6.4±9%, P<0.01 versus day 0) or e-EPDC (3.7±8%, P<0.01 versus day 0) compared with rats treated with e-EPC (16.4±8%).
Embryonic-Endothelial Progenitor Cells Augment Basal and Leptomeningeal Collateral Vessel Growth
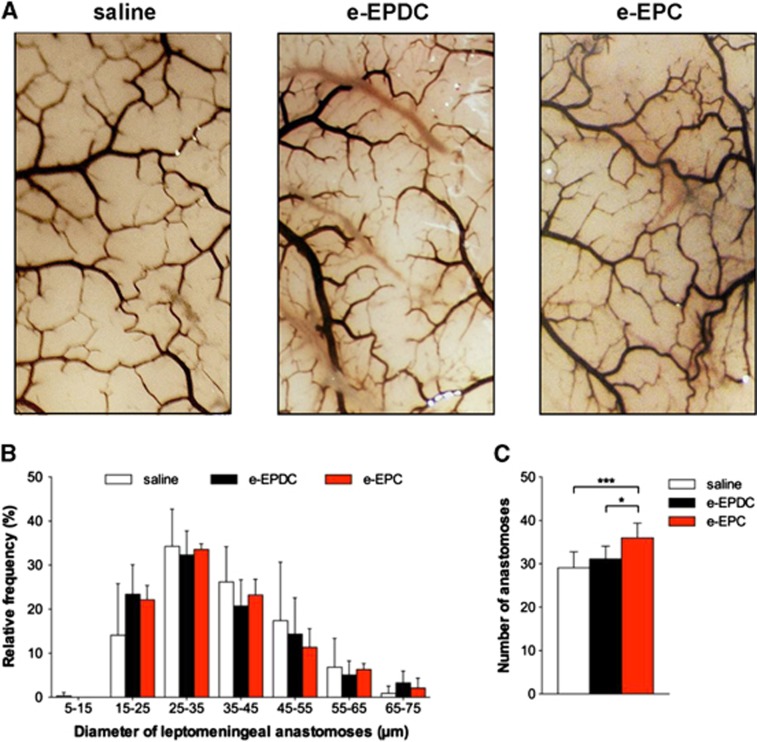
On day 21, treatment with e-EPC resulted in a significant increase of the external vessel diameters of the MCA and ICA segments in the anterior circulation compared with animals treated with saline, whereas e-EPDC-treated animals only showed a significant increase of the external vessel diameter of the P1 segment of the posterior cerebral artery (Figure 3; *P<0.05 versus saline). Neither e-EPC nor e-EPDC treatment resulted in diameter changes of the leptomeningeal anastomoses. However, animals treated with e-EPC had a significantly higher number of anastomoses (36±3) compared with animals treated with e-EPDC (31±3, P<0.05 versus e-EPC) or saline (29±4, P<0.001 versus e-EPC) (Figure 4).
Figure 3.
Collateral vessel growth—basal vasculature. (A) Latex/carbon black angiograms of the basal vasculature on day 21 after 3-vessel occlusion (3-VO) in animals treated with saline (n=6), embryonic-endothelial progenitor-derived cells (e-EPDC) (n=6) and embryonic-endothelial progenitor cells (e-EPC) (n=6). (B) Mean diameters of the basal vasculature (*P<0.05 versus saline) and (C) bar graph illustrating the augmented external diameter increase of the basal vasculature in the anterior circulation after the treatment with e-EPC (*P<0.05). ACA, anterior cerebral artery; BA, basilar artery; ICA, internal carotid artery; MCA, middle cerebral artery; PComA, posterior communicating artery.
Figure 4.
Collateral vessel growth—leptomeningeal anastomoses. (A) Latex/carbon black angiograms of the leptomeningeal vasculature on day 21 after 3-vessel occlusion (3-VO) in animals treated with saline (n=6), embryonic-endothelial progenitor-derived cells (e-EPDC) (n=6) and embryonic-endothelial progenitor cells (e-EPC) (n=6). (B) Frequency distribution of leptomeningeal anastomoses diameters. (C) Bar graph illustrating the 20% increase in the number of leptomeningeal anastomoses after treatment with e-EPC compared with e-EPDC and saline (*P<0.05, ***P<0.001).
Increased Cortical Parenchymal Capillary Density after Treatment with Embryonic-Endothelial Progenitor Cells
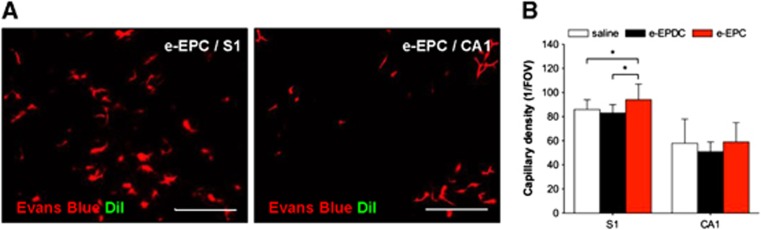
On day 21, treatment with e-EPC led to a significantly higher density of patent capillaries within the cortical S1 area compared with animals treated with e-EPDC or saline. In the subcortical CA1 region of the hippocampus, no difference in capillary density was observed (Figure 5).
Figure 5.
Parenchymal microvascular density and embryonic-endothelial progenitor cells (e-EPCs) incorporation on day 21. (A) Photomicrographs of the S1 (left) and CA1 (right) territory after 3-vessel occlusion and treatment with e-EPC visualize the patent cortical (S1) and subcortical (CA1) microvasculature (red) but fail to show DiI-positive e-EPC (green) incorporation into the cerebral vasculature or the surrounding parenchyma. (B) Capillary density within the S1 and CA1 territories in animals treated with saline (n=6), embryonic-endothelial progenitor-derived cells (e-EPDC) (n=6) and e-EPC (n=6) expressed as capillaries per field of view (FOV). (*P<0.05). S1=primary sensory cortex, CA1=hippocampus. Bar=50 μm.
Embryonic-Endothelial Progenitor Cells do not Incorporate into the Cerebral Vasculature In Vivo in Chronic Cerebral Hypoperfusion
On day 21, histologic cryosections failed to show DiI-positive e-EPC within the cerebral vasculature or the perivascular niche (Figure 5). Interestingly, intravital fluorescence video microscopy detected a transit of circulating e-EPC in the cortical microvascular environment immediately after e-EPC injection after 3-VO on day 0 (Figure 6). Although interaction of the DiI-labeled cells with postcapillary venules was noted, e-EPC transmigration into the perivascular niche or incorporation into the existing arterial/arteriolar vasculature was not observed.
Figure 6.
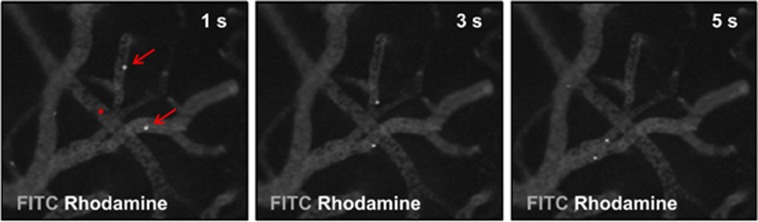
In vivo assessment of cerebrovascular embryonic-endothelial progenitor cell (e-EPC) interaction. Intravital fluorescence video microscopy after cranial window preparations and 3-vessel occlusion (3-VO) before injection of DiI-labeled e-EPC (n=2). The depicted images were obtained 1 (left), 3 (center), and 5 (right) seconds after e-EPC injection. Positive interaction of e-EPC (Rhodamine excitation) with fluorescein isothiocyanate (FITC)-illuminated postcapillary venules can be noted (arrow) but no interaction was observed between e-EPC and the arterial/arteriolar bed (asterisk). s, seconds.
Discussion
In the present study, we demonstrate that e-EPC have potential for therapeutic stimulation of collateral vessel growth in the cerebrovascular system. Compared with their differentiated biologic controls (e-EPDC) and saline-treated animals, e-EPC (I) restored hemodynamic impairment, (II) increased basal and leptomeningeal collateralization and parenchymal capillary density but (III), did not transmigrate and incorporate into existing arterial/arteriolar brain vessels despite positive in vitro and in vivo interaction of e-EPC with the endothelial cell layer of the vasculature. Although the definite mechanism for functional and morphologic rescue remains unclear, these findings pave the way for future clinical treatment studies for chronic cerebral ischemia via artificial stimulation of collateral vessel growth.
Therapeutic Stimulation of Collateral Vessel Growth in Chronic Cerebral Hypoperfusion
Hemodynamic impairment is mirrored in an abolished CVRC, which correlates with the risk of subsequent ischemic stroke.16 Hemodynamic rescue is a result of augmented collateral flow and expressed as an improvement of CVRC. According to the literature, bypass grafting remains controversial and does not appear to be the way to effectively improve hemodynamic reserve in patients with typical arteriosclerotic symptomatic ICA occlusion.17, 18 Thus, there is a need to develop safe and technically robust revascularization techniques.
The cerebrovascular system underlies permanent vascular remodeling to maintain adequate tissue perfusion under physiologic and pathologic conditions but the development of new blood vessels in the chronically hypoperfused brain is poorly understood. Adaptation via neovascularization may be achieved by different mechanisms. Sprouting of newly formed capillaries from preexisting ones (angiogenesis) is mainly driven by hypoxia and does not significantly contribute to the gross tissue perfusion. Arteriogenesis, however, is characterized as the positive outgrowth of preexisting collaterals at the level of the conductance or resistance vasculature, which coincides with an augmentation of collateral flow.4, 5, 19 Against this background, we investigated EPC as a novel treatment modality for chronic cerebral hypoperfusion because EPC secrete various proangiogenic growth factors,20, 21, 22 which may be beneficial in chronic steno-occlusive cerebrovascular disease where neovascularization in terms of collateral vessel growth is required in regions apart from the ischemic tissue. Moreover, EPC are characterized by high plasticity and promigratory activity with the potential for active incorporation into a developing vasculature.9, 23, 24 In an experimental model, this hypothesis must be tested against differentiated endothelial cells; although saline may serve as a sufficient control from a purely therapeutic point of view, it does not from a biologic/mechanistic one. In the present study, we therefore used e-EPC because they harbor the advantage of easy proliferation and maintenance in culture, possible genetic manipulation and the hallmark of ex vivo differentiation by cyclic AMP to obtain differentiated biologic control cells (e-EPDC). The use of differentiated e-EPDC also controlled for potentially species-related immunologic effects on our results. In this regard, however, previous findings demonstrated that the mouse e-EPC used in our study are immuno-privileged owing to a lack of MHC I expression and resistance to natural killer cells.25 Further, xenotransplantation of e-EPC into rabbits demonstrated that e-EPC survived for at least 10 days within the circulatory system of a xenograft host without inducing an inflammatory response.20
Functional Recovery of Hemodynamic Impairment after Treatment with Embryonic-Endothelial Progenitor Cells
The perfusion response (CVRC) after application of a vasodilatory stimulus has previously been used as a functional readout by others and our group with comparable results.4, 26 Occlusion of three of the four major brain-feeding arteries leads to a completely suppressed CVRC, which continued for the observation period of 3 weeks. Intravenous application of e-EPC, however, improved CVRC within 1 week of treatment. This time interval might seem short, taking into account that for functional recovery structural remodeling is necessary. Some previous studies evaluated arteriogenic effects after longer time intervals—for example, after systemic administration of growth factors—suggesting a long-term recovery effect with the requirement of a longer time interval to permit sufficient structural remodeling.4, 5 Other studies, however, also demonstrated structural changes as soon as 1 week after conductance vessel occlusion27, 28 and recent findings from our group on vascular remodeling in a mouse model of chronic cerebral hypoperfusion are in line with these results.19 Next to the structural remodeling, functional improvement of the vasodilation response could additionally contribute to the higher CVRC after treatment with e-EPC.29, 30
Augmentation of Basal and Leptomeningeal Collateralization after Treatment with Embryonic-Endothelial Progenitor Cells
In an experimental model of peripheral artery disease, rabbits with chronic hind limb ischemia treated with the mouse e-EPC from the present study showed significantly better collateralization, higher capillary density and better perfusion.20 In the brain, therapeutic stimulation of collateral vessel growth at the level of the basal vasculature was initially reported by Buschmann et al. and later confirmed by our group.4, 5 Overall, treatment with e-EPC but not e-EPDC compared favorably with these results and led to a significant increase of the ICA and MCA vessel diameters in the anterior circulation. This, together with the positive remodeling of the leptomeningeal and microcirculatory vasculature in e-EPC-treated animals (see below) could account for their improved CVRC and reduced need for compensational cross-flow via the P1 segment, which serves as the natural conduit between the anterior and posterior circulation. The isolated increase of the P1 segment and poor CVRC recovery in e-EPDC-treated animals is in line with this hypothesis and supported by previous observations that collateral outgrowth at the level of the P1 segment does not relevantly influence hemodynamic rescue in the anterior circulation in rats.31 Compared with previous studies where a single hematopoietic stem cell factor (granulocyte macrophage colony-stimulating factor) was administered,4, 5 the augmented collateralization after treatment with e-EPC could be due to the different treatment modalities, since e-EPC harbor the potential for expression of multiple protein growth factors.20 Nevertheless, the application of a different vessel occlusion model (2-VO) in our previous study5 as well as differences in the observation time points somewhat limit comparability.
Leptomeningeal anastomoses are the main collaterals to compensate for restricted blood flow distal to the Circle of Willis. Beyond their function of regulating vascular resistance,32, 33 these vessel segments also improve functional recovery of an impaired CVRC after granulocyte macrophage colony-stimulating factor treatment in rat and mouse models of chronic cerebral hypoperfusion.5, 34, 35 In our study, e-EPC resulted in a significant 20% increase of the total number of anastomoses, whereas the diameter of these vessel segments remained unchanged. This increase of the number of anastomoses leads to an increase of the total cross-sectional vessel area and thus, may improve the total conductance of the brain vasculature. Although this finding is in line with our previous report,5 it differs from published mouse model studies,34, 35 where a primary increase in the diameter but not the number of anastomoses was detected. Possibly, this difference in leptomeningeal outgrowth may be due to differences in the animal strain but more importantly, different vessel occlusion techniques in these studies may influence collateral outgrowth due to different perfusion pressure gradients across the leptomeningeal vasculature.
Next, we investigated the cerebral angioarchitecture beyond the basal and leptomeningeal vasculature and detected a higher density of patent capillaries in the cortical S1 area of the MCA territory. Although the functional contribution of parenchymal microcirculatory flow redistribution remains unknown, parenchymal angiogenesis was also observed in our mouse model of chronic cerebral hypoperfusion.19 Regardless, the present findings should be interpreted with caution because an increased microvascular density could also be a sign of post-ischemic angiogenesis. However, hypoxic tissue injury after 3-VO was previously excluded and thus, the increased microvascular density more likely suggests an activation of parenchymal angiogenesis.
In Vivo and In Vitro Recruitment of Embryonic-Endothelial Progenitor Cells
After intravenous injection, EPCs mainly accumulate in the liver, lung, spleen, and kidney but not in the brain.36 To rule out an influence of the injection site on this e-EPC distribution pattern, we compared intravenous (tail vein, jugular vein) and intraarterial (carotid artery) e-EPC injections in preliminary experiments in animals without 3-VO. In all cases, we found similar e-EPC distribution patterns demonstrating positive e-EPC cell clusters in the liver and the lung but not in the brain (data not shown). In this regard, a number of publications has provided evidence that EPC exert their effect via paracrine/endocrine signaling mechanisms and that direct EPC incorporation into sites of neovascularization and collateral outgrowth is dependent on the underlying pathology and may not be required in all cases.20, 21, 22 Our observed functional and morphologic improvement despite negative e-EPC incorporation into the cerebral vasculature or the perivascular niche supports this hypothesis.
Still, we were somewhat surprised that we detected no sign of e-EPC incorporation, because experimental stroke studies reported EPC incorporation into sites of neovascularization as a sign of postnatal vasculogenesis.37, 38, 39 However, this effect was observed in the ischemic zone and within the penumbra of the infarct, an area with ischemic/hypoxic damage and altered blood–brain barrier function. The blood–brain barrier might be one explanation for this phenomenon, because an intact blood–brain barrier could hamper e-EPC host vessel incorporation and vascular transmigration. The fact that we were able to confirm e-EPC interaction with the endothelium of a preformed vascular bed in vitro is in line with this hypothesis. To get a better understanding whether e-EPC interaction and recruitment may be observed during first passage or recirculation of the cells in vivo, we then performed intravital fluorescence video microscopy through cranial window preparations in animals treated with e-EPC. Although we did note some adherence of e-EPC to the intima of postcapillary venules, direct transmigration or e-EPC interaction within the arterial/arteriolar bed was not observed, which confirmed our histologic findings. Alternatively, the single dose/administration and observation protocol or the xenograft design of our model may also hamper the detection of e-EPC incorporation into the host.
Together, our findings underline that treatment of chronic cerebral hypoperfusion with e-EPC appears to rely on mechanisms other than direct e-EPC integration alone. Although some interaction of e-EPC with the cerebral endothelium was observed in vivo, it seems that direct e-EPC incorporation into the vasculature of the host is not mandatory for mediation of the functional and morphologic effects we observed. In chronic cerebral hypoperfusion, an indirect paracrine/endocrine signaling mechanism may therefore have a more predominant role in mediating collateral outgrowth and subsequent functional hemodynamic recovery after treatment with e-EPC.
The authors declare no conflict of interest.
Footnotes
Johannes Woitzik received funding from the Deutsche Forschungsgemeinschaft (DFG-WO 1704/11).
References
- Markus HS, Khan U, Birns J, Evans A, Kalra L, Rudd AG, et al. Differences in stroke subtypes between black and white patients with stroke: the South London Ethnicity and Stroke Study. Circulation. 2007;116:2157–2164. doi: 10.1161/CIRCULATIONAHA.107.699785. [DOI] [PubMed] [Google Scholar]
- Schaper W. Arteriogenesis, the good and bad of it. Cardiovasc Res. 1999;43:835–837. doi: 10.1016/s0008-6363(99)00191-1. [DOI] [PubMed] [Google Scholar]
- Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol. 2003;23:1143–1151. doi: 10.1161/01.ATV.0000069625.11230.96. [DOI] [PubMed] [Google Scholar]
- Buschmann IR, Busch HJ, Mies G, Hossmann KA. Therapeutic induction of arteriogenesis in hypoperfused rat brain via granulocyte-macrophage colony-stimulating factor. Circulation. 2003;108:610–636. doi: 10.1161/01.CIR.0000074209.17561.99. [DOI] [PubMed] [Google Scholar]
- Schneider UC, Schilling L, Schroeck H, Nebe CT, Vajkoczy P, Woitzik J. Granulocyte-macrophage colony-stimulating factor-induced vessel growth restores cerebral blood supply after bilateral carotid artery occlusion. Stroke. 2007;38:1320–1328. doi: 10.1161/01.STR.0000259707.43496.71. [DOI] [PubMed] [Google Scholar]
- Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, et al. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- Schlager O, Giurgea A, Schuhfried O, Seidinger D, Hammer A, Groger M, et al. Exercise training increases endothelial progenitor cells and decreases asymmetric dimethylarginine in peripheral arterial disease: a randomized controlled trial. Atherosclerosis. 2011;217:240–248. doi: 10.1016/j.atherosclerosis.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J-I, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- Hatzopoulos AK, Folkman J, Vasile E, Eiselen GK, Rosenberg RD. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development. 1998;125:1457–1468. doi: 10.1242/dev.125.8.1457. [DOI] [PubMed] [Google Scholar]
- Vajkoczy P, Blum S, Lamparter M, Mailhammer R, Erber R, Engelhardt B, et al. Multistep nature of microvascular recruitment of ex vivo-expanded embryonic endothelial progenitor cells during tumor angiogenesis. J Exp Med. 2003;197:1755–1765. doi: 10.1084/jem.20021659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- Vajkoczy P, Schilling L, Ullrich A, Schmiedek P, Menger MD. Characterization of angiogenesis and microcirculation of high-grade glioma: an intravital multifluorescence microscopic approach in the athymic nude mouse. J Cereb Blood Flow Metab. 1998;18:510–520. doi: 10.1097/00004647-199805000-00006. [DOI] [PubMed] [Google Scholar]
- Grubb RL, Jr., Derdeyn CP, Fritsch SM, Carpenter DA, Yundt KD, Videen TO, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA. 1998;280:1055–1060. doi: 10.1001/jama.280.12.1055. [DOI] [PubMed] [Google Scholar]
- Powers WJ, Clarke WR, Grubb RL, Jr., Videen TO, Adams HPJ, Derdeyn CP, et al. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the carotid occlusion surgery study randomized trial. JAMA. 2011;306:1983–1992. doi: 10.1001/jama.2011.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin-Hanjani S, FG 2nd Barker, Charbel FT, Connolly ESJ, Jr., Morcos JJ, Thompson BG. Extracranial-intracranial bypass for stroke-is this the end of the line or a bump in the road. Neurosurgery. 2012;71:557–561. doi: 10.1227/NEU.0b013e3182621488. [DOI] [PubMed] [Google Scholar]
- Hecht N, He J, Kremenetskaia I, Nieminen M, Vajkoczy P, Woitzik J. Cerebral hemodynamic reserve and vascular remodeling in C57/BL6 mice are influenced by age. Stroke. 2012;43:3052–3062. doi: 10.1161/STROKEAHA.112.653204. [DOI] [PubMed] [Google Scholar]
- Kupatt C, Horstkotte J, Vlastos GA, Pfosser A, Lebherz C, Semisch M, et al. Embryonic endothelial progenitor cells expressing a broad range of proangiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J. 2005;19:1576–1578. doi: 10.1096/fj.04-3282fje. [DOI] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- Heil M, Ziegelhoeffer T, Mees B, Schaper W. A different outlook on the role of bone marrow stem cells in vascular growth: bone marrow delivers software not hardware. Circ Res. 2004;94:573–574. doi: 10.1161/01.RES.0000124603.46777.EB. [DOI] [PubMed] [Google Scholar]
- Rookmaaker MB, Tolboom H, Goldschmeding R, Zwaginga J-J, Rabelink TJ, Verhaar MC. Bone-marrow-derived cells contribute to endothelial repair after thrombotic microangiopathy. Blood. 2002;99:1095. doi: 10.1182/blood.v99.3.1095. [DOI] [PubMed] [Google Scholar]
- Werner N, Priller J, Laufs U, Endres M, Bohm M, Dirnagl U, et al. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 2002;22:1567–1572. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- Wei J, Blum S, Unger M, Jarmy G, Lamparter M, Geishauser A, et al. Embryonic endothelial progenitor cells armed with a suicide gene target hypoxic lung metastases after intravenous delivery. Cancer Cell. 2004;5:477–488. doi: 10.1016/s1535-6108(04)00116-3. [DOI] [PubMed] [Google Scholar]
- Duelsner A, Gatzke N, Glaser J, Hillmeister P, Li M, Lee EJ, et al. Acetylsalicylic acid, but not clopidogrel, inhibits therapeutically induced cerebral arteriogenesis in the hypoperfused rat brain. J Cereb Blood Flow Metab. 2011;32:105–114. doi: 10.1038/jcbfm.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch HJ, Schirmer SH, Jost M, van Stijn S, Peters SL, Piek JJ, et al. Leptin augments cerebral hemodynamic reserve after three-vessel occlusion: distinct effects on cerebrovascular tone and proliferation in a nonlethal model of hypoperfused rat brain. J Cereb Blood Flow Metab. 2011;31:1085–1092. doi: 10.1038/jcbfm.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duelsner A, Gatzke N, Glaser J, Hillmeister P, Li M, Lee E-J, et al. Granulocyte colony-stimulating factor improves cerebrovascular reserve capacity by enhancing collateral growth in the Circle of Willis. Cerebrovasc Dis. 2012;33:419–429. doi: 10.1159/000335869. [DOI] [PubMed] [Google Scholar]
- Hiasa K-I, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, et al. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–2461. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- Zhou J, Cheng M, Liao Y-H, Hu Y, Wu M, Wang Q, et al. Rosuvastatin enhances angiogenesis via eNOS-dependent mobilization of endothelial progenitor cells. PLoS One. 2013;8:e63126. doi: 10.1371/journal.pone.0063126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch HJ, Buschmann IR, Mies G, Bode C, Hossmann KA. Arteriogenesis in hypoperfused rat brain. J Cereb Blood Flow Metab. 2003;23:621–628. doi: 10.1097/01.WCB.0000057741.00152.E4. [DOI] [PubMed] [Google Scholar]
- Tariq N, Khatri R. Leptomeningeal collaterals in acute ischemic stroke. J Vasc Interv Neurol. 2008;1:91–95. [PMC free article] [PubMed] [Google Scholar]
- Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- Todo K, Kitagawa K, Sasaki T, Omura-Matsuoka E, Terasaki Y, Oyama N, et al. Granulocyte-macrophage colony-stimulating factor enhances leptomeningeal collateral growth induced by common carotid artery occlusion. Stroke. 2008;39:1875–1882. doi: 10.1161/STROKEAHA.107.503433. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Yagita Y, Oyama N, Terasaki Y, Omura-Matsuoka E, Sasaki T, et al. Granulocyte colony-stimulating factor enhances arteriogenesis and ameliorates cerebral damage in a mouse model of ischemic stroke. Stroke. 2011;42:770–775. doi: 10.1161/STROKEAHA.110.597799. [DOI] [PubMed] [Google Scholar]
- Moubarik C, Guillet B, Youssef B, Codaccioni J-L, Piercecchi M-D, Sabatier F, et al. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev. 2011;7:208–220. doi: 10.1007/s12015-010-9157-y. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu W-C, Lin S-Z, Chiang M-F, Su C-Y, Li H. Intracerebral peripheral blood stem cell (CD34+) implantation induces neuroplasticity by enhancing beta1 integrin-mediated angiogenesis in chronic stroke rats. J Neurosci. 2006;26:3444–3453. doi: 10.1523/JNEUROSCI.5165-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90:284–288. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]