Abstract
White matter hyperintensities (WMHs) and lacunes are magnetic resonance imaging hallmarks of cerebral small-vessel disease, which increase the risk of stroke, cognitive, and mobility impairment. Although most studies of cerebral small-vessel disease have focused on white matter abnormalities, the gray matter (GM) is also affected, as evidenced by frequently observed lacunes in subcortical GM. Diffusion tensor imaging (DTI) is sensitive to subtle neurodegenerative changes in deep GM structures. We explored the relationship between baseline DTI characteristics of the thalamus, caudate, and putamen, and the volume and subsequent accrual of WMHs over a 4-year period in 56 community-dwelling older (⩾75 years) individuals. Baseline thalamic fractional anisotropy (FA) was an independent predictor of WMH accrual. WMH accrual also correlated with baseline lacune count and baseline WMH volume, the latter showing the strongest predictive power, explaining 27.3% of the variance. The addition of baseline thalamic FA in multivariate modeling increased this value by 70%, which explains 46.5% of the variance in WMH accrual rate. Thalamic FA might serve as a novel predictor of cerebral small-vessel disease progression in clinical settings and trials. Furthermore, our findings point to the possibility of a causal relationship between thalamic damage and the accrual of WMHs.
Keywords: aging, deep gray matter, diffusion tensor imaging, magnetic resonance imaging, small-vessel disease, white matter hyperintensities
Introduction
Cerebral white matter hyperintensities (WMHs) are common magnetic resonance imaging (MRI) findings in older people, with prevalence as high as 90% depending on the defining criteria.1 Associations with cerebrovascular risk factors, such as increasing age, hypertension, and prior stroke or myocardial infarction,2, 3 have suggested a vascular origin of age-related WMHs, which are considered MRI correlates of cerebral small-vessel disease (SVD).4 However, the etiology of WMHs and the pathophysiologic mechanisms leading to their accrual are still debated. WMH accrual over time has significant clinical impact in terms of increased risk of mobility impairment, stroke, dementia, and death.5, 6 Although WMH progression occurs mostly in subjects showing larger WMH burden at baseline,7, 8, 9 WMH volume alone does not explain the whole extent of variability in WMH accrual over time. Significant efforts have been made in the last decade to identify other predictors of cerebral SVD progression. Recently the Leukoaraiosis And DISability (LADIS) study group showed that WMH accrual was more likely to occur in subjects with larger number of lacunes in gray matter (GM) and white matter (WM),9 reemphasizing the relevance of GM damage in the context of SVD.
In this study we assessed deep GM damage in a 4-year, longitudinal MRI study of WMHs in older community-dwelling subjects, by using diffusion tensor imaging (DTI), an MRI technique that provides sensitive indicators of tissue microarchitecture based on the diffusion behavior of water molecules within the tissues of interest.10 The method has been used for characterizing the microstructural properties of WM tracts, and only more recently has been explored as a means to detect microstructural abnormalities in the deep GM. Changes in tissue anisotropy of the deep GM have been reported in older subjects,11, 12 cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL),13 as well as multiple sclerosis,14, 15 and appear to represent a sensitive, and clinically relevant biomarker of GM damage.
In this longitudinal MRI study of older individuals, we investigated the relationship between DTI characteristics of the thalamus, caudate, and putamen, and the severity and future accrual of WMHs. We found that baseline tissue anisotropy of the thalamus independently predicted WMH accrual over a 4-year period.
Materials and methods
Subjects
The subjects included in this analysis represent a subset of a cohort of 99 community-dwelling elderly subjects (age range: 75–90 years) enrolled in a 4-year prospective study on the relationship between brain MRI abnormalities, cardiovascular risk factors, cognitive, and motor performance, about which we have reported previously.16, 17, 18, 19 Recruitment methods and eligibility criteria have been described elsewhere.16 Briefly, subject enrollment was aimed at uniformly populating a 3 × 3 grid stratified by age (75–80; 80–85; >85) and performance on the short physical performance battery (SPPB),20 a composite score mostly reflective of gait and mobility function (normal, SPPB=11–12; intermediate, SPPB=9–10; impaired, SPPB<9). An expert neurologist (LW) performed neurologic examination on each subject, which included evaluation of sensory function (touch, pin position sense, and vibration) and motor function (strength, tone, coordinated/rapid alternating hand movements, finger to nose, heel–knee–shin tests, and observation of gait/balance). The subjects were also administered the Mini-Mental State Examination (MMSE). All the subjects included in this study were asymptomatic for major neurologic diseases impairing motor or cognitive functions (MMSE⩾24). All the subjects were investigated by 3 Tesla (3T) brain MRI. Data for the present longitudinal analysis included 56 subjects who had both baseline and 4-year follow-up conventional MRI scans, and baseline DTI data of sufficient quality. This subpopulation was representative of the overall population studied for baseline demographics and clinical characteristics (see the comparison in Table 1). The following cerebrovascular risk factors were collected and included in the analyses: history of hypertension and/or diabetes mellitus, average 24-hour systolic and diastolic blood pressure, and serum lipoprotein levels (total, high-density lipoprotein and low-density lipoprotein (LDL) cholesterol). The 24-hour blood pressure data were obtained and analyzed as previously described.18 The Institutional Review Board of the Brigham and Women's Hospital (known as Partners Human Research Committee) and the Institutional Review Board of University of Connecticut Health Center approved the study protocol before the start of the study. All participants provided written informed consent.
Table 1. Baseline characteristics of the study subjects.
| All participants (n=99) | Subjects included in this study (n=56) | |
|---|---|---|
| Demographics | ||
| Age (years) | 83±4 | 82±4 |
| Sex (F) | 57 (58%) | 32 (57%) |
| Clinical | ||
| MMSE | 28±1 | 29±1 |
| SPPB | 9±2 | 9±2 |
| Cardiovascular risk | ||
| Hypertension | 71 (72%) | 41 (73%) |
| 24-hour SBP (mm Hg) | 131±13 | 130±11 |
| 24-hour DBP (mm Hg) | 68±7 | 66±7 |
| Diabetes | 6 (6%) | 5 (9%) |
| BMI (kg/m2) | 26±4 | 26±5 |
| Total cholesterol (mg/dL) | 199±40 | 197±41 |
| LDL (mg/dL) | 126±36 | 124±37 |
| HDL (mg/dL) | 57±15 | 57±15 |
| MRI | ||
| WMH burden (mL) | 13.7±12.4 | 13.2±12.9 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; MMSE, Mini-Mental State Examination; MRI, magnetic resonance imaging; SBP, systolic blood pressure; SPPB, short physical performance battery; WMH, white matter hyperintensity.
Data are expressed as mean±s.d. or n (%).
MRI acquisition and image analysis
Brain MR images were acquired on a 3T Siemens Allegra scanner (Erlangen, Germany) using the following sequences and parameters: three dimensional (3D) T1-weighted magnetization prepared rapid gradient echo (MPRAGE; repetition time/echo time/inversion time=2,500/2.74/900 ms, 176 contiguous 1-mm-thick axial slices, matrix size=256 × 208, nominal in-plane pixel dimensions=1 mm × 1 mm), 3D fast spin echo (T2; repetition time/echo time=2,500/353 ms, 176 contiguous 1-mm-thick sagittal slices, matrix size=256 × 220, nominal in-plane pixel dimensions=1 mm × 1 mm), and 3D fluid attenuated inversion recovery (FLAIR; repetition time/echo time/inversion time=6,000/353/2,200 ms, 128 contiguous 1.3-mm-thick sagittal slices, matrix size=256 × 208, nominal in-plane pixel dimensions=1 mm × 1 mm). Preprocessing included correction of signal inhomogeneities (bias field),21 and linear affine registration of FLAIR and T2-weighted images to the MPRAGE images.22 DTI was performed using a standard echo planar imaging sequence with repetition time/echo time=5,800/87 ms, 45 contiguous 3-mm-thick axial slices, field of view=20 cm, acquisition and reconstruction matrices=128 × 96 and 128 × 128, diffusion-sensitizing orientations in 12 directions with one B0, and 8 averages for each direction at b=1,000 s/mm2. DTI data were checked for excessive background noise, motion, and other artifacts; significant artifacts resulted in subject exclusion. FSL software (MMRIB software library, www.fmrib.ox.ac.uk/fsl) was used for analysis. All DTI images were coregistered to the B0 image, with gradient directions corrected for the applied rotation. The 3D extent of the thalamus, caudate head, and the putamen was manually outlined on baseline DTI images by a trained physician (MC) masked to demographic, clinical, and other MRI data, using axial lambda-1 maps as guidance, as previously described.15 These regions of interest were then superimposed on the fractional anisotropy (FA) and mean diffusivity (MD) maps to extract FA and MD values for each structure of interest. Mean axial diffusivity (AD) and radial diffusivity (RD) values were calculated for each region of interest from the lambda-1, lambda-2, and lambda-3 maps. Intrarater reliability for FA measurements was tested on a subsample of 10 cases, and showed an intraclass correlation coefficient of 96% (95% confidence interval (CI)=86–99%) for the caudate, 94% (95% CI=79–99%) for the putamen, and 96% (95% CI=84–99%) for the thalamus. Mean FA, MD, AD, and RD values at baseline of each region of interest (left and right combined) were included in the statistical analyses.
Total T2-weighted WMH volume (mL) at baseline and after 4 years were obtained by computer-assisted, user-interactive image segmentation of FLAIR images, and have been previously published in the context of their association with functional outcomes (see, e.g., Moscufo et al17). WMH accrual was calculated by subtracting baseline WMH volume from WMH volume at 4 years, and expressed as annualized accrual rate of WMHs (mL/year). 3D Slicer (www.slicer.org) was used for region of interest outlining, DTI metrics analysis, and WMH volume measurement.
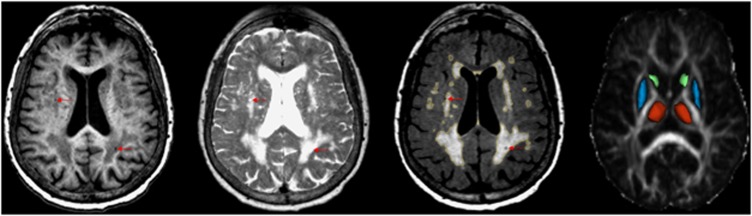
Lacunes were identified as 3–15 mm diameter areas showing cerebrospinal fluid intensity, i.e., hypointense on T1 and FLAIR, and hyperintense on T2-weighted images. Lower and upper dimension limits were applied to differentiate lacunes from smaller perivascular spaces and larger infarcts, respectively. Lacunes were distinguished from enlarged perivascular spaces by relying on a hyperintense rim that often surrounds them on FLAIR images, and the fact that the shape and location of perivascular spaces usually follow the vessel path. T2-weighted images were used to increase the detection sensitivity for lacunes occurring in the deep GM, and to differentiate lacunes from microbleeds, the latter being hypointense on T2-weighted images. Total baseline lacune count was considered in the analyses. MRI examples of lacunes, WMHs, and the segmented deep GM structures are illustrated in Figure 1.
Figure 1.
Magnetic resonance imaging examples of lacunes, white matter hyperintensities (WMHs), and the segmented deep gray matter structures. Definitions of lacunes, WMHs, and anatomic regions of interest are illustrated on a set of magnetic resonance images from a 77-year-old female study participant. From left to right: T1-weighted, T2-weighted, fluid attenuated inversion recovery (FLAIR), and fractional anisotropy (FA) map images. Lacunes (arrows) were defined as circumscribed small areas in brain parenchyma showing cerebrospinal fluidlike intensity (hypointense on T1 and FLAIR, and hyperintense on T2-weighted images). The output of computer-assisted segmentation of WMHs is shown on FLAIR. The three dimensional models of the manually outlined thalamus (red), caudate (green), and putamen (blue) are overlaid on the FA map of a single section containing these structures.
Statistical analysis
Statistical analysis was performed using STATA 11 (www.stata.com). Normal distribution of the data was assessed with the Kolmogorov–Smirnov test. Baseline FA values of the three structures of interest were normally distributed. Since most of the other variables had nonnormal distribution, we measured associations by calculating Spearman's rho correlation coefficients. To assess the predictive value of baseline MRI and clinical variables with regard to subsequent WMH accrual, we performed linear regression as follows. Variables with nonnormal distribution (i.e., baseline WMHs and accrual, MD values, body mass index (BMI), and total cholesterol and LDL levels) were transformed with square root to fit a normal distribution. FA and MD values of the thalamus, caudate, and putamen were the main variables of interest for this study. FA and MD of each structure of interest and other baseline variables that showed significant correlation with annualized accrual rate of WMHs were tested individually in univariate linear regression analysis. In a subsequent step, the variables that showed significant correlation in univariate analysis were entered together in a linear regression model, controlling for age as a covariate, to identify the best combination of predictors of WMH accrual. Post hoc linear regression analyses were used to investigate the relationship of AD and RD values with FA, as well as to assess which DTI parameter/s drove the observed association of FA with WMH accrual. We also assessed the relationship between baseline DTI changes and WMH accrual by linear mixed model analysis. Given that the study aim was to investigate the relationship of DTI metrics of the thalamus, caudate, and putamen with the severity and future accrual of WMHs, we applied a threshold for statistical significance ≤0.0083 for the analyses involving FA and MD measurements of the three structures of interest, according to Bonferroni correction for multiple comparisons (0.05/6). The threshold of statistical significance for analyses involving other variables was P≤0.05.
Results
Of the 99 subjects originally included in the study, 85 had acceptable DTI at baseline, and 56 of these subjects completed the 4-year clinical and neuroimaging follow-up. The average interval between baseline and 4-year follow-up MRI scans was 3.9±0.1 years (mean±s.d.). Baseline characteristics of the study subjects are summarized in Table 1. All subjects showed WMHs of presumed vascular origin at baseline. According to the Fazekas score,23 baseline WMH burden ranged from mild to severe, with ∼2/3 (n=37/56=66% 95% CI=53–77%) of the study subjects showing mild lesion load. Baseline WMH burden ranged from 0.50 to 56.63 mL. Average WMH accrual over the 4-year follow-up period was 2.79±2.13 mL/year (mean±s.d.). Lacunes were present in approximately half of the subjects at baseline (n=27/56=48% 95% CI=36–61%) and occurred in the following subcortical regions: frontal WM in 7 subjects, parieto-occipital WM in 7 subjects, temporal WM in 2 subjects, and deep GM in 15 subjects (basal ganglia in all of them and thalamus in one subject). Out of the 56 subjects included, 21 (38% 95% CI=25–51%) had 1–3 lacunes, while 6 subjects (11% 95% CI=4–22%) had >3 lacunes.
Mean FA values (±s.d.) of the three structures of interest were 0.299±0.026 for the thalamus, 0.181±0.038 for the caudate, and 0.163±0.035 for the putamen. Average MD values (±s.d.) were 0.827±0.048 μm2/s for the thalamus, 0.782±0.095 μm2/s for the caudate, and 0.863±0.144 μm2/s for the putamen.
We found a positive cross-sectional correlation of thalamus and putamen MD values with WMH burden at baseline (rho=0.335, P=0.0117 for the thalamus; rho=0.471, P=0.0003 for the putamen), while there were no significant correlations of caudate MD and FA values of the three structures of interest with baseline WMH volume. MD values of the striatal nuclei correlated with total lacune count (rho=0.333, P=0.012 for the caudate; rho=0.361, P=0.006 for the putamen). After Bonferroni correction for multiple comparisons, only the associations of putamen MD with baseline WMH burden and lacune count remained significant (P≤0.0083).
Separately, we also assessed the direct impact of lacunes within the striatum and found increased MD values in the caudate and putamen in subjects with striatal lacunes compared with those without (P=0.052 for the caudate; P=0.0009 for the putamen; Mann–Whitney test).
There were no correlations between FA of the thalamus, caudate, or putamen and lacune count, nor were there differences in FA values between the subjects with lacunes in the deep GM and those without.
Among the structures of interest only baseline thalamic FA and putamen MD showed a positive correlation with the annualized accrual rate of WMHs (Table 2). After Bonferroni correction for multiple comparisons, only the association between thalamic FA and WMH accrual remained significant (P≤0.0083). Baseline WMH volume and total lacune count also showed significant positive correlation with WMH accrual rate (Table 2). Baseline cardiovascular risk factors, age, and sex showed no significant association with WMH accrual. Total cholesterol and LDL levels were positively correlated with putamen and thalamus FA (putamen FA/total cholesterol: rho=0.411, P=0.002; putamen FA/LDL: rho=0.352, P=0.008; thalamus FA/total cholesterol: rho=0.318, P=0.017; thalamus FA/LDL: rho=0.272, P=0.043). We also found an inverse correlation between BMI and thalamic FA (rho=−0.286, P=0.032). No significant associations were found between deep GM FA and any other cardiovascular risk factors, age, or sex, as well as between MD values and all cardiovascular risk factors, age, or sex. The baseline variables showing significant correlations with the annualized accrual rate of WMHs—i.e., thalamic FA, putamen MD, WMH burden, and lacune count—were tested first individually and then entered simultaneously into a linear regression model as candidate predictors of WMH accrual, controlling for age as covariate. Baseline WMHs and thalamic FA were significant predictors of WMH accrual in univariate analyses. Among these variables, baseline WMHs was the strongest predictor (Table 3). According to a linear regression model, thalamic FA was an independent predictor of WMH accrual and contributed to increase the predictive power of baseline WMH volume. When included into the model, lacune count also contributed to the prediction of WMH accrual. The linear regression model including both thalamus FA and WMH volume at baseline explained 46.5% of the population's variability in annualized accrual rate of WMHs, while all three variables together explained 49.6%. Baseline WMH volume explained only 27.3% of the variance in WMH accrual rates (Table 3). Linear mixed model results were very similar to those of linear regression (see Supplementary Table).
Table 2. Spearman's correlations of baseline clinical and MRI indices with annual accrual rate of WMH.
| Variables | rho | P |
|---|---|---|
| Thalamus FA | 0.366 | 0.006* |
| Thalamus MD | 0.173 | 0.202 |
| Caudate FA | 0.230 | 0.088 |
| Caudate MD | 0.237 | 0.079 |
| Putamen FA | 0.034 | 0.806 |
| Putamen MD | 0.283 | 0.035 |
| Age | −0.042 | 0.761 |
| Sex | −0.197 | 0.147 |
| 24-hour SBP (mm Hg) | −0.022 | 0.881 |
| 24-hour DBP (mm Hg) | 0.099 | 0.490 |
| Diabetes | −0.149 | 0.273 |
| BMI (kg/m2) | −0.220 | 0.104 |
| Total cholesterol (mg/dL) | 0.035 | 0.799 |
| LDL (mg/dL) | −0.002 | 0.989 |
| HDL (mg/dL) | −0.094 | 0.490 |
| Baseline WMH volume (mL) | 0.577 | 0.00001 |
| Lacunes count | 0.270 | 0.044 |
Abbreviations: BMI, body mass index; DPB, diastolic blood pressure; DTI, diffusion tensor imaging; FA, fractional anisotropy; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; MD, mean diffusivity; MRI, magnetic resonance imaging; SBP, systolic blood pressure; WMH, white matter hyperintensity.
Threshold for statistical significance for analyses involving other variables was 0.05. Statistical significance is indicated in bold. According to the primary study aim, we applied Bonferroni correction for multiple comparisons, which resulted in a threshold for statistical significance ≤0.0083 (0.05/6) for the analyses involving DTI measurements of the three structures of interest. P-values ≤0.05 are indicated in bold; (*) indicates P-values ≤0.0083.
Table 3. Baseline predictors of annual accrual rate of WMH.
| Baseline variables | β (95% CI) | P | r2 |
|---|---|---|---|
| Thalamus FA | 8.225 (2.268 to 14.181) | 0.0077 | 0.126 |
| Thalamus MD | 4.185 (−2.466 to 10.835) | 0.212 | 0.029 |
| Caudate FA | 4.749 (−0.240 to 9.738) | 0.062 | 0.064 |
| Caudate MD | 2.189 (−1.134 to 5.522) | 0.193 | 0.032 |
| Putamen FA | 1.397 (−3.841 to 6.635) | 0.595 | 0.005 |
| Putamen MD | 1.792 (−0.513 to 4.098) | 0.125 | 0.044 |
| Age | 0.005 (−0.042 to 0.051) | 0.838 | 0.001 |
| WMH volume | 0.211 (0.116 to 0.306) | 0.00001 | 0.273 |
| Lacune count | 0.084 (−0.006 to 0.174) | 0.065 | 0.063 |
| Linear regression models | |||
| Model 1 | 0.0002 | 0.273 | |
| WMH volume | 0.211 (0.115 to 0.307) | 0.0001 | |
| Age | 0.003 (−0.037 to 0.043) | 0.865 | |
| Model 2 | 0.00001 | 0.465 | |
| WMH volume | 0.228 (0.142 to 0.313) | 0.0001 | |
| Thalamus FA | 9.532 (4.647 to 14.417) | 0.0001 | |
| Age | 0.003 (−0.033 to 0.038) | 0.887 | |
| Model 3 | 0.00001 | 0.496 | |
| WMH volume | 0.215 (0.133 to 0.298) | 0.0001 | |
| Thalamus FA | 10.023 (5.317 to 14.730) | 0.0001 | |
| Lacunes | 0.082 (0.011 to 0.1532) | 0.024 | |
| Age | 0.013 (−0.002 to 0.048) | 0.466 | |
Abbreviations: CI, confidence interval; FA, fractional anisotropy; MD, mean diffusivity; WMH, white matter hyperintensity.
To fit them to a normal distribution we derived the square roots of baseline WMH volume, MD values, and annual WMH accrual rate before performing linear comparisons.
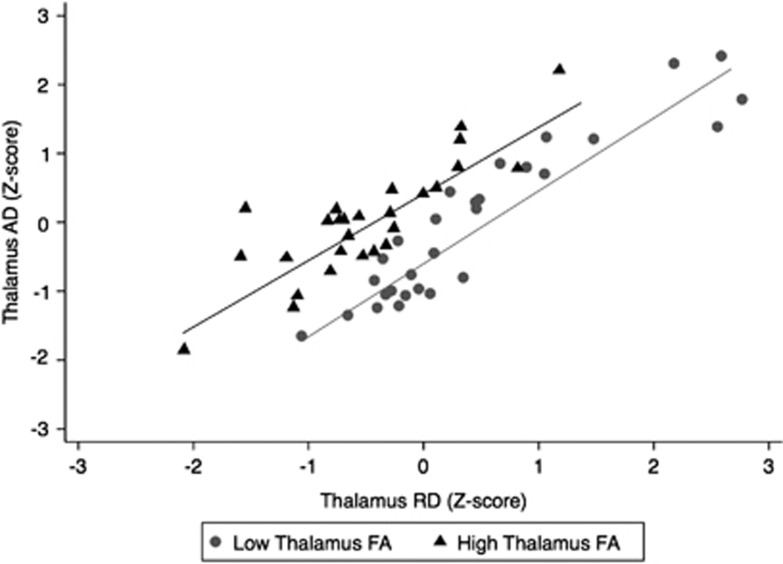
To assess whether the observed positive correlation between thalamic FA and WMH accrual was driven by AD or RD changes we looked at the relationship between thalamic AD and RD relative to thalamic FA (Figure 2). The plot shows a clear left shift (corresponding to increased AD and decreased RD) of the regression line between thalamic AD and RD in the subjects with relatively higher FA (z-score>0). In a linear regression model thalamic FA showed a positive correlation with thalamic AD (β=0.523, P<0.0001), and a negative correlation with thalamic RD (β=−0.824, P=0.0001). We also investigated the relationship between WMH accrual and thalamic AD or RD. Increased thalamic AD, but not RD, was a significant predictor of the annual rate of WMH accrual (Table 4). On controlling for age and baseline WMH volume, the correlation between AD and WMH accrual lost statistical significance, and the inclusion of thalamic AD did not provide additional power compared with the predictive model that included only baseline WMH volume (Tables 3 and 4).
Figure 2.
Scatterplot showing the relationship between thalamic axial diffusivity (AD) and radial diffusivity (RD) relative to thalamic fractional anisotropy (FA). Black triangles indicate the subjects with relatively higher FA (thalamic FA z-score >0); gray circles indicate the subjects with relatively lower thalamic FA (thalamic FA z-score <0). All the z-scores were calculated using the mean and standard deviation of each thalamic index in the whole study population. The plot shows a clear left shift (corresponding to increased AD and decreased RD) of the linear regression fit in the subjects with relatively higher FA compared with the corresponding fit in the subjects with FA below the mean value of the whole population.
Table 4. Post hoc analysis of the thalamic DTI predictors of WMH accrual by linear regression.
| Baseline variables | β (95% CI) | P | r2 |
|---|---|---|---|
| Thalamus AD | 4.984 (1.868 to 8.099) | 0.0023 | 0.163 |
| Thalamus RD | 0.439 (−3.098 to 3.977) | 0.8042 | 0.001 |
| Linear regression model | |||
| Model | 0.0003 | 0.302 | |
| Thalamus AD | 2.408 (−0.930 to 5.746) | 0.154 | |
| Baseline WMH volume | 0.173 (0.064 to 0.281) | 0.002 | |
| Age | 0.001 (−0.039 to 0.040) | 0.466 | |
Abbreviations: AD, axial diffusivity; CI, confidence interval; DTI, diffusion tensor imaging; RD, radial diffusivity; WMH, white matter hyperintensity.
To fit them to a normal distribution we derived the square roots of baseline WMH volume and annual WMH accrual rate before performing linear comparisons.
Discussion
We investigated the microstructural integrity of the deep GM nuclei by using DTI, to assess the relationship between WM and GM changes, and the predictive value of GM changes on WMH accrual over a subsequent 4-year period in community-dwelling older subjects. We identified thalamus FA as a novel candidate biomarker of WMH progression. Specifically, increased FA of the thalamus independently predicted the annualized accrual rate of WMHs.
The number of studies on cerebral SVD focused on GM abnormalities is very limited.
Brain parenchymal damage is usually assessed by quantifying WMH burden, as WMHs are easily detectable as bright signal alterations on conventional T2-weighted MR images, while GM areas are not conspicuously abnormal on conventional MRI. A few studies reported DTI abnormalities in the deep GM. Increased FA of caudate and putamen has been reported in a few cross-sectional studies on aging.11, 12 A study of CADASIL, the most common inherited form of cerebral SVD, used different indices of tissue anisotropy (i.e., trace and volume ratio) to show increased trace and decreased volume ratio in the patients compared with controls.13 To our knowledge, no studies have reported on the longitudinal relationship between GM anisotropy and progression of WM changes. While the interpretation of this finding will require further study, it is clear that GM changes should be assessed in conjunction with those in the WM, when studying cerebral SVD with MRI. More specifically, DTI appears to be a sensitive tool for this purpose.
Given the scarcity of MRI–pathology association studies, interpretation of the observed association between thalamic FA and WMH accrual in terms of its histologic correlates is still controversial. Among the studies reporting DTI abnormalities in the deep GM the findings have been interpreted as microstructural damage because of neuroaxonal injury, dendritic stripping, gliosis, or iron accumulation.11 Post hoc analyses showed that thalamic FA was positively correlated with thalamic AD, and negatively correlated with thalamic RD (Figure 2). In addition, increased thalamic AD, but not RD, was a significant predictor of the annual rate of WMH accrual (Table 4). These findings suggest that the relationship between thalamic FA and WMH accrual is associated with increased AD and relatively low RD. In recent animal experiments of traumatic brain injury a similar DTI pattern has been associated with gliosis at the histopathologic level.24 Unlike FA, AD did not provide added predictive value with regard to WMH accrual, compared with a regression model that included only baseline WMH volume (Table 4). Future studies using more sophisticated DTI techniques employing multiple or higher b-values compared with the standard DTI, such as high angular resolution diffusion imaging25 or composite hindered and restricted model of diffusion,26 as well as DTI–pathology combination studies may provide meaningful information to better understand the histopathologic substrate of DTI changes in the GM. Further, the addition of iron-sensitive sequences (e.g., T2*-weighted gradient-recalled echo MRI or susceptibility-weighted imaging) in future studies might clarify the potential role of iron accumulation in our findings, as well as enable the concomitant study of microbleeds, which are also considered a relevant independent MRI feature of SVD. As to the thalamus, the coexistence of WM and GM damage, which results in competing influences on diffusion tensor signal, requires even more careful biologic interpretation of the findings. Nevertheless, increased FA in the thalamus is considered a reliable correlate of GM injury, and is thought to reflect microstructural damage within the thalamic nuclei, as opposed to the relatively abundant thalamic WM.15 In contrast, increased MD values can result from either GM or WM damage within this structure. Therefore, the cross-sectional correlation between thalamic MD and cerebral WMHs at baseline could be driven by either or both GM and WM components of the thalamus. However, the absence of cross-sectional correlation between thalamic FA and WMH volume suggests that the above correlation is driven by damage to thalamic WM. Although reduced, rather than increased FA is considered the hallmark DTI correlate of WM damage, a more restricted intracellular (intraaxonal) water compartment associated with cytotoxic edema could lead to a high FA with relatively low MD or no change of MD, thus supporting the hypothesis that the detected DTI changes in the thalamus might reflect WM injury. However, the positive correlation between AD and WMH accrual and the lack of correlation between RD and WMH accrual (Table 4) would not support this interpretation.27 Increased FA with little or no MD changes can also be explained by extraaxonal degeneration in GM nuclei.15 This would support the interpretation that thalamic GM rather than thalamic WM abnormalities drive the association with subsequent cerebral WMH accrual. Future studies using more sophisticated techniques to measure the diffusion tensor signal in the thalamic nuclei separately from the WM tracts28, 29 may clarify the relative contribution of GM and WM damage to the observed association between baseline thalamic FA and WMH accrual.
Consistently with previous work,8, 9, 30 both baseline WMH volume and number of lacunes predicted WMH accrual. Baseline WMH volume was the strongest predictor of WMH progression, in line with previous studies, including our own reports on the very same cohort.31 The primary novel finding of our study is that thalamic FA is an independent predictor of WMH accrual. In fact, the addition of thalamic FA to baseline WMH volume led to a relative increase in predictive power of 70% (from 27.3% for WMHs alone, to 46.5% for WMH+thalamus FA; 46.5/27.3=1.70). The further inclusion of the number of lacunes into this predictive model increased the percentage of explained variance to 49.6%. Notwithstanding the strong association between baseline WM damage and its subsequent accrual, our findings suggest that assessment of deep GM damage, and especially damage to thalamic GM, is relevant for the prediction of WMH accrual over time.
There are two possible interpretations of our principal findings that associate thalamic FA to the accrual of WMHs. First, it is possible that there is thalamic involvement in cerebral blood flow regulation, and that the detected thalamic damage evidenced by the DTI findings has a pathogenic role in accelerating the accrual of WMHs. Alternatively, the increase in thalamic FA improves the prediction of WMH accrual as ‘innocent bystander', because of heightened susceptibility to ischemic injury. Thalamic damage might accelerate WMH accrual through impairment of its regulatory function on cerebral blood flow. Under physiologic conditions, cerebral autoregulation is designed to protect the brain from larger fluctuations in blood pressure occurring in the periphery. Within a certain range of fluctuations in systemic blood pressure (i.e., mean arterial pressure 50–150 mmHg), the perfusion of the brain parenchyma is stabilized through different mechanisms, including myogenic vasomotor activity, chemical responses to hyper/hypocapnia, and alkalosis/acidosis, as well as neurogenic control involving the sympathetic nervous system.32 Very little is known about the involvement of the thalamus in the neural network underpinning the neurogenic regulation of cerebral blood flow. Our speculation about a possible causative role of thalamic damage with regard to WMH accrual relies on a limited number of pioneering reports showing that some of the thalamic nuclei (e.g., the dorsomedial and the intrathalamic relay nuclei) are involved in cerebral blood flow regulation.33, 34 A recent study indicating colocalization of increased FA in cerebral GM areas showing decreased cerebral blood flow35 is consistent with our interpretation that the observed association between thalamic FA changes and WMH accrual is related to cerebral blood flow dysregulation. In addition, a study showing autonomic dysfunction in patients with lacunar stroke in either the thalamus or the putamen further supports this hypothesis.36 Future studies directly investigating the relationship between thalamic FA changes and hemodynamic measures of cerebral blood flow (e.g., transcranial Doppler, perfusion MRI, or positron emission tomography) are needed to further support this conceptual link. However, the relatively frequent occurrence of hypertensive intracerebral hemorrhage in the thalamus of older subjects37 indicates that the thalamus is especially vulnerable to vascular damage in an older population, similar in age range to the one we studied. Hence, the presence of thalamic damage may be a particularly sensitive indicator of more widespread cerebral SVD that leads to progression of WMHs.
The high prevalence of WMHs and lacunes in our study population is in line with comparable subpopulations (age ⩾75 years old) in other cohorts.1, 9 As to the pathogenesis leading to the observed thalamic FA changes related to WMH accrual, we speculate that the abnormalities in thalamic tissue anisotropy reflect microstructural tissue damage, due to either direct—likely ischemic—injury or Wallerian degeneration, secondary to damage to WM pathways projecting to the thalamic nuclei. The lack of cross-sectional correlation between FA changes and WMHs would support the former mechanism, i.e., a primary, rather than a secondary (Wallerian) thalamic injury. These interpretations, however, are based on indirect evidence, and remain speculative, especially because of lack of a reference, age-matched, control group. Future DTI studies using tractography techniques to investigate the characteristics of thalamocortical connections are needed to specifically address this issue.38 Given its original primary aims, the present study design did not include subject selection based on presence or absence of SVD MRI correlates, which would have enabled such case–control comparisons. Further investigations, especially MRI–pathologic combination studies, are warranted to clarify the biologic and pathogenetic substrates of the observed DTI changes.
Among the cardio/cerebrovascular risk factors included in the analyses total cholesterol and LDL levels correlated positively with FA values of the putamen and thalamus. Increased cholesterol/LDL levels may facilitate the injury process in brain structures that are particularly vulnerable to vascular injury, such as the deep GM structures that are served by end arteries. Baseline lipid levels did not associate with WMH volumes, in keeping with the inconsistent association with WMHs reported in previous studies.30, 39 This might suggest a differential impact of vascular risk factors on GM compared with WM. However, our study did not account for statin use, which is widespread in this age group. This severely limits the ability to interpret these findings. Surprisingly, we also found an inverse correlation between BMI and thalamic FA. Based on the notion that FA may be influenced by head size, which is proportional to height (divisor in the BMI formula),40, 41 we found a post hoc correlation between thalamic FA and intracranial cavity volume (rho=0.260, P=0.050). Normalization of the FA values by intracranial cavity volume resulted in the loss of correlation between normalized thalamic FA and BMI (data not shown), supporting a geometric rather than pathophysiologic basis for the observed association. Indeed, recent studies have found a similar association between head size and FA in certain WM areas.42
A limitation of this study is the relatively small sample size. However, the limited subjectivity of the quantitative volumetric methodology used to measure WMH progression, together with the longitudinal study design, may compensate for the relatively small number of subjects included in this study. Although the high prevalence of WMHs in older individuals (⩾75 years)1 accounted for lack of an ‘SVD-free', age-matched control group in this study; this issue may limit the histopathologic and pathophysiologic interpretation of our findings. Specifically, without a control group or histopathologic data we are unable to assess whether FA values of the deep GM reflected SVD pathology. In addition, because of the reported direct correlation between iron content and FA values,12, 42 age-related iron accumulation in the striatum might have concealed possible associations of FA changes in the caudate and putamen with WMH burden and accrual. There is emerging scientific evidence that the iron content increases with age also within the thalamus.43 However, since iron content in the thalamus is lower than in the basal ganglia,44 this issue is unlikely to have significantly affected the main finding of our study. We also note that we failed to fit lacune count values into a normal distribution. Thus, linear regression analysis results regarding this variable mandate a more cautious interpretation. Confidence in the results, however, is supported by the significant correlation between lacune count and WMH accrual in nonparametric tests, together with the consistency of our finding with a previous study.9 Because of low anisotropy of GM, the first two eigenvalues (i.e., lambda-1 and lambda-2) are of similar magnitude, and cannot be distinguished with sufficient confidence.45 Therefore, we reported only the rotationally invariant FA in primary analyses, and used indices such as axial and RD only for post hoc analyses.
In conclusion, the observed association between DTI changes in the thalamus at baseline and the rate of WMH accrual singles out thalamic FA as a promising candidate surrogate marker of cerebral SVD progression, and points to a possible causal role of thalamic damage in the accrual of WMHs. Further studies with longitudinal assessment of thalamic FA and WMH burden at multiple time points are needed to support this hypothesis and clarify the time course of events leading to WMH accrual.
Acknowledgments
The authors thank Julie Schmidt for her contribution to recruitment and study coordination, and Dorothy Wakefield and Brian Healy for statistical advice.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by the National Institute on Aging—2R01AG22092-06A1; University of Connecticut Health Center General Clinical Research Center Grant M01 RR06192; and NIH 5 P41 RR13218.
Supplementary Material
References
- De Leeuw F-E, De Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, Van Den Hout JH, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44:1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- Verhaaren BFJ, Vernooij MW, De Boer R, Hofman A, Niessen WJ, Van Der Lugt A, et al. High blood pressure and cerebral white matter lesion progression in the general population. Hypertension. 2013;61:1354–1359. doi: 10.1161/HYPERTENSIONAHA.111.00430. [DOI] [PubMed] [Google Scholar]
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- Guttmann CR, Benson R, Warfield SK, Wei X, Anderson MC, Hall CB, et al. White matter abnormalities in mobility-impaired older persons. Neurology. 2000;54:1277–1283. doi: 10.1212/wnl.54.6.1277. [DOI] [PubMed] [Google Scholar]
- Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman GT, Tang Y, Lin A, Baloh RW, Tang T. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001;57:990–994. doi: 10.1212/wnl.57.6.990. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Enzinger C, Ropele S, Schmidt H, Fazekas F. Progression of cerebral white matter lesions: 6-year results of the Austrian Stroke Prevention Study. Lancet. 2003;361:2046–2048. doi: 10.1016/s0140-6736(03)13616-1. [DOI] [PubMed] [Google Scholar]
- Gouw AA, Van Der Flier WM, Fazekas F, Van Straaten ECW, Pantoni L, Poggesi A, et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the Leukoaraiosis and Disability study. Stroke. 2008;39:1414–1420. doi: 10.1161/STROKEAHA.107.498535. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Halphen C, Boska MD, Narayana PA. Diffusion tensor metrics, T2 relaxation, and volumetry of the naturally aging human caudate nuclei in healthy young and middle-aged adults: possible implications for the neurobiology of human brain aging and disease. Magn Reson Med. 2008;59:7–13. doi: 10.1002/mrm.21434. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. Diffusion tensor imaging of deep gray matter brain structures: effects of age and iron concentration. Neurobiol Aging. 2010;31:482–493. doi: 10.1016/j.neurobiolaging.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molko N, Pappata S, Mangin JF, Poupon C, Vahedi K, Jobert A, et al. Diffusion tensor imaging study of subcortical gray matter in CADASIL. Stroke. 2001;32:2049–2054. doi: 10.1161/hs0901.094255. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Halphen C, Kamali A, Nelson FM, Wolinsky JS, Narayana PA. Caudate nuclei volume, diffusion tensor metrics, and T(2) relaxation in healthy adults and relapsing-remitting multiple sclerosis patients: implications for understanding gray matter degeneration. J Magn Reson Imaging. 2009;29:70–77. doi: 10.1002/jmri.21648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannoun S, Durand-Dubief F, Confavreux C, Ibarrola D, Streichenberger N, Cotton F, et al. Diffusion tensor-MRI evidence for extra-axonal neuronal degeneration in caudate and thalamic nuclei of patients with multiple sclerosis. Am J Neuroradiol. 2012;33:1363–1368. doi: 10.3174/ajnr.A2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield DB, Moscufo N, Guttmann CR, Kuchel GA, Kaplan RF, Pearlson G, et al. White matter hyperintensities predict functional decline in voiding, mobility, and cognition in older adults. J Am Geriatr Soc. 2010;58:275–281. doi: 10.1111/j.1532-5415.2009.02699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscufo N, Guttmann CRG, Meier D, Csapo I, Hildenbrand PG, Healy BC, et al. Brain regional lesion burden and impaired mobility in the elderly. Neurobiol Aging. 2011;32:646–654. doi: 10.1016/j.neurobiolaging.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White WB, Wolfson L, Wakefield DB, Hall CB, Campbell P, Moscufo N, et al. Average daily blood pressure, not office blood pressure, is associated with progression of cerebrovascular disease and cognitive decline in older people. Circulation. 2011;124:2312–2319. doi: 10.1161/CIRCULATIONAHA.111.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari M, Moscufo N, Skudlarski P, Meier D, Panzer VP, Pearlson GD, et al. Mobility impairment is associated with reduced microstructural integrity of the inferior and superior cerebellar peduncles in elderly with no clinical signs of cerebellar dysfunction. Neuroimage Clin. 2013;2:332–340. doi: 10.1016/j.nicl.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, Zimmerma A, June M. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Budde MD, Janes L, Gold E, Turtzo LC, Frank JA. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain. 2011;134:2248–2260. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48:577–582. doi: 10.1002/mrm.10268. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Basser PJ. Composite hindered and restricted model of diffusion (CHARMED) MR imaging of the human brain. Neuroimage. 2005;27:48–58. doi: 10.1016/j.neuroimage.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Anderson AW, Zhong J, Petroff OA, Szafer A, Ransom BR, Prichard JW, et al. Effects of osmotically driven cell volume changes on diffusion-weighted imaging of the rat optic nerve. Magn Reson Med. 1996;35:162–167. doi: 10.1002/mrm.1910350206. [DOI] [PubMed] [Google Scholar]
- Wiegell MR, Tuch DS, Larsson HB, Wedeen VJ. Automatic segmentation of thalamic nuclei from diffusion tensor magnetic resonance imaging. Neuroimage. 2003;19:391–401. doi: 10.1016/s1053-8119(03)00044-2. [DOI] [PubMed] [Google Scholar]
- Wakana S, Nagae-Poetscher LM, Jiang H, van Zijl P, Golay X, Mori S. Macroscopic orientation component analysis of brain white matter and thalamus based on diffusion tensor imaging. Magn Reson Med. 2005;53:649–657. doi: 10.1002/mrm.20386. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Arnold AM, Beauchamp NJ, Manolio TA, Lefkowitz D, Jungreis C, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- Wolfson L, Wakefield DB, Moscufo N, Kaplan RF, Hall CB, Schmidt Ja, et al. Rapid buildup of brain white matter hyperintensities over 4 years linked to ambulatory blood pressure, mobility, cognition, and depression in old persons. J Gerontol A Biol Sci Med Sci. 2013;68:1387–1394. doi: 10.1093/gerona/glt072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Laan M, van Dijk JMC, Elting JWJ, Staal MJ, Absalom AR. Sympathetic regulation of cerebral blood flow in humans: a review. Br J Anaesth. 2013;111:361–367. doi: 10.1093/bja/aet122. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Teraura T, Sakamoto K, Kondo A. Central neurogenic control of cerebral blood flow. Neurology. 1971;21:247–262. doi: 10.1212/wnl.21.3.247. [DOI] [PubMed] [Google Scholar]
- Kullberg G, Risberg J. Changes in cerebral blood flow after stereotactic thalamotomy. Appl Neurophysiol. 1985;48:362–366. doi: 10.1159/000101158. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Ali H, Shad MU. Atlas-based and DTI-guided quantification of human brain cerebral blood flow: feasibility, quality assurance, spatial heterogeneity and age effects. Magn Reson Imaging. 2013;31:1445–1452. doi: 10.1016/j.mri.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Kuriyama N, Mizuno T, Niwa F, Watanabe Y, Nakagawa M. Autonomic nervous dysfunction during acute cerebral infarction. Neurol Res. 2010;32:821–827. doi: 10.1179/016164109X12464612122696. [DOI] [PubMed] [Google Scholar]
- Chiquete E, Ruiz-Sandoval MC, Alvarez-Palazuelos LE, Padilla-Martínez JJ, González-Cornejo S, Ruiz-Sandoval JL. Hypertensive intracerebral hemorrhage in the very elderly. Cerebrovasc Dis. 2007;24:196–201. doi: 10.1159/000104477. [DOI] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Cross AH, Song SK. Evolving Wallerian degeneration after transient retinal ischemia in mice characterized by diffusion tensor imaging. Neuroimage. 2008;40:1–10. doi: 10.1016/j.neuroimage.2007.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AD, Staff RT, Shenkin SD, Deary IJ, Starr JM, Whalley LJ. Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly people. Radiology. 2005;237:251–257. doi: 10.1148/radiol.2371041496. [DOI] [PubMed] [Google Scholar]
- Bale SJ, Amos CI, Parry DM, Bale AE. Relationship between head circumference and height in normal adults and in the nevoid basal cell carcinoma syndrome and neurofibromatosis type I. Am J Med Genet. 1991;40:206–210. doi: 10.1002/ajmg.1320400217. [DOI] [PubMed] [Google Scholar]
- Takao H, Hayashi N, Inano S, Ohtomo K. Effect of head size on diffusion tensor imaging. Neuroimage. 2011;57:958–967. doi: 10.1016/j.neuroimage.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Zhang J, Tao R, Liu C, Wu W, Zhang Y, Cui J, et al. Possible effects of iron deposition on the measurement of DTI metrics in deep gray matter nuclei: an in vitro and in vivo study. Neurosci Lett. 2013;551:47–52. doi: 10.1016/j.neulet.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Hagemeier J, Dwyer MG, Bergsland N, Schweser F, Magnano CR, Heininen-Brown M, et al. Effect of age on MRI phase behavior in the subcortical deep gray matter of healthy individuals. Am J Neuroradiol. 2013;34:2144–2151. doi: 10.3174/ajnr.A3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim IAL, Faria AV, Li X, Hsu JTC, Airan RD, Mori S, et al. Human brain atlas for automated region of interest selection in quantitative susceptibility mapping: application to determine iron content in deep gray matter structures. Neuroimage. 2013;82:449–469. doi: 10.1016/j.neuroimage.2013.05.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Kingshott C, Cercignani M. About ‘axial' and ‘radial' diffusivities. Magn Reson Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.