Abstract
This study aimed to examine the cortical microvessel diameter response to hypercapnia in misery perfusion using two-photon laser scanning microscopy (TPLSM). We evaluated whether the vascular response to hypercapnia could represent the cerebrovascular reserve. Cerebral blood flow (CBF) during normocapnia and hypercapnia was measured by laser-Doppler flowmetry through cranial windows in awake C57/BL6 mice before and at 1, 7, 14, and 28 days after unilateral common carotid artery occlusion (UCCAO). Diameters of the cortical microvessels during normocapnia and hypercapnia were also measured by TPLSM. Cerebral blood flow and the vascular response to hypercapnia were decreased after UCCAO. Before UCCAO, vasodilation during hypercapnia was found primarily in arterioles (22.9%±3.5%). At 14 days after UCCAO, arterioles, capillaries, and venules were autoregulatorily dilated by 79.5%±19.7%, 57.2%±32.3%, and 32.0%±10.8%, respectively. At the same time, the diameter response to hypercapnia in arterioles was significantly decreased to 1.9%±1.5%. A significant negative correlation was observed between autoregulatory vasodilation and the diameter response to hypercapnia in arterioles. Our findings indicate that arterioles play main roles in both autoregulatory vasodilation and hypercapnic vasodilation, and that the vascular response to hypercapnia can be used to estimate the cerebrovascular reserve.
Keywords: cerebral blood flow, cerebrovascular reserve, misery perfusion, two-photon laser scanning microscopy, vascular response
Introduction
Cerebral blood flow (CBF) is stably maintained over a wide range of cerebral perfusion pressure (CPP) by the mechanism of cerebral autoregulation.1, 2 Regional CBF is maintained by the autoregulatory vasodilation of resistance vessels when regional CPP is decreased as a result of steno-occlusive lesions in cerebral arteries, resulting in an increase in regional cerebral blood volume (stage I hemodynamic compromise).3, 4 As vessels dilate maximally and lead to a decline in regional CBF, the oxygen extraction fraction increases to maintain normal oxygen metabolism and neuronal function. This is known as misery perfusion or stage II hemodynamic compromise.5 Misery perfusion has been reported to be a predictor of subsequent stroke in both medically treated patients and surgically treated patients with symptomatic major cerebral artery disease.5, 6 Misery perfusion is interpreted as a status of decreased cerebrovascular reserve, defined as the maximum increase in CBF from a baseline value after vasodilator stimuli, with decreased regional CBF. In recent years, the vascular response to hypercapnia or acetazolamide challenge has also been used to assess the cerebrovascular reserve in these patients.7, 8 Although the decreased vascular response to hypercapnia or acetazolamide challenge observed in the vascular territory affected by chronic cerebrovascular disease has been ascribed to the exhaustion of the cerebrovascular reserve because of maximal autoregulatory vasodilation in response to decreased CPP,9 it is not well understood whether evaluation of the vascular response is valid as a quantitative assessment of the cerebrovascular reserve.
In the present study, we directly examined the cortical microvessel diameter response to hypercapnia using two-photon laser scanning microscopy (TPLSM) in a mouse model of misery perfusion induced by chronic hypoperfusion. Chronic hypoperfusion was achieved by permanent unilateral common carotid artery occlusion (UCCAO), and misery perfusion was confirmed as a chronic mild decrease in CBF and a decrease in the vascular response to hypercapnia. We thereby evaluated whether the vascular response to hypercapnia could represent the cerebrovascular reserve.
Materials and methods
Animal Preparation
Animal use and experimental protocols were approved by the Institutional Animal Care and Use Committee in the National Institute of Radiological Sciences (Inage, Chiba, Japan). All experiments were performed in accordance with the guidelines on the humane care and use of laboratory animals at the National Institute of Radiological Sciences. A total of 18 male C57BL/6J mice (20 to 30 g, 8 to 10 weeks; Japan SLC, Hamamatsu, Japan) were used in four separate experiments: laser-Doppler flowmetry (LDF) experiments (N=6), TPLSM experiments (N=6), magnetic resonance imaging (MRI) experiments (N=6), and blood gas experiments (N=6). TPLSM and MRI experiments were conducted using the same animals. The mice were housed in a room with a 12-hour dark/light cycle at a temperature of 25°C and were provided with water and food ad libitum.
For the surgical procedure, mice were anesthetized with a mixture of air, oxygen, and isoflurane (3% for induction and 1.5% for surgery) using a facemask. The animals were fixed in a stereotactic frame, and rectal temperature was maintained at 37°C with a heating pad (ATC-210, Unique Medical, Tokyo, Japan). The methods used for preparing the cranial window, the ‘Seylaz–Tomita method', have been previously reported in detail.10, 11, 12 A midline incision (10 mm) was made to expose the skull. Two cranial windows were attached over the left and right side of the cerebral cortex (∼3 mm in diameter, centered at 1.8 mm caudal and 2.5 mm lateral from the bregma). A custom-made aluminum U-shaped plate was affixed to the front of the skull.12 After completion of the surgery, the animals were allowed to recover from anesthesia and were housed for 1 to 2 weeks before initiation of the experiments.
For the UCCAO surgical procedure, the mice were administered a mixture of air, oxygen, and isoflurane (3% for induction and 1.5% for surgery) using a facemask. A midline cervical incision was made and the right common carotid artery was isolated from the adjacent vagus nerve and double ligated using 6-0 silk sutures.
Experimental Protocols
The head plate mounted on the animal's skull was attached to a custom-made stereotactic apparatus. Animals were placed in a box that padded their bodies (with the exception of the head) with cotton sheets.12
In the first experiment, the CBF during normocapnia (baseline CBF) and hypercapnia was measured using LDF through chronic cranial windows in awake mice. The CBF measurements were repeated before and at 1, 7, 14, and 28 days after UCCAO. In the second experiment, the diameter of arterioles, capillaries, and venules during normocapnia and hypercapnia was measured by TPLSM through chronic cranial windows in awake mice using the schedule applied to the LDF experiments. In the third experiment, MRI studies were performed at 16 and 30 days after UCCAO. In the fourth experiment, systemic arterial blood gas analyses were performed. The animals were kept in their cage between the scheduled experiments.
Hypercapnia (5% CO2 Inhalation)
The experimental protocol used to conduct 5% CO2 inhalation has been previously reported.11 A hypercapnic gas mixture of 5% CO2, 21% O2, and residual N2 was inhaled by awake-behaving mice using a facemask (300 mL/minutes). At all times, except during the CO2 inhalation, the mice inhaled room air (300 mL/minutes). CO2 gas was given to the mice for 20 seconds using a Master-8 apparatus (A.M.P.I, Jerusalem, Israel). As we previously reported, 10 to 20 seconds of the CO2 inhalation period was used to establish the vascular response to hypercapnia.11
Laser-Doppler Flowmetry Experiments
CBF was measured with an LDF system (laser wavelength, 780 nm; FLO-C1, OMEGAWAVE, Tokyo, Japan) using an LDF probe with a 0.46-mm diameter tip (Type NS, OMEGAWAVE) placed perpendicular to the cerebral cortex through a guide tube. The guide tube was attached to the cranial window above the barrel cortex in the somatosensory cortex using dental cement (Luxaflow, DMG, Hamburg, Germany), avoiding areas with large blood vessels (Supplementary Figure S1).12, 13 The time course of the LDF signal changes was recorded using a polygraph data acquisition system (MP150, BIOPAC Systems, Goleta, CA, USA) at a sampling rate of 200 Hz and analyzed offline. All LDF measurements were simultaneously obtained for both hemispheres.
CO2 inhalation was repeated 10 times with an onset-to-onset interval of 120 seconds. For each trial of the experiment, the LDF signal was normalized using the baseline level of 20-second pre-CO2 inhalation (baseline CBF) and averaged across 10 trials. The baseline CBF on each experimental day was expressed as a percentage relative to the preoperative value. The vascular response to hypercapnia was defined as the percentage change in CBF during hypercapnia, which was calculated from 10 to 20 seconds of the inhalation period.11 This was because CBF increased gradually and reached a plateau at ∼10 to 15 seconds after inhalation and remained stable for ∼10 seconds during inhalation of 5% CO2. Statistical analysis was performed to compare the baseline CBF and the vascular response to hypercapnia across different experimental days using one-way analysis of variance with repeated measures, followed by Tukey's test. Statistical significance was assumed for values of P<0.05.
Two-Photon Laser Scanning Microscopy Experiments
Sulforhodamine 101 (SR101; MP Biomedicals, Irvine, CA, USA) dissolved in saline (10 mmol/L) was injected intraperitoneally (8 μL/g of body weight) just before starting the imaging experiments.14 The cortical microvasculature was imaged using a two-photon microscope (TCS-SP5 MP, Leica Microsystems GmbH, Wetzlar, Germany) with an excitation of 900 nm. An emission signal was detected through a band-pass filter for SR101 (610/75 nm). We used a × 20 water-immersion objective (NA=1.0, Leica Microsystems) to obtain a high-resolution image (field of view, 1,024 × 1,024 pixels; in-plane pixel resolution, 0.25 μm).
The methods used for dynamic imaging of vasodilation have been reported in detail by Sekiguchi et al.15 Briefly, dynamic imaging was conducted in arterioles, capillaries, and venules of the parietal cortex at a rate of 1 frame/second for 40 seconds (i.e., 10-second pre-CO2 inhalation, 20-second CO2 inhalation, and 10-second post-CO2 inhalation). The timings of image acquisition and 5% CO2 gas inhalation were controlled with a Master-8 apparatus (A.M.P.I.). Images were collected randomly at the cortical surface and at a depth of 300 μm from the cortical surface. The regions sampled by TPLSM were within a circle ∼2 mm in diameter, covering the primary somatosensory barrel cortex (Supplementary Figure S1). Inasmuch as the awake mice moved rarely, one to four inhalation trials were repeated in each location. Thereafter, only one session, in which motion was absent, was used for the analysis mentioned below. Between inhalation trials, 2-minute intervals were taken to allow for recovery of the responses.
The diameters of the blood vessels were measured offline using LAS AF software (Leica Microsystems). The diameter of single vessels during normocapnia was defined as the mean diameter of the image made by averaging 10-second pre-CO2 inhalation periods, whereas that during hypercapnia was defined as the mean diameter 10 to 20 seconds after CO2 inhalation. Capillaries were defined as vessels with an internal diameter of <8 μm during normocapnia.16 The calculated arterioles and venules were chosen at the cortical surface exclusively from pial arterioles and venules, while capillaries were chosen randomly at a depth of 300 μm from the cortical surface. Before the image acquisition, we traced penetrating arterioles to distal in order to confirm that the measured vessels were not the first branches from penetrating arterioles. Owing to this process, it was improbable that the vessels we defined as capillaries were parenchymal arterioles. Most arterioles, capillaries, and venules were in a range of 10 to 25, 5 to 7, and 15 to 30 μm, respectively. On each experimental day, the same vessels were measured repeatedly.
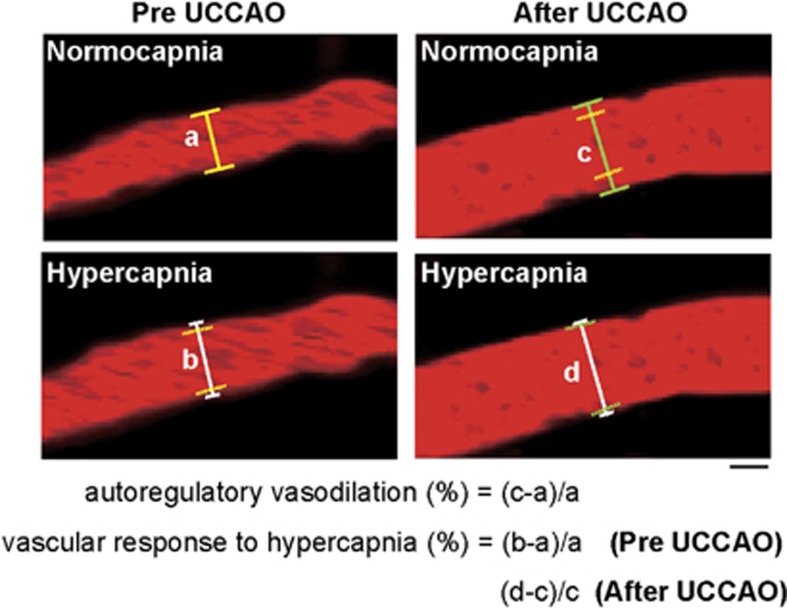
The relationships between the vessel diameter during normocapnia and that during hypercapnia in the bilateral hemisphere before and at 14 days after UCCAO were examined by linear regression. The percentage change in diameter of each vessel from the preoperative value was calculated on each experimental day (Figure 1). Subsequently, those values of all measured six to eight different vessels were averaged in each mouse. We defined the above average in the hemisphere ipsilateral to UCCAO for each mouse as autoregulatory vasodilation on each experimental day. The cortical microvessel diameter response to hypercapnia was also calculated as the percentage change in diameter of each vessel during hypercapnia in reference to normocapnia within a session (Figure 1). Subsequently, those values of all measured six to eight different vessels were averaged in each mouse. We defined the above average of each mouse as vascular response to hypercapnia on each experimental day. Data were presented as mean±standard deviation tested over the animals.
Figure 1.
Two-photon laser scanning microscopy images of a pial arteriole before and after unilateral common carotid artery occlusion (UCCAO) during both normocapnia and hypercapnia taken at the cerebral cortical surface. Scale bar, 10 μm. After UCCAO, red blood cell speed seems to have dramatically decreased. Autoregulatory vasodilation was calculated as the percentage change in diameter from the preoperative value on each experimental day. Vascular response to hypercapnia was calculated as the percentage change in diameter during hypercapnia in reference to normocapnia. Solid lines perpendicular to the vessels indicate the diameter of the arterioles in each condition. Yellow dashed lines indicate the position of the vessel wall during normocapnia before UCCAO (a). Green dashed lines indicate the position of the vessel wall during normocapnia after UCCAO (c). (a–d) Indicate the vessel diameter during normocapnia before UCCAO, during hypercapnia before UCCAO, during normocapnia after UCCAO, and during hypercapnia after UCCAO, respectively.
Statistical analyses were performed to compare the diameter of each component of the cerebral microvessels during normocapnia and the vascular responses to hypercapnia across different experimental days using one-way analysis of variance with repeated measures, followed by Tukey's test. Furthermore, the relationship between the vascular responses to decreased CPP and those to hypercapnia in the arterioles in the hemisphere ipsilateral to UCCAO in all mice during the entire experimental period was examined by linear regression (n=30). A value of P<0.05 was considered statistically significant.
Systemic Arterial Blood Gas Analysis
The experimental protocols used for systemic arterial blood gas sampling in awake mice have been previously reported.17 The tail artery of the mice was cannulated for systemic arterial blood gas sampling under isoflurane anesthesia (3.0% for induction and 2.0% during surgery). Body temperature was monitored with a rectal probe and maintained at ∼37°C with a heating pad (ATC-210; Unique Medical). The mice were fixed in a custom-made stereotactic apparatus. Sampling at normocapnia (room air) was performed 60 minutes after the cessation of anesthesia, during which the mice had enough time to recover. A second sampling was performed 20 seconds after the induction of 5% CO2. These blood samples were analyzed by means of a blood analyzer (i-STAT; Abbott, Green Oaks, IL, USA). Partial pressure of oxygen in arterial blood, partial pressure of carbon dioxide in arterial blood, and pH were measured at every sampling. All values recorded at normocapnia were statistically compared with those recorded at hypercapnia (paired t-test).
Magnetic Resonance Imaging Experiments
To determine whether abnormal changes such as cerebral infarction existed in the brain, we acquired MRI data. All MRI experiments were performed with a 7.0T horizontal MRI scanner (Magnet: Kobelco and JASTEC, Kobe, Japan; Console: Bruker Biospin, Ettlingen, Germany), with a volume coil for transmission (Bruker Biospin) and a 2-channel phased array surface coil for reception (Rapid Biomedical, Rimpar, Germany). The mice were initially anesthetized with 3.0% isoflurane (Escain, Mylan Japan, Tokyo, Japan) and then anesthetized with 1.5% to 2.0% isoflurane and 1:5 oxygen/room-air mixture during the MRI experiments. Rectal temperature was continuously monitored using an optical fiber thermometer (FOT-M, FISO, Quebec, QC, Canada) and maintained at 37.0°C±0.5°C using a heating pad (Temperature control unit, Rapid Biomedical), and warm air was provided by a homemade automatic heating system based on an electric temperature controller (E5CN, Omron, Kyoto, Japan) throughout all experiments. During MRI scanning, the mice lay in a prone position on an MRI-compatible cradle and were held in place by handmade ear bars. The first imaging slices were carefully set at the rhinal fissure with reference to the mouse brain atlas.18 Each image set consisted of two different kinds of MRI measurements taken in the following order: T2-weighted imaging and diffusion-weighted imaging.
MRIs were acquired at 16 and 30 days after UCCAO. Transaxial T2-weighted images were acquired by rapid acquisition with a relaxation enhancement (RARE) sequence as follows: TR/effective TE=4,200/36 ms, slice thickness=1.0 mm, slice gap=0.0 mm, Fat-Sup=on, matrix=256 × 256, FOV=25.6 × 25.6 mm2, number of acquisitions (NA)=4, RARE factor=8, number of slices=13, and scan time=6 minutes 43 seconds. Frequency selective saturation pulses and crusher magnetic field gradients were used for fat suppression.
Transaxial diffusion-weighted images were acquired using a multislice SE sequence as follows: TR/TE=2,500/27 ms, slice thickness=1.0 mm, slice gap=0.0 mm, Fat-Sup=on, matrix=128 × 128, FOV=25.6 × 25.6 mm2, number of slices=9 (a part of the T2-weighted images), NA=1, b values=0 and 1,000 seconds/mm2, diffusion direction=z, and scan time=10 minutes 40 seconds.
Results
Systemic Arterial Blood Gas Analysis
Table 1 shows the arterial blood gases during normocapnia and at 20 seconds of hypercapnia in awake mice. Partial pressure of carbon dioxide in arterial blood increased significantly after 5% CO2 inhalation (10.1±1.9 mm Hg increase).
Table 1. Parameters of systemic arterial blood gas sampling under normocapnia and hypercapnia.
| Parameters | Normocapnia | Hypercapnia |
|---|---|---|
| PaO2 (mm Hg) | 99.8±7.5 | 110.5±12.6 |
| PaCO2 (mm Hg) | 37.4±3.1 | 47.5±4.5** |
| pH | 7.35±0.02 | 7.30±0.02** |
PaCO2, partial pressure of carbon dioxide in arterial blood; PaO2, partial pressure of oxygen in arterial blood.
**P<0.01.
Baseline CBF after UCCAO
Baseline CBF in the cerebral hemisphere ipsilateral to UCCAO decreased to 80.0%±9.1%, 81.4%±6.0%, 82.9%±6.7%, and 84.0%±4.7% of the preoperative value at 1, 7, 14, and 28 days after UCCAO, respectively. Statistically significant differences were found at 1, 7, 14, and 28 days (P<0.01) after occlusion (Supplementary Figure S2). There were no statistically significant differences in baseline CBF in the cerebral hemisphere contralateral to UCCAO throughout the experimental period (Supplementary Figure S2).
Cerebral Blood Flow Response to Hypercapnia after UCCAO
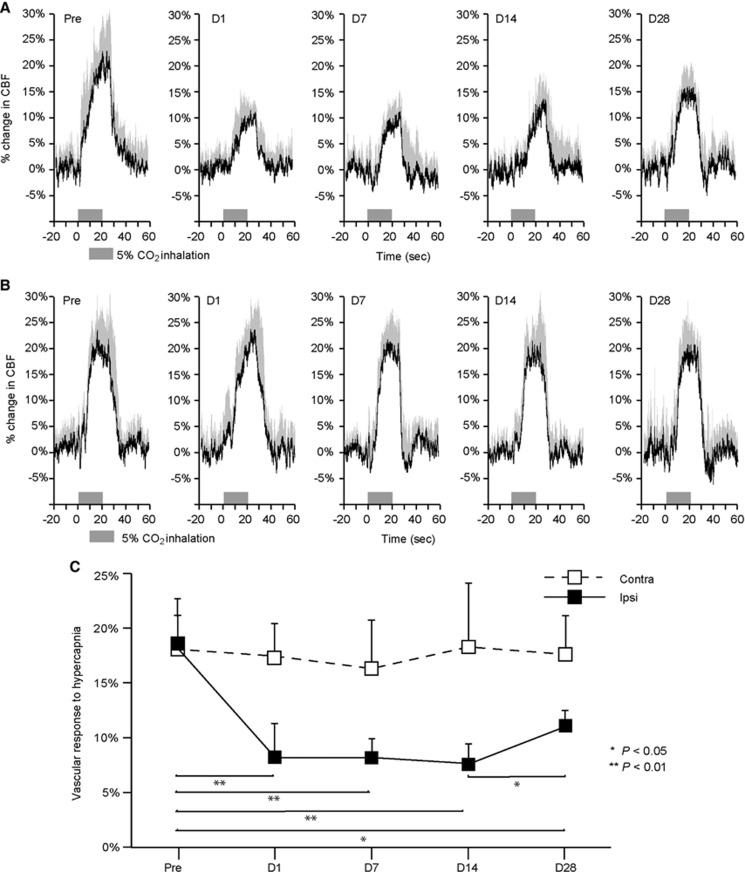
The longitudinal time–response curves of CBF during 5% CO2 inhalation are shown in Figures 2A and B. The vascular responses to hypercapnia in the cerebral hemisphere ipsilateral to UCCAO were 18.9%±4.9%, 8.5%±3.5%, 8.5%±1.7%, 7.9%±2.2%, and 11.5%±0.8% before and at 1, 7, 14, and 28 days after UCCAO, respectively. Statistically significant differences were found at 1, 7, and 14 days (P<0.01) and 28 days (P<0.05) after UCCAO compared with the values observed before UCCAO (Figure 2C). There were no significant differences in the vascular responses to hypercapnia in the cerebral hemisphere contralateral to UCCAO throughout the experimental period (Figure 2C).
Figure 2.
Vascular responses to hypercapnia during chronic hypoperfusion in the bilateral cerebral hemisphere. (A, B) Time–response curves for the normalized increase in cerebral blood flow (CBF) response to hypercapnia during chronic hypoperfusion in the hemisphere ipsilateral to unilateral common carotid artery occlusion (UCCAO) (A) and in the hemisphere contralateral to UCCAO (B). The horizontal bars indicate the 5% CO2 inhalation period. These data were normalized to the pre-CO2 inhalation level (20 seconds before 5% CO2 inhalation). Each response curve represents the mean for all animals on each measurement day. The error bars indicate standard deviation. (C) Mean percentage increase in CBF in the bilateral hemisphere within 10 to 20 seconds of 5% CO2 inhalation. Ipsilateral hemisphere (Ipsi, black); contralateral hemisphere (Contra, white). The error bars indicate standard deviation. *P<0.05, **P<0.01.
Cortical Microvessel Diameter Response to Decreased CPP
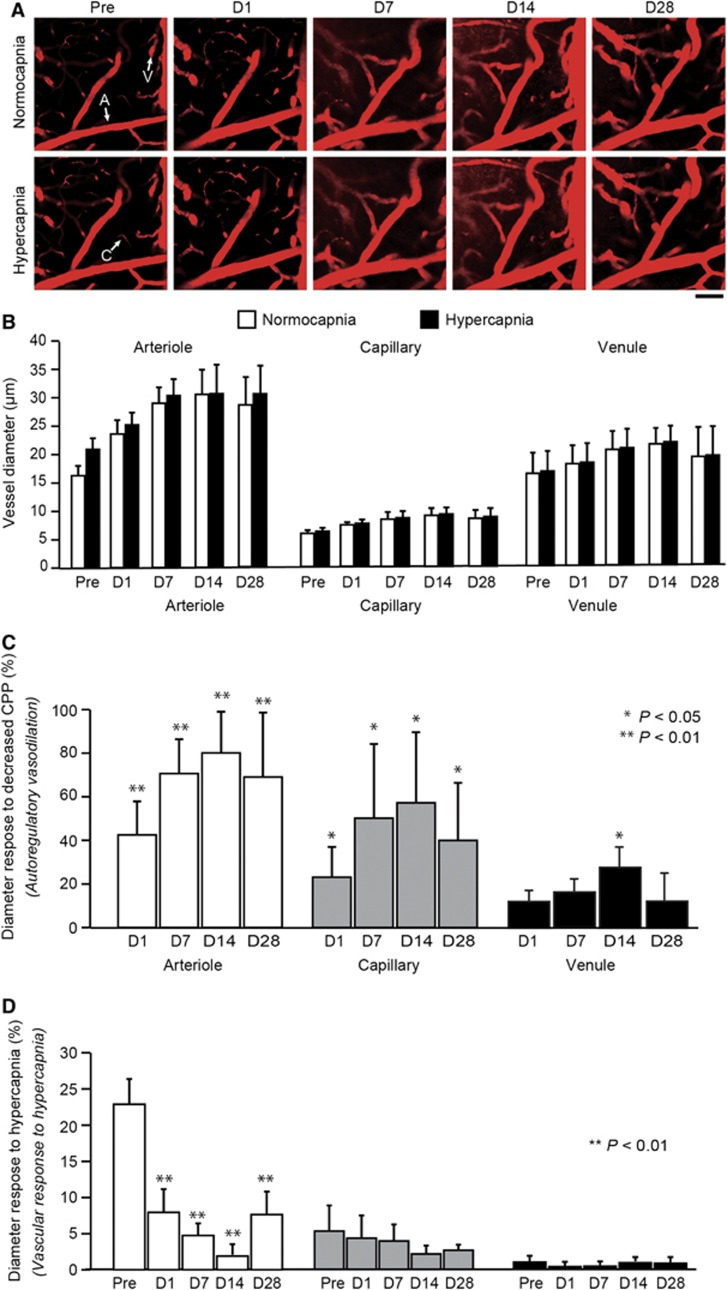
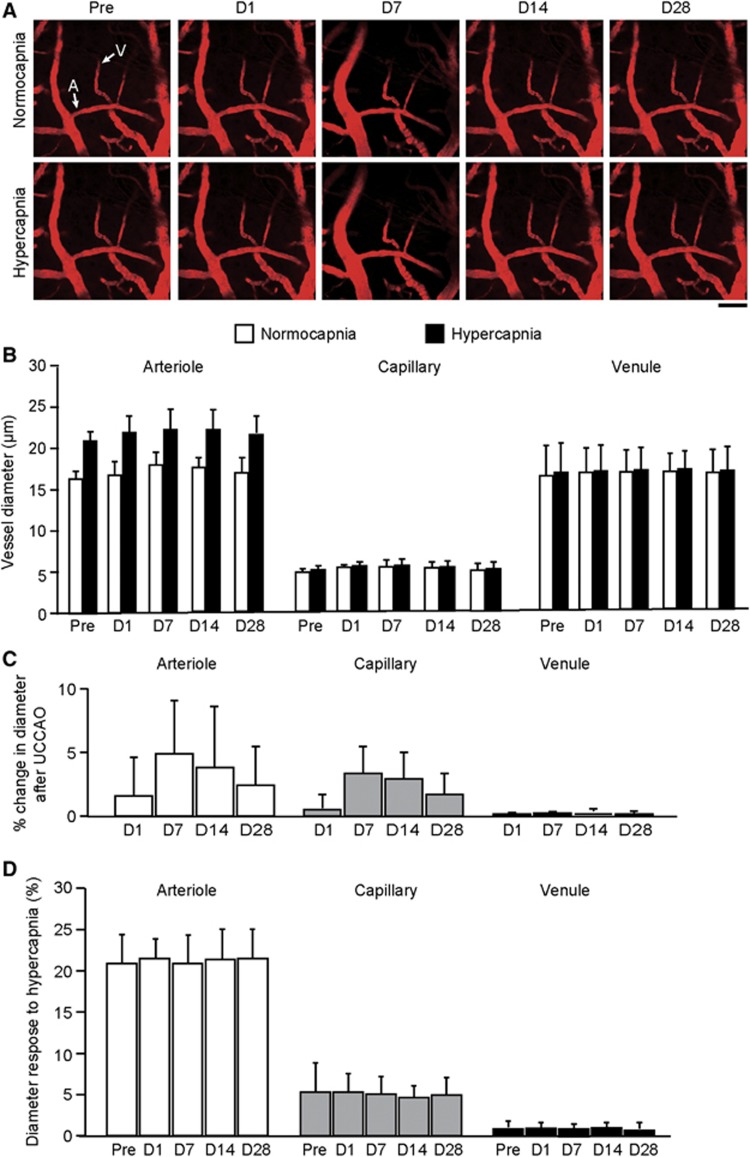
After UCCAO, autoregulatory vasodilation was observed in all three components of the cerebral microvessels (i.e., arterioles, capillaries, and venules) in the hemisphere ipsilateral to UCCAO (Figure 3A and Supplementary Figure S3). Before UCCAO, the diameters in arterioles, capillaries, and venules were 16.6±1.3, 4.8±0.5, and 19.7±3.0 μm, respectively (Figure 3B). After UCCAO, the microvessels dilated progressively and reached a maximum diameter at 14 days after UCCAO: 29.9±4.8, 7.5±1.7, and 25.9±3.3 μm in arterioles, capillaries, and venules, respectively (Figure 3B). The calculated autoregulatory vasodilation in arterioles, capillaries, and venules was 79.5%±19.7%, 57.2%±32.3%, and 32.0%±10.8%, respectively (Figure 3C). At 28 days after UCCAO, the vasodilation subsided slightly. Conversely, there were no statistically significant differences in vessel diameter in all three components of the cerebral hemisphere contralateral to UCCAO throughout the experimental period (Figures 4A and B).
Figure 3.
(A) Longitudinal imaging of cortical vessels at the cortical surface in the hemisphere ipsilateral to unilateral common carotid artery occlusion (UCCAO). The arterioles are dilated significantly after UCCAO. Before UCCAO, the arterioles were dilated during hypercapnia. The dilation observed during hypercapnia was unnoticeable after UCCAO. Scale bar, 50 μm. (B) Longitudinal diameters in the cortical microvessels during normocapnia and hypercapnia in the hemisphere ipsilateral to UCCAO. (C) Longitudinal percentage changes in cortical microvessel diameter from the preoperative value. These findings represent the diameter response to decreased cerebral perfusion pressure (CPP; i.e., autoregulatory vasodilation). The microvessels dilated progressively and reached a maximum diameter at 14 days after UCCAO. (D) Longitudinal cortical microvessel diameter responses to hypercapnia. The diameter responses to hypercapnia in the arterioles were significantly decreased after UCCAO. The error bars indicate standard deviation. A, arteriole; C, capillary; V, venule. Normocapnia (white); hypercapnia (black). *P<0.05, **P<0.01 (against preoperative value).
Figure 4.
(A) Longitudinal imaging of cortical vessels at the cortical surface in the hemisphere contralateral to unilateral common carotid artery occlusion (UCCAO). Scale bar, 50 μm. (B) Longitudinal diameters in cortical microvessels during normocapnia and hypercapnia in the hemisphere contralateral to UCCAO. (C) Longitudinal percentage changes in cortical microvessel diameter from the preoperative value in the hemisphere contralateral to UCCAO. Although arterioles were slightly dilated 7 days after UCCAO, statistically significant dilation was not found in any of the three microvasculature components throughout the experimental period. (D) Longitudinal vascular responses to hypercapnia in cortical microvessels in the hemisphere contralateral to UCCAO. There were no significant differences in responses throughout the experimental period. The error bars indicate standard deviation. A, arteriole; V, venule. Normocapnia (white); hypercapnia (black).
Cortical Microvessel Diameter Responses to Hypercapnia
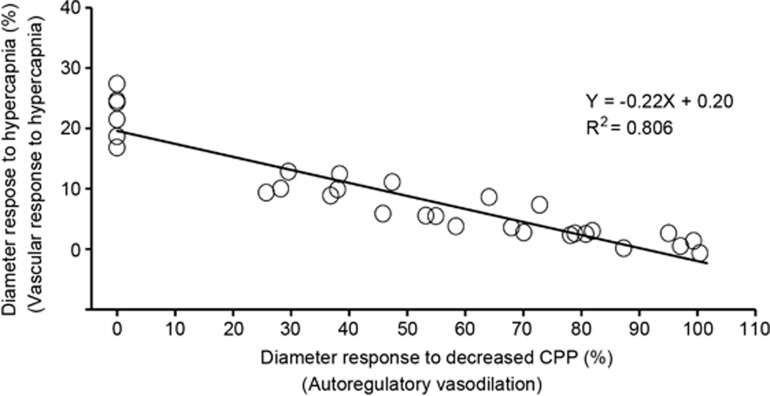
Before UCCAO, the diameter responses to hypercapnia in arterioles, capillaries, and venules were 22.9%±3.5%, 6.9%±3.9%, and 1.8%±0.9%, respectively (Figure 3D). After UCCAO, the diameter responses to hypercapnia were depressed significantly in arterioles of the cerebral hemisphere ipsilateral to UCCAO, while autoregulatory vasodilation occurred. The vessel diameter responses to hypercapnia in these vessels were 22.9%±3.5%, 8.0%±3.2%, 4.3%±1.5%, 1.9%±1.5%, and 6.9%±4.0% before and at 1, 7, 14, and 28 days after UCCAO, respectively (Figure 3D). Moreover, a significant negative correlation was observed between the diameter responses to decreased CPP and to hypercapnia in arterioles (R2=0.806; P<0.0001; Figure 5). Conversely, there were no significant differences in the diameter responses to hypercapnia in the cerebral hemisphere contralateral to UCCAO throughout the experimental period (Figure 4C).
Figure 5.
Correlation between the diameter responses to decreased cerebral perfusion pressure (CPP) and those to hypercapnia in the arterioles for the hemisphere ipsilateral to unilateral common carotid artery occlusion (UCCAO) in all mice during the entire experimental period. There was a significant negative correlation (R2=0.806; P<0.0001).
MRI Experiments
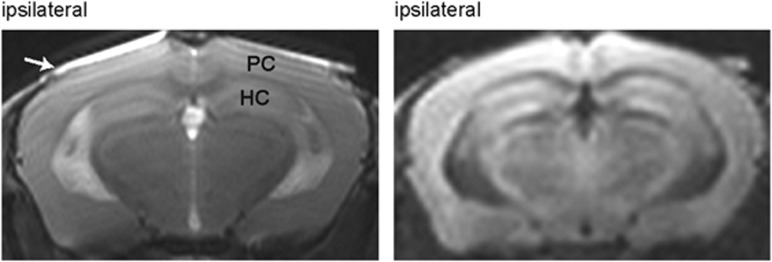
No abnormal changes in signal intensity and in morphology were observed on both T2-weighted and diffusion-weighted images at 16 and 30 days after UCCAO in any of the mice. Figure 6 shows representative transaxial MR images at the level of the parietal cortex and hippocampus acquired 30 days after UCCAO.
Figure 6.
Magnetic resonance imaging experiments obtained 30 days after unilateral common carotid artery occlusion. The panel on the left shows a T2-weighted image. The panel on the right shows a diffusion-weighted image. The images were acquired at the area including the parietal cortex (PC) and hippocampus (HC). No abnormal changes in signal intensity were observed. The white arrow indicates the cover glass.
Discussion
To the best of our knowledge, this is the first study to examine the cortical microvessel diameter response to hypercapnia directly under misery perfusion in awake animals. In addition, we performed LDF experiments and TPLSM experiments not only in the affected hemisphere but also in the non-affected hemisphere. The results showed that there were no significant differences in values in the non-affected hemisphere obtained by these experiments throughout the experimental period (Figure 4 and Supplementary Figure S2). These findings show that the experimental protocol followed using the invasive cranial window itself did not have a major impact on the repeated recordings of LDF and TPLSM performed in our study. In addition, no abnormal changes in signal intensity and morphology were detected for both T2-weighted and diffusion-weighted MR images in any of the mice (Figure 6). The findings further showed that our occlusion model developed no detectable infarction in the brains, thus preserving the normal mechanism of cerebral autoregulation.19
It is well known that LDF measurement is very sensitive to the position of the LDF probe in the head of animals.20 To reproduce the LDF probe attachment over long periods, we affixed the polyvinyl chloride guide tube to the cranial window for longitudinal LDF recording.12 Using this guide tube, we previously showed a stable day-to-day recording of the LDF in single animals.12 However, the TPLSM experiments could not be performed on the same animals measured with LDF because of the presence of the fixed guide tube on the cranial window. In the present study, the LDF probe was therefore consistently placed on the center of the area on which the TPLSM experiments were performed (Supplementary Figure S1), which could allow for direct comparisons of the CBF measured with LDF and vascular diameters measured with TPLSM between the two animal groups.
The baseline CBF measured with LDF in the cerebral hemisphere ipsilateral to UCCAO decreased to ∼80% of the preoperative value at 1 day after UCCAO, and remained significantly lower than the preoperative value (Supplementary Figure S2). This finding is consistent with the result reported previously using anesthetized mice.21 After UCCAO, the vascular response to hypercapnia in the cerebral hemisphere ipsilateral to UCCAO was decreased and remained lower than that observed before UCCAO (Figure 2C). These results led us to conclude that a C57BL/6J mouse that underwent UCCAO represents a model of misery perfusion.
A previous study showed that there was variable patency of the posterior communicating artery in the C57/BL6 mouse, and this variability influenced the degree of decrease in CBF after bilateral common carotid artery occlusion and middle cerebral artery occlusion.22 Nevertheless, Hecht et al reported that the perfusion after a mild reduction of flow in the anterior circulation by unilateral internal carotid artery (ICA) occlusion was not influenced by the variable patency of the posterior communicating artery.23 This was mainly because all C57/BL6 mice showed a prominent interconnection between both hemispheres by the anterior communicating artery.23 Although our experimental model differed from that study (UCCAO versus unilateral internal carotid artery occlusion), the anterior communicating artery present in this animal brain might have a critical role in the unilateral cerebral hypoperfusion model. As a matter of fact, the present study showed only minor variability in autoregulatory vasodilation and CBF responses across the animals tested, indicating that the effect of the variable patency of the posterior communicating artery on the above might be insignificant in our model.
Previous studies on pial vascular responses have consistently reported dilation of arterioles and capillaries during decreases in CPP,24, 25, 26 but no study has measured diameter changes in all three components of the cerebral microvasculature: arterioles, capillaries, and venules. Although these previous studies support the notion that the myogenic response of the vascular smooth muscle plays a major role in cerebral autoregulation by dilating the arteries and arterioles in response to decreased CPP,27 the present study additionally showed that not only the arterioles and capillaries but also venules dilated in response to decreased CPP (Figures 3B and C). According to Poiseuille's law, the resistance of blood flow is inversely proportional to the fourth power of the vessel diameter. Once the arterioles dilate, the arteriolar resistance could decrease significantly. Mandeville et al modeled the cerebral circulation using active arteriolar resistance elements that are placed proximal to passive capillary and venous compartments with constant resistance and variable compliance.28 In their model, the venous compliance increased slowly when the arteriolar resistance decreased, and this was followed by venous dilation. Considering these potential mechanisms of vasodilation during decreases in CPP, we tentatively concluded that the arterioles act as major flow regulators and adjust resistance to maintain CBF, and capillaries and venules may dilate in responses to changes in the upstream resistances. However, other mechanisms could also contribute to the vascular responses to decreases in CPP. For example, decreases in CPP may directly stimulate the release of vasoactive substances from the cortical neurons, such as NO, hydrogen, potassium, and adenosine, which eventually cause vasodilation.4, 29 Pericytes have also been considered to be critical cells supporting and controlling brain capillary tones, which mimic functions of smooth muscle cells in the arterioles.30 Because of their contracting functions, CBF can be controlled at capillaries with pericytes.31 It is therefore likely that the active mechanisms of capillary dilation may participate in the CBF response to decreases in CPP.
In contrast to the results of the autoregulatory vasodilation, the vessel diameter response to hypercapnia showed that the arterioles are the primal microvessels that dilate significantly, but no significant dilation occurred in capillaries and venules (Figures 3D and 4C). These results are consistent with a previous report that showed increases in blood volume and vessel diameter in arteries, but not in veins, during hypercapnia in the rat brain.32 In addition, human positron emission tomography studies revealed that changes in cerebral blood volume during hypercapnia were induced by changes in arterial blood volume without changes in venous blood volume.33 However, change in capillary diameter during hypercapnia has also been observed in animals.34 Furthermore, a study using confocal microscopy showed significant dilation in veins and venules during hypercapnia.35 These conflicting results may have arisen from differences in the experimental methods (e.g., duration of hypercapnia inhalation and modalities for measurements) or in subject characteristics, such as animal species.
Overall, our results consistently showed that both autoregulatory vasodilation and the diameter responses to hypercapnia predominantly occur in cortical arterioles. These findings support the previous notion that cerebral arterioles have considerable basal tone and contribute significantly to vascular resistance in the brain.36 Moreover, we found that a significant negative linear relationship exists between autoregulatory vasodilation and the diameter response to hypercapnia in the arterioles (Figure 5). This observation further indicates that increased dilation of the arterioles due to cerebral autoregulation leads to a decrease in dilation for hypercapnia stimuli, and thus the vessel diameter response to hypercapnia (i.e., vascular response to hypercapnia) can be used to estimate the cerebrovascular reserve.
For translation to clinical application, our finding is consistent with a human-based study performed by Yamauchi et al that showed the presence of a significant negative linear relationship between the vascular response to acetazolamide and cerebral blood volume, suggesting that the vascular response may be decreased according to the degree of autoregulatory vasodilation.37
However, the reason why the autoregulatory vasodilation in arterioles subsided at 28 days after UCCAO and why the vascular response to hypercapnia was increased at the same time, as observed in the present study, remains unclear. One possible explanation for these observations is that the cerebrovascular reserve might recover in response to chronic hypoperfusion. Another explanation is that some changes in neuronal activity or selective neuronal loss might occur when the chronic hypoperfusion becomes persistent, despite the absence of cerebral ischemic changes on MRI. If these changes occurred, the metabolic demand for oxygen would be reduced, leading to a normalization of the hypercapnic response in spite of a low baseline CBF.38 Further studies are required to evaluate the changes in neuronal activity or neuronal loss during misery perfusion. In addition, another important mechanism, called neurovascular coupling, controls local cerebral perfusion according to local brain activity through a release of vasoactive substances that is potentially coupled to glucose uptake and oxygen consumption.39 Determining whether the vascular response to hypercapnia can predict that to neuronal activation is beyond the scope of the present study. It was shown that normal CBF response to neural activation exists despite a decreased vascular response to acetazolamide stress at a steno-occlusive lesion of a major cerebral artery.40 This finding indicates that the mechanism of the vascular response to neural activation is not necessarily the same as that for hypercapnia or acetazolamide challenges. Further studies measuring the diameter response to neuronal activation during misery perfusion need to be performed.
There are some limitations and caveats in the present study. First, we measured vessel diameters manually using LAS AF software. This could have caused biased errors in the measurement of the vessel diameters. As the fluorescent signals appearing in the cortical microvessels ∼5 minutes after the intraperitoneal injection of SR101 provided a well-definable boundary between the labeled vessel and non-labeled tissue, the measurement errors could have been minimal in our experiments. However, the intravascular signal weakened over time,14 and thus the vascular imaging was completed within 120 minutes from the injection. This hampered the acquisition of a large number of imaging volumes. Second, LDF and TPLSM measurements under awake conditions are susceptible to the motion artifacts caused by animal movements during the recordings. Although extensive movement of animals during 40-second recordings in single trials was not a matter of major concern, if motion was present, the trial was repeated several times. This effectively minimized measurement errors derived from motion artifacts. Third, we did not measure the microvessel velocity using two-photon microscopy. In view of the microvessel flux, both the diameter and velocity are very important factors. Actually, the red blood cell speed in the arterioles seems to have been dramatically decreased after UCCAO (Figure 1). Therefore, a further study focusing on those values needs to be performed. Finally, we defined both autoregulatory vasodilation and hypercapnic vasodilation of each mouse as the average of those values of all measured vessels in one mouse in accordance with the methods that have been used in positron emission tomography/single-photon emission computed tomography studies.7, 8, 33 However, this analytical method used in the present study is likely to neglect intrasubject variabilities. In this regard, the segmental and spatial heterogeneity of autoregulatory vasodilation has been reported during acute decreases in systemic arterial pressure in cats.24 Thus, to avoid these sampling biases, we focused on vessels having certain ranges of diameters and maintained consistent locations of the measurement across the animals tested. To further determine the segmental and spatial heterogeneity of the responses of cortical microvasculature to misery perfusion, future studies evaluating the responses of cortical microvessels over varying diameters and regions will be required.
In conclusion, we found that the arteriolar diameter response to hypercapnia was significantly decreased because of autoregulatory vasodilation in misery perfusion. These findings indicate that the arterioles play the main roles in both autoregulatory vasodilation and hypercapnic vasodilation and that the vascular response to hypercapnia can be used to estimate the cerebrovascular reserve.
Acknowledgments
The assistance of members of the National Institute of Radiological Sciences in performing the LDF experiments and the TPLSM experiments is gratefully acknowledged. The authors thank Ms Sayaka Shibata, Ms Aiko Sekita, and Mr Nobuhiro Nitta for the MRI experiments.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Lassen NA.BrainIn: Johnson PC (eds.). Peripheral Circulation John Wiley & Sons: New York; 1978337–358. [Google Scholar]
- Strandgaard S, Paulson OB. Cerebral autoregulation. Stroke. 1984;15:413–416. doi: 10.1161/01.str.15.3.413. [DOI] [PubMed] [Google Scholar]
- Powers WJ, Grubb RL, Jr., Raichle ME. Physiological responses to focal cerebral ischemia in humans. Ann Neurol. 1984;16:546–552. doi: 10.1002/ana.410160504. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Yamauchi H, Fukuyama H, Nagahama Y, Nabatame H, Nakamura K, Yamamoto Y, et al. Evidence of misery perfusion and risk for recurrent stroke in major cerebral arterial occlusive diseases from PET. J Neurol Neurosurg Psychiatry. 1996;61:18–25. doi: 10.1136/jnnp.61.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi H, Higashi T, Kagawa S, Nishii R, Kudo T, Sugimoto K, et al. Is misery perfusion still a predictor of stroke in symptomatic major cerebral artery disease. Brain. 2012;135:2515–2526. doi: 10.1093/brain/aws131. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Houkin K, Kamiyama H, Mitsumori K, Iwasaki Y, Abe H. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it. Stroke. 2001;32:2110–2116. doi: 10.1161/hs0901.095692. [DOI] [PubMed] [Google Scholar]
- Ogasawara K, Ogawa A, Yoshimoto T. Cerebrovascular reactivity to acetazolamide and outcome in patients with symptomatic internal carotid or middle cerebral artery occlusion: a xenon-133 single-photon emission computed tomography study. Stroke. 2002;33:1857–1862. doi: 10.1161/01.str.0000019511.81583.a8. [DOI] [PubMed] [Google Scholar]
- Derdeyn CP, Videen TO, Fritsch SM, Carpenter DA, Grubb RL, Jr., Powers WJ. Compensatory mechanisms for chronic cerebral hypoperfusion in patients with carotid occlusion. Stroke. 1999;30:1019–1024. doi: 10.1161/01.str.30.5.1019. [DOI] [PubMed] [Google Scholar]
- Tomita Y, Kubis N, Calando Y, Tran Dinh A, Meric P, Seylaz J, et al. Long-term in vivo investigation of mouse cerebral microcirculation by fluorescence confocal microscopy in the area of focal ischemia. J Cereb Blood Flow Metab. 2005;25:858–867. doi: 10.1038/sj.jcbfm.9600077. [DOI] [PubMed] [Google Scholar]
- Takuwa H, Masamoto K, Yamazaki K, Kawaguchi H, Ikoma Y, Tajima Y, et al. Long-term adaptation of cerebral hemodynamic response to somatosensory stimulation during chronic hypoxia in awake mice. J Cereb Blood Flow Metab. 2013;33:774–779. doi: 10.1038/jcbfm.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima Y, Takuwa H, Kawaguchi H, Masamoto K, Ikoma Y, Seki C, et al. Reproducibility of measuring cerebral blood flow by laser-Doppler flowmetry in mice. Front Biosci (Elite Ed) 2014;6:62–68. doi: 10.2741/e691. [DOI] [PubMed] [Google Scholar]
- Matsuura T, Kashikura K, Kanno I. Hemodynamics of local cerebral blood flow induced by somatosensory stimulation under normoxia and hyperoxia in rats. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:363–372. doi: 10.1016/s1095-6433(00)00354-8. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Tomita Y, Toriumi H, Aoki I, Unekawa M, Takuwa H, et al. Repeated longitudinal in vivo imaging of neuro-glio-vascular unit at the peripheral boundary of ischemia in mouse cerebral cortex. Neuroscience. 2012;212:190–200. doi: 10.1016/j.neuroscience.2012.03.034. [DOI] [PubMed] [Google Scholar]
- Sekiguchi Y, Masamoto K, Takuwa H, Kawaguchi H, Kanno I, Ito H, et al. Measuring the vascular diameter of brain surface and parenchymal arteries in awake mouse. Adv Exp Med Biol. 2013;789:419–425. doi: 10.1007/978-1-4614-7411-1_56. [DOI] [PubMed] [Google Scholar]
- Klein B, Kuschinsky W, Schrock H, Vetterlein F. Interdependency of local capillary density, blood flow, and metabolism in rat brains. Am J Physiol. 1986;251:H1333–H1340. doi: 10.1152/ajpheart.1986.251.6.H1333. [DOI] [PubMed] [Google Scholar]
- Takuwa H, Matsuura T, Obata T, Kawaguchi H, Kanno I, Ito H. Hemodynamic changes during somatosensory stimulation in awake and isoflurane-anesthetized mice measured by laser-Doppler flowmetry. Brain Res. 2012;1472:107–112. doi: 10.1016/j.brainres.2012.06.049. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Gulf Professional Publishing; 2004. [Google Scholar]
- Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991;29:231–240. doi: 10.1002/ana.410290302. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Kaplan B, Jacewicz M, Pulsinelli W. Continuous measurement of cerebral cortical blood flow by laser-Doppler flowmetry in a rat stroke model. J Cereb Blood Flow Metab. 1989;9:589–596. doi: 10.1038/jcbfm.1989.84. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K, Adachi K, Kataoka S, Watanabe A, Tabira T, Takahashi K, et al. Chronic cerebral hypoperfusion induced by right unilateral common carotid artery occlusion causes delayed white matter lesions and cognitive impairment in adult mice. Exp Neurol. 2008;210:585–591. doi: 10.1016/j.expneurol.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, et al. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab. 1998;18:570–579. doi: 10.1097/00004647-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Hecht N, He J, Kremenetskaia I, Nieminen M, Vajkoczy P, Woitzik J. Cerebral hemodynamic reserve and vascular remodeling in C57/BL6 mice are influenced by age. Stroke. 2012;43:3052–3062. doi: 10.1161/STROKEAHA.112.653204. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL., Jr Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol. 1978;234:H371–H383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- MacKenzie ET, Farrar JK, Fitch W, Graham DI, Gregory PC, Harper AM. Effects of hemorrhagic hypotension on the cerebral circulation. I. Cerebral blood flow and pial arteriolar caliber. Stroke. 1979;10:711–718. doi: 10.1161/01.str.10.6.711. [DOI] [PubMed] [Google Scholar]
- Guo H, Itoh Y, Toriumi H, Yamada S, Tomita Y, Hoshino H, et al. Capillary remodeling and collateral growth without angiogenesis after unilateral common carotid artery occlusion in mice. Microcirculation. 2011;18:221–227. doi: 10.1111/j.1549-8719.2011.00081.x. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Raper AJ, Rosenblum WI, Navari RM, Patterson JL., Jr. Role of tissue hypoxia in local regulation of cerebral microcirculation. Am J Physiol. 1978;234:H582–H591. doi: 10.1152/ajpheart.1978.234.5.H582. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Ayata C, Zaharchuk G, Moskowitz MA, Rosen BR, et al. Evidence of a cerebrovascular postarteriole windkessel with delayed compliance. J Cereb Blood Flow Metab. 1999;19:679–689. doi: 10.1097/00004647-199906000-00012. [DOI] [PubMed] [Google Scholar]
- Talman WT, Nitschke Dragon D. Neuronal nitric oxide mediates cerebral vasodilatation during acute hypertension. Brain Res. 2007;1139:126–132. doi: 10.1016/j.brainres.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamouchi M, Ago T, Kuroda J, Kitazono T. The possible roles of brain pericytes in brain ischemia and stroke. Cell Mol Neurobiol. 2012;32:159–165. doi: 10.1007/s10571-011-9747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Suzuki N. Control of brain capillary blood flow. J Cereb Blood Flow Metab. 2012;32:1167–1176. doi: 10.1038/jcbfm.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SP, Duong TQ, Yang G, Iadecola C, Kim SG. Relative changes of cerebral arterial and venous blood volumes during increased cerebral blood flow: implications for BOLD fMRI. Magn Reson Med. 2001;45:791–800. doi: 10.1002/mrm.1107. [DOI] [PubMed] [Google Scholar]
- Ito H, Kanno I, Ibaraki M, Hatazawa J, Miura S. Changes in human cerebral blood flow and cerebral blood volume during hypercapnia and hypocapnia measured by positron emission tomography. J Cereb Blood Flow Metab. 2003;23:665–670. doi: 10.1097/01.WCB.0000067721.64998.F5. [DOI] [PubMed] [Google Scholar]
- Duelli R, Kuschinsky W. Changes in brain capillary diameter during hypocapnia and hypercapnia. J Cereb Blood Flow Metab. 1993;13:1025–1028. doi: 10.1038/jcbfm.1993.129. [DOI] [PubMed] [Google Scholar]
- Hutchinson EB, Stefanovic B, Koretsky AP, Silva AC. Spatial flow-volume dissociation of the cerebral microcirculatory response to mild hypercapnia. NeuroImage. 2006;32:520–530. doi: 10.1016/j.neuroimage.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. SKCa and IKCa Channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: effect of ischemia and reperfusion. Stroke. 2009;40:1451–1457. doi: 10.1161/STROKEAHA.108.535435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi H, Okazawa H, Kishibe Y, Sugimoto K, Takahashi M. Reduced blood flow response to acetazolamide reflects pre-existing vasodilation and decreased oxygen metabolism in major cerebral arterial occlusive disease. Eur J Nucl Med Mol Imaging. 2002;29:1349–1356. doi: 10.1007/s00259-002-0899-x. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Shiga T, Houkin K, Ishikawa T, Katoh C, Tamaki N, et al. Cerebral oxygen metabolism and neuronal integrity in patients with impaired vasoreactivity attributable to occlusive carotid artery disease. Stroke. 2006;37:393–398. doi: 10.1161/01.STR.0000198878.66000.4e. [DOI] [PubMed] [Google Scholar]
- Dunn KM, Nelson MT. Potassium channels and neurovascular coupling. Circ J. 2010;74:608–616. doi: 10.1253/circj.cj-10-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inao S, Tadokoro M, Nishino M, Mizutani N, Terada K, Bundo M, et al. Neural activation of the brain with hemodynamic insufficiency. J Cereb Blood Flow Metab. 1998;18:960–967. doi: 10.1097/00004647-199809000-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.