Abstract
Long-term exposure of mice to mild heat (34°C±1°C) confers neuroprotection against traumatic brain injury (TBI); however, the underling mechanisms are not fully understood. Heat acclimation (HA) increases hypothalamic angiotensin II receptor type 2 (AT2) expression and hypothalamic neurogenesis. Accumulating data suggest that activation of the brain AT2 receptor confers protection against several types of brain pathologies, including ischemia, a hallmark of the secondary injury occurring following TBI. As AT2 activates the same pro-survival pathways involved in HA-mediated neuroprotection (e.g., Akt phosphorylation, hypoxia-inducible factor 1α (HIF-1α), and brain-derived neurotrophic factor (BDNF)), we examined the role of AT2 in HA-mediated neuroprotection after TBI. Using an AT2-specific antagonist PD123319, we found that the improvements in motor and cognitive recovery as well as reduced lesion volume and neurogenesis seen in HA mice were all diminished by AT2 inhibition, whereas no significant alternations were observed in control mice. We also found that nerve growth factor/tropomyosin-related kinase receptor A (TrkA), BDNF/TrkB, and HIF-1α pathways are upregulated by HA and inhibited on PD123319 administration, suggesting that these pathways play a role in AT2 signaling in HA mice. In conclusion, AT2 is involved in HA-mediated neuroprotection, and AT2 activation may be protective and should be considered a novel drug target in the treatment of TBI patients.
Keywords: angiotensin receptor type 2, heat acclimation, neurogenesis, neuroprotection, PD123319, traumatic brain injury
Introduction
Heat acclimation (HA), conferred by long-term exposure to mild heat, leads to the development of a neuroprotective phenotype against traumatic brain injury (TBI).1 After TBI, HA mice display hypothermia, improved motor recovery, and cognitive state as well as reduced lesion volume and edema formation.1, 2
The molecular mechanisms underlying neuroprotection include reduced inflammation and apoptosis,1, 2 along with induction of hypoxia-inducible factor 1α (HIF-1α), brain-derived neurotrophic factor (BDNF), Akt phosphorylation, and erythropoietin signaling. Given the indispensable role of HIF-1α in HA-mediated neuroprotection,1 we hypothesized that an upstream mediator of HIF-1α may coordinate neuroprotective elements. In the current study, we examined the nomination of angiotensin receptor type 2 (AT2) signaling as an upstream candidate. Brain AT2 is abundant during development and during chronic stress.3, 4 In the brain, most studies focused on brain angiotensin receptor type 1; however, accumulating evidence suggests that the beneficial effects of blocking angiotensin receptor type 1 are in fact caused by the shifting of endogenous angiotensin II to bind AT2 receptors.5 The roles of AT2 in post-ischemic protection,4, 6 as well as in axonal regeneration, spinal cord injury,7 and regeneration of sciatic or optic nerve,4, 8 have been described. However, the underlying mechanisms by which AT2 stimulation enhances protection are incompletely understood. In vitro experiments showed that AT2 activation involves subsequent activation of tropomyosin-related kinase receptor A (TrkA) and its downstream effectors p42/p44mapk (ERK1/2), NO release and subsequent Akt activation,4, 9 and increased BDNF and TrkA/B.7 Other in vitro studies imply a role of AT2 in neuronal proliferation,10 differentiation, and regeneration.4, 8, 11, 12 Furthermore, it was recently suggested that AT2 mediates HIF-1α upregulation, a key factor in HA-mediated neuroprotection.1, 13
The observation that the increase in hypothalamic AT2 after HA14 coincides with increased hypothalamic neurogenesis15 may imply that AT2 upregulation is one of the HA-induced changes that contribute to the orchestrated neuroprotective mechanisms. As some neuroprotective cellular downstream pathways of AT2 are also upregulated in HA (e.g., Akt, HIF-1α, and BDNF), we aim to determine whether AT2 is involved in the development of HA-mediated neuroprotection after TBI, which will promote its relevance as a drug target to confer neuroprotection and regeneration after TBI.
Materials and methods
Animals and Maintenance
The study was approved by the Institutional Animal Ethics Committee of the Hebrew University and complied with the guidelines of ARRIVE (Animal Research: Reporting In Vivo Experiments) and the National Research Council Guide for the Care and Use of Laboratory Animals (NIH Publication no. 85-23, revised 1996). Male Sabra mice weighing 39 to 52 g were used; all were 9 to 10 weeks old when subjected to closed head injury. Animals were kept under HA (34°C±1°C) or normothermic (NT) temperature (24°C±1°C) for 4 weeks before the injury, under a 12-hour/12-hour light/dark cycle. Food and water were provided ad libitum.
Trauma Model
Experimental closed head injury was induced under isoflurane anesthesia using a modified weight drop device developed in our laboratory.16 Briefly, after anesthesia, a midline longitudinal incision exposing the skull was made. A Teflon-tipped cone (2 mm diameter) was placed 1 to 2 mm lateral of the midline in the midcoronal plane. The head was held in place and a 95 g weight was allowed to fall on the cone from a preestablished height, causing focal injury to the left hemisphere. Motor and neurobehavioral tests, histopathology, and protein markers of neurogenesis were measured as described below.
Pharmacological Treatments
At the time of surgery and while anesthetized, a group of NT and HA mice were randomly allocated to receive the AT2 antagonist PD123319 (Tocris Bioscience, Bristol, UK) at a dose of 10 mg/kg/day dissolved in sterile isotonic saline17, 18 that was delivered for 3 days after injury using Alzet osmotic mini pumps 1003D (Alzet Cupertino, CA, USA). Pumps were transplanted subcutaneously. After recovery from anesthesia, the mice were given postoperative care in their cages with free access to food and water. Sham control mice underwent anesthesia and skin incisions. HA mice returned to the climatic chamber after the first 24 hours post injury, to preserve the HA phenotype and avoid deacclimation.
BrdU Injections
From the first day after injury or sham operation, for 7 consecutive days, all animals received an intraperitoneal injection twice daily of the tracer 5-bromo-2-deoxyuridine (BrdU; 50 mg/kg), which labels dividing cells.
Neurobehavioral Evaluation
The functional status of the mice was evaluated according to the Neurological Severity Score (NSS) by an observer unaware of the treatment. This score is a 10-point scale assessing functional neurologic status based on the presence of some reflexes and the ability to perform motor and behavioral tasks, such as beam walking, beam balance, and spontaneous locomotion.16 Mice are awarded one point for failure to perform a task—i.e., scores increase with the severity of dysfunction. The NSS obtained 1 hour after closed head injury reflects initial injury severity. NSS values were measured for 1 hour once daily for the first 3 days and once weekly for 6 weeks post injury (n=9 to 10 mice/group).
Novel Object Recognition Test
The novel object recognition test (NORT) was performed 3 and 30 days after injury as previously described.19 This is a sensitive and reproducible test for measuring cognitive abnormalities in this model as was employed in several studies testing neuroprotective drugs.20 Each mouse was placed in an open glass aquarium-like transparent box, in a sound-isolated room, and allowed a 1-hour habituation period. The next day they were placed in the same box for 5 minutes with identical clean plaster objects placed in two separate corners of the box. Four hours later, one of the objects was replaced with a new one of the same size and texture, and the mice were reintroduced for an additional 5 minutes to the same cage. Time spent by the mouse in object exploration was recorded by a person blinded to the treatments, and the cumulative time spent at each object was recorded. Exploration of an object was defined as directing the nose to the object at a distance of 2 cm and/or touching it with the nose. The percentage of the total exploration time that the animal spent investigating the new object out of total exploration time was used as a measure of recognition memory (n=9 to 10 mice/group).
Lesion Volume
Forty-two days after injury, the mice were deeply anesthetized and perfusion-fixed with 4% paraformaldehyde. Brains were frozen-sectioned at 10 μm. Brain slices 200 μm apart between bregma +1.78 mm and bregma −2.54 mm were obtained. Giemsa stain-modified solution (1:1; Fluka, Sigma-Aldrich Corporation, St Louis, MO, USA) was used for staining and the sections were photographed using stereoscope Stemi SV11 (Zeiss, Jena Germany) equipped with a digital photo camera (Coolpix 4500, Nikon, Tokyo, Japan). The volume of injured tissue was measured with ImageJ 1.40 software (National Institutes of Health, Bethesda, MD, USA). Damaged tissue volume was calculated as previously described by dividing the volume of the injured left hemisphere by that of the right, undamaged hemisphere.20 Results are expressed as a percentage of hemispheric tissue (n=9 to 10 mice/group).
Western Immunoblotting
Mice treated with saline were killed at 6, 24, or 72 hours after injury and surgical procedure (n=6/group). PD123319-treated mice were killed after the entire PD123319 had released from the minipump—namely, 72 hours post injury. After decapitation, brains were rapidly removed and frontal segments (40–60 mg) from the left injured hemisphere were separated and stored at −80 °C until analysis. Sample preparation was performed as previously described.2 After homogenization in a buffer containing sucrose 0.25 mol/L, Tris 20 mmol/L (pH=7.6), MgCl2 1.5 mmol/L, glycerol 10%, and ethylenediaminetetraacetic acid 1 mmol/L, samples were centrifuged at 5,000 r.c.f. for 10 minutes, and supernatants were stored at −80 °C until analysis. Protein concentration was determined using a Pierce BSA Protein Assay Kit (Thermo scientific Rockford, IL, USA). Equal protein samples (40 μg) were separated on 10% sodium dodecyl sulfate–polyacrylamide gels with a 4.5% sodium dodecyl sulfate stacking gel and electrotransferred onto nitrocellulose membranes (0.2 μm, Schleicher and Schuell, Dessel, Germany). Blots were probed with anti-NGF (anti-nerve growth factor) 1:200 (Almone Labs, Jerusalem, Israel), anti-HIF-1α (1:1,000; Abcam, Cambridge, UK), anti-BDNF (1:500), anti-TrkA (1:1,000; Abcam, Cambridge, UK), anti-TrkB (1:1,000; Abcam, Cambridge, UK), and anti-AT2 (1:1,000; Santa Cruz, Dallas, TX, USA). Anti-β-actin antibody (1:1,000; Cell Signaling Technology Danvers, MA, USA) was used to confirm equal protein loading. Appropriate peroxidase-coupled immunoglobulin G (1:10,000; Jackson Immunoresearch, Soham, Cambridgeshire, UK) was used as the secondary antibody. Reactive bands were visualized using the enhanced chemiluminescence system (Biological Industries, Beit Haemek, Israel). Optical density of the reactive bands was quantified using Tina software (Raytest, Straubenhardt, Germany) and protein levels were expressed as the optical density of the examined factor relative to β-actin in the same lane.
Immunohistochemistry
Evenly spaced (200 μm) slices were counted for each mouse, between bregma +1.78 mm and bregma −2.54 mm. In each slice all the relevant fields from the subventricular zone (SVZ), dentate gyrus, and from the area surrounding the lesion were counted. Considering that the lesion core had already liquefied by 42 days after TBI, the boundary of this area was considered to represent the border zone that survived. This area included cortical and subcortical tissue but not the striatum. Brain slices were double stained for immunohistochemical evaluation using fate-specific antibodies anti-BrdU (2 μg/mL; Calbiochem, Darmstadt, Germany) and anti-NeuN (1:750; Merck Millipore, Darmstadt, Germany). Dylight 488 (1:300; Abcam, Cambridge, UK) and NorthernLights 557 (R&D systems Emeryville, CA, USA) conjugates were used as secondary antibodies. UltraCruz mounting medium (Santa Cruz, Dallas, TX, USA) that contains 46-diamidino-2-phenyl indole was used to visualize nuclei in order to avoid artifacts.
Statistical Analysis
For statistical analyses, we used commercially available computer software (SigmaStat 2.03, Systat Software, San Jose, CA, USA). Treatments were the independent variables and the outcomes of the TBI parameters were the dependent variables. Significance was tested using two-way analysis of variance followed by the Tukey posttest for protein levels and immunohistochemistry. For NSS, NORT and lesion volume calculations, Mann–Whitney with Bonferroni correction was used. P values <0.05 were considered significant for all comparisons. Data are expressed as mean±s.e.m.
Results
PD123319 Inhibits the Improved Motor Recovery and Cognitive Performance of Heat-Acclimated Mice after Traumatic Brain Injury
To determine the effect of AT2 blockade on HA-induced neuroprotection, we examined the impact of PD123319 on the motor recovery profile of treated mice, as determined by the NSS test.
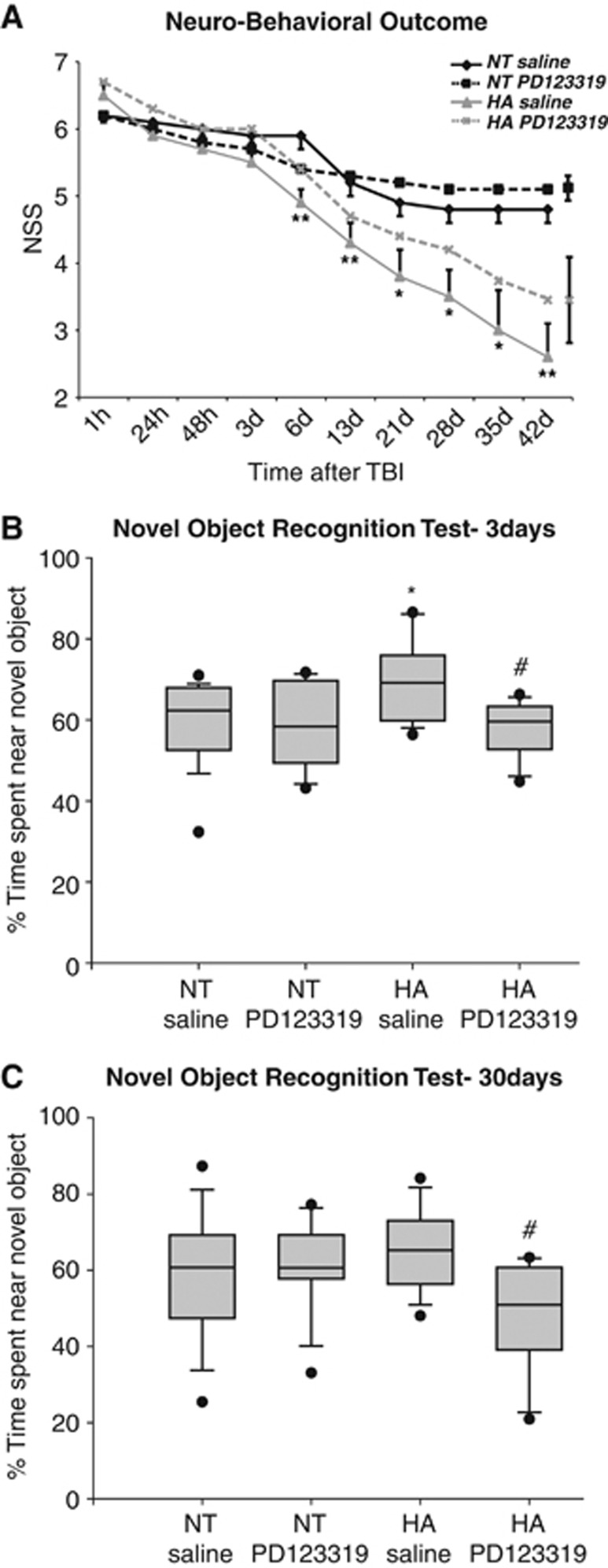
The neurologic deficits were measured at predetermined time points after TBI (Figure 1A). Heat-acclimated mice exhibited improved motor recovery from day 6, as shown by attenuated NSS score, compared with the NT controls. Although HA mice continued to improve for 6 weeks after injury, control mice showed signs of recovery only for 3 weeks. Administration of PD123319 compromised the enhanced recovery of HA mice, which was no longer significant as compared with NT control mice at all time points after the treatment. However, PD123319 treatment did not alter the spontaneous recovery demonstrated by NT mice.
Figure 1.
Heat acclimation (HA)-mediated neuroprotection and cognitive performance are compromised by angiotensin receptor type 2 blockade after traumatic brain injury (TBI). PD123319 (PD, 10 mg/kg/day) or saline-treated mice were subjected to TBI, and neurobehavioral outcome was evaluated by the Neurological Severity Score (NSS) at 1 hour post injury to assess initial disability and at multiple time points thereafter. PD treatment eliminated the statistically significant improvement in neurobehavioral outcome of HA mice (A; *P<0.05; **P<0.01 HA treated with saline versus normothermic (NT) treated with saline, determined by two-way analysis of variance for repeated measures, n=9 to 10 per group). For the sake of clarity, maximal error bars of the groups treated with PD123319 are presented on the right end of the representative line. In the box plots, mice were subjected to the novel object recognition test (NORT) 3 days (B) and 30 days (C) after TBI. The graph shows the median (band), 25th and 75th quantiles (bottom and top of box), sample minimum and maximum (whiskers), and outliers (dots). The longer exploration time of the novel object of HA mice decreased after PD123319 (PD, 10 mg/kg/day) treatment. *P<0.05 HA treated with saline versus NT treated with saline; #P<0.05 HA treated with saline versus HA treated with PD123319, determined by Mann–Whitney, n=9 to 10 per group.
The effect of PD123319 on cognitive function was evaluated using NORT at 3 (Figure 1B) and 30 days post injury (Figure 1C). At 3 days after injury, HA mice spent significantly more time at the novel object, indicating better memory function compared with NT mice (P=0.03). PD123319 eliminated the improved performance; i.e., it reduced the recognition function of HA mice (P=0.86 versus NT saline, P=0.02 versus HA saline). At 30 days after TBI, no significant effect was observed in HA mice; nevertheless, the PD123319-treated mice kept the attenuated exploration time as compared with HA mice that were given saline (P=0.045).
PD123319 Abolishes Heat Acclimation-Mediated Reduction in Lesion Volume after Traumatic Brain Injury
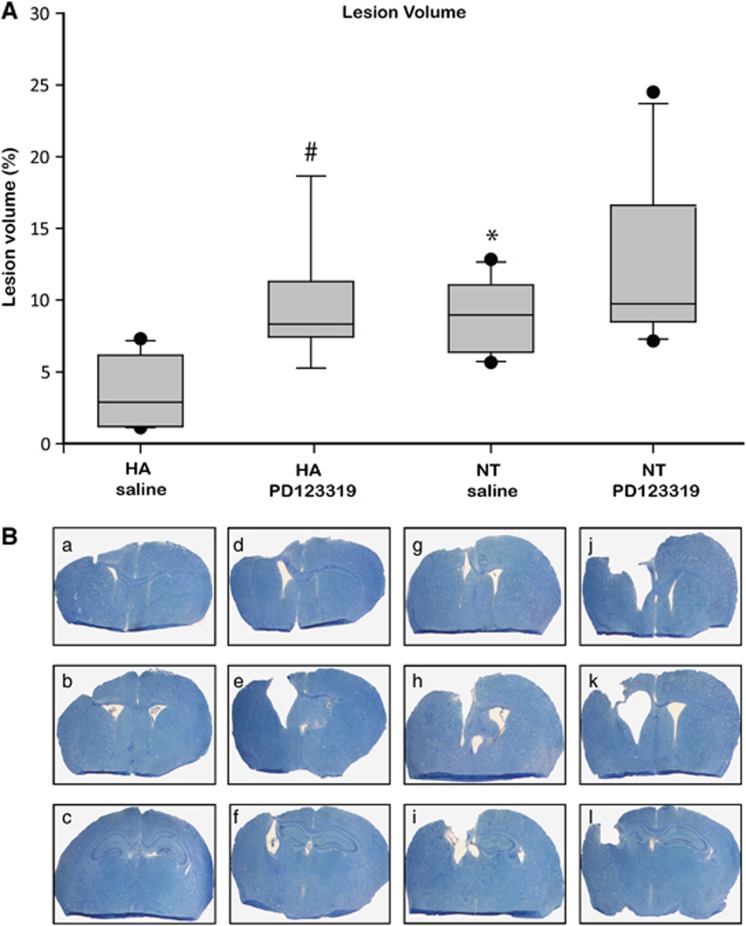
To examine whether AT2 inhibition affects lesion volume, we measured tissue loss in the injured brains at 42 days post injury using Giemsa staining (Figure 2). Attenuated lesion volume was observed in the HA mice, as compared with NT mice (P<0.01). Treatment with the antagonist PD123319 abolished this effect, with a dramatic increase in lesion volume (P=0.03 versus saline HA-treated mice), and HA PD123319-treated mice had the same extent of lesion volume as NT mice. PD123319 also tended to increase the lesion volume in the NT mice; however, this change was statistically insignificant.
Figure 2.
Heat acclimation (HA)-mediated reduced lesion volume is eliminated by angiotensin receptor type 2 blockade after traumatic brain injury (TBI). Mice were killed 42 days after TBI, and evenly separated 10-μm slices were stained with Giemsa. (A) The graph shows the median (band), 25th and 75th quantiles (bottom and top of box), sample minimum and maximum (whiskers), and outliers (dots). (B) The reduced lesion volume measured in HA mice (panels a–c) was abolished by PD123319 treatment (panels d–f, PD123319, 10 mg/kg/day). *P<0.05 HA treated with saline versus normothermic (NT) treated with saline (panels g–i); #P<0.05 HA treated with saline versus HA treated with PD123319, as determined by Mann–Whitney (n=6 per group).
Angiotensin Receptor Type 2 Expression Levels are not Affected by Heat Acclimation but are Reduced in Heat-Acclimated Mice after PD123319 Treatment
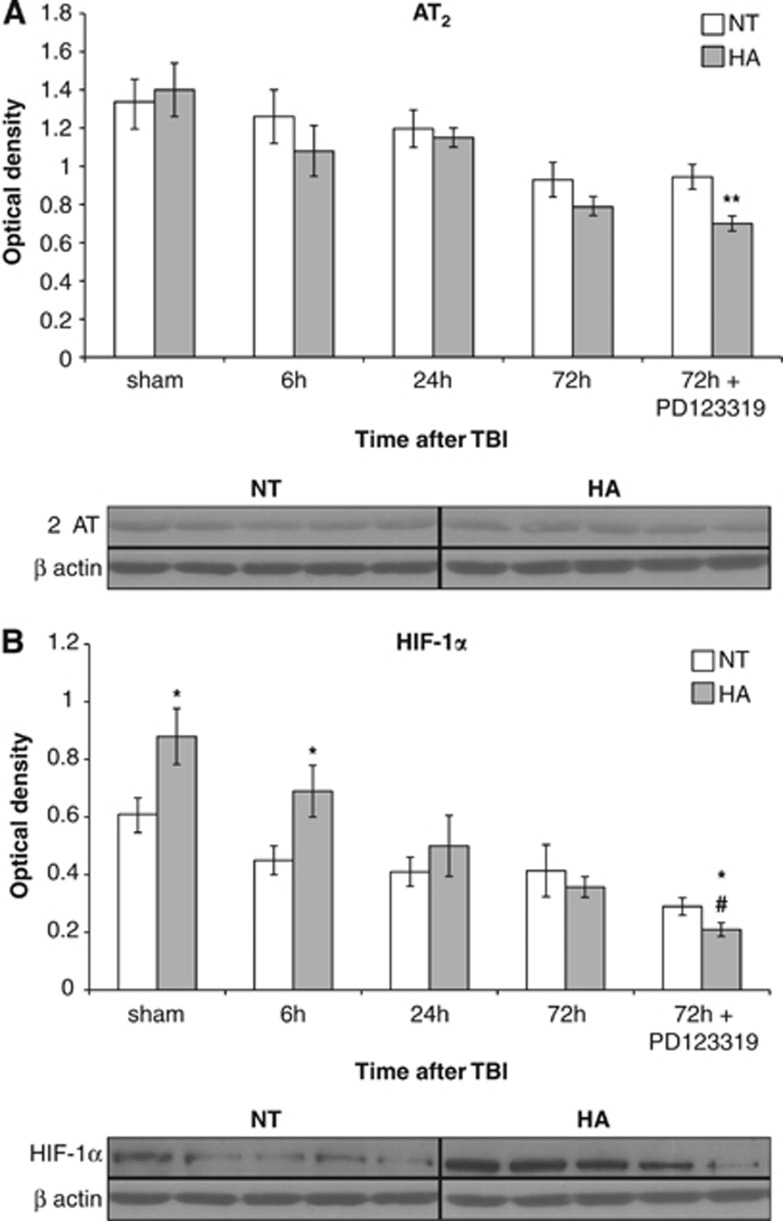
We hypothesized that HA increases AT2 levels and thus mediates neuroprotection after TBI, and that this increase is attenuated after PD123319 treatment. Western blot analysis revealed no differences in AT2 protein levels between HA and NT mice (Figure 3A). Interestingly, PD123319 did not alter AT2 expression levels in NT mice but reduced expression levels in HA mice, resulting in significant differences between NT and HA injured mice 72 hours after PD123319 administration as seen in Figure 3A (P=0.04).
Figure 3.
Angiotensin receptor type 2 (AT2) blockade decreases protein levels of AT2 and hypoxia-inducible factor 1α (HIF-1α) in heat-acclimated (HA) mice. Mice were subjected to traumatic brain injury (TBI) after treatment with saline or PD123319 (PD, 10 mg/kg/day), and killed at 6, 24, or 72 hours post TBI. (A) HA did not alter AT2 levels; however, PD123319 treatment lowered AT2 levels in HA mice. (B) Elevated HIF-1α levels were measured in HA mice and were reduced after PD123319 treatment. *P<0.05 between HA treated with saline versus normothermic (NT) treated with saline; **P<0.05 between HA treated with PD123319 versus NT treated with PD123319; #P<0.05 HA treated with saline versus HA treated with PD123319, determined by two-way analysis of variance followed by Tukey's test, n=6 per group.
Attenuated Hypoxia-Inducible Factor 1α Protein Levels may Underlie the Susceptibility of Heat-Acclimated Mice to PD123319 Treatment
To further investigate the molecular mechanisms affecting recovery, we examined HIF-1α protein levels, which are essential for HA-mediated neuroprotection.21 Levels of HIF-1α were ∼50% higher in sham HA mice compared with sham NT mice. Six hours after TBI, HA mice still had higher levels (P<0.05), which showed a decline for up to 72 hours. In contrast, in NT mice a moderate, nonsignificant reduction in HIF-1α was observed during the first 72 hours post TBI. AT2 antagonist PD123319 administration further reduced HIF-1α protein levels in HA injured mice, and 72 hours after PD123319 administration the HIF-1α levels were lower than those in NT mice (P=0.04) and HA saline-treated mice (P=0.03; Figure 3B).
Heat Acclimation Induces Neurotrophins and their Receptors
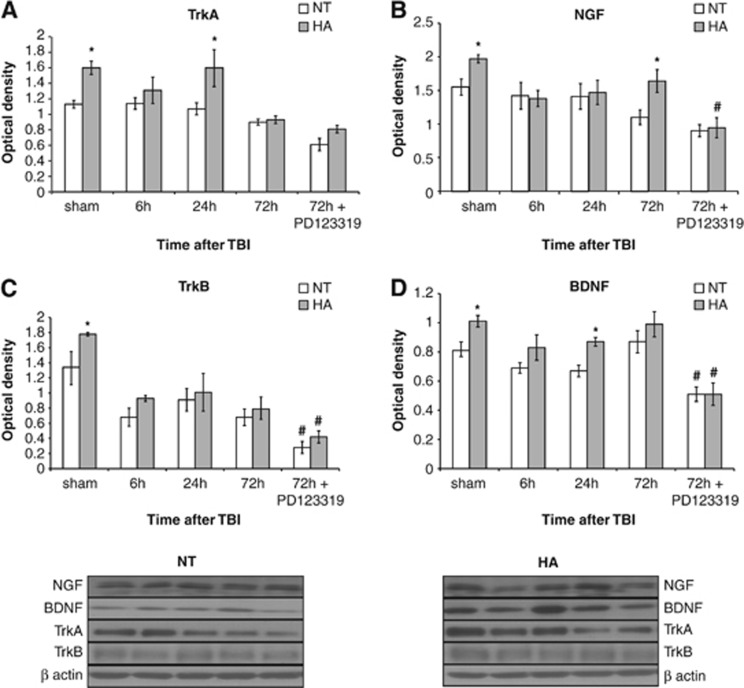
As no difference was observed in AT2 protein levels, we postulated that TrkA, which is transactivated by AT2, may be induced in HA mice. Western blot analysis (Figure 4A) revealed higher levels of TrkA receptors in HA mice (P=0.02) and injury-induced elevation of the receptor 24 hours post TBI (P<0.01) compared with NT mice. PD123319 did not significantly alter TrkA levels. NGF (the endogenous ligand of TrkA) levels are presented in Figure 4B. NGF levels were higher in HA sham mice (P=0.04) and in HA mice 72 hours post TBI compared with NT mice (P=0.02). NGF induction was inhibited by PD123319 treatment only in HA mice (P=0.01).
Figure 4.
Angiotensin receptor type 2 is involved in heat acclimation (HA)-mediated enhanced neurotrophin signaling. Mice were subjected to traumatic brain injury (TBI) after treatment with saline or PD123319 (PD, 10 mg/kg/day), and were killed at 6, 24, or 72 hours post TBI. HA elevated levels of tropomyosin-related kinase receptor A (TrkA, A) as well as its endogenous ligand, nerve growth factor (NGF). PD123319 treatment decreased NGF levels in HA mice (B). Elevated TrkB levels were seen in HA mice and were reduced after PD123319 treatment in both normothermic (NT) and HA mice (C). HA induced brain-derived neurotrophic factor (BDNF) levels, whereas PD123319 treatment reduced BDNF levels, in both NT and HA mice (D). *P<0.05 HA treated with saline versus NT treated with saline; #P<0.05 mice treated with saline versus mice treated with PD123319 of the same group (NT/HA), determined by two-way analysis of variance followed by Tukey's test, n=6 per group.
In light of these findings, we measured the levels of TrkB and BDNF (its endogenous ligand). HA increased the levels of both proteins compared with the protein levels in NT sham mice (P<0.05). Although BDNF levels were higher in HA than in NT mice also at 24 hours post injury (P=0.04), no changes were observed in TrkB levels. TrkB (Figure 4C) and BDNF (Figure 4D) protein levels were dramatically lower in HA and NT mice 3 days after PD123319 treatment (P<0.01 in BDNF for both HA and NT mice; P=0.02 in TrkB for both HA and NT mice).
Heat Acclimation Induces Post-Traumatic Brain Injury Neurogenesis in the Neurogenic Niches, Which is Blocked by PD123319 Treatment
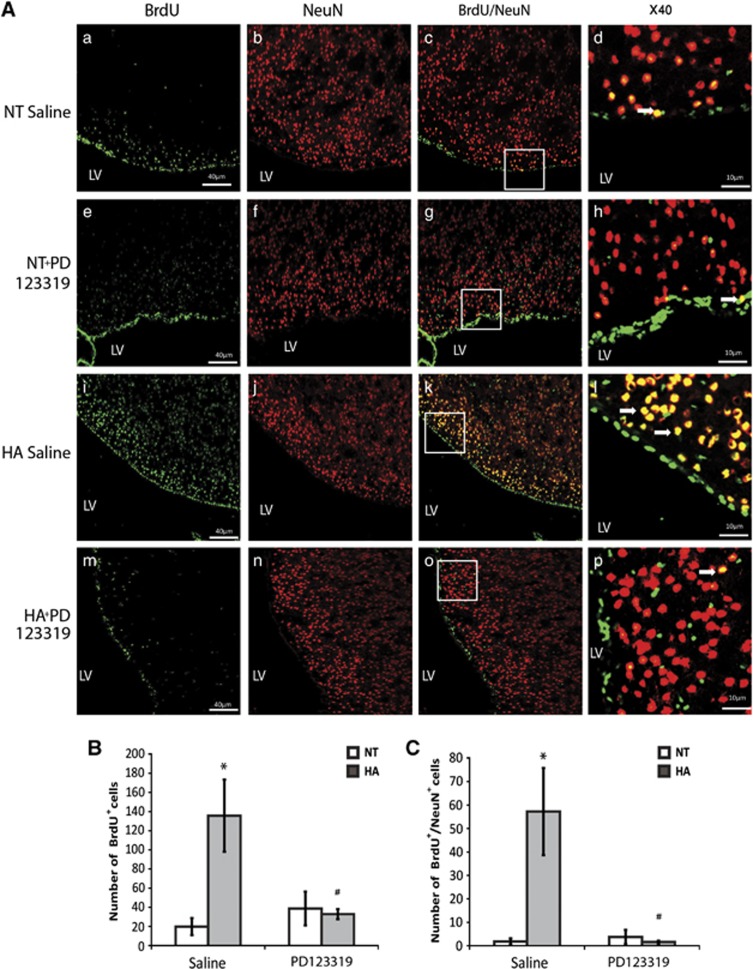
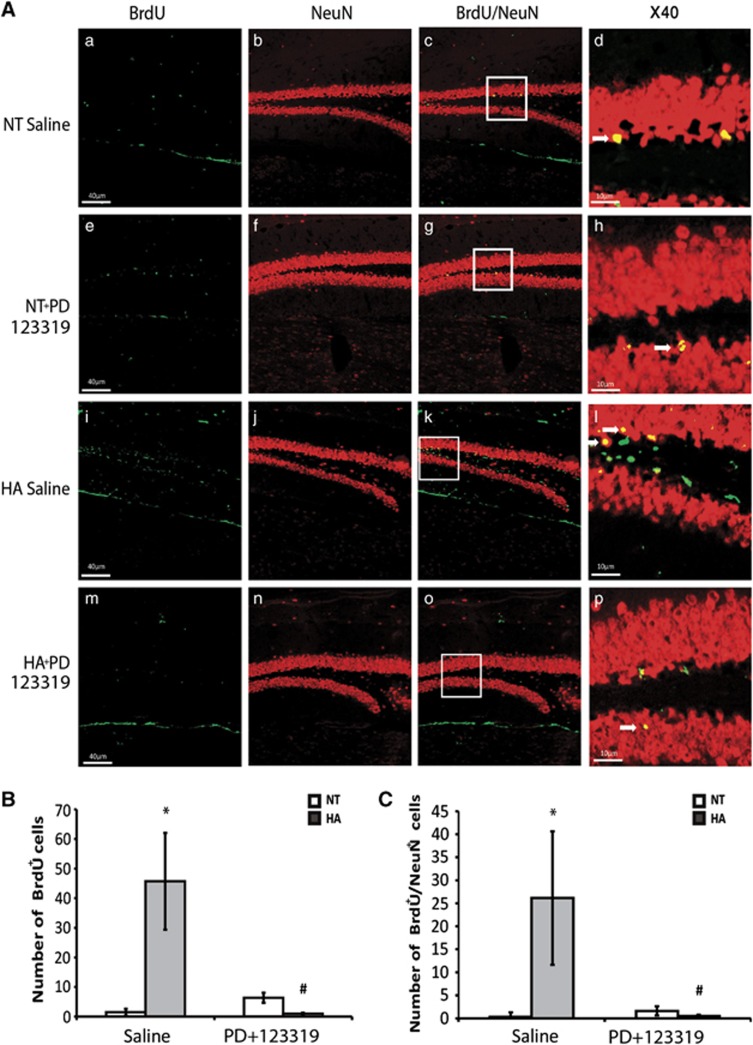
To investigate whether the sustained neuroprotection seen in injured HA is partially due to enhanced neurogenesis and to determine the role of AT2 in this process, we stained brain slices for BrdU and NeuN. We found that, in the SVZ, HA dramatically increased the proliferation of neuronal precursor cells (NPCs) after TBI (P<0.01) and PD123319 blocked this effect, as is depicted in Figure 5B (P<0.01 between HA saline and HA PD123319-treated mice). Although almost no newborn neurons were seen in the SVZ of NT mice, the SVZ of HA mice was full of newly mature neurons double stained with BrdU and NeuN, Figure 5C (P<0.01), and PD123319 also blocked this effect (P<0.01 between HA saline and HA PD123319-treated mice). In the dentate gyrus (Figure 6) we also found that HA induced proliferation with an increase in BrdU-positive cells after TBI (P<0.04 versus NT) and an increase in newborn mature neurons (P<0.02 versus NT mice). PD123319 treatment blocked this enhancement in HA mice (P<0.03 between HA saline and HA PD123319-treated mice).
Figure 5.
Angiotensin receptor type 2 is involved in heat acclimation (HA)-mediated enhanced neurogenesis in the subventricular zone (SVZ). Mice were subjected to traumatic brain injury (TBI) after treatment with saline or PD123319 (PD, 10 mg/kg/day) for 3 days, and killed 42 days post TBI. Evenly sliced 10-μm sections were incubated with anti-NeuN for mature neurons and with anti-BrdU (anti-5-bromo-2-deoxyuridine) for newborn cells. (A) HA induced neurogenesis in the SVZ (i, j) and PD123319 (PD, 10 mg/kg/day) treatment blocked the observed neurogenesis (m–p). PD treatment (e–h) did not reduce neurogenesis in normothermic (NT) mice measured after saline treatment (a–d). Stereological quantification of the fields was used to count the number of BrdU-positive cells (BrdU+). Results show the average number of BrdU+ per field (B), and the average number of double-positive cells for NeuN and for BrdU (BrdU+/NeuN+) per field (C). *P<0.01 HA treated with saline versus NT treated with saline; #P<0.01 HA mice treated with saline versus HA mice treated with PD123319, determined by two-way analysis of variance followed by Tukey's test, n=6 per group.
Figure 6.
Angiotensin receptor type 2 is involved in heat acclimation (HA)-mediated enhanced neurogenesis in the dentate gyrus. Mice were subjected to traumatic brain injury (TBI) and treated with saline or PD123319 (PD, 10 mg/kg/day) for 3 days, and killed 42 days post TBI. Evenly sliced 10-μm sections were incubated with anti-NeuN for mature neurons and with anti-BrdU (anti-5-bromo-2-deoxyuridine) for newborn cells. (A) HA induced neurogenesis in the dentate gyrus subgranular zone (i, j) and PD123319 (PD, 10 mg/kg/day) treatment blocked the observed neurogenesis (m–p). PD treatment (e–h) did not reduce neurogenesis in normothermic (NT) mice (a–d). Stereological quantification of the fields was used to count the number of BrdU-positive cells (BrdU+). Results show the average number of BrdU+ per field (B), and the average number of double-positive cells for NeuN and for BrdU (BrdU+/NeuN+) per field (C). *P<0.05 HA treated with saline versus NT treated with saline; #P<0.05 HA mice treated with saline versus HA mice treated with PD123319, determined by two-way analysis of variance followed by Tukey's test, n=6 per group.
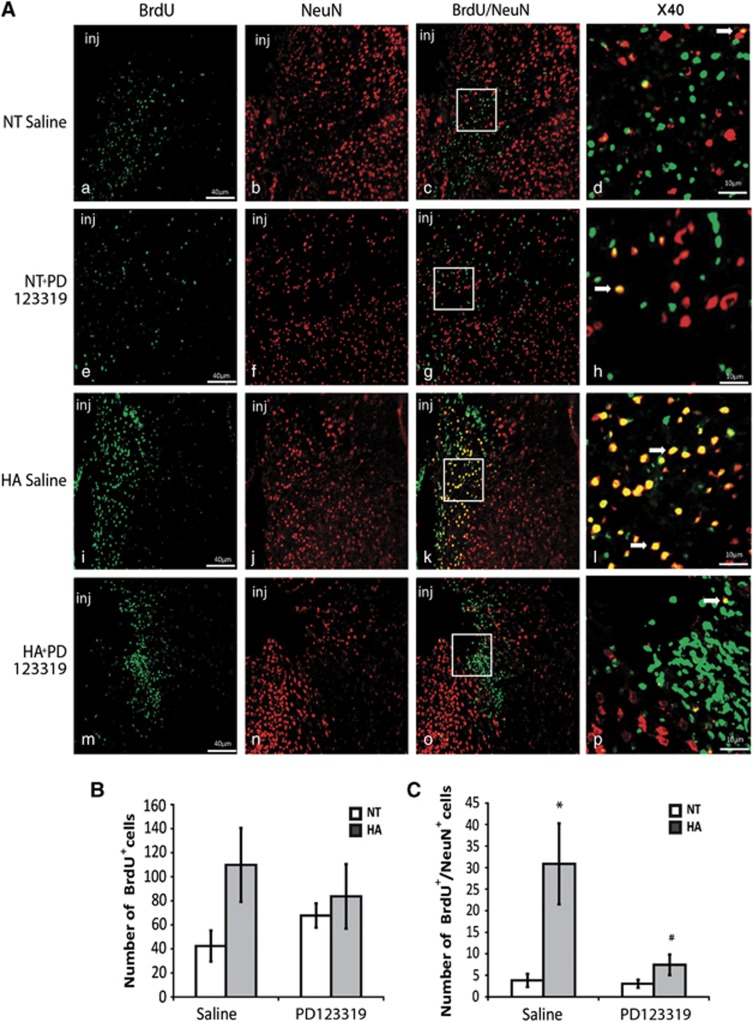
In the region surrounding the injury, HA insignificantly increased the number of newborn cells. Furthermore, PD123319 treatment had no effect on the number of newborn cells surrounding the injured area (Figure 7B). The number of newborn mature neurons increased in HA mice in the injured area (P<0.01 versus NT mice), and this effect was abolished by PD123319 treatment (P<0.01 versus HA mice treated with saline; Figure 7C).
Figure 7.
Angiotensin receptor type 2 is involved in heat acclimation (HA)-mediated enhanced neurogenesis in the injury region (inj). Mice were subjected to traumatic brain injury (TBI) and treated with saline or PD123319 (PD, 10 mg/kg/day) for 3 days, and killed 42 days post TBI. Evenly sliced 10-μm sections were incubated with anti-NeuN for mature neurons and with anti-BrdU (anti-5-bromo-2-deoxyuridine) for newborn cells. (A) HA induced neurogenesis in the injury region (i, j) and PD123319 (PD, 10 mg/kg/day) treatment blocked the observed neurogenesis (m–p). PD treatment (e–h) did not reduce neurogenesis in normothermic (NT) mice (a–d). Stereological quantification of the fields was used to count the number of BrdU-positive cells (BrdU+). Results show the average number of BrdU+ per field (B), and the average number of double-positive cells for NeuN and for BrdU (BrdU+/NeuN+) per field (C). *P<0.01 HA treated with saline versus NT treated with saline; #P<0.01 HA mice treated with saline versus HA mice treated with PD123319, determined by two-way analysis of variance followed by Tukey's test, n=6 per group.
Discussion
In this study we showed, for the first time, that HA-induced long-term neuroprotection is accompanied by neurorepair and mediated at least partly by AT2. This study suggests that AT2 mediates two distinctive beneficial pleiotropic effects in HA mice: (i) neuroprotection by reducing cell death and therefore lesion volume and (ii) enhancing delayed neurorepair events such as neurogenesis. Those effects may at least in part be mediated by neurotrophin signaling.
Heat Acclimation-Mediated Long-Term Neuroprotection and Improved Cognitive Performance after Traumatic Brain Injury—the role of Angiotensin Receptor Type 2
This study is the first to follow up HA mice for 6 weeks post TBI. HA mice showed sustained spontaneous recovery throughout the entire experimental period. Blocking AT2 using PD123319 compromised the accelerated recovery of HA mice after TBI, which was no longer statistically different from the spontaneous recovery represented by NT mice. PD123319 did not significantly affect the spontaneous recovery of control NT mice.
We hypothesize that these results imply that HA enhances AT2 signaling, which has a role in HA-induced neuroprotection after TBI, but is not important in the spontaneous recovery after TBI. This is reinforced by the histopathological findings that PD123319 did not significantly increase lesion volume in NT mice after TBI, whereas the HA mice treated with PD123319 had lesions twice as large as those given saline. Our data are in accordance with previous studies on brain ischemia, showing that PD123319 treatment did not increase infarct volume, whereas AT2 activation attenuated the infarct volume.6 Moreover, our unpublished results imply that AT2 activation, using a specific agonist in NT control mice, also improves motor and cognitive outcome after TBI. Previously, we demonstrated that HA mice perform significantly better in the NORT, indicating improved recognition memory after TBI. However, PD123319 diminished this effect. Interestingly, PD123319-treated HA mice failed to recognize the novel object as compared with saline-treated HA after 30 days. The NSS and the NORT are two distinguished behavioral tests that examine different physiologic abilities. These data may imply that the cognitive effects of AT2 inhibition take place at an earlier time point than the motor effects. It is possible that cognitive function is more susceptible to the inhibition in neuroprotection compared with motor performance, whereas motor performance may be compromised mostly by the inhibition of neuroregeneration, which takes place at a much later time point. Moreover, it was previously shown that after TBI the hippocampal neurons are extremely vulnerable, which may also underlie the differential effect of PD123319 on cognitive and motor function.22 A growing body of evidence has linked AT2 to learning and memory processes—e.g., findings that mice lacking the AT2 gene have significantly impaired spatial memory performance.23 Furthermore, AT2 activation improved spatial memory,23 and AT2 was suggested to underlie the beneficial effects of angiotensin receptor type 1 blockers in memory impairment.24 Collectively, current data suggest that AT2 may have a critical role in memory processes. PD123319 failed to worsen the cognitive function of NT mice after TBI; however, it did block the improved cognitive function noted in HA mice post TBI. Together with the similar results on motor recovery, our data suggest that AT2 signaling may be a part of the defense mechanisms recruited by HA mice, yet has a negligible role in spontaneous motor and cognitive recovery processes.
Heat Acclimation-Induced Angiotensin Receptor Type 2 Signaling Amplification: Involvement of Neurotrophins and their Receptors
The susceptibility of HA mice to AT2 blockage cannot be explained by enhanced AT2 levels, as HA did not alter AT2 levels. However, we found that TrkA, which is transactivated by AT2, is induced after HA. TrkA expression levels increase after HA and remain elevated after TBI. Moreover, we found that the TrkA ligand, NGF, is also upregulated in HA mice, implying enhanced central NGF signaling in HA mice. Furthermore, PD123319 inhibited the NGF induction. These results may imply that HA does not induce a direct increase of AT2 receptor levels but mediates AT2 signaling amplification by the induction of AT2-transactivated TrkA signaling. It is also noteworthy to cite a recently published paper that showed that the commonly used AT2 antibodies lack specificity,25 which challenges the findings of previous work based on AT2 receptor levels, including our own.
It has also been suggested that central AT2 increases BDNF signaling.7 In our study, we found that HA elevates TrkB and BDNF levels. Moreover, PD123319 attenuated expression levels of BDNF and TrkB in HA mice and in NT mice, suggesting that AT2 also mediates BDNF signaling involved in spontaneous recovery, but this effect is insufficient to prevent recovery of motor or cognitive function in our study. Previous TBI experiments indicated that neurotrophic factors can prevent neuronal death and dysfunction.26 Recovery from TBI may be facilitated by induction of NGF and BDNF: NGF may play a critical role in the regeneration of injured neurons post TBI,27 and increased NGF levels in cerebral spinal fluid after TBI correlate with improved clinical outcome.28 NGF expression may be necessary to promote neuron survival and function post TBI during repair.29 Similarly, the neuroprotective effects of BDNF have also been demonstrated in animal models of ischemia30, 31 and TBI.32 BDNF also has a pivotal role in learning and memory.33 This highlights the potentially neuroprotective effect of elevated NGF and BDNF signaling seen in HA mice and may partially explain the improved motor and cognitive performance of HA mice and the loss of this effect when PD123319 was administered.
A Role of Angiotensin Receptor Type 2 in Heat Acclimation-Induced Neurogenesis
We provide first evidence that HA induces neurogenesis. These results are in accordance with the enhanced progenitor cell proliferation found in the hypothalamus of HA rats.15
Adult neurogenesis occurs in the SVZ and in the subgranular zone of the dentate gyrus.34 These neurogenic niches are enriched with NPCs, and NPC proliferation is altered by different brain pathologies. After injury, the NPC proliferation rate increases and the newborn cells migrate toward the injury site.34 Here we not only demonstrate that HA enhances NPC proliferation but also suggest that AT2 is crucial for this process, as PD123319 blocked NPC proliferation. Furthermore, no alternations in the number of BrdU-positive cells after PD123319 treatment was observed in the NT mice, implying that AT2 is not necessary for spontaneous NPC proliferation after TBI.
The enhanced neurogenesis after HA may be partially due to the increased NGF and BDNF signaling observed in the present study. Both NGF and BDNF mediate differentiation and proliferation,35 and may contribute to enhanced postinjury neurogenesis. Although detecting mature newborn neurons in the dentate gyrus was expected,36 we were surprised to find these neurons in the injured SVZ. Nevertheless, we are not the first to observe this phenomenon, and BrdU+/NeuN+ have been observed in murine SVZ.37 It is reasonable to speculate that migratory properties and guidance of those cells were compromised by TBI because of vessel damage.38 Intriguingly, HA mice had a greater number of newborn cells around the injury area, which was unaffected by PD123319. Nevertheless, when counting newborn mature neurons, we observed impressive neurogenesis in the lesion area of HA mice, which were eliminated when PD123319 was administered, suggesting a role of AT2 in this process. Our results correlate with those of others describing that AT2 may mediate neuronal differentiation and migration.39
Taken together, the remarkable increase in NPC proliferation and differentiation in distinct brain regions, exhibited by the HA mice and mediated by AT2, may underlie the sustained enhanced motor recovery of HA mice.
Hypoxia-Inducible Factor 1α: a Potential Link between Angiotensin Receptor Type 2 and Heat Acclimation-Mediated Neuroprotection
The mechanisms by which AT2 mediates neuroprotection and neurogenesis are yet to be elucidated. This study reconfirms our previous results of HIF-1α involvement in neuroprotection. We recently demonstrated that HA-induced HIF-1α elevation is indispensable for the acquired neuroprotection by HA, and HIF-1α inhibition abolished HA-mediated neuroprotection.1 As in vitro experiments suggest that HIF-1α is a downstream target of AT2,13 we examined the effect of PD123319 on HIF-1α levels. We showed that PD123319 decreases HIF-1α levels after injury, suggesting that, in our model of murine TBI, HIF-1α is at least partly regulated by AT2. However, it is noteworthy to mention that in injured HA mice HIF-1α peaks at earlier time points than the measured (72 hours) and it is possible that PD123319 attenuated HIF-1α levels more prominently at an earlier time point post injury, which was not examined in this study.
AT2 transactivates TrkA4 to induce Akt phosphorylation,40 which may be followed by HIF-1α induction. HA enhances NGF signaling owing to increased TrkA and NGF levels, which coincide with the upregulation of Akt and HIF-1α levels, leading to a protected basal state. HIF-1α regulation by HA may contribute to the enhanced neurogenesis seen in injured HA mice,41 in part via the Wnt/β catenin pathway,42, 43 which is also altered by HA.44 TrkB, BDNF, TrkA, NGF, and HIF-1α upregulation in HA mice enables these mice to inhibit damaging signals and renders these mice susceptible to inhibition of those factors. Indeed, blocking AT2 inhibits these signals in HA mice, which may underlie the observed deterioration in neuroprotection and neurogenesis.
Conclusions and Remarks
In this study we nominate AT2 as an upstream regulator of HA-induced neuroprotection and neurogenesis. We demonstrate that by inhibiting AT2 all beneficial effects of HA that are observed after TBI are lost. In light of our findings, it is possible that activation of AT2 in NT mice will enhance recovery as seen in HA mice after TBI as well as from brain ischemia,4, 6 thus providing a novel therapeutic target for TBI.
The authors declare no conflict of interest.
Footnotes
This study was partially supported by grants from Brettler Foundation at the School of Pharmacy, and by the Dr Miriam and Sheldon G Adelson Medical Research Foundation (AMRF).
References
- Horowitz M. Heat acclimation, epigenetics, and cytoprotection memory. Compr Physiol. 2014;4:199–230. doi: 10.1002/cphy.c130025. [DOI] [PubMed] [Google Scholar]
- Umschwief G, Shein NA, Alexandrovich AG, Trembovler V, Horowitz M, Shohami E. Heat acclimation provides sustained improvement in functional recovery and attenuates apoptosis after traumatic brain injury. J Cereb Blood Flow Metab. 2010;30:616–627. doi: 10.1038/jcbfm.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Rafrafi S, Laforest S, Drolet G. Involvement of central angiotensin receptors in stress adaptation. Neuroscience. 1999;93:877–884. doi: 10.1016/s0306-4522(99)00206-7. [DOI] [PubMed] [Google Scholar]
- Guimond MO, Gallo-Payet N. The angiotensin II type 2 receptor in brain functions: an update. Int J Hypertens. 2012;2012:351758. doi: 10.1155/2012/351758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra JM, Benicky J, Zhou J. Mechanisms of the anti-ischemic effect of angiotensin II AT( 1 ) receptor antagonists in the brain. Cell Mol Neurobiol. 2006;26:1099–1111. doi: 10.1007/s10571-006-9009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy CA, Vinh A, Broughton BR, Sobey CG, Callaway JK, Widdop RE. Angiotensin II type 2 receptor stimulation initiated after stroke causes neuroprotection in conscious rats. Hypertension. 2012;60:1531–1537. doi: 10.1161/HYPERTENSIONAHA.112.199646. [DOI] [PubMed] [Google Scholar]
- Namsolleck P, Boato F, Schwengel K, Paulis L, Matho KS, Geurts N, et al. AT2-receptor stimulation enhances axonal plasticity after spinal cord injury by upregulating BDNF expression. Neurobiol Dis. 2013;51:177–191. doi: 10.1016/j.nbd.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Reinecke K, Lucius R, Reinecke A, Rickert U, Herdegen T, Unger T. Angiotensin II accelerates functional recovery in the rat sciatic nerve in vivo: role of the AT2 receptor and the transcription factor NF-kappaB. FASEB J. 2003;17:2094–2096. doi: 10.1096/fj.02-1193fje. [DOI] [PubMed] [Google Scholar]
- Day RT, Cavaglieri RdeC, Tabatabaimir H, Mantravadi V, Lee MJ, Barnes JL, et al. Acute hyperglycemia rapidly stimulates VEGF mRNA translation in the kidney. Role of angiotensin type 2 receptor (AT2) Cell Signal. 2010;22:1849–1857. doi: 10.1016/j.cellsig.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J, Yang L, Buch S, Gao L. Angiotensin II increased neuronal stem cell proliferation: role of AT2R. PLoS One. 2013;8:e63488. doi: 10.1371/journal.pone.0063488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffert S, Stoll M, Steckelings UM, Bottari SP, Unger T. The angiotensin II AT2 receptor inhibits proliferation and promotes differentiation in PC12W cells. Mol Cell Endocrinol. 1996;122:59–67. doi: 10.1016/0303-7207(96)03873-7. [DOI] [PubMed] [Google Scholar]
- Laflamme L, Gasparo M, Gallo JM, Payet MD, Gallo-Payet N. Angiotensin II induction of neurite outgrowth by AT2 receptors in NG108-15 cells. Effect counteracted by the AT1 receptors. J Biol Chem. 1996;271:22729–22735. doi: 10.1074/jbc.271.37.22729. [DOI] [PubMed] [Google Scholar]
- Wolf G, Schroeder R, Stahl RA. Angiotensin II induces hypoxia-inducible factor-1 alpha in PC 12 cells through a posttranscriptional mechanism: role of AT2 receptors. Am J Nephrol. 2004;24:415–421. doi: 10.1159/000080086. [DOI] [PubMed] [Google Scholar]
- Schwimmer H, Gerstberger R, Horowitz M. Heat acclimation affects the neuromodulatory role of AngII and nitric oxide during combined heat and hypohydration stress. Brain Res Mol Brain Res. 2004;130:95–108. doi: 10.1016/j.molbrainres.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K, Katakura M, Hara T, Li G, Hashimoto M, Shido O. Proliferation of neuronal progenitor cells and neuronal differentiation in the hypothalamus are enhanced in heat-acclimated rats. Pflugers Arch. 2009;458:661–673. doi: 10.1007/s00424-009-0654-2. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Stahel PF, Beauchamp KM, Morgan SJ, Smith WR, Shohami E. Mouse closed head injury model induced by a weight-drop device. Nat Protoc. 2009;4:1328–1337. doi: 10.1038/nprot.2009.148. [DOI] [PubMed] [Google Scholar]
- Macova M, Pavel J, Saavedra JM. A peripherally administered, centrally acting angiotensin II AT2 antagonist selectively increases brain AT1 receptors and decreases brain tyrosine hydroxylase transcription, pituitary vasopressin and ACTH. Brain Res. 2009;1250:130–140. doi: 10.1016/j.brainres.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse M, Tanabe A, Sato A, Takagi S, Tsuchiya K, Imaki T, et al. Aldosterone breakthrough during angiotensin II receptor antagonist therapy in stroke-prone spontaneously hypertensive rats. Hypertension. 2002;40:28–33. doi: 10.1161/01.hyp.0000022606.52221.2f. [DOI] [PubMed] [Google Scholar]
- Tsenter J, Beni-Adani L, Assaf Y, Alexandrovich AG, Trembovler V, Shohami E. Dynamic changes in the recovery after traumatic brain injury in mice: effect of injury severity on T2-weighted MRI abnormalities, and motor and cognitive functions. J Neurotrauma. 2008;25:324–333. doi: 10.1089/neu.2007.0452. [DOI] [PubMed] [Google Scholar]
- Thau-Zuchman O, Shohami E, Alexandrovich AG, Leker RR. Vascular endothelial growth factor increases neurogenesis after traumatic brain injury. J Cereb Blood Flow Metab. 2010;30:1008–1016. doi: 10.1038/jcbfm.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umschweif G, Alexandrovich AG, Trembovler V, Horowitz M, Shohami E. Hypoxia-inducible factor 1 is essential for spontaneous recovery from traumatic brain injury and is a key mediator of heat acclimation induced neuroprotection. J Cereb Blood Flow Metab. 2013;33:524–531. doi: 10.1038/jcbfm.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes DM, LaPlaca MC, Cargill RS. Susceptibility of hippocampal neurons to mechanically induced injury. Exp Neurol. 2003;184:420–427. doi: 10.1016/s0014-4886(03)00254-1. [DOI] [PubMed] [Google Scholar]
- Mogi M, Horiuchi M. Effect of angiotensin II type 2 receptor on stroke, cognitive impairment and neurodegenerative diseases. Geriatr Gerontol Int. 2013;13:13–18. doi: 10.1111/j.1447-0594.2012.00900.x. [DOI] [PubMed] [Google Scholar]
- Tota S, Hanif K, Kamat PK, Najmi AK, Nath C. Role of central angiotensin receptors in scopolamine-induced impairment in memory, cerebral blood flow, and cholinergic function. Psychopharmacology (Berl) 2012;222:185–202. doi: 10.1007/s00213-012-2639-7. [DOI] [PubMed] [Google Scholar]
- Hafko R, Villapol S, Nostramo R, Symes A, Sabban EL, Inagami T, et al. Commercially available angiotensin II At2 receptor antibodies are nonspecific. PLoS One. 2013;8:e69234. doi: 10.1371/journal.pone.0069234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Flinn P, Bao J, Venya R, Hayes RL. Nerve growth factor attenuates cholinergic deficits following traumatic brain injury in rats. Exp Neurol. 1997;146:479–490. doi: 10.1006/exnr.1997.6557. [DOI] [PubMed] [Google Scholar]
- Varon S, Conner JM. Nerve growth factor in CNS repair. J Neurotrauma. 1994;11:473–486. doi: 10.1089/neu.1994.11.473. [DOI] [PubMed] [Google Scholar]
- Chiaretti A, Barone G, Riccardi R, Antonelli A, Pezzotti P, Genovese O, et al. NGF, DCX, and NSE upregulation correlates with severity and outcome of head trauma in children. Neurology. 2009;72:609–616. doi: 10.1212/01.wnl.0000342462.51073.06. [DOI] [PubMed] [Google Scholar]
- Lu J, Frerich JM, Turtzo LC, Li S, Chiang J, Yang C, et al. Histone deacetylase inhibitors are neuroprotective and preserve NGF-mediated cell survival following traumatic brain injury. Proc Natl Acad Sci USA. 2013;110:10747–10752. doi: 10.1073/pnas.1308950110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Krupinski J, Goutan E, Martí E, Ambrosio S, Arenas E. Brain-derived neurotrophic factor reduces cortical cell death by ischemia after middle cerebral artery occlusion in the rat. Acta Neuropathol. 2001;101:229–238. doi: 10.1007/s004010000268. [DOI] [PubMed] [Google Scholar]
- Müller HD, Hanumanthiah KM, Diederich K, Schwab S, Schäbitz WR, Sommer C. Brain-derived neurotrophic factor but not forced arm use improves long-term outcome after photothrombotic stroke and transiently upregulates binding densities of excitatory glutamate receptors in the rat brain. Stroke. 2008;39:1012–1021. doi: 10.1161/STROKEAHA.107.495069. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Vasterling JJ, Vedak PC. Brain-derived neurotrophic factor in traumatic brain injury, post-traumatic stress disorder, and their comorbid conditions: role in pathogenesis and treatment. Behav Pharmacol. 2010;21:427–437. doi: 10.1097/FBP.0b013e32833d8bc9. [DOI] [PubMed] [Google Scholar]
- Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Hagg T. From neurotransmitters to neurotrophic factors to neurogenesis. Neuroscientist. 2009;15:20–27. doi: 10.1177/1073858408324789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C, et al. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci USA. 2001;98:5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan I, Barhum Y, Melamed E, Offen D. Mesenchymal stem cells stimulate endogenous neurogenesis in the subventricular zone of adult mice. Stem Cell Rev. 2011;7:404–412. doi: 10.1007/s12015-010-9190-x. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Guimond MO, Gallo-Payet N. How does angiotensin AT(2) receptor activation help neuronal differentiation and improve neuronal pathological situations. Front Endocrinol (Lausanne) 2012;3:164. doi: 10.3389/fendo.2012.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Zhang P, Li W, Li L, Wang N, Li X, Gao M, et al. Treatment with edaravone attenuates ischemic brain injury and inhibits neurogenesis in the subventricular zone of adult rats after focal cerebral ischemia and reperfusion injury. Neuroscience. 2012;201:297–306. doi: 10.1016/j.neuroscience.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9:210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- Cunningham LA, Candelario K, Li L. Roles for HIF-1α in neural stem cell function and the regenerative response to stroke. Behav Brain Res. 2012;227:410–417. doi: 10.1016/j.bbr.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umschweif G, Alexandrovich AG, Trembovler V, Horowitz M, Shohami E. The role and dynamics of β-catenin in precondition induced neuroprotection after traumatic brain injury. PLoS One. 2013;8:e76129. doi: 10.1371/journal.pone.0076129. [DOI] [PMC free article] [PubMed] [Google Scholar]