Abstract
A mild insult to the brain can sometimes trigger secondary brain injury, causing severe postconcussion syndrome, but the underlying mechanism is ill understood. We show here that secondary brain injury occurs consistently in mice lacking immediate early responsive gene X-1 (IEX-1), after a gentle impact to the head, which closely simulates mild traumatic brain injury in humans. The pathologic lesion was characterized by extensive cell death, widespread leukocyte infiltrates, and severe tissue loss. On the contrary, a similar insult did not induce any secondary injury in wild-type mice. Strikingly, noninvasive exposure of the injured head to a low-level laser at 4 hours after injury almost completely prevented the secondary brain injury in IEX-1 knockout mice. The low-level laser therapy (LLLT) suppressed proinflammatory cytokine expression like interleukin (IL)-1β and IL-6 but upregulated TNF-α. Moreover, although lack of IEX-1 compromised ATP synthesis, LLLT elevated its production in injured brain. The protective effect of LLLT may be ascribed to enhanced ATP production and selective modulation of proinflammatory mediators. This new closed head injury model provides an excellent tool to investigate the pathogenesis of secondary brain injury as well as the mechanism underlying the beneficial effect of LLLT.
Keywords: IEX-1, inadequate mitochondrial function, LLLT, mTBI
Introduction
Traumatic brain injury (TBI) represents a serious public health problem that affects millions of people in the United States every year.1 Although most of these brain injuries are clinically defined as mild TBI (mTBI) and are fully recovered within a year after concussion, approximately 15% of patients with mTBI experience short-term or prolonged neurologic dysfunctions known as postconcussion syndrome, manifested by persistent physical, behavioral, cognitive, and emotional difficulties.2 It is generally believed that both the primary events and the secondary alterations contribute to the resulting persistent neurologic dysfunction. The primary injury comprises direct and mechanical damage to brain tissue, including neurons, axons, glia, blood vessels, and skull, whereas the secondary injury encompasses diverse and reversible molecular, cellular, metabolic, and structural changes at and around the primary injured site.3 The primary injury of mTBI is usually mild and is healed naturally in most of the cases and thus neurologic impairments occurring after mTBI in a proportion of the victims are most likely attributed to secondary brain injury.
Secondary brain injury usually takes days to evolve from the primary injury, which offers a golden window of a time for intervention or prevention against postconcussion syndrome development. Indeed, a number of therapeutic modalities have been shown capable of preventing secondary brain injury, among which transcranial low-level laser therapy (LLLT), either a single dose or multiple doses, significantly improve neurobehavioral performance after moderate and severe TBI in mice,4 ischemic strokes in rabbits,5 neurodegenerative disease,6 and spinal cord injuries.7 Low-level laser therapy refers to the use of red or near-infrared lights with a wavelength at 600 to 1,100 nm and an output power of 1 to 500 mW, either a continuous or pulsed wave at a relatively low energy density of 0.04 to 50 J/cm2. The reaction of light energy to modulate biologic function is nonthermal, also called cold or soft light therapy. The underlying mechanisms are not fully understood, one of which may rely on the ability of LLLT to directly activate the mitochondrial cytochrome c oxidase that is one of the primary photoacceptors operating in LLLT.8, 9 In accordance to this, we have shown that 810-nm laser can significantly enhance adenosine-5'-triphosphate (ATP) generation in cell culture.10 Exposure of the injured head to the laser greatly improved the neurologic performance of the injured mice as compared with untreated mice.11, 12
It is generally believed that the pathogenesis of secondary brain injury involves a complex cellular and molecular cascade in association with inadequate mitochondrial function, blood–brain barrier disruption, neuroinflammation, oxidative stress, cell death, and so on.3 Owing to the complicated pathogenesis, no single animal model can recapitulate all aspects of the pathogenesis observed in humans so far. The immediate early responsive gene X-1 (IEX-1) has a pivotal role in regulation of mitochondrial F1F0-ATPase activity, in protection against apoptosis, and in resolution of inflammation.13, 14, 15 Null mutation of IEX-1 enhanced apoptosis,16 reduced ATP synthase activity,17 and prolonged inflammatory responses in a tissue-dependent manner in mice.15, 18 Furthermore, IEX-1 is upregulated by NF-κB and in turn inhibits NF-κB activation as a negative feedback mechanism contributing to inflammation resolution.19, 20
To gain insights into a possible role for inadequate mitochondrial function in the initiation and progression of secondary brain injury, effects of IEX-1 deficiency on the pathogenesis of secondary brain injury were investigated. We found that IEX-1 knockout (KO) mice were more susceptible to secondary brain injury than wild-type (WT) littermates after mTBI, concurrent with extensive cell death, prolonged neuroinflammation, and severe brain tissue loss at and around the impact site. Strikingly, a single dose of LLLT at 4 hours after brain injury effectively prevented the secondary brain injury induced by IEX-1 deficiency. The finding underscores mitochondria to be a key player in both the pathogenesis of secondary brain injury and the effectiveness of LLLT.
Materials and Methods
Animals
Wild-type (WT) and IEX-1 knockout (KO) mice at 8 weeks of age on 129Sv/C57BL/6 background were generated in our laboratory14 and used in this study unless otherwise indicated. Mice were maintained in a 12-hour light/dark cycle. The animal protocol was approved by the subcommittee on Research Animal Care of the Massachusetts General Hospital. All animal experiments were performed according to the National Institutes of Health guidelines for the Care and Use of Laboratory Animals.
Mild Traumatic Brain Injury Procedures
Eight-week-old mice of 20 to 22 g in weight were subjected to mTBI by a standard controlled impact on the left lateral area with an intact skull and scalp. In brief, mice were anesthetized with isoflurane and placed on the mobile plate with head hair removed. A flat-face tip with a 2-mm diameter in size, of the pneumatic impact device (AMS 201, AmScien Instruments, Richmond, VA, USA) was positioned on the left hemisphere center, lowered gradually down to touch the scalp, and recorded as zero depth (sham control). The punch depth was then set at 2.0 mm using a screw-mounted adjustment. A 4.9±0.2 m/s velocity and an 80 ms contact time were specified by setting 150 psi for high pressure and 30 psi for low pressure. To transiently induce a low degree of systemic inflammation,21 WT mice were given lipopolysaccharide (LPS) intraperitoneally at a dose of 4 mg/kg body weight in 1 hour and 7 days after brain injury. The mice were returned to cages with postoperative care, after recovery from anesthesia.
Low-Level Laser Therapy
Low-level laser therapy was performed at 4 hours after mTBI using an infrared diode laser of 810 nm (Acculaser, PhotoThera, Carlsbad, CA, USA). The mice were positioned on a plate and covered by aluminum sheet with a hole of 1 cm diameter to expose the contusion site on the hairless head. The pulse frequency of laser was 10 Hz, a pulse duration 50 ms, an average irradiance 150 mW/cm2, a total exposure time of 4 minutes, and an energy density of 36 J/cm2. Because of the barrier of the skull and scalp over the head, the transmitted laser energy to the cortex was ∼5.9±0.98% of total input irradiance,11 and the fluence that reached the cortex tissue was ∼1.8 to 2.5 J/cm2.
Neurologic Severity Score
The neurologic severity score (NSS) was assessed as described.22 Ten individual clinical parameters were used to evaluate the motor ability, physiologic behavior, and alertness of the mice as shown in Table 1 in which 1 point was assigned for the inability to perform the tasks, and no point for successful performance of the task. The NSS was measured at 1 hour and 1, 3, 5, 7, 14, and 28 days after mTBI by blind test.
Table 1. Neurologic severity score (NSS).
| Task description | Score |
|---|---|
| Failure to exit a 30-cm-diameter circle within 3 min | 1 |
| Loss of interest to environment (seeking behavior) | 1 |
| Monoparesis/hemiparesis | 1 |
| Failure to walk straight | 1 |
| Loss of startle reflex | 1 |
| Inability to balance on a 7-mm width beam | 1 |
| Inability to walk on a 3-cm width beam | 1 |
| Inability to walk on a 2-cm width beam | 1 |
| Inability to walk on a 1-cm width beam | 1 |
| Inability to balance on a 5-mm round stick | 1 |
| Maximum score | 10 |
Histologic Examination
Mice were anesthetized and fixed by cardiac perfusion with cold phosphate-buffered saline, followed by 10% formalin. Brains were carefully removed, postfixed overnight in 10% formalin, and embedded in paraffin. A cross-sectional tissue block of 4-mm thickness encompassing the whole injured region of the brain was taken from each sample, and three sections were cut at 5-μm each from the top, middle, and bottom of the block. The sections were stained with hematoxylin and eosin and analyzed by Nanozoomer Slide Scanner (Olympus America, Center Valley, PA, USA) and Image J software as previously described.4, 12 Cavity lesion was measured by average percentages of the lesion area relatively to the whole brain area of the three sections prepared from each mouse with six mice in each group. Ratios of morphologically normal cells in cortex, and necrotic cells in hippocampus over a total number of cells in the same field were attained by counting a total of 200 cells in three randomly selective fields in each section with three sections per mouse and six mice per group.
Real-Time Quantitative Reverse Transcription–Polymerase Chain Reaction
Total RNA isolated from mouse cortex beneath the impact site or perilesional area was used to determine the short-term and long-term inflammatory responses at indicated times after treatment. RNA (1 μg) was reverse transcribed with a high capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA, USA). Real-time quantitative PCR (qRT–PCR) was performed on Roche Lightcycler 480 with a SYBR Green I Master kit (Roche Diagnostics, Indianapolis, IN, USA). The PCR program was preincubation at 95 °C, 5 minutes, followed by 45 cycles of 95 °C, 10 seconds, 60 °C, 10 seconds, and 72 °C, 10 seconds. Relative quantification of each target gene was normalized to endogenous β-actin, and calculated using comparative Ct method (ΔΔCt method).23 The primer sequences used in this study are as follows: interleukin (IL)-1β, 5′-GAAGAGCCCATCCTCTGTGA-3′ (forward) and 5′- TTCATCTCGGAGCCTGTAGTG-3′ (reverse); IL-6, 5′-ACAAAGCCAGAGTCCTTCAGG-3′ (forward) and 5′-CATTGGAAATTGGGGTAGGA-3′ (reverse); CCL2, 5′-GGCTCAGCCAGATGCAGTTAA-3′ (forward) and 5′-CCTACTCATTGGGATCATCTTGT-3′ (reverse); CXCL10, 5′- GCCGTCATTTTCTGCCTCA-3′ (forward) and 5′-CGTCCTTGCGAGAGGGATC-3′ (reverse); TNF-α, 5′-GTCTACTGAACTTCGGGGTGAT-3′ (forward) and 5′-ATGATCTGAGTGTGAGGGTCTG-3′ (reverse); β-actin, 5′-CGAGGCCCAGAGCAAGAGAG-3′ (forward) and 5′-CGGTTGGCCTTAGGGTTCAG-3′ (reverse).
Measurement of Adenosine-5'-Triphosphate
Total ATP levels in the cortex of WT and KO mice were measured using an ATP assay kit (Promega, Madison, WI, USA). Briefly, at indicated time points after mTBI and LLLT, the cortical tissues were carefully isolated and homogenized in 10% (weight/volume) extraction buffer (Pierce, Rockford, IL, USA). The resultant homogenate (10 μL) was added into 96-well luminescence plate containing 100 μL of assay reagent. The luminescence signal was measured with a microplate reader (Molecular Devices, Sunnyvale, CA, USA). Relative ATP levels were normalized to the total protein concentration measured by a Bio-Rad protein assay kit.
Statistical Analysis
The data were expressed as mean±standard error of measurement (s.e.m.) and statistical significance was assessed with two-way analysis of variance. A value of P<0.05 was considered statistically significant. The relationship between NSS and ATP level in WT and KO injured mice with and without LLLT was tested by regression and correlation analysis, and coefficient of determination (R2) was calculated using Graphpad Prism 4.0 (GraphPad Software, La Jolla, CA, USA).
Results
Immediate Early Responsive Gene X-1 Knockout Mice Fail to Fully Recover from Mild Traumatic Brain Injury
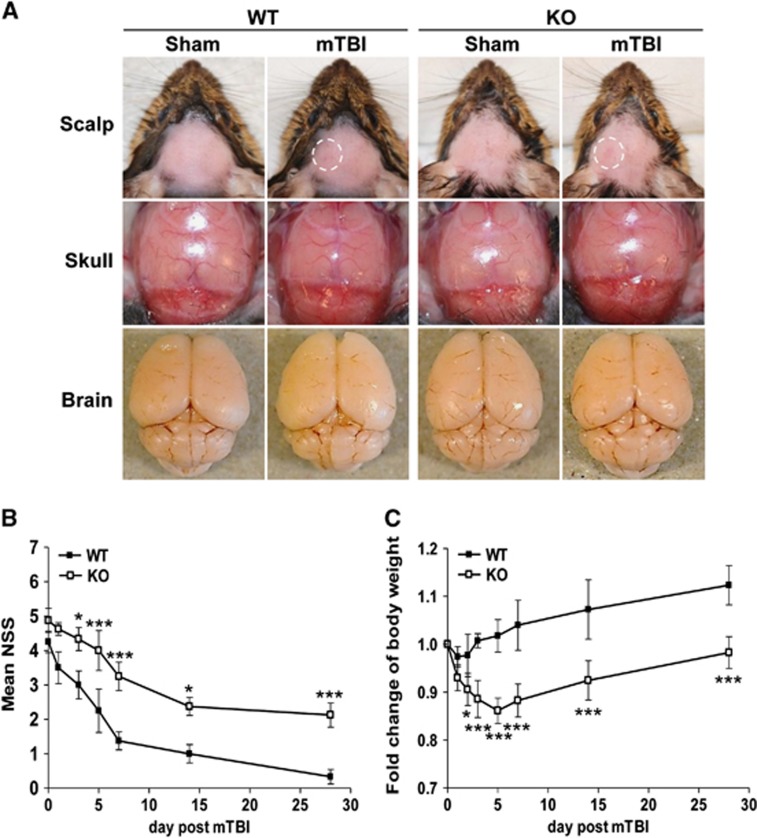
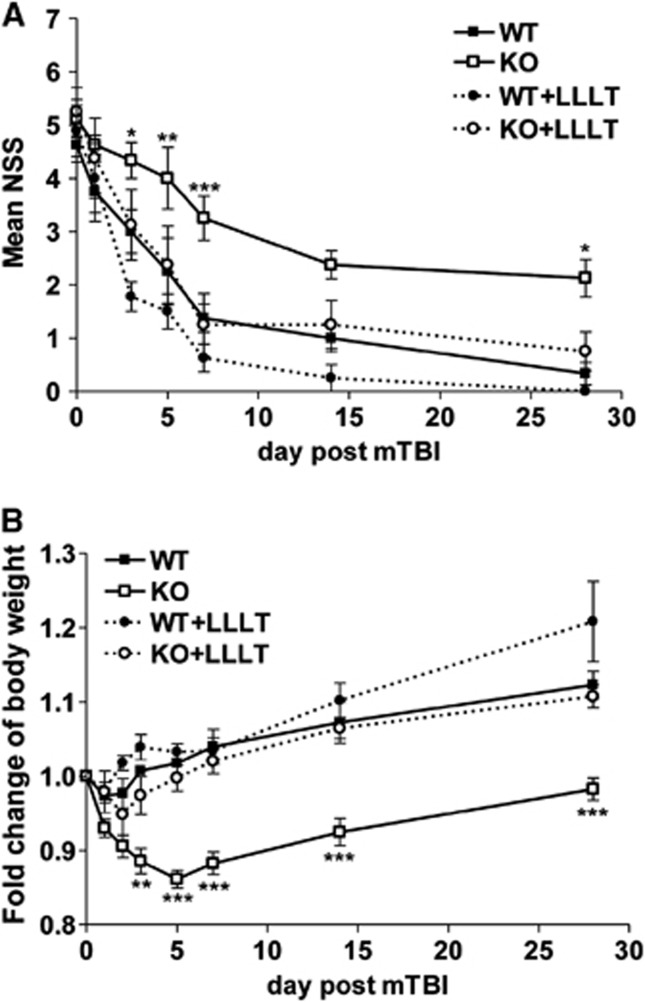
To mimic mTBI in humans, we set up a closed head impact model in which hairless mouse head was directly hit by a pneumatic impact device at a 2.0 mm punch depth. Severity of initial injury was assessed by NSS at 1 hour after TBI and mice with scores of 4 to 6 were enrolled in the study. The impact force on this closed head brought about mild erythema on the impact site of the scalp (Figure 1A, a white dashed cycle), but did not produce any structural damage to the skull, brain, or blood–brain barrier visibly when compared with sham controls (Figure 1A). There was no evidence of acute hemorrhage either on the cortical surface or in the cortical matter underneath (Figure 1A), resembling mild TBI described in humans. Assessment of neurologic behavior of these mice revealed that NSS diminished significantly from 4.3±0.9 at 1 hour after mTBI, to 1.0±0.8 on day 14, and continuously to 0.3±0.5 on day 28 in WT mice (Figure 1B), demonstrating a complete recovery of the neurologic behavior in most of the mice. In contrast, although IEX-1 KO mice had similar NSS at 1 hour after mTBI (4.9±1.0), their recovery was largely delayed, and their NSS remained significantly high at the end of the experimental period of 28 days (2.1±1.0 versus 0.3±0.5, P<0.001; Figure 1B).
Figure 1.
A failure of IEX-1 KO mice to fully recover from mTBI. (A) Representative photos of sham and mildly injured brains of WT and KO mice. The photos were taken at 6 hours after the mild injury showing erythema, marked in a white dash line circle, on the impact scalp (upper), with normal skull (middle) and brain (lower) underneath. (B) A time course of NSS after mTBI. NSS was assessed in WT and KO mice at 1 hour and indicated days after mTBI and expressed as means±s.e.m. (C) Body weight changes over time in injured mice. A health status of the animals was monitored by weighing the animals at the indicated days after mTBI and expressed as means±s.e.m. of body weight changes relative to a preinjury level that sets arbitrarily as 1. *P<0.05 and ***P<0.001 in the presence or absence of IEX-1 (n=9 mice per group). IEX-1, immediate early responsive gene X-1; KO, knockout; mTBI, mild traumatic brain injury; NSS, neurologic severity score; WT, wild-type.
Moreover, body weight of WT mice decreased only slightly and briefly in the first day or two after injury (Figure 1C). After the brief weight drop, the animals started gaining weight on day 3 and reached preinjury levels on day 4, followed by a steady body weight gain above the preinjury level thereafter. In contrast, KO mice displayed a precipitous loss of body weight during the first 5 consecutive days after the injury (Figure 1C). Once having reached a minimum body weight, the KO mice began to gain body weight on day 7, 4 days later than WT mice, and gradually increased their body weight from then on, but never reached preinjury levels at the end of the experiment (Figure 1C).
Histologic Alteration in Immediate Early Responsive Gene X-1 Knockout Mice after Mild Traumatic Brain Injury
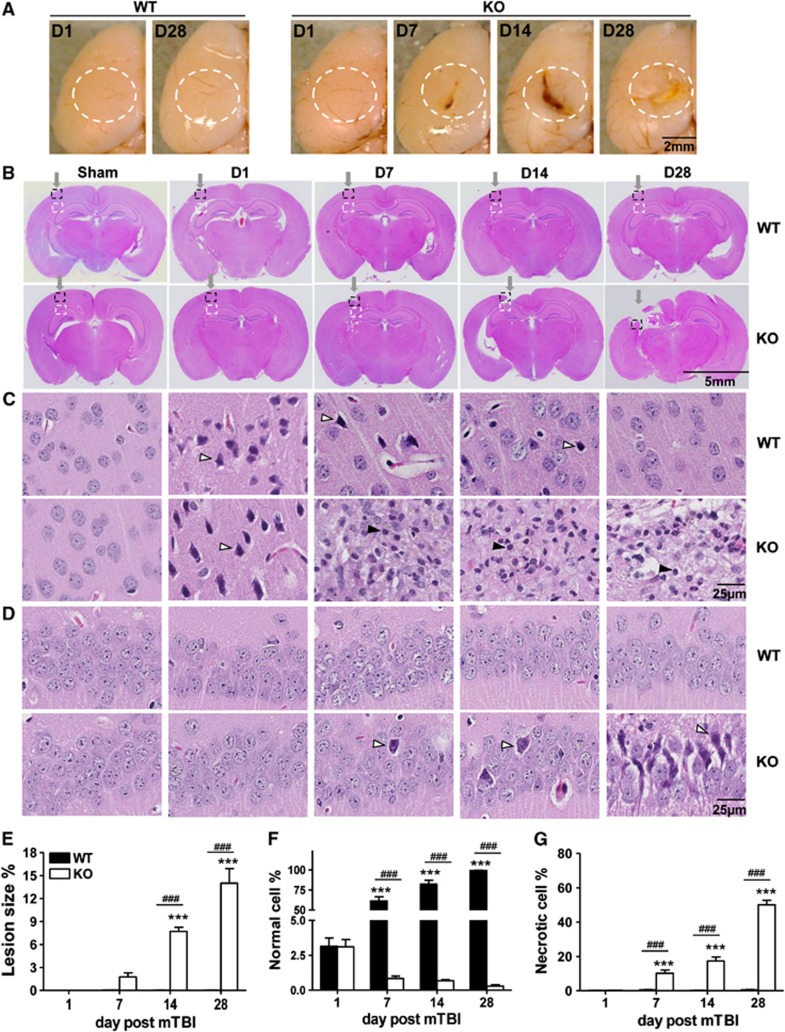
Concurrent with a poor body weight recovery from the injury, the gross morphology of the brain started to show an overt lesion at the affected site on day 7, which was exacerbated over time in KO mice (Figure 2A). The lesion expanded and deepened on day 14, forming a big scar on day 28 (Figure 2A). The discernible aggravation of the lesion in KO mice was in sharp contrast to a normal gross appearance of the WT brain during the entire experimental period (Figure 2A). Moreover, there was no evidence of any lesion at the impact site over the course of the study when the mouse brain sections were histologically examined at a low magnification (Figure 2B, upper panel). In contrast, KO mice demonstrated a steady increase in the lesion size, which was hardly seen on day 1, but clearly evidenced on day 7, greatly expanded on day 14, and aggressively spread from cortex into hippocampus, resulting in a profound loss of brain tissue on day 28 (Figure 2B, lower panel). Quantitatively, average lesion areas relative to the whole brain section were increased to 1.8±1.2% on day 7, 7.7±1.3% on day 14, or 14.0±4.7% on day 28, from 0% on day 1 (Figure 2E).
Figure 2.
Secondary brain injury occurs in KO mice but not in WT mice after gentle insult. (A) Gross morphology of the impact, left hemisphere of the brain. Note, a normal appearance on day 1 (D1) and day 28 (D28) of WT brain after trauma, but a lesion deteriorating over time in KO brain, marked by white line dash circles. (B–D) Histologic examination of coronal sections stained with hematoxylin and eosin from WT and KO brains at indicated days after mTBI. The impact site in sections panel B is pointed by an arrow; the injured neocortex is highlighted in a dash black line square and enlarged in panel C; and the hippocampus underneath is outlined by a dashed white line square and magnified in panel D. Unfilled arrows indicate one of the necrotic cells in each field and filled arrows indicate one of the infiltrated leukocytes. All data in the figures were representative of six mice per group. Percentages of a lesion size over the whole brain section in panel B were determined by Image J and expressed as means±s.e.m. in panel E. Percentages of normal cells in panel C or necrotic cells in panel D relative to a total number of cells counted in the same fields were determined as detailed in Materials and Methods and shown as means±s.e.m. in panel F and panel G, respectively. ***P<0.001 compared with Day 1 and ###P<0.001 in the presence or absence of IEX-1. IEX-1, immediate early responsive gene X-1; KO, knockout; mTBI, mild traumatic brain injury; WT, wild-type.
On a high magnification, healthy cell nuclei were relatively large consisting of several discernible nucleoli in the nucleoplasm in sham-treated cerebral neocortex, regardless of whether IEX-1 was expressed (Figure 2C, first panel). Upon mTBI, abundant necrotic cells (unfilled arrows) with pyknotic and condensed nuclei were present uniformly beneath the contusion site at 1 day after injury and indistinguishable in the presence or absence of IEX-1 (Figure 2C, second panel). The discernible nucleoli were no longer seen in these necrotic cells, probably owing to nuclear condensation. The injured cortical cells in WT mice appeared to mostly recover from the damage on day 7, evidenced by a drastic decrease in the number of necrotic cells, concomitant with a robust increase in the number of the cells morphologically resembling those in the sham brain (Figure 2C, third upper panel). The percentage of morphologically normal cells arose from 3.1±1.5% on day 1, to 61.2±13.0% on day 7 or 82.3±11.5% on day 14, and reached 99.4±0.4% on day 28 after injury at the impact site in WT mice (Figure 2F), clearly indicating vigorous regeneration of brain cells after the injury. In marked contrast to the robust recovery in WT mice, there was little evidence of cell morphologic recovery in injured IEX-1 KO brain (Figure 2C, third to fifth lower panels). On the contrary, percentages of normal cells at the impact area diminished steadily in the KO mice over the course of the investigation (Figure 2F). Notably, neuroinflammation, manifested by infiltration of a large number of leukocytes (filled arrows) was initiated and aggravated over the 7-day period at the impact site. On days 14 and 28, the cortical tissue at the direct impact area was lost either partially or completely (Figure 2, fourth to fifth panels), and perilesional tissues were thus examined in hematoxylin and eosin staining, once again showing leukocyte infiltrates. The data suggest aggressive spreading of the lesion into the surrounding tissue.
Beneath the impact site, the necrotic cell death started to show in the hippocampus 7 days after trauma, and exacerbated over time, leading to severe hippocampal lesions on day 28 in KO mice (Figure 2D, fifth lower panel). As shown in Figure 2G, necrotic hippocampal cells increased from 0.3±0.1% on day 1, to 10.2±4.8% on day 7, further to 17.4±5.7% on day 14, and reached as high as 50.1±6.3% on day 28 after mTBI. Similar to initiation of neuroinflammation in the cortical area, we also observed necrotic cell death in the hippocampus before leukocyte infiltration, hinting that some diffuse inflammatory mediators may be toxic, causing cell death in the surrounding tissue. There was no cell death or leukocyte infiltrate in the hippocampal region in WT mice, confirming that injury-induced cellular responses were well confined in the cortical area in the animals (Figure 2D, upper panel). The result strongly argues an importance of IEX-1 in protection against secondary brain injury, either through its ability to protect cell death, resolve inflammation, or both.
Inflammatory Responses after Mild Traumatic Brain Injury
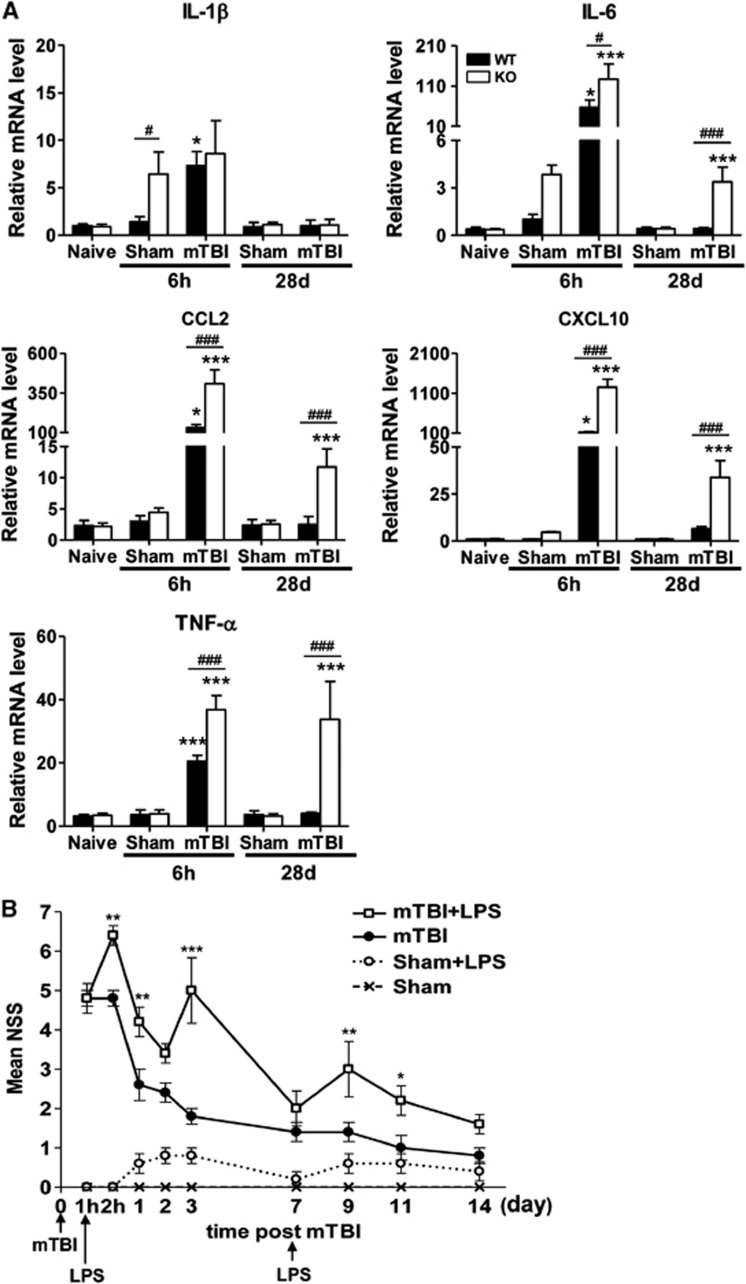
To verify a high level of inflammatory responses in the absence of IEX-1, the levels of inflammatory mediators were assayed by qRT–PCR at the impact site at varying times after mTBI. mTBI resulted in upregulation of IL-1β, IL-6, CCL2, CXCL10, and TNF-α in both WT and KO cortex at 6 hours after mTBI (Figure 3A), similar to previous investigations showing that neuroinflammation could be acutely provoked by TBI.24, 25 In comparison with WT mice, KO mice expressed significantly higher levels of IL-6, CCL2, CXCL10, and TNF-α, corroborating more vigorous inflammatory responses in KO brain described in Figure 2C. All these cytokines and chemokines dwindled down to sham levels in WT mice in 28 days of the injury. However, four out of the five inflammatory mediators tested remained significantly elevated in injured KO cortex relatively to injured WT cortex or sham KO cortex in 28 days of the injury, despite the fact that these proinflammatory mediators were decreased considerably when compared with the levels attained at 6 hours after injury (Figure 3A). It was noticed that IL-1β, and to a lesser degree, IL-6 were higher in IEX-1 KO mice than WT mice 6 hours after sham-treatment (Figure 3A). The sham-treatment, including anesthesia, hair removal, and sham impact by setting the punch depth of device tip at 0 mm might briefly stress the mice, which might be well tolerated by WT mice but not IEX-1 KO mice. IEX-1 is a stress-inducible gene required for dealing with a variety of stress and its loss can compromise the stress-management capacity of the mice.18, 20 In support, no differences in transcription of these cytokines were found in naive WT and KO mice (Figure 3A, naive columns). In spite of increasing transcription of inflammatory cytokines, there was no evidence of leukocyte infiltration in sham brains (Figure 2C), suggesting that sham-induced inflammation is transient in the mice.
Figure 3.
Exaggerated inflammatory responses in injured KO brain. (A) Real-time quantitative reverse transcription–polymerase chain reaction (qRT–PCR) analysis of proinflammatory mediators at indicated times after mTBI. Total RNA was isolated from the impact cortex in 6 hours (6 h) and/or perilesional area in 28 days (28 d) after injury. IL-1β, IL-6, CCL2, CXCL10, and TNF-α mRNA levels were analyzed by qRT–PCR and normalized to β-actin. Results are shown as mean±s.e.m. (n=5 mice per group). *P<0.05 and ***P<0.001 between injured brains and sham controls; and #P<0.05 and ###P<0.001 in the presence or absence of IEX-1. (B) LPS-mediated detrimental effects on neurobehavior after mTBI. LPS was intraperitoneally administered into WT mice at 1 hour and 7 days after mTBI. NSS was assessed at indicated time points. Results are presented as means±s.e.m. (n=5 mice per group). *P<0.05, **P<0.01 and ***P<0.001 in the presence or absence of LPS. IEX-1, immediate early responsive gene X-1; IL, interleukin; KO, knockout; LPS, lipopolysaccharide; mTBI, mild traumatic brain injury; NSS, neurologic severity score; TNF-α, tumor necrosis factor-alpha; WT, wild-type.
The finding that IL-6, CCL2, CXCL10, and TNF-α were not restored to a WT level in 28 days of mTBI in KO mice suggested impaired resolution of inflammatory responses in the mice.15, 18 To corroborate that the aggravated neurologic pathogenesis was ascribed, at least in part, to elevated inflammatory responses induced by the absence of IEX-1, LPS at a dose of 4 mg/kg body weight, which is capable of inducing a low degree of inflammatory responses,21 was administered into WT mice 1 hour after mTBI. LPS deteriorated the neurobehavioral performance, and the deterioration was apparent as early as 1 hour after LPS injection, increasing NSS from 4.8±0.4 to 6.4±0.5 (Figure 3B, P<0.01). The deteriorated effect lasted for more than 3 days in injured WT mice (5.0±1.9 versus 1.8±0.5 on day 3, P<0.001, Figure 3B), and subsided on day 7, but could be re-provoked by another LPS injection (Figure 3B). LPS alone did not interfere with the neurologic function significantly in control mice under similar conditions (Figure 3B). These data indicate that neuroinflammation is aggravated by null mutation of IEX-1, resulting in leukocyte infiltrates that can contribute significantly to initiation and progression of secondary brain injury.
Low-Level Laser Therapy Suppresses Inflammatory Responses in Immediate Early Responsive Gene X-1 Knockout Mice
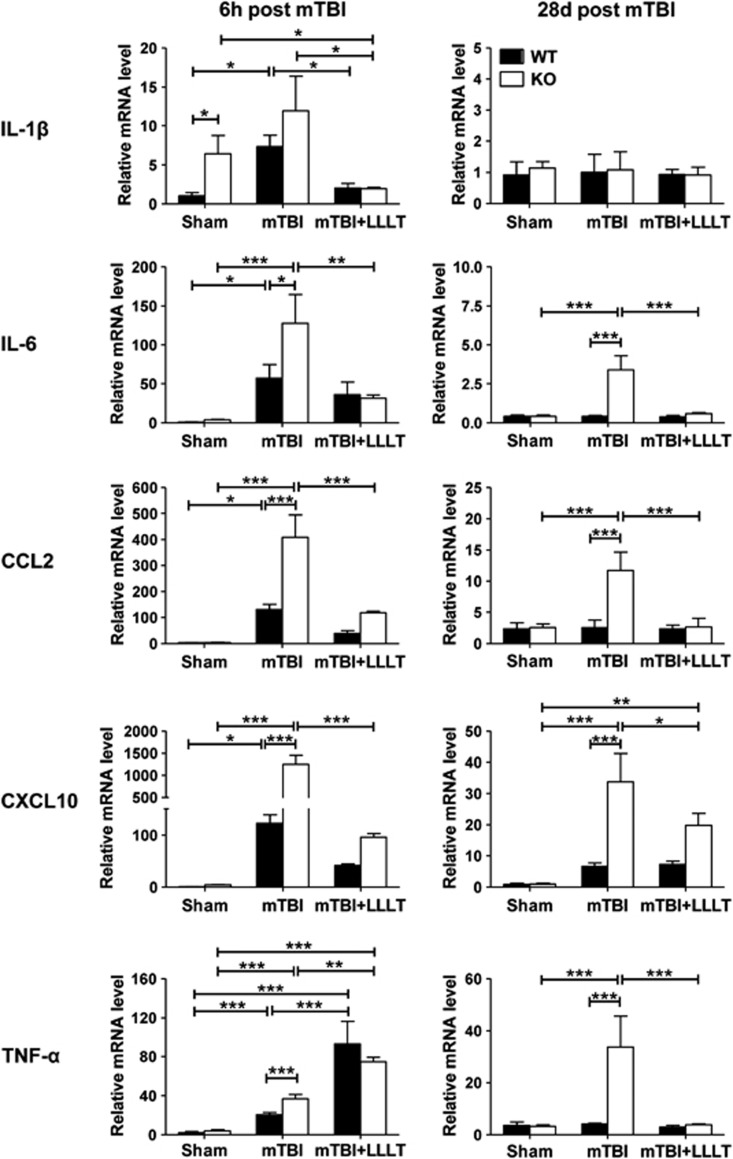
Several studies have shown antiinflammatory and therapeutic effects of low-level laser on neurodegenerative diseases in some animal models.26 We thus addressed whether LLLT could prevent the neuroinflammation triggered by IEX-1 deficiency and protect the mice from secondary brain injury. A single dose of low-level laser was noninvasively applied onto the hairless scalp of injured area 4 hours after mTBI. The injured brain tissues with or without exposure to the light, alone with sham brain tissues, were dissected 2 hours later from WT and KO mice. Levels of inflammatory cytokines and chemokines were measured by qRT–PCR in the tissues as shown in Figure 3A. IL-1β, IL-6, CCL2, and CXCL10, but not TNF-α, all diminished drastically in the presence compared with the absence of LLLT in these injured mice regardless of IEX-1 expression (Figure 4, left panel). Although the level of CCL2 and CXCL10 expression appeared to be higher in KO mice than in WT after LLLT when analyzed by Student's t-test (data not shown), it was without statistical significance if the difference was attested by two-way analysis of variance (Figure 4, left panel). In sharp contrast to decreases of these inflammatory mediators after LLLT, TNF-α was upregulated by LLLT with more prominent effects on WT mice (4.5-fold) than KO mice (2.0-fold) at 6 hours after mTBI (Figure 4, left panel). The disparate effect of LLLT on TNF-α expression gave rise to comparable levels of TNF-α in WT and KO mice after the light exposure. The high level of TNF-α expression was transient and returned to a sham level in 28 days in both WT and KO mice (Figure 4, right panel). Besides TNF-α, IL-1β, IL-6, and CCL2, but not CXCL10, all diminished to the sham level in 28 days after LLLT in both WT and KO mice. CXCL10 remained elevated in comparison with the sham controls on day 28, with an approximate eightfold increase in WT mice and a 21-fold increase in KO mice (Figure 4, right panel, P<0.01).
Figure 4.
Disparate influences of LLLT in the expression of various inflammatory mediators. Proinflammatory cytokines and chemokines were analyzed by real-time quantitative reverse transcription–polymerase chain reaction in 2 hours after LLLT or 6 hours (6 h) after mTBI as well as 28 days (28 d) after injury as shown in Figure 3A. The data are expressed as means±s.e.m. (n=5 mice per group). *P<0.05, **P<0.01 and ***P<0.001 between indicated groups. IL, interleukin; KO, knockout; LLLT, low-level laser therapy; mTBI, mild traumatic brain injury; WT, wild-type.
Low-Level Laser Therapy Effectively Rescues Neurologic Deficits and Brain Tissue Damage in Immediate Early Responsive Gene X-1 Knockout Mice
Low-level laser therapy-mediated suppression of neuroinflammation was clearly associated with improved neurologic performance as reflected by greatly lower NSS of the mice (Figure 5A). Similar to what was described in Figure 1B, the initial NSS assessed at 1 hour after mTBI was comparable in the presence or absence of IEX-1 (Figure 5A). The NSS of KO mice was significantly lower in LLLT-treated group than in sham-treated group (Figure 5A). The NSS reduction was seen on day 3 (P<0.05), became highly significant on day 7 (P<0.001), and continued throughout the 28-day duration of this study. This recovery trend was comparable with the one attained with WT mice in the absence of LLLT (Figure 5A). The change in body weight in these KO mice followed a similar pattern with complete normalization to a WT level by LLLT (Figure 5B).
Figure 5.
LLLT significantly improves neurobehavioral performance in KO animals. A time course of NSS (A) and fold changes of body weight (B) after mTBI and LLLT. The NSS and relative body weight changes were measured and expressed as Figures 1B and 1C with nine mice in each group. *P<0.05, **P<0.01, and ***P<0.001 compared in the presence versus absence of LLLT in KO mice. KO, knockout; LLLT, low-level laser therapy; mTBI, mild traumatic brain injury; NSS, neurologic severity score; WT, wild-type.
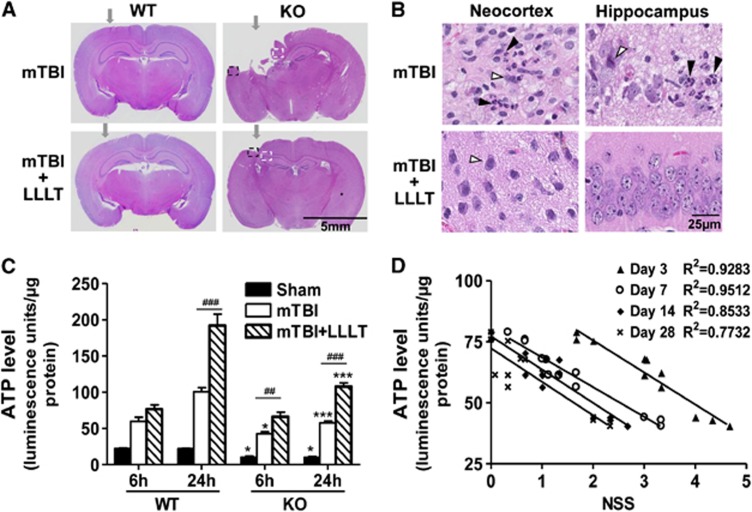
Histologically, brain tissue loss was reduced drastically in injured IEX-1 KO mice receiving LLLT as opposed to the mice without LLLT when examined at 28 days after injury (5.5±2.5% versus14.0±4.7%, P<0.01, Figure 6A). Importantly, the hippocampus was well preserved by LLLT in the mice. On the contrary, a great number of necrotic (unfilled arrows) and apoptotic cells (filled arrows) were observed not only in the neocortex but also in the hippocampus of KO mice if left untreated (Figure 6B). The hippocampal region of the brain is considered to be essential for memory and spatial navigation, which is likely to be the reason behind a decline of NSS in the KO mice receiving LLLT (Figure 5A). Moreover, LLLT significantly alleviated neuroinflammation, as manifested by diminished infiltration of leukocytes (Figure 6B, low panel) and decreased expression of proinflammatory mediators at the impact site in KO mice (Figure 4).
Figure 6.
Prevention of IEX-1 deficiency-induced cortical tissue loss by one dose of LLLT. (A) Representative histologic coronal sections from WT and KO mice with mTBI (upper) or the injured mice receiving LLLT (lower). The sections were prepared in 28 days after injury and arrows indicate the impact sites. Highlighted areas in the neocortex (a black dash line square) and hippocampus (a white dash line square) in KO mice are shown on a high magnification (B). Representative necrotic cells with pyknotic and condensed nuclei are marked with unfilled arrows and apoptotic cells with pyknotic and fragmented nuclei are labeled with filled arrows, respectively, in panel B. The data are representative of six mice per group. (C and D) Cortical ATP production was low in the absence compared with the presence of IEX-1, but higher after LLLT, regardless of IEX-1 expression (C). WT and KO mice were subjected to mTBI, followed 4 hours later by LLLT. At 6 hours and 24 hours after mTBI, the cortical lysate were collected for ATP measurement, and relative ATP levels were expressed as means±s.e.m. (n=6 mice per group). *P<0.05 and ***P<0.001 in the presence or absence of IEX-1; and ##P<0.01 and ###P<0.001 in the presence or absence of LLLT. Correlations between 6 hours ATP levels and NSS measured at indicated days regardless of IEX-1 expression are analyzed by coefficient of determination (R2) in panel D. Each symbol represents the data from individual groups with 12 mice in each group. Among the 12 animals, 6 animals were sacrificed at 6 hours after mTBI for ATP measurement, and remaining 6 mice were monitored for neurologic performance on the indicated days. ATP, adenosine-5'-triphosphate; IEX-1, immediate early responsive gene X-1; KO, knockout; LLLT, low-level laser therapy; mTBI, mild traumatic brain injury; NSS, neurologic severity score; WT, wild-type.
Finally, we confirmed the ability of LLLT to increase ATP production in injured WT or KO mice. As can be seen in Figure 6C, a relatively high level of ATP production was detected at the cortical impact site after mTBI, which was further elevated by LLLT in WT mice. The increment became significantly higher at 24 hours after LLLT, although it did not reach statistical significance at an early (6 hours) time point (Figure 6C). Lack of IEX-1 reduced ATP production in the sham controls as well as in injured brains in comparison with WT counterparts when ATP was assayed 6 hours after injury (Figure 6C, P<0.05). The compromised ATP production was more prominent 24 hours after the injury (Figure 6C, P<0.001). The result corroborates that lack of IEX-1 impairs oxidative phosphorylation in injured brain. Similar to what happened in WT mice, LLLT also augmented ATP generation in injured brain lacking IEX-1, increasing ATP production in the KO mice to a level comparable with that of injured WT brains not given LLLT at either time point (Figure 6C). Interestingly, the levels of ATP synthesis were found to reciprocally correlate with NSS in these mice regardless of LLLT or IEX-1 expression. Highly statistical correlations were attained between an ATP level measured at 6 hours of the injury and better neurobehavioral performance at varying days, with a coefficient of determination R2=0.9283 (P<0.0001) on day 3, R2=0.9512 (P<0.0001) on day 7, R2=0.8533 (P<0.0001) on day 14, or R2=0.7732 (P<0.001) on day 28 after injury (Figure 6D). Similarly, an ATP level measured at 24 hours after injury was also highly correlated with a reduced NSS in these animals, with a coefficient of determination R2=0.9271 (P<0.0001) on day 3, R2=0.7165 (P<0.001) on day 7, R2=0.8086 (P<0.0001) on day 14, or R2=0.6004 (P<0.01) on day 28 after injury, respectively (data not shown). These observations demonstrate a causal relationship between sufficient ATP production at the early stages after mTBI and a long-term neurologic recovery.
Discussion
The current study shows that a mild brain injury can progress into a severe TBI in the absence, but not in the presence of IEX-1. The primary injury is attained by a gentle hit on the hairless head with closed skull and scalp, which is well known not to produce significant secondary brain injury in WT mice and therefore is not commonly used, despite the fact that this closed head trauma mostly simulates the head injuries suffered by humans. The commonly used preclinical TBI models, including controlled cortical impact and fluid percussion injury, all involve a craniotomy at different locations of the brain,27 and may not concur with increased skull pressure and insufficient oxygen supply at the injured site as most of the mTBIs do in humans. Recently, a blast TBI model was also induced with an intact skull, but it was intended to mimic those injuries from the combat field activities in Iraq and Afghanistan,28 distinct from the current brain injury that occurs in the absence of any explosion. This new mTBI model can potentially provide us with a unique opportunity to address the mechanism underlying secondary brain injury separating from the primary injury.
One of the major differences in the histologic study is that leukocyte infiltrates after initial cell injury take place at the impact site only in KO mice and not in WT mice. The same sequential pattern was also observed in the hippocampus where cell death was present before leukocyte infiltration. The observation raises an intriguing possibility that diffusible inflammatory cytokines trigger cell death in the vicinity of the impact site, which in turn stimulates inflammation. This vicious cycle of cell death stimulating inflammation, which then causes cell injury in the neighboring cells, followed by more inflammation may be one of the mechanisms resulting in a perpetuating cycle of brain cell loss.29, 30 A deleterious role of neuroinflammation in neuropathogenesis after brain injury has been demonstrated by a number of studies.30 Our study also confirms that TBI-induced neuron deficit could be aggravated each time a small amount of LPS is administered into injured WT mice (Figure 3B) or by null mutation of IEX-1. Lack of IEX-1 gave rise to relatively high levels of proinflammatory cytokines at the impact site. Among these proinflammatory mediators, TNF-α, IL-1β, and IL-6 are known to be neurotoxic, and CCL2 and CXCL10 are able to actively recruit leukocytes into the injured region,31 which explains histologic findings of heavy infiltrates of leukocytes and cell death extending beyond the impact site in the absence of IEX-1.
Early responsive gene X-1 is consistently expressed in the brain at a low level.32 Its expression was enhanced by brain injury as early as 2 hours after trauma, reaching a peak level at 6 hours after TBI in WT perilesional cortex (data not shown). Lack of IEX-1 has been shown to render cells vulnerable to cell death17 and prolong inflammation, both of which probably contribute to secondary brain injury. Mechanistically, we have demonstrated that IEX-1 controls oxidative phosphorylation at mitochondria by facilitation of F1F0-ATPase inhibitor (IF1) degradation.13 Null mutation of IEX-1 stabilized IF1 giving rise to its accumulation that reduced oxidative phosphorylation while increasing aerobic glycolysis, as suggested by more active glucose uptake and lactate production in hypoxic conditions, and accelerated cell death upon glucose-deprivation in IEX-1-deficient cells compared with WT counterparts.17 The current investigation extends this observation demonstrating that ATP synthesis is compromised in injured KO cortex (Figure 6C). Yan et. al33 showed that brain injury immediately followed by brief hypoxia deteriorated neurologic performance and increased production of proinflammatory cytokines IL-1β, IL-6, and TNF-α. Systemic hypoxia after TBI exacerbated neuronal death and the lesion, and increased neurologic deficit and brain edema.34 It has been also shown in clinical studies that posttraumatic hypoxia is associated with poor neurologic outcomes and a prolonged recovery period.35 The adverse effect of hypoxia on the protection against secondary brain damage argues strongly for an importance of ATP in the healing process after brain injury.
One of the mechanisms for the beneficial effects of LLLT relies on its ability to increase oxidative phosphorylation and ATP synthesis by direct activation of mitochondrial cytochrome c oxidase.36 Our previous investigations showed that LLLT could increase ATP synthesis in mouse embryonic fibroblasts and other cell types in vitro.10 The current investigations validate that LLLT stimulates ATP production in injured brains in a manner independent on IEX-1 expression. Moreover, ATP generation was compromised in injured IEX-1 KO brain but was restored by LLLT at a level comparable with that in injured WT brains. ATP takes center part in clearance of injured cells, in cell proliferation, migration, and signaling, and in many enzymatic reactions. In accordance with this, the level of ATP generation was found to highly correlate with improved neurologic behaviors in the animals. Hence, the compromised ATP generation may make IEX-1 KO mice prone to secondary brain injury, whereas LLLT-augmented ATP production may protect the mice from secondary brain injury. To the best of our knowledge, this is the first study demonstrating a causal relationship between ATP and recovery of brain injury. Our study also confirms an effective delivery of LLLT to the damaged area through closed scalp and skull in mice. These investigations suggest that LLLT may benefit TBI patients with inadequate levels of mitochondrial function or increased levels of glycolysis such as in the elderly.
It has been long appreciated that neuroinflammation is responsible for both beneficial and detrimental effects on secondary brain injury, because neither antiinflammatory nor enhanced inflammatory treatment can significantly ameliorate the injury experimentally and clinically.37 Low-level laser therapy-mediated suppression of inflammation may be one of the key factors contributing to the protection against secondary brain damage in mice. But, it is not known whether the protection is associated with selective upregulation of TNF-α expression by LLLT at an early stage of the disorder, while inhibiting the expression of other antiinflammatory mediators. TNF-α-deficient mice exhibited an early functional improvement but failed to fully recover over time after TBI,38 raising an intriguing possibility that an outcome of TNF-α on brain injury depends on the presence of other proinflammatory cytokines. For instance, TNF-α was found to synergistically enhance the neurotoxic effects of IL-1β as both shared many of the same physiologic effects.39 Therefore, inhibition of IL-1β but upregulation of TNF-α may tilt the balance towards beneficiary of TNF-α. The selective modulation of inflammatory cytokine production is hardly achieved by current antiinflammation-based therapies, emphasizing a unique property of LLLT in the prevention of secondary brain damage.
To date, pharmacological treatments that can confer neuroprotection against secondary brain injury are still critically lacking. Further investigation of the molecular basis underlying the therapeutic benefit of LLLT is urgently needed. A systemic biologic study in human samples could potentially unravel which set of genes are up- or downregulated by LLLT, leading to more sufficient rational combination therapies for TBI. Our current investigations extend previous findings confirming the beneficial effects of LLLT on a novel head trauma model with closed skull and scalp.11, 12 The results argue strongly for the great potential of LLLT as a convenient preventative strategy for TBI in the clinics. This is particularly of clinical significance for mTBI. Unlike severe TBI victims that would be treated immediately, victims suffering with mTBI are difficult to diagnose even using magnetic resonance imaging or computer-assisted tomography scans and most of them leave the emergency room untreated. LLLT is a simple, noninvasive, safe, convenient, and cost-effective modality and could be used routinely for patients at their first hospital visit to prevent or slow down neurologic dysfunction after mTBI.
Acknowledgments
The authors thank Dr Jenny Zhao, Julia LaGraves, Margaret Sherwood, and Danny Cao of the Wellman Center Photopathology Core for experimental assistance with histopathology.
Author contributions
QZ designed and performed the research, analyzed data, and wrote the manuscript. CZ performed the research. MRH participated in the experimental design, analysis of data and manuscript writing. MXW designed and supervised the research and wrote the manuscript.
The authors declare no conflict of interest.
Footnotes
This work is supported by Air Force Office of Scientific Research Militory Phtomedicine Program (FA9550-11-1-0331) and the National Institutes of Health Grants CA158756 to MW and National Institutes of Health Grants R01AI050875 to MR.
References
- Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD, et al. Surveillance for traumatic brain injury-related deaths: United States, 1997–2007. MMWR Surveill Summ. 2011;60:1–32. [PubMed] [Google Scholar]
- Margulies S. The postconcussion syndrome after mild head trauma: is brain damage overdiagnosed? Part 1. J Clin Neurosci. 2000;7:400–408. doi: 10.1054/jocn.1999.0681. [DOI] [PubMed] [Google Scholar]
- Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan W, Vatansever F, Huang L, Wu Q, Xuan Y, Dai T, et al. Transcranial low-level laser therapy improves neurological performance in traumatic brain injury in mice: effect of treatment repetition regimen. PLoS One. 2013;8:e53454. doi: 10.1371/journal.pone.0053454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Salgado KF, Chao CH, Zivin JA. Transcranial near-infrared light therapy improves motor function following embolic strokes in rabbits: an extended therapeutic window study using continuous and pulse frequency delivery modes. Neuroscience. 2007;148:907–914. doi: 10.1016/j.neuroscience.2007.07.002. [DOI] [PubMed] [Google Scholar]
- De Taboada L, Yu J, El Amouri S, Gattoni-Celli S, Richieri S, McCarthy T, et al. Transcranial laser therapy attenuates amyloid-beta peptide neuropathology in amyloid-beta protein precursor transgenic mice. J Alzheimers Dis. 2011;23:521–535. doi: 10.3233/JAD-2010-100894. [DOI] [PubMed] [Google Scholar]
- Wu X, Dmitriev AE, Cardoso MJ, Viers-Costello AG, Borke RC, Streeter J, et al. 810 nm Wavelength light: an effective therapy for transected or contused rat spinal cord. Lasers Surg Med. 2009;41:36–41. doi: 10.1002/lsm.20729. [DOI] [PubMed] [Google Scholar]
- Karu T. Laser biostimulation: a photobiological phenomenon. J Photochem Photobiol B. 1989;3:638–640. doi: 10.1016/1011-1344(89)80088-0. [DOI] [PubMed] [Google Scholar]
- Pastore D, Greco M, Passarella S. Specific helium-neon laser sensitivity of the purified cytochrome c oxidase. Int J Radiat Biol. 2000;76:863–870. doi: 10.1080/09553000050029020. [DOI] [PubMed] [Google Scholar]
- Chen AC, Arany PR, Huang YY, Tomkinson EM, Sharma SK, Kharkwal GB, et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS One. 2011;6:e22453. doi: 10.1371/journal.pone.0022453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando T, Xuan W, Xu T, Dai T, Sharma SK, Kharkwal GB, et al. Comparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury in mice. PLoS One. 2011;6:e26212. doi: 10.1371/journal.pone.0026212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Xuan W, Ando T, Xu T, Huang L, Huang YY, et al. Low-level laser therapy for closed-head traumatic brain injury in mice: effect of different wavelengths. Lasers Surg Med. 2012;44:218–226. doi: 10.1002/lsm.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella M, Casswell E, Chong S, Farah Z, Wieckowski MR, Abramov AY, et al. Regulation of mitochondrial structure and function by the F1Fo-ATPase inhibitor protein, IF1. Cell Metab. 2008;8:13–25. doi: 10.1016/j.cmet.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Shahid M, Shen L, Seldin DC, Lu B, Ustyugova IV, Chen X, et al. Impaired 3',5'-cyclic adenosine monophosphate-mediated signaling in immediate early responsive gene X-1-deficient vascular smooth muscle cells. Hypertension. 2010;56:705–712. doi: 10.1161/HYPERTENSIONAHA.110.154880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi L, Ustyugova IV, Chen X, Zhang Q, Wu MX. Enhanced Th17 differentiation and aggravated arthritis in IEX-1-deficient mice by mitochondrial reactive oxygen species-mediated signaling. J Immunol. 2012;189:1639–1647. doi: 10.4049/jimmunol.1200528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Guo J, Santos-Berrios C, Wu MX. Distinct domains for anti- and pro-apoptotic activities of IEX-1. J Biol Chem. 2006;281:15304–15311. doi: 10.1074/jbc.M600054200. [DOI] [PubMed] [Google Scholar]
- Shen L, Zhi L, Hu W, Wu MX. IEX-1 targets mitochondrial F1Fo-ATPase inhibitor for degradation. Cell Death Differ. 2009;16:603–612. doi: 10.1038/cdd.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt A, Schafer H. Role of the immediate early response 3 (IER3) gene in cellular stress response, inflammation and tumorigenesis. Eur J Cell Biol. 2011;90:545–552. doi: 10.1016/j.ejcb.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Lehoux S, Tedgui A. All strain, no gain: stretch keeps proliferation at bay via the NF-kappaB response gene iex-1. Circ Res. 2003;93:1139–1141. doi: 10.1161/01.RES.0000108693.79326.A8. [DOI] [PubMed] [Google Scholar]
- Wu MX, Ao Z, Prasad KV, Wu R, Schlossman SF. IEX-1L, an apoptosis inhibitor involved in NF-kappaB-mediated cell survival. Science. 1998;281:998–1001. doi: 10.1126/science.281.5379.998. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flierl MA, Stahel PF, Beauchamp KM, Morgan SJ, Smith WR, Shohami E. Mouse closed head injury model induced by a weight-drop device. Nat Protoc. 2009;4:1328–1337. doi: 10.1038/nprot.2009.148. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK. Experimental brain injury induces expression of interleukin-1 beta mRNA in the rat brain. Brain Res Mol Brain Res. 1995;30:125–130. doi: 10.1016/0169-328x(94)00287-o. [DOI] [PubMed] [Google Scholar]
- Shohami E, Novikov M, Bass R, Yamin A, Gallily R. Closed head injury triggers early production of TNF alpha and IL-6 by brain tissue. J Cereb Blood Flow Metab. 1994;14:615–619. doi: 10.1038/jcbfm.1994.76. [DOI] [PubMed] [Google Scholar]
- Assis L, Moretti AI, Abrahao TB, Cury V, Souza HP, Hamblin MR, et al. Low-level laser therapy (808 nm) reduces inflammatory response and oxidative stress in rat tibialis anterior muscle after cryolesion. Lasers Surg Med. 2012;44:726–735. doi: 10.1002/lsm.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund N, Hillered L. Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here. Br J Pharmacol. 2011;164:1207–1229. doi: 10.1111/j.1476-5381.2010.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4:134ra60. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Carvey PM, Chang Q, Lipton JW, Ling Z. Prenatal exposure to the bacteriotoxin lipopolysaccharide leads to long-term losses of dopamine neurons in offspring: a potential, new model of Parkinson's disease. Front Biosci. 2003;8:s826–s837. doi: 10.2741/1158. [DOI] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Feldmann KA, Pittelkow MR, Roche PC, Kumar R, Grande JP. Expression of an immediate early gene, IEX-1, in human tissues. Histochem Cell Biol. 2001;115:489–497. doi: 10.1007/s004180100284. [DOI] [PubMed] [Google Scholar]
- Yan EB, Hellewell SC, Bellander BM, Agyapomaa DA, Morganti-Kossmann MC. Post-traumatic hypoxia exacerbates neurological deficit, neuroinflammation and cerebral metabolism in rats with diffuse traumatic brain injury. J Neuroinflammation. 2011;8:147. doi: 10.1186/1742-2094-8-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita Y, Bramlett HM, Alonso O, Dietrich WD. Posttraumatic hypothermia is neuroprotective in a model of traumatic brain injury complicated by a secondary hypoxic insult. Crit Care Med. 2001;29:2060–2066. doi: 10.1097/00003246-200111000-00004. [DOI] [PubMed] [Google Scholar]
- Chesnut RM, Marshall SB, Piek J, Blunt BA, Klauber MR, Marshall LF. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir Suppl (Wien ) 1993;59:121–125. doi: 10.1007/978-3-7091-9302-0_21. [DOI] [PubMed] [Google Scholar]
- Sutherland JC. Biological effects of polychromatic light. Photochem Photobiol. 2002;76:164–170. doi: 10.1562/0031-8655(2002)076<0164:beopl>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Finnie JW. Neuroinflammation: beneficial and detrimental effects after traumatic brain injury. Inflammopharmacology. 2013;21:309–320. doi: 10.1007/s10787-012-0164-2. [DOI] [PubMed] [Google Scholar]
- Khuman J, Meehan WP, III, Zhu X, Qiu J, Hoffmann U, Zhang J, et al. Tumor necrosis factor alpha and Fas receptor contribute to cognitive deficits independent of cell death after concussive traumatic brain injury in mice. J Cereb Blood Flow Metab. 2011;31:778–789. doi: 10.1038/jcbfm.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Hu S, Ehrlich L, Peterson PK. Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-D-aspartate receptors. Brain Behav Immun. 1995;9:355–365. doi: 10.1006/brbi.1995.1033. [DOI] [PubMed] [Google Scholar]