Abstract
In addition to delayed vasospasm also early brain injury, which occurs during the first few days after subarachnoid hemorrhage (SAH) when large cerebral arteries are still fully functional, plays an important role for the outcome after SAH. In the current study, we investigated the hypothesis that carbon dioxide (CO2), a strong cerebral vasodilator, has a therapeutic potential against early posthemorrhagic microvasospasm. C57BL/6 mice (n=36) and Sprague-Dawley rats (n=23) were subjected to sham surgery or SAH by filament perforation. The pial microcirculation in the mice was visualized 3 and 24 hours after SAH using intravital fluorescence microscopy. Partial pressure of CO2 (PaCO2) was modulated by hyper- or hypoventilation or by inhalation of 10% CO2. In rats, CO2-mediated changes in cerebral blood flow (CBF) were measured at the same time points using laser Doppler fluxmetry. Increased PaCO2 caused vasodilatation in sham-operated animals. Following SAH, however, cerebral arterioles were nonreactive to CO2. This lack of microvascular CO2 reactivity was accompanied by a complete loss of CO2-induced hyperemia. Our data show that CO2 is not able to dilate spastic microvessels and to increase CBF early after SAH. Future therapeutic approaches will therefore need to address mechanisms beyond CO2.
Keywords: CO2 reactivity, intravital microscopy, mice, microcirculation, microvasospasm, subarachnoid hemorrhage
Introduction
Subarachnoid hemorrhage (SAH) underlies 6% to 10% of all stroke events yet accounts for 22% to 25% of cerebrovascular-related deaths.1 Only ∼10% of patients who experience SAH recover completely, and ∼70% of initially comatose patients die within the first 6 months of the SAH.2 In addition to the severity of the disease also the lack of causal therapeutic options is responsible for this less than optimal situation.
The pathophysiology of SAH can be divided into two distinct phases: (1) delayed brain injury associated with delayed macrovasospasm, which occurs later than 5 days after SAH3, 4, 5 and (2) early brain injury (EBI) that occurs within the first few days after SAH when large cerebral vessels are still fully functional. Nevertheless, EBI is associated with acute cerebral hypoperfusion.6 Recently, several laboratories, including ours, showed that spasms of the cerebral microcirculation may be responsible for cerebral hypoperfusion/ischemia during EBI. Using histology, Sehba et al7 described macro- and microvascular spasms after the induction of experimental SAH, and microvasospasms were recently observed in vivo early after SAH both in patients8, 9 and in experimental animals.10, 11 Accordingly, the appealing hypothesis was put forward that the application of vasodilators, ideally cerebral vasodilators, may be an appropriate way to dilate spastic posthemorrhagic microvessels thereby mitigating or even preventing EBI after SAH. Accordingly, the aim of the current study was to address this hypothesis by applying carbon dioxide (CO2), a potent cerebral vasodilator, to mice and rats after experimental SAH and investigate whether this intervention was able to resolve posthemorrhagic microvasospasm and increase cerebral blood flow (CBF).
Materials and methods
Male 7 to 11 weeks old C57BL/6 mice (20 to 23 g, Charles River, Germany) and Sprague-Dawley rats (270 to 300 g, own breeding), were used for these experiments. All procedures were performed in accordance with and were approved by the respective regulatory bodies in Germany (Regierung von Oberbayern, Approval No. 6/04) and Poland (Nencki Institute of Experimental Biology Local Commission for the Care and Use of Laboratory Animals for Experimental Procedures, Approval No. 191/2002).
Induction of Subarachnoid Hemorrhage in Mice
The mice were anesthetized with 0.5 mg/kg BW medetomidine, 5 mg/kg BW midazolam, and 0.05 mg/kg BW fentanyl (intraperitoneally), intubated and ventilated under control of end-tidal partial pressure of CO2 (PaCO2) by microcapnometry. SAH was induced using the filament perforation model and monitored by the measurement of intracranial pressure in each animal as previously described.12 Sham-operated mice were treated in the same way only that the filament was not advanced so far that hemorrhage was induced. Eleven mice died after SAH (9/47 operated animals; total mortality: 23%) and could therefore not be investigated further.
Intravital Microscopy
The mice were randomly assigned to one of the six treatment groups (n=6 mice each) and reanesthetized 3 or 24 hours after SAH as described above. The pial microcirculation was visualized with the intravascular dye fluorescein isothiocyanate (FITC)-dextran (MW 150,000 Da) as previously described (Figures 1A and 1B).10, 13 The vessel diameter of three arterioles (30 to 50 μm diameter) surrounded by blood and therefore spastic (see Figure 1C and Friedrich et al10 for details) were recorded every 15 minutes for 1 hour to establish the baseline values. The animals were then sequentially normoventilated, hyperventilated, normoventilated, and then finally hypoventilated (for 10 minutes each) to assess the responsiveness of the vessels to hypo- and hypercapnia. In three additional experimental groups (no SAH, SAH+3 hours, and SAH+24 hours; n=6 mice each), the animals were ventilated with 10% CO2 for 15 minutes, and vessel diameter was recorded as described above. The intravital microscopy images were analyzed offline by an investigator who was masked with respect to the treatment of the animals.13
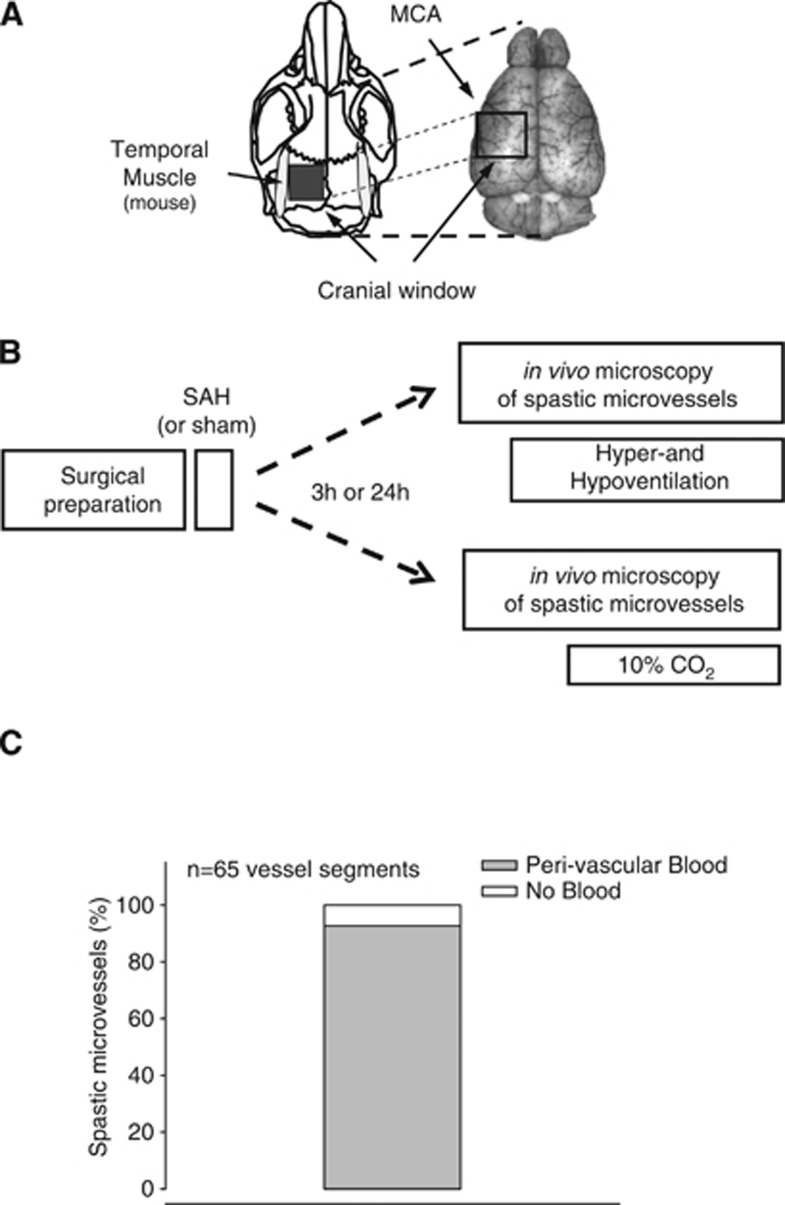
Figure 1.
(A) Schematic drawing of the rodent skull (left) and a dorsal image of the brain (right) indicating the location of the cranial window used for intravital microscopy in mice. MCA, middle cerebral artery. (B) Schematic drawing of the experimental protocol used in this study. SAH, subarachnoid hemorrhage. (C) Percentage of spastic microvessels ensheathed by blood.
Induction of Subarachnoid Hemorrhage in Rats
Rats were anesthetized with chloral hydrate (3.6% solution intraperitoneally, 1 mL/100 g BW), intubated and ventilated with 30% O2. Catheters were placed in the femoral or tail artery and in the femoral or tail vein. SAH was induced by the filament perforation technique and monitored by a sudden drop in regional CBF (rCBF) as previously described.14
CO2 Reactivity in Rats
CO2 reactivity in rats was evaluated 3 and 24 hours after SAH or sham surgery by ventilating the animals with 5% CO2 for 5 minutes and measuring rCBF over the middle cerebral artery territory ipsilateral to the hemorrhage by laser Doppler fluxmetry. The results are expressed as a chemical index (CICVR) as previously described.14 This CI is independent of changes in arterial blood pressure, as it is based on the change in cerebrovascular resistance (the ratio of arterial blood pressure to rCBF). Thus, CICVR is expressed as the relative change in cerebrovascular resistance per 1 mm Hg PaCO2.
Statistical Analysis
Statistical analysis including calculation of sample size was performed using SigmaStat 3.0 (SPSS Science, Chicago, IL, USA). A one-way analysis of variance on ranks followed by the Dunn's or Tukey's test was used. Differences with a P value <0.05 and a power of >0.8 were considered to be statistically significant. Unless otherwise indicated, summary data are presented as the mean±the standard error of the mean.
Results
Physiologic Parameters
Body temperature (data not shown), blood pressure, and PaCO2 were all constant throughout the baseline measurements in all groups (Tables 1 and 2). As expected, the mice that underwent SAH had lower blood pressure than sham-operated animals (Table 1). In both the sham and SAH-induced mice, blood pressure increased and decreased, and PaCO2 decreased and increased, during hyperventilation and hypoventilation, respectively. During inhalation of 10% CO2, both groups exhibited a drop in blood pressure and an increase in PaCO2 to high levels (Table 1).
Table 1. Physiologic parameters measured 3 hours after SAH or sham surgery in mice.
| Baseline | 10% CO2 | Hyperventilation | Hypoventilation | |
|---|---|---|---|---|
| Sham | ||||
| BP | 92±5 mm Hg | 70±8 mm Hg | 92±5 mm Hg | 52±5 mm Hg |
| PaCO2 | 39±3 mm Hg | 274±11 mm Hg | 22±2 mm Hg | 49±2 mm Hg |
| SAH | ||||
| BP | 64±3 mm Hga | 53±3 mm Hg | 72±4 mm Hg | 65±5 mm Hg |
| PaCO2 | 38±2 mm Hg | 278±12 mm Hg | 23±3 mm Hg | 47±4 mm Hg |
BP, blood pressure; SAH, subarachnoid hemorrhage.
P<0.05 versus sham.
Table 2. Physiologic parameters measured 3 hours after SAH or sham surgery in rats.
| Baseline | 5% CO2 | |
|---|---|---|
| Sham | ||
| BP | 105±3 mm Hg | 112±3 mm Hg* |
| PaCO2 | 37±1 mm Hg | 56±1 mm Hg** |
| SAH | ||
| BP | 103±8 mm Hg | 110±7 mm Hg |
| PaCO2 | 38±1 mm Hg | 57±2 mm Hg** |
BP, blood pressure; SAH, subarachnoid hemorrhage.
*P<0.05, **P<0.005.
The rats that underwent SAH had the same blood pressure as sham-operated rats. During inhalation of 5% CO2, blood pressure slightly increased in the sham-operated rats, but not in the SAH-induced rats (Table 2).
Relationship Between Microvasospasm and Perivascular Blood
As previously shown, 70% of pial microvessels become spastic after SAH.10 To investigate whether perivascular blood was a possible cause for microvascular constriction, we determined the percentage of spastic microvessels surrounded by blood. Indeed, 93% of all spastic vessels were ensheathed by blood (Figure 1C) and no spasms were detected in vessels not being in contact with perivascular blood (data not shown). This strongly suggests that microvessels become only spastic when coming in direct contact with blood. Therefore only vessels covered with blood (and hence spastic) were used for the current study.
Responses to Changes in PaCO2 by Variation of Ventilation
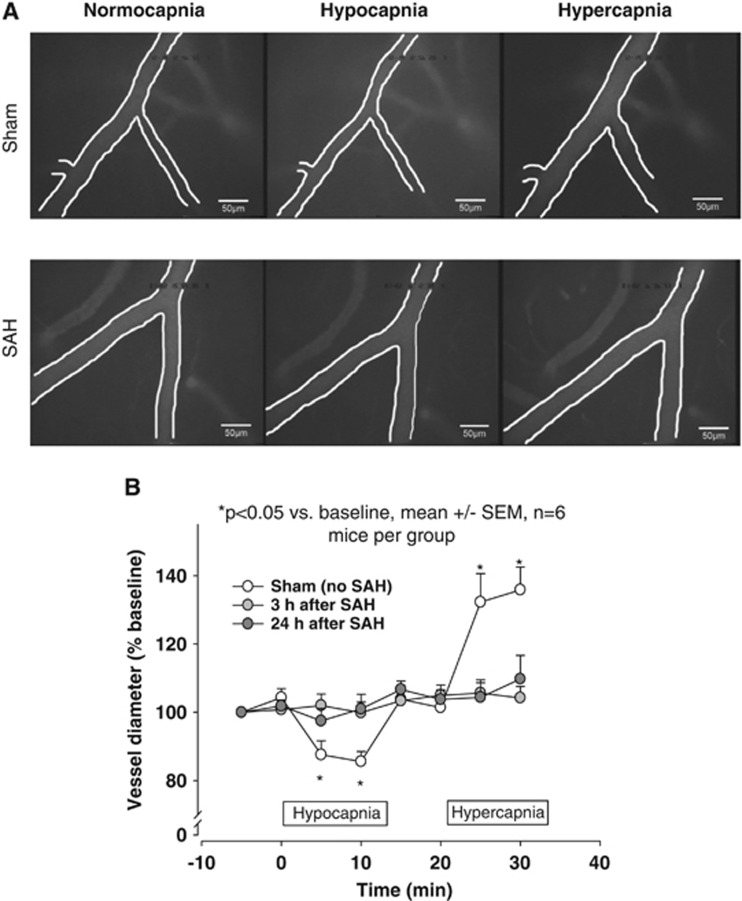
During baseline measurements, the diameter of the pial resistance vessels (i.e., arterioles) was constant. Following hypo- and hyperventilation, arterioles of sham-operated mice reacted as expected; specifically, vessels contracted during hyperventilation, returned to baseline upon normoventilation, and then dilated upon hypoventilation (Figure 2A, upper panels and Figure 2B, open circles). In the SAH-induced animals, the vessels that were affected by microvasospasm did not react to changes in ventilation either 3 or 24 hours after SAH (Figure 2A, lower panels and Figure 2B, light and dark gray circles). These findings indicate a complete loss of vessel reactivity in the cerebral microvessels that were affected by microvasospasm after SAH.
Figure 2.
(A) In vivo microscopy images of mouse pial arterioles and their response to various ventilation frequencies 3 hours after sham surgery (upper panels) or SAH (lower panels). For each animal, the left, middle, and right panels show the same vessel. (B) Quantification of these data show that sham-operated mice exhibited a significant decrease and increase in vessel diameter during hypocapnia and hypercapnia, respectively (open circles). In contrast, vessels in mice that underwent SAH showed no change in vessel diameter in response to various ventilation frequencies (gray circles). SAH, subarachnoid hemorrhage; SEM, standard error of the mean. *P<0.05 versus baseline; n=6 mice per group.
Inhalation with 10% CO2
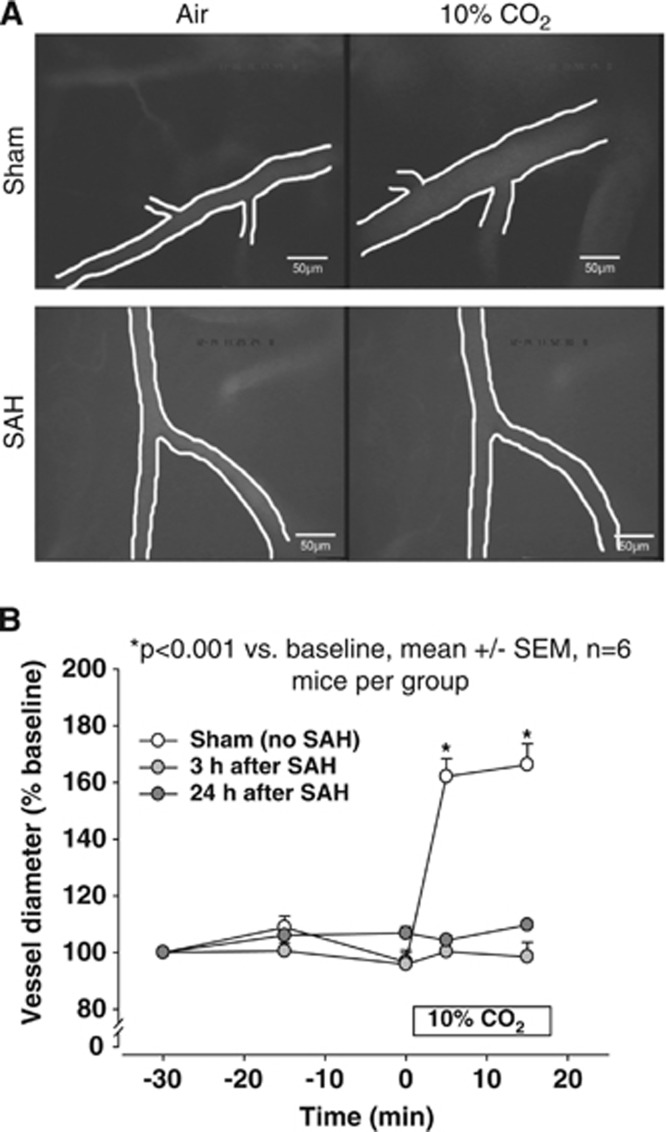
To exclude the possibility that the observed lack of CO2 reactivity after SAH was not simply caused by a reduced sensitivity of posthemorrhagic vessels to CO2 (i.e., dependent on the dose of the applied CO2), PaCO2 was increased to supraphysiologic levels by ventilation with 10% CO2. In the sham-operated mice, inhalation of 10% CO2 triggered an immediate and significant dilatation of the pial resistance vessels to 162% of baseline (P=0.002 versus baseline; Figure 3A, upper panels and Figure 3B, open circles). Again, the spastic microvessels in the SAH-induced mice showed no reaction to inhaled CO2 either 3 or 24 hours after SAH, indicating that spastic microvessels were indeed nonresponsive to CO2 (Figure 3A, lower panels and Figure 3B, gray circles).
Figure 3.
(A) Representative in vivo microscopy images of mouse pial arterioles and their response to inhalation of 10% CO2 3 hours after sham surgery (upper panels) or SAH (lower panels). For each animal, the left and right panels show the same vessel. (B) Quantification of experiments shown above. Vessel diameter in the sham-operated animals increased immediately upon ventilation with 10% CO2. In contrast, the diameter of cerebral arterioles of mice subjected to SAH showed no change in response to elevated PaCO2. SAH, subarachnoid hemorrhage; SEM, standard error of the mean. *P<0.001 versus baseline; n=6 mice per group.
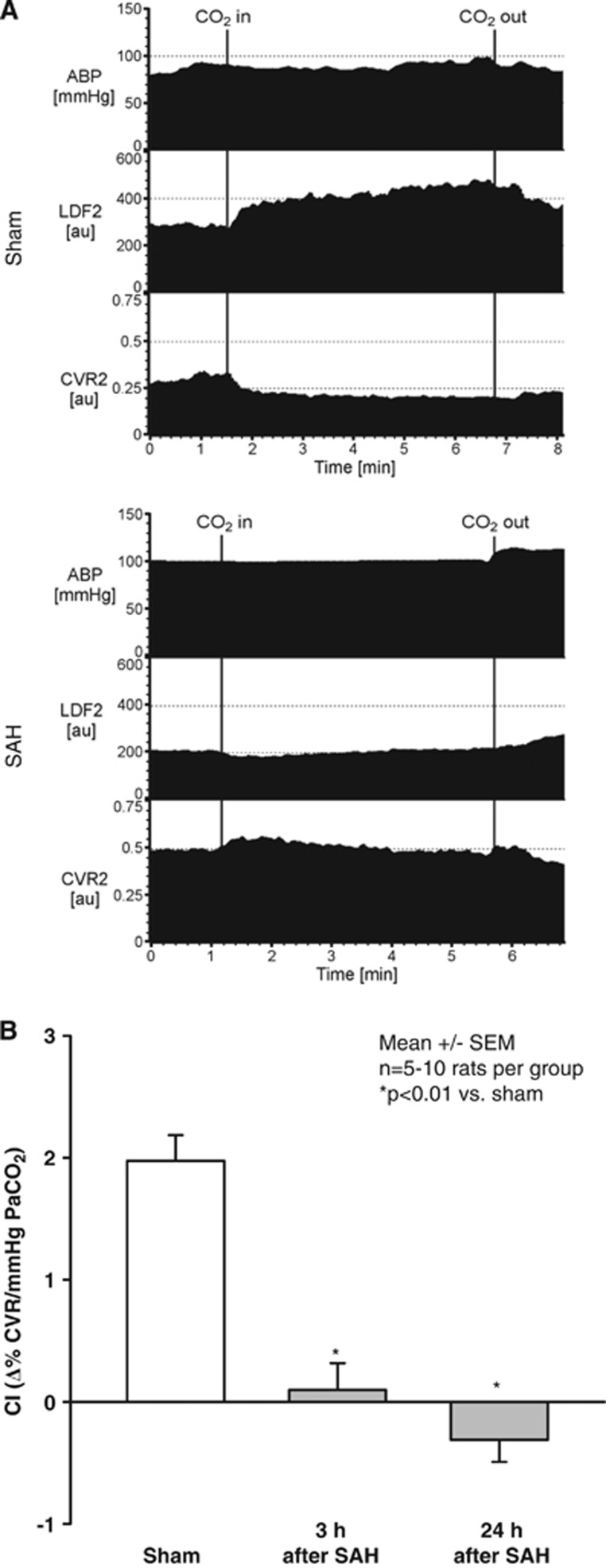
To determine whether the changes that were observed at the microcirculation level also had an effect on CBF, and to evaluate whether the same effect also occurs in another species, similar experiments—with the addition of rCBF measurements using laser Doppler flowmetry—were performed in rats as showed by an original recording 3 hours after sham surgery or SAH (Figure 4A). Inhalation of 5% CO2 triggered a large increase in rCBF and a decrease in cerebrovascular resistance in sham-operated rats (CI=1.9±0.1%/mm Hg PaCO2). However, 3 and 24 hours after SAH, CO2 reactivity was completely lost, as CI was 0.1±0.2 and −0.3±0.2%/mm Hg PaCO2, respectively (Figure 4B).
Figure 4.
(A) Representative original recording of arterial blood pressure (ABP), regional cerebral blood flow (LDF), and cerebrovascular resistance (CVR) 3 hours after sham operation (upper recordings) or subarachnoid hemorrhage (SAH; lower recordings) in rats. Upon carbon dioxide (CO2) inhalation regional cerebral blood increased and CVR decreased in sham-operated animals, while no significant changes were observed after SAH. (B) Chemical index (CI) of rats subjected to SAH or sham surgery. CI is indicated as a relative change of CVR per 1 mm Hg partial pressure of CO2 (PaCO2) during inhalation of CO2. Sham-operated rats showed a normal reactivity to 5% inhaled CO2 (open bar; CI=1.9±0.1%/1 mm Hg PaCO2). Three and twenty-four hours after SAH the cerebral cortex of rats subjected to SAH showed a complete lack of CO2 reactivity as showed by CI values close to zero. *P<0.01 versus sham-operated rats; n=5 to 10 rats per group.
Discussion
The aim of this study was to investigate whether early posthemorrhagic microvasospasm after SAH, which is associated with microthrombosis, cerebral ischemia, and the development of early-onset brain injury could be reduced or resolved by increasing the plasma concentration of CO2, a potent cerebral vasodilator. Our results show that cerebral arterioles that are covered with blood and therefore affected by posthemorrhagic microvasospasm undergo a complete loss of CO2 reactivity both 3 and 24 hours after SAH, and this loss of reactivity results in the inability of the brain to increase its blood flow in response to elevated PaCO2 levels. This lack of microvascular CO2 reactivity suggests that the cerebral autoregulation is severely compromised after SAH and this may render the brain vulnerable to decreases in blood pressure and/or increases in brain metabolism, thereby causing additional brain damage. In addition, our results show that increasing PaCO2 is not a viable therapeutic option against posthemorrhagic microvasospasm after SAH and that hyperventilation clinically used to reduce increased intracranial pressure by constricting cerebral vessels may not be appropriate after SAH.
In the present study, we induced SAH using the intraluminal perforation model, a well-characterized model that does not require opening of the skull and mimics quite nicely the acute processes that are triggered by the rupture of an intracranial aneurysm (e.g., elevated intracranial pressure, cerebral ischemia, and hippocampal damage) as well as the long-term squeals of SAH in patients (e.g., mortality, cerebral edema, and hydrocephalus). The induction of SAH was confirmed in each animal by a typical posthemorrhagic increase in intracranial pressure in mice and a sharp bilateral decrease of rCBF in the territory of the middle cerebral artery in rats. Importantly, the physiologic parameters were monitored closely and kept constant during all procedures including intravital microscopy.12 Thus, we believe that our setup—though technically demanding—minimized the likelihood of potential experimental artifacts.
Data regarding cerebral vessel reactivity were collected in vivo and in real time using epi-fluorescence microscopy. Although this technique has a limited penetration depth and therefore does not allow the investigation of deep parenchymal vessels, it is sufficient for the current study that focused on spastic arterioles in the subarachnoid space.
CO2 is one of the most important local regulators of cerebral vessel diameter and thus CBF. Accordingly, several clinical and experimental trials investigated whether CO2 could be used to treat delayed cerebral vasospasm after SAH.3, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 In patients suffering from delayed vasospasm CO2 reactivity was decreased and was found to be an accurate predictor of delayed cerebral ischemia.3, 16 However, so far, CO2 reactivity and the potential therapeutic benefits of CO2 on the recently described early, that is EBI associated, posthemorrhagic microvasospasm has not been investigated.
Our current and previous results suggest that the cerebral microvessels that are located in the subarachnoid space and which come into contact with sufficient amounts of blood become spastic and nonresponsive to CO2.10 Although, the mechanisms by which SAH causes microvasospasm are not yet fully understood, the cooccurrence of microvasospasm together with an attenuated CO2 response in the very same microvessels strongly suggests that a common mechanism may underlie both phenomena. Experiments by Iadecola and colleagues revealed that changes in CBF because of hypercapnia in the physiologic range are mediated primarily by nitric oxide (NO),26, 27 and these findings are supported by the fact that CO2 elevates the classic NO-induced messenger molecule cyclic guanosine monophosphate in the smooth-muscle cells of cerebral vessels.28 Because hemoglobin is a potent NO scavenger,29 and because NO is required for both CO2-mediated vasodilatation and to maintain physiologic arteriolar diameter, we suggest that hemoglobin in the blood surrounding cerebral microvessels after SAH scavenges vascular (and/or perivascular) NO, thereby causing microvasospasm and a complete lack of CO2 reactivity in these vessels. This hypothesis is supported, among others, by a recent nonhuman primate study that showed that replenishing vascular (or perivascular) NO by administering intravenously sodium nitrite—a strategy that is often used to deliver NO to endothelial cells—reversed SAH-induced macrovasospasm.30 A possible argument against this interpretation of our findings may be provided by a recent study performed on brain slices obtained from rats subjected to SAH, showing that on electrical stimulation of the neighboring parenchyma not perfused and hence not pressurized vessels show inverse neurovascular coupling, i.e., rather constriction than dilatation.31 Which interpretation represents the situation after SAH in vivo will have to be showed by ongoing studies.
Here, we report that the cerebral microvessels that are affected by early posthemorrhagic microvasospasms lose their ability to dilate in the face of increased levels of CO2. These findings suggest that post-SAH microvasospasm does not impair cerebral perfusion only by vasoconstriction and the formation of microthrombi; the impaired perfusion may also be because of either a lack of responsiveness to increased metabolic demand and/or reduced cerebral perfusion pressure. Our data also suggest that CO2 has no therapeutic effect against early microvasospasm and, hence, against EBI. Mechanistically, our results point to the possibility that the severe SAH-induced microvascular dysfunction is caused by a lack of vascular NO. Therefore, our negative results concerning CO2 may suggest that increasing vascular NO could be a promising strategy for treating patients who have EBI after SAH.
The authors declare no conflict of interest.
References
- Weaver JP, Fisher M. Subarachnoid hemorrhage: an update of pathogenesis, diagnosis and management. J Neurol Sci. 1994;125:119–131. doi: 10.1016/0022-510x(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Hop JW, Rinkel GJ, Algra A, van GJ. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997;28:660–664. doi: 10.1161/01.str.28.3.660. [DOI] [PubMed] [Google Scholar]
- Frontera JA, Rundek T, Schmidt JM, Claassen J, Parra A, Wartenberg KE, et al. Cerebrovascular reactivity and vasospasm after subarachnoid hemorrhage: a pilot study. Neurology. 2006;66:727–729. doi: 10.1212/01.wnl.0000200777.96896.3d. [DOI] [PubMed] [Google Scholar]
- Robertson E. Cerebral lesions due to intracranial aneurysms. Brain. 1949;72:150–185. doi: 10.1093/brain/72.2.150. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Weir BK. A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke. 1991;22:971–982. doi: 10.1161/01.str.22.8.971. [DOI] [PubMed] [Google Scholar]
- Schubert GA, Seiz M, Hegewald AA, Manville J, Thome C. Acute hypoperfusion immediately after subarachnoid hemorrhage: a xenon contrast-enhanced CT study. J Neurotrauma. 2009;26:2225–2231. doi: 10.1089/neu.2009.0924. [DOI] [PubMed] [Google Scholar]
- Sehba FA, Friedrich V, Jr., Makonnen G, Bederson JB. Acute cerebral vascular injury after subarachnoid hemorrhage and its prevention by administration of a nitric oxide donor. J Neurosurg. 2007;106:321–329. doi: 10.3171/jns.2007.106.2.321. [DOI] [PubMed] [Google Scholar]
- Uhl E, Lehmberg J, Steiger HJ, Messmer K. Intraoperative detection of early microvasospasm in patients with subarachnoid hemorrhage by using orthogonal polarization spectral imaging. Neurosurgery. 2003;52:1307–1315. doi: 10.1227/01.neu.0000065154.04824.9e. [DOI] [PubMed] [Google Scholar]
- Pennings FA, Bouma GJ, Ince C. Direct observation of the human cerebral microcirculation during aneurysm surgery reveals increased arteriolar contractility. Stroke. 2004;35:1284–1288. doi: 10.1161/01.STR.0000126039.91400.cb. [DOI] [PubMed] [Google Scholar]
- Friedrich B, Muller F, Feiler S, Scholler K, Plesnila N. Experimental subarachnoid hemorrhage causes early and long-lasting microarterial constriction and microthrombosis: an in-vivo microscopy study. J Cereb Blood Flow Metab. 2012;32:447–455. doi: 10.1038/jcbfm.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun BL, Zheng CB, Yang MF, Yuan H, Zhang SM, Wang LX. Dynamic alterations of cerebral pial microcirculation during experimental subarachnoid hemorrhage. Cell Mol Neurobiol. 2009;29:235–241. doi: 10.1007/s10571-008-9316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiler S, Friedrich B, Scholler K, Thal SC, Plesnila N. Standardized induction of subarachnoid hemorrhage in mice by intracranial pressure monitoring. J Neurosci Methods. 2010;190:164–170. doi: 10.1016/j.jneumeth.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Terpolilli NA, Kim SW, Thal SC, Kataoka H, Zeisig V, Nitzsche B, et al. Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ Res. 2012;110:727–738. doi: 10.1161/CIRCRESAHA.111.253419. [DOI] [PubMed] [Google Scholar]
- Jarus-Dziedzic K, Czernicki Z, Kozniewska E. Acute decrease of cerebrocortical microflow and lack of carbon dioxide reactivity following subarachnoid haemorrhage in the rat. Acta Neurochir Suppl. 2003;86:473–476. doi: 10.1007/978-3-7091-0651-8_97. [DOI] [PubMed] [Google Scholar]
- Britz GW, Meno JR, Park IS, Abel TJ, Chowdhary A, Nguyen TS, et al. Time-dependent alterations in functional and pharmacological arteriolar reactivity after subarachnoid hemorrhage. Stroke. 2007;38:1329–1335. doi: 10.1161/01.STR.0000259853.43084.03. [DOI] [PubMed] [Google Scholar]
- Carrera E, Kurtz P, Badjatia N, Fernandez L, Claassen J, Lee K, et al. Cerebrovascular carbon dioxide reactivity and delayed cerebral ischemia after subarachnoid hemorrhage. Arch Neurol. 2010;67:434–439. doi: 10.1001/archneurol.2010.43. [DOI] [PubMed] [Google Scholar]
- Diringer MN, Heffez DS, Monsein L, Kirsch JR, Hanley DF, Traystman RJ. Cerebrovascular CO2 reactivity during delayed vasospasm in a canine model of subarachnoid hemorrhage. Stroke. 1991;22:367–372. doi: 10.1161/01.str.22.3.367. [DOI] [PubMed] [Google Scholar]
- Diringer MN, Kirsch JR, Hanley DF, Traystman RJ. Altered cerebrovascular CO2 reactivity following subarachnoid hemorrhage in cats. J Neurosurg. 1993;78:915–921. doi: 10.3171/jns.1993.78.6.0915. [DOI] [PubMed] [Google Scholar]
- Diringer MN, Kirsch JR, Traystman RJ. Reduced cerebral blood flow but intact reactivity to hypercarbia and hypoxia following subarachnoid hemorrhage in rabbits. J Cereb Blood Flow Metab. 1994;14:59–63. doi: 10.1038/jcbfm.1994.9. [DOI] [PubMed] [Google Scholar]
- Hauerberg J, Juhler M, Rasmussen G. Cerebral blood flow autoregulation after experimental subarachnoid hemorrhage during hyperventilation in rats. J Neurosurg Anesthesiol. 1993;5:258–263. doi: 10.1097/00008506-199310000-00006. [DOI] [PubMed] [Google Scholar]
- Jakubowski J, Bell BA, Symon L, Zawirski MB, Francis DM. A primate model of subarachnoid hemorrhage: change in regional cerebral blood flow, autoregulation carbon dioxide reactivity, and central conduction time. Stroke. 1982;13:601–611. doi: 10.1161/01.str.13.5.601. [DOI] [PubMed] [Google Scholar]
- Mendelow AD, McCalden TA, Hattingh J, Coull A, Rosendorff C, Eidelman BH. Cerebrovascular reactivity and metabolism after subarachnoid hemorrhage in baboons. Stroke. 1981;12:58–65. doi: 10.1161/01.str.12.1.58. [DOI] [PubMed] [Google Scholar]
- Park IS, Meno JR, Witt CE, Chowdhary A, Nguyen TS, Winn HR, et al. Impairment of intracerebral arteriole dilation responses after subarachnoid hemorrhage. Laboratory investigation. J Neurosurg. 2009;111:1008–1013. doi: 10.3171/2009.3.JNS096. [DOI] [PubMed] [Google Scholar]
- Schatlo B, Glasker S, Zauner A, Thompson GB, Oldfield EH, Pluta RM. Correlation of end-tidal CO2 with transcranial Doppler flow velocity is decreased during chemoregulation in delayed cerebral vasospasm after subarachnoid haemorrhage—results of a pilot study. Acta Neurochir Suppl. 2008;104:249–250. doi: 10.1007/978-3-211-75718-5_49. [DOI] [PubMed] [Google Scholar]
- Schmieder K, Jarus-Dziedzic K, Wronski J, Harders A. CO2 reactivity in patients after subarachnoid haemorrhage. Acta Neurochir (Wien ) 1997;139:1038–1041. doi: 10.1007/BF01411557. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Does nitric oxide mediate the increases in cerebral blood flow elicited by hypercapnia. Proc Natl Acad Sci USA. 1992;89:3913–3916. doi: 10.1073/pnas.89.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Zhang F. Nitric oxide-dependent and -independent components of cerebrovasodilation elicited by hypercapnia. Am J Physiol. 1994;266:R546–R552. doi: 10.1152/ajpregu.1994.266.2.R546. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- Owusu BY, Stapley R, Patel RP. Nitric oxide formation versus scavenging: the red blood cell balancing act. J Physiol. 2012;590:4993–5000. doi: 10.1113/jphysiol.2012.234906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi AR, Pluta RM, Bakhtian KD, Qi M, Lonser RR. Reversal of cerebral vasospasm via intravenous sodium nitrite after subarachnoid hemorrhage in primates. J Neurosurg. 2011;115:1213–1220. doi: 10.3171/2011.7.JNS11390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide M, Bonev AD, Nelson MT, Wellman GC. Inversion of neurovascular coupling by subarachnoid blood depends on large-conductance Ca2+-activated K+ (BK) channels. Proc Natl Acad Sci USA. 2012;109:E1387–E1395. doi: 10.1073/pnas.1121359109. [DOI] [PMC free article] [PubMed] [Google Scholar]