Abstract
Background:
Venous thromboembolic prophylaxis (VTEp) is often delayed following traumatic brain injury (TBI), yet animal data suggest that it may reduce cerebral inflammation and improve cognitive recovery. We hypothesized that earlier VTEp initiation in severe TBI patients would result in more rapid neurologic recovery and reduced progression of brain injury on radiologic imaging.
Study Design:
Medical charts of severe TBI patients admitted to a level 1 trauma center in 2009-2010 were queried for admission Glasgow Coma Scale (GCS), head Abbreviated Injury Scale, Injury Severity Score (ISS), osmotherapy use, emergency neurosurgery, and delay to VTEp initiation. Progression (+1 = better, 0 = no change, −1 = worse) of brain injury on head CTs and neurologic exam (by bedside MD, nurse) was collected from patient charts. Head CT scan Marshall scores were calculated from the initial head CT results.
Results:
A total of 22, 34, and 19 patients received VTEp at early (<3 days), intermediate (3-5 days), and late (>5 days) time intervals, respectively. Clinical and radiologic brain injury characteristics on admission were similar among the three groups (P > 0.05), but ISS was greatest in the early group (P < 0.05). Initial head CT Marshall scores were similar in early and late groups. The slowest progression of brain injury on repeated head CT scans was in the early VTEp group up to 10 days after admission.
Conclusion:
Early initiation of prophylactic heparin in severe TBI is not associated with deterioration neurologic exam and may result in less progression of injury on brain imaging. Possible neuroprotective effects of heparin in humans need further investigation.
Keywords: Heparin, intracranial hemorrhage, traumatic brain injury, VTE prophylaxis
INTRODUCTION
The Centers for Disease Control and Prevention (CDC)[1] estimates that 1.7 million people sustain a traumatic brain injury (TBI) annually, of whom 52,000 die and 275,000 are hospitalized. TBI is a contributing factor to a third (30.5%) of all injury-related deaths in the United States. The mortality of brain injury, particularly in those with severe TBI (sTBI - presenting Glasgow Coma Scale [GCS] < 9), exceeds that of most other body system injuries and has been estimated at 23%.[2]
While the initial structural damage to brain tissue is the primary determinant of outcome, subsequent or persistent brain injury progression or expansion may be equally, if not more important in determining ultimate neurological recovery.[3,4]
Moderate and severe TBI patients are also at an increased risk of developing venous thromboembolic (VTE) complications including extremity deep vein thrombosis (DVT) and pulmonary embolism (PE). Thus, TBI patients, as other trauma populations, require anticoagulant prophylaxis (VTEp) to prevent these complications.[5,6,7,8] Heparin derivatives (such as unfractionated heparin [UFH] or low molecular weight heparin [LMWH]) are often administered subcutaneously as prophylactic treatment when considered safe in the injured patient.[9] However, clinicians caring for patients with brain injury may be hesitant to administer these agents early after injury given the perceived risk of worsening intracranial hemorrhage.[10,11]
Recent human studies suggest that early VTEp in patients with severe TBI is not associated with increased risk of worsening brain hemorrhage and confers benefit from lower rates of VTE complications.[12,13,14] Current Brain Trauma Foundation guidelines[15] recommend chemical prophylaxis for the prevention of VTE complications in severe TBI, but remain noncommittal on timing of initiation following injury.
In animal studies, a handful of reports indicate that heparin derivatives may, seemingly paradoxically, reduce progression of anatomic brain injury through anti-inflammatory effects.[16,17,18,19] To date, no human studies have evaluated the effects of chemical VTEp on the brain following injury. We hypothesized that early VTEp in patients with severe TBI is associated with reduced radiological progression of brain injury and more rapid clinical neurological recovery.
PATIENTS AND METHODS
Subject inclusion and exclusion criteria
This retrospective review was conducted in accordance with the ethical standards of the Perelman School of Medicine at the University of Pennsylvania and was approved by the Institutional Review Board.
The trauma registry of the Hospital of the University of Pennsylvania, an academic, tertiary care level 1 trauma center, was used to find eligible subjects. Electronic medical records (EMRs) of all consecutive injured patients who presented with traumatic brain injury (TBI) from January 1, 2009 to December 31, 2010 were queried. All eligible subjects were confirmed to have intracranial pathology on initial head computed tomography (CT). To restrict analysis to severely brain injured patients only, inclusion criteria included: Admission GCS ≤ 8, blunt mechanism of injury, hospital length of stay (LOS) >3 days, head Abbreviated Injury Scale (AIS) score ≥3, and Injury Severity Scale (ISS) score ≥11. Patients with preexisting thromboembolic disease, age <16 or transferred from an outside institution, were excluded, as were patients who died or were discharged prior to 72 h after hospital presentation. The primary outcome measurements of the study were neurologic deterioration as determined by bedside neurologic exam and worsening of intracranial bleeding as determined by serial head CT imaging.
Classification of groups: Timing of VTE prophylaxis initiation
Patients were classified into one of three groups: Those who received the first dose of prophylactic subcutaneous heparin (UFH three times a day or LMWH twice per day) in the first 72 hours after admission (early) or 5 days or more after admission (late). Patients receiving initial prophylactic heparin analogs after 72 h and before 120 h after admission were classified into the intermediate group. Exposure to therapeutic anticoagulation including intravenous UFH, therapeutic LMWH, and warfarin in the first 10 days of admission was also recorded.
Patient characteristics and ED records
Medical records of all study patients were reviewed to determine patient demographics (age, sex, and race), course of hospitalization (hospital LOS, mechanism of injury, and mortality). Emergency department and ICU records were queried for need for urgent interventions (osmotherapy) and procedures (emergent craniotomy/craniectomy, placement of ventriculostomy). Although not uniform, the practice of neurosurgeons at the Hospital of the University of Pennsylvania is to routinely insert parenchymal intracranial monitors rather than ventriculostomies except as mandated by head injury severity or specific clinical characteristics.
Head computed tomography: Admission and subsequent serial studies
Radiology records were examined for official results of head CTs (admission and subsequent studies) obtained in the first 10 days following admission. Per inclusion criteria, only subjects in whom the admission head CT was reported to demonstrate acute trauma-related pathology were included in the study. Such findings included epidural, subdural, subarachnoid, intraparenchymal and intraventricular hemorrhage as well as cerebral contusions, cerebral edema (shift, sulci effacement), and signs of diffuse axonal injury. The attending radiologist's description of all admission head CTs was used in the calculation of the Marshall score.[12] The official descriptions of subsequent serial head CT scans obtained after admission were graded for radiological signs of deterioration (−1: New or increased edema, increased hemorrhage, increased shift, new or additional areas of hemorrhage), improvement (+1: Reduced edema, resorbing hemorrhage, improved edema), or lack of change (+0). For each patient, sum totals for each time interval were added to the subsequent interval change in score (0, +1, or −1) and carried cumulatively to the subsequent time intervals. Results were gathered in 4-h windows for the first 12 h, a 12-h window for the second 12 h, 24-h windows for postadmission-day 2 through 3, and 48-h windows for days 4 through 7. Any head CT obtained on days 8-10 was grouped together into one 72-h interval. Abstractors were blinded to the timing of initiation of VTE prophylaxis.
Clinical neurological progression
Progress notes documented by bedside providers (MD, nurse, and advanced practice providers) during the first 10 days of admission were reviewed and entries were scored at 8-h intervals for the first 48 h and every 24 h for the subsequent 8 days. The conglomerate of notes entered during each time interval were used to obtain a score of −1 for clinical neurologic deterioration, +1 for improvement, or 0 for no change. Entries interpreted as neurologic deterioration included: Initiating/re-administering/increasing osmotherapy, reduced responsiveness, reduced motor/sensory response, need for ventriculostomy, initiation of cooling or neuromuscular blockade for intracranial hypertension, emergent craniotomy/craniectomy, and reintubation. Notes judged as indicators of improvement were increased wakefulness, more communicative, more eye opening, new onset of following commands, improved orientation, increased responsiveness, and improved motor/sensory function. When conflicting notes were found documented by the various providers, the final score reflected the note written by the bedside nurse.
Bleeding, VTE, and other complications
Development of DVTs and PEs were extracted from progress notes and radiological studies (extremity duplex scans, chest CT, ventilation perfusion lung scans). Serial white blood cell counts were also recorded.
Statistical analysis
The Mann–Whitney test was used to determine differences between means at each time interval. The Kruskal–Wallis test was used to analyze demographic data and differences in injury severity at the time of admission. Means ± standard error of the mean (SEM) are reported unless otherwise stated and differences were considered statistically significant if P < 0.05.
RESULTS
Patient population
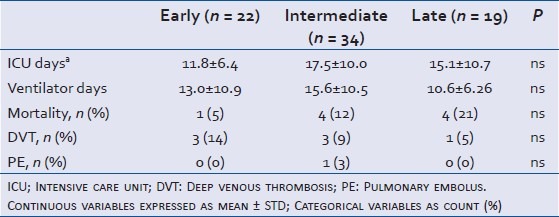
Seventy-five subjects sustained a blunt TBI and were admitted for longer than 3 days, fulfilling all inclusion and exclusion criteria over the designated study period. Most subjects were males (79%) and Caucasian (71%) with a mean age of 44 ± 19.5 years of age [Table 1]. Of these, 68 patients received chemical VTE prophylaxis during their admission. VTEp was initiated within the first 72 h (early) in 22 cases (32%), between day 3-5 (intermediate) in 34 (50%), and from days 6-10 (late) in 14 (18%) cases. Five patients received heparin therapy after 10 days, and were included in the "late" group. Three patients received LMWH (4%: 1 in the early group, 2 in the intermediate group, and 0 in the late group); the remainder received UFH. Sixty-five (96%) patients were also placed on sequential leg compression devices. No patient received therapeutic anticoagulation during the first 10 days of hospitalization. Early and late groups had a similar ICU LOS, ventilator days, and mortality. VTE complications were similar in all groups [Table 2].
Table 1.
Demographics and admission outcome data in severe TBI patients admitted in 2009-2010
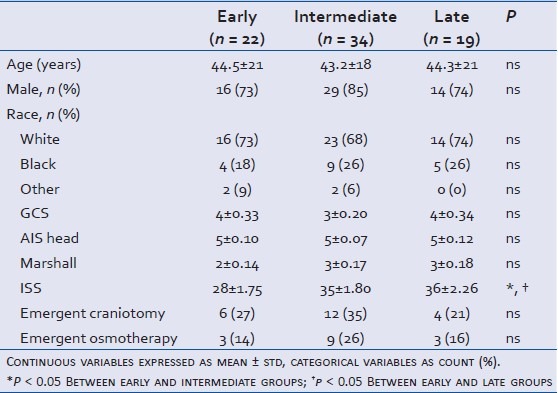
Table 2.
Admission outcome data in severe TBI patients admitted in 2009-2010
Severity of brain and global injury at admission
Clinical (GCS), anatomical (head AIS), and radiological (head CT Marshall score) severity grades at admission did not differ between groups [Table 1 and Figure 1]. However, patients in the early cohort were less globally injured with a significantly lower ISS score than those in either the intermediate or late cohorts (P < 0.05) [Table 1 and Figure 2]. Severity of brain injury upon ED presentation was similar between groups as demonstrated by comparable need for emergent treatment (need for osmotherapy in the ED) and need for immediate neurosurgical intervention (operative craniotomy/craniectomy directly from ED) in all groups [Table 1 and Figure 3]. Twenty-two patients needed emergent craniotomies/craniectomies upon ED presentation. Nonimmediate craniectomies/craniotomies were much less frequent occurring only in four patients (early 2, intermediate 1, and late 1) after ICU admission. Need for ventriculostomy placement and administration of osmotherapy after admission tended to be more frequent in patients receiving early vs. later VTEp, though these differences were not significant [Figure 4]. White blood cell count trends did not significantly differ over the 10-day period [Table 3].
Figure 1.
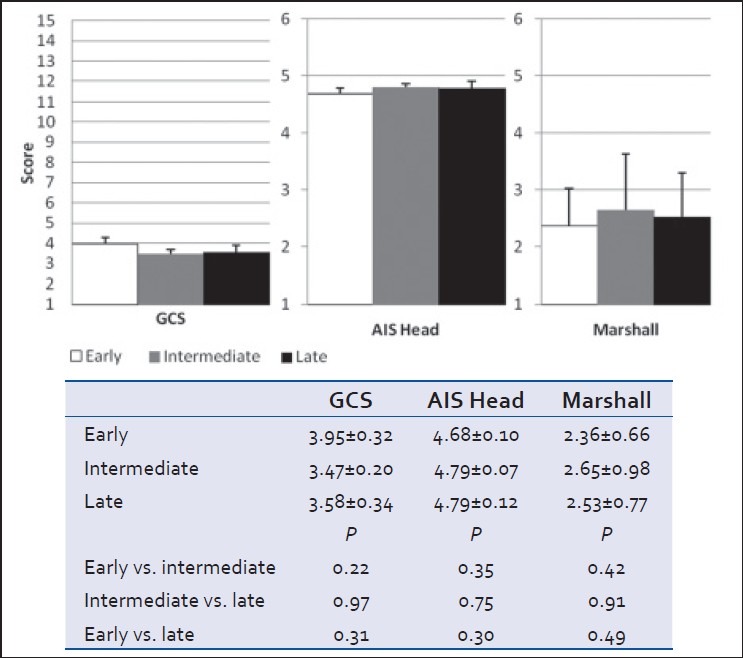
Glasgow coma scale (GCS), head abbreviated Injury Scale (AIS), and head CT Marshall Score at admission to hospital (mean ± SEM, P > 0.05)
Figure 2.
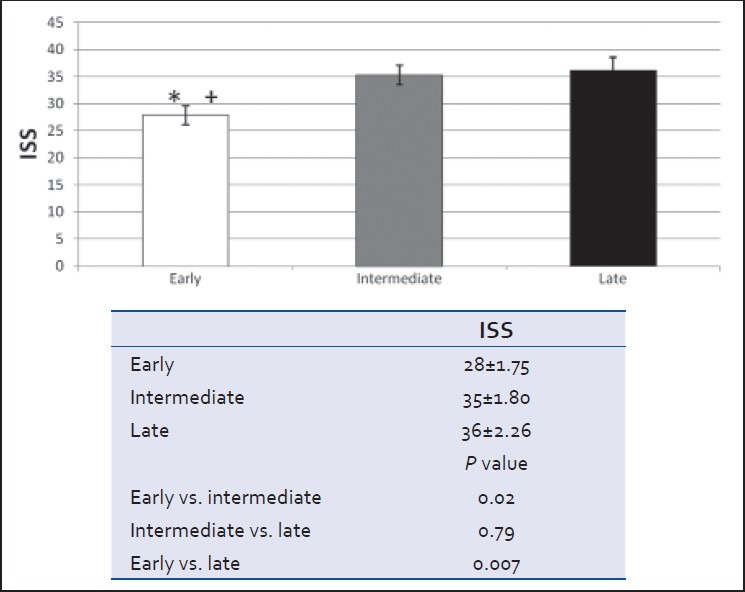
Injury severity score at admission to hospital (mean ± SEM, *P < 0.05 early vs. intermediate, †P < 0.05 early vs. late)
Figure 3.
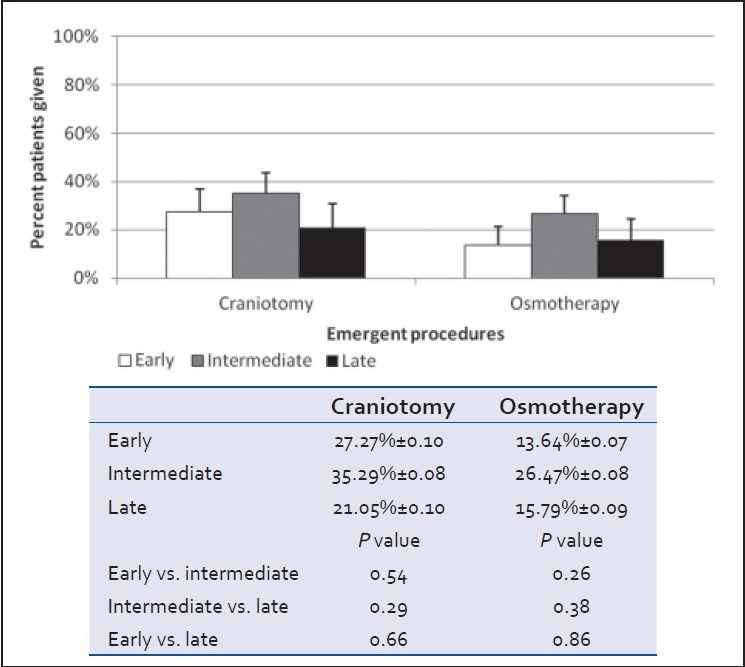
Emergent need for osmotherapy and surgery while the patient in the ED (mean ± SEM, P > 0.05)
Figure 4.
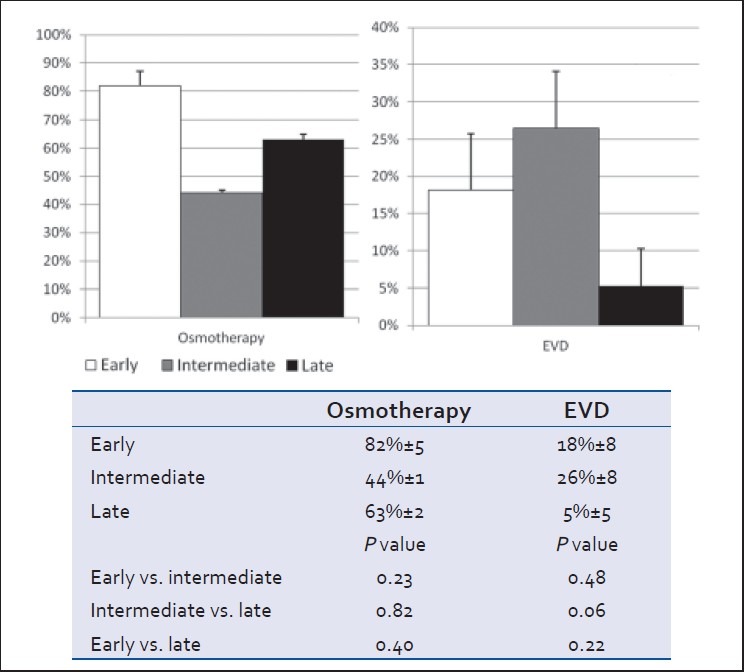
Proportion of patients requiring osmotherapy and placement of a ventriculostomy (EVD) in the 10 days following admission (mean ± SEM, P > 0.05)
Table 3.
White blood cell count over 10-day period (mean ± SEM)

Clinical neurological change as recorded by bedside provider
All three groups demonstrated cumulative clinical neurologic improvement during the first 10 days of admission [Figure 5]. Neurologic exam in patients of the early group improved at a faster rate than that in the late group, particularly on days 4-9 (P < 0.05). Changes in neurological recovery in the intermediate cohort did not differ significantly from those in either early or late cohorts.
Figure 5.
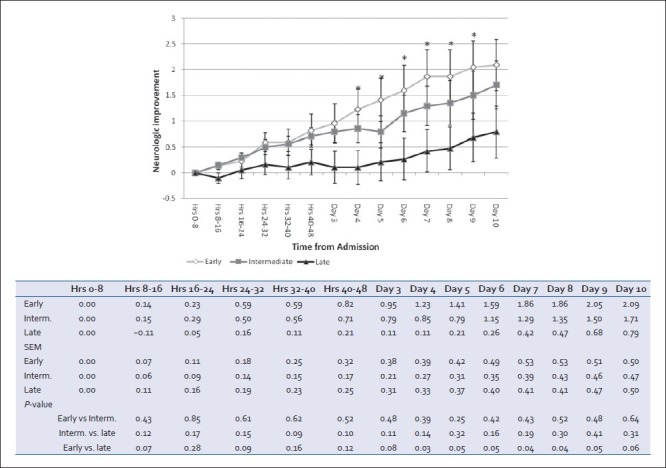
Cumulative clinical neurological changes based on serial nursing and physician documentation on daily progress notes. Positive deflection implies improvement, negative defl ection implies deterioration. For each patient at each time interval, progress notes were evaluated for change in neurological exam and a score of +1 (improvement), 0 (no change/no note documented), or −1 (deterioration) were averaged for the group (mean ± SEM, *P < 0.05 early vs. late)
Progression of head CT brain injury in official neuroradiologist report
In all three groups, head CTs demonstrated worsening of the injury during the first 24 h. Thereafter, scans tended to stabilize and finally demonstrate signs of resolution in all groups. The slowest progression of radiologic brain injury occurred in the early group, which was significantly slower than in the late group in hours 8-48 postadmission (P < 0.05) [Figure 6].
Figure 6.
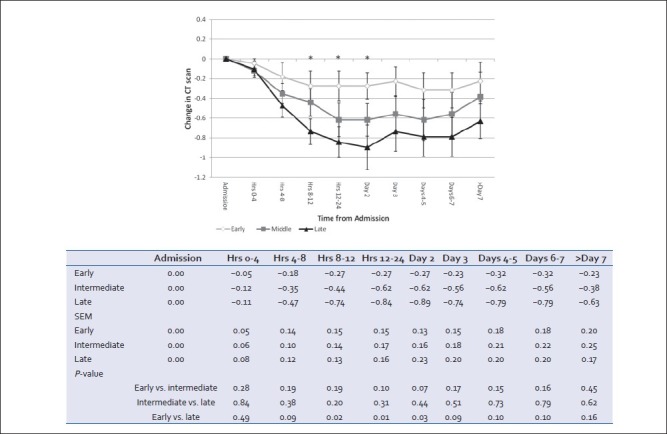
Radiological progression of intracranial lesion as described in serial head CTs by attending neuroradiologists. Positive deflection implies improvement, negative deflection implies worsening. For each patient at each time interval, radiology records were queried for change in neurological exam and a score of +1 (improvement), 0 (no change/no scan done during this time frame) or −1 (worsening) were averaged for the group (mean ± SEM, *P < 0.05 early vs. late)
DISCUSSION
The present study uses retrospective registry data to compare radiological and neurological recovery in subgroups of patients with severe TBI stratified according to timing of chemical VTE prophylaxis initiation. The data demonstrated that initiation of prophylactic heparin within three days of admission did not result in deterioration of the clinical neurologic exam, but was associated with a more rapid radiographic improvement in brain injury imaging on repeated head CT scans.
VTE prophylaxis forms the standard of care in all injured patients, particularly those with TBI as their risk of thromboembolic complications exceeds that associated with trauma to other systems.[5,6] Additionally, increasing evidence suggests that initiation of chemical VTE prophylaxis is safe and efficacious in preventing VTE events in patients with severe TBI.[10,20] Scudday et al.[13] reported that TBI patients receiving VTEp had half the incidence of venous thromboembolic events than those who did not. They further showed a nonsignificant trend toward a reduced rate of brain injury progression in those receiving VTE prophylaxis. Other large series have also suggested that progression of brain injury occurs at similar rates (1-4% per day) regardless of the presence or absence of chemical prophylaxis.[13,14] The current study is congruous with these results and suggests that earlier initiation of heparin prophylaxis is associated with more rapid improvement of radiological signs of brain injury in the first week after injury.
Optimal timing of chemical prophylaxis remains uncertain in recommendations from the Brain Trauma Foundation and other published studies. In a report by Norwood et al.,[10] initiation of heparin within 48 hours of blunt TBI resulted in <1% risk of progression of injury affecting neurologic outcome or any change in management. This study demonstrates radiologic worsening early after admission regardless of whether chemical prophylaxis is initiated or not. Concordant with the above studies, it also demonstrated that earlier VTEp initiation did not result in worsening radiologic injury progression.
The Marshall score[12] is used to compare head CT findings and ultimate neurologic recovery. Higher scores on admission CT have been associated with greater subsequent radiological worsening in both spontaneous subarachnoid hemorrhage and TBI populations.[21,22] In the present study, the Marshall scores were similar in early and late groups, further adding radiological evidence that initial severity of brain injury was similar in both groups.
Evolution of radiologic changes in head CT is a useful method to gauge improvement that parallels neurological recovery. Several studies have shown that when evaluating TBI patients with serial head CT scans an important number (18-40%) will develop progressive hemorrhagic injury and worse outcome.[23,24,25] The practice of using serial head CT to follow patients has become standard practice at ours and other centers.[16,26] Using these serial CT results, we further were able to characterize the cumulative change in radiologic injury progression which paralleled daily clinical findings of neurologic recovery in the early prophylaxis group. Neurological improvement associated with the early group compared to the intermediate administration seemed to start after day 4. However, these results were not significant and thus could not be seen as a clear indication that early prophylaxis had any benefit over intermediate administration.
Brain edema is a major component of TBI progression making the prevention of edema an important element in the treatment of traumatic intracranial lesions. Heparin analogs are known anticoagulants, but also possess anti-inflammatory and trophic properties that are also present in other anticoagulants, such as protein C.[27] In rodent studies, heparins have been shown to diminish brain edema, reduce cerebral lesion size, and improve cognitive impairment and neurological deficit following both experimental TBI and stroke.[16,17] In particular, Stuzmann and colleagues demonstrated how starting a LMWH in the first 24 hours after an ischemic insult significantly reduced brain contusion volume as observed 1 week after injury.[17] One proposed mechanism by which heparin components reduce brain edema is through reduction of polymorphonuclear neutrophil (PMN) trafficking in areas of contused brain. In experimental cerebral injury, PMN infiltration into the cerebral parenchyma due to hemorrhage and endothelial disruption has been shown to aggravate tissue injury by prolonging or intensifying the existing tissue ischemia.[18,19] One LMWH was found to reduce brain myeloperoxidase, a marker of PMN sequestration, by greater than 50% following rat TBI. Finally, blocking of the classic pathways of complement activation may also occur with certain anticoagulants in TBI relating to their ability to not only block coagulation but also inflammation.[28]
Other markers of brain edema have been studied in the process of TBI.[29,30] Preclinical models of TBI show an elevation of white blood cells within 4-48 hours after induced injury, suggesting that leukocytosis may interfere with cerebral microcirculation and contribute to secondary brain damage.[31] Additionally, Di Napoli et al. showed higher levels of C-reactive protein within a few hours of symptom onset of intracranial hemorrhage, and found higher levels of C-reactive protein associated with higher mortality and poor functional outcome.[29] The acutely injured brains also release interleukin-6, the extent of which correlates with subsequent enlargement of the hematoma.[32] However, our study did not find any significant difference in white blood cell count among the three groups, and C-reactive protein and fibrinogen levels were not regularly measured. Ultimately, according to our study white blood cells do not appear to be strongly correlated with TBI progression. However, other studies reporting white blood cell accumulation and elevated C-reactive protein suggest that brain edema plays a critical role in TBI, and that these markers are also potentially useful in evaluating the progression of TBI.
There are several limitations of this study. Being a retrospective chart review, it was impossible to confirm without a doubt that patients ordered VTE prophylaxis received all doses prescribed. Serial head CT scans were not re-read by blinded study radiologists but since official CT results used were created by attending neuroradiologists without knowledge of anticoagulation status, this effectively renders the radiological results blinded. Official interpretations of the scans were categorized into either worsened, no change, or improved, regardless of the magnitude of change, and as such, the subtleties and nuances in impressions may have been lost by this rigid categorization. Nonetheless, this categorization was conducted by a study team member without prior knowledge of VTEp timing. Concomitant administration of other nonheparin agents such as ASA and clopidogrel was not ascertained and may not have been evenly distributed among groups. In the daily bedside assessment of neurological function, we did not factor presence or absence of sedatives, narcotics, or medications that affect neurological recovery. Furthermore, clinical recovery scores were derived from provider notes that at times may have been vague or imprecise and may not have consisted in a strict neurological exam equally conducted in all subjects. It is conceivable that the late group, which had a more severe global injury, may have had greater exposure to sedatives than the early group. Due to the retrospective nature of the study, it could be argued that patients receiving earlier prophylaxis were judged to have a lesser brain injury by clinicians than those in the late group. However, patients in the early and late cohorts demonstrated similar admission GCS and head AIS score. Furthermore, the early group tended to require osmotherapy more often (both in the ED and in the subsequent 10 days), as well as emergent neurosurgical intervention and salvage ventriculostomy though these differences were not significant. Of all available methods employed to compare brain injury severity, none demonstrated differences in severity at presentation between groups. Interestingly however, ISS was greatest in the group where prophylaxis was initiated late. Since ISS is a composite sum of the squares of the three greatest AIS scores (head, face, neck chest, abdomen, spine, extremities) and head AIS scores did not differ among groups, this indicates a greater global injury severity in the late group without a greater head injury severity. This greater severity of injury likely contributed to render clinicians more reluctant to administer earlier prophylaxis though this cannot be confirmed from the EMR. Finally, the study was restricted to the first 10 days of admission and as such did not explore long-term neurological recovery.
To our knowledge, this study is the first to evaluate both the radiological and clinical progression of neurological injury after early or late initiation of VTE prophylaxis following severe TBI. While early initiation of an anticoagulant in patients with acute brain hemorrhage may appear counterintuitive, experimental animal data suggest that heparin possesses antinflammatory properties that may affect ongoing brain inflammation and edema. These pilot data in humans suggest that randomized, prospective, and blinded evaluation of the neuroprotective effects of VTE prophylaxis in patients with TBI is warranted.
Footnotes
Source of Support: There were no funding sources associated with any authors in this study.
Conflict of Interest: None declared.
REFERENCES
- 1.Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002-2006. Atlanta. 2010 [Google Scholar]
- 2.Hyam JA, Welch CA, Harrison DA, Menon DK. Case mix, outcomes and comparison of risk prediction models for admissions to adult, general and specialist critical care units for head injury: A secondary analysis of the ICNARC Case Mix Programme Database. Crit Care. 2006;10(Suppl 2):S2. doi: 10.1186/cc5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helmy A, Vizcaychipi M, Gupta AK. Traumatic brain injury: Intensive care management. Br J Anaesth. 2007;99:32–42. doi: 10.1093/bja/aem139. [DOI] [PubMed] [Google Scholar]
- 4.Pascual JL, Georgoff P, Maloney-Wilensky E, Sims C, Sarani B, Stiefel MF, et al. Reduced brain tissue oxygen in traumatic brain injury: Are most commonly used interventions successful.? J Trauma. 2011;70:535–46. doi: 10.1097/TA.0b013e31820b59de. [DOI] [PubMed] [Google Scholar]
- 5.Reiff DA, Haricharan RN, Bullington NM, Griffin RL, McGwin G, Jr, Rue LW, 3rd, et al. Traumatic brain injury is associated with the development of deep vein thrombosis independent of pharmacological prophylaxis. J Trauma. 2009;66:1436–40. doi: 10.1097/TA.0b013e31817fdf1c. [DOI] [PubMed] [Google Scholar]
- 6.Denson K, Morgan D, Cunningham R, Nigliazzo A, Brackett D, Lane M, et al. Incidence of venous thromboembolism in patients with traumatic brain injury. Am J Surg. 2007;193:380–3. doi: 10.1016/j.amjsurg.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331:1601–6. doi: 10.1056/NEJM199412153312401. [DOI] [PubMed] [Google Scholar]
- 8.Ekeh AP, Dominguez KM, Markert RJ, McCarthy MC. Incidence and risk factors for deep venous thrombosis after moderate and severe brain injury. J Trauma. 2010;68:912–5. doi: 10.1097/TA.0b013e3181b21cad. [DOI] [PubMed] [Google Scholar]
- 9.Jamjoom AA, Jamjoom AB. Safety and efficacy of early pharmacological thromboprophylaxis in traumatic brain injury: Systematic review and meta-analysis. J Neurotrauma. 2013;30:503–11. doi: 10.1089/neu.2012.2584. [DOI] [PubMed] [Google Scholar]
- 10.Norwood SH, Berne JD, Rowe SA, Villarreal DH, Ledlie JT. Early venous thromboembolism prophylaxis with enoxaparin in patients with blunt traumatic brain injury. J Trauma. 2008;65:1021–6. doi: 10.1097/TA.0b013e31818a0e74. [DOI] [PubMed] [Google Scholar]
- 11.Carlile MC, Yablon SA, Mysiw WJ, Frol AB, Lo D, Diaz-Arrastia R. Deep venous thrombosis management following traumatic brain injury: A practice survey of the traumatic brain injury model systems. J Head Trauma Rehabil. 2006;21:483–90. doi: 10.1097/00001199-200611000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Marshall LF, Marshall SB, Klauber MR, Van Berkum Clark M, Eisenberg H, Jane JA, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9(Suppl 1):S287–92. [PubMed] [Google Scholar]
- 13.Scudday T, Brasel K, Webb T, Codner P, Somberg L, Weigelt J, et al. Safety and efficacy of prophylactic anticoagulation in patients with traumatic brain injury. J Am Coll Surg. 2011;213:148–53. doi: 10.1016/j.jamcollsurg.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 14.Koehler DM, Shipman J, Davidson MA, Guillamondegui O. Is early venous thromboembolism prophylaxis safe in trauma patients with intracranial hemorrhage. J Trauma. 2011;70:324–9. doi: 10.1097/TA.0b013e31820b5d22. [DOI] [PubMed] [Google Scholar]
- 15.Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS. Bratton SL, et al. Guidelines for the management of severe traumatic brain injury. V. Deep vein thrombosis prophylaxis. J Neurotrauma. 2007;24(Suppl 1):S32–6. doi: 10.1089/neu.2007.9991. [DOI] [PubMed] [Google Scholar]
- 16.Wahl F, Grosjean-Piot O, Bareyre F, Uzan A, Stutzmann JM. Enoxaparin reduces brain edema, cerebral lesions, and improves motor and cognitive impairments induced by a traumatic brain injury in rats. J Neurotrauma. 2000;17:1055–65. doi: 10.1089/neu.2000.17.1055. [DOI] [PubMed] [Google Scholar]
- 17.Stutzmann JM, Mary V, Wahl F, Grosjean-Piot O, Uzan A, Pratt J. Neuroprotective profile of enoxaparin, a low molecular weight heparin, in in vivo models of cerebral ischemia or traumatic brain injury in rats: A review. CNS Drug Rev. 2002;8:1–30. doi: 10.1111/j.1527-3458.2002.tb00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoettle RJ, Kochanek PM, Magargee MJ, Uhl MW, Nemoto EM. Early polymorphonuclear leukocyte accumulation correlates with the development of posttraumatic cerebral edema in rats. J Neurotrauma. 1990;7:207–17. doi: 10.1089/neu.1990.7.207. [DOI] [PubMed] [Google Scholar]
- 19.Soares HD, Hicks RR, Smith D, McIntosh TK. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J Neurosci. 1995;15:8223–33. doi: 10.1523/JNEUROSCI.15-12-08223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Gearhart MM, Zurick A, Zuccarello M, James L, Luchette FA. Preliminary report on the safety of heparin for deep venous thrombosis prophylaxis after severe head injury. J Trauma. 2002;53:38–42. doi: 10.1097/00005373-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Chieregato A, Fainardi E, Morselli-Labate AM, Antonelli V, Compagnone C, Targa L, et al. Factors associated with neurological outcome and lesion progression in traumatic subarachnoid hemorrhage patients. Neurosurgery. 2005;56:671–80. doi: 10.1227/01.neu.0000156200.76331.7a. [DOI] [PubMed] [Google Scholar]
- 22.Smith JS, Chang EF, Rosenthal G, Meeker M, von Koch C, Manley GT, et al. The role of early follow-up computed tomography imaging in the management of traumatic brain injury patients with intracranial hemorrhage. J Trauma. 2007;63:75–82. doi: 10.1097/01.ta.0000245991.42871.87. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi S, Nakazawa S, Otsuka T. Clinical value of serial computed tomography with severe head injury. Surg Neurol. 1983;20:25–9. doi: 10.1016/0090-3019(83)90101-5. [DOI] [PubMed] [Google Scholar]
- 24.Kaups KL, Davis JW, Parks SN. Routinely repeated computed tomography after blunt head trauma: Does it benefit patients.? J Trauma. 2004;56:475–80. doi: 10.1097/01.ta.0000114304.56006.d4. [DOI] [PubMed] [Google Scholar]
- 25.Oertel M, Kelly DF, McArthur D, Boscardin WJ, Glenn TC, Lee JH, et al. Progressive hemorrhage after head trauma: Predictors and consequences of the evolving injury. J Neurosurg. 2002;96:109–16. doi: 10.3171/jns.2002.96.1.0109. [DOI] [PubMed] [Google Scholar]
- 26.Cooper PR, Maravilla K, Moody S, Clark WK. Serial computerized tomographic scanning and the prognosis of severe head injury. Neurosurgery. 1979;5:566–9. doi: 10.1227/00006123-197911000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Bernard GR. Drotrecogin alfa (activated) (recombinant human activated protein C) for the treatment of severe sepsis. Crit Care Med. 2003;31(1 Suppl):S85–93. doi: 10.1097/00003246-200301001-00012. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Pinzon MA, Maier CM, Yoon EJ, Sun GH, Giffard RG, Steinberg GK. Correlation of CGS 19755 neuroprotection against in vitro excitotoxicity and focal cerebral ischemia. J Cereb Blood Flow Metab. 1995;15:865–76. doi: 10.1038/jcbfm.1995.108. [DOI] [PubMed] [Google Scholar]
- 29.Di Napoli M, Godoy DA, Campi V, Masotti L, Smith CJ, Parry Jones AR, et al. C-reactive protein in intracerebral hemorrhage: Time course, tissue localization, and prognosis. Neurology. 2012;79:690–9. doi: 10.1212/WNL.0b013e318264e3be. [DOI] [PubMed] [Google Scholar]
- 30.Sun W, Peacock A, Becker J, Phillips-Bute B, Laskowitz DT, James ML. Correlation of leukocytosis with early neurological deterioration following supratentorial intracerebral hemorrhage. J Clin Neurosci. 2012;19:1096–100. doi: 10.1016/j.jocn.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartl R, Medary MB, Ruge M, Arfors KE, Ghajar J. Early white blood cell dynamics after traumatic brain injury: Effects on the cerebral microcirculation. J Cereb Blood Flow Metab. 1997;17:1210–20. doi: 10.1097/00004647-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Silva Y, Leira R, Tejada J, Lainez JM, Castillo J, Dávalos A. Stroke Project, Cerebrovascular Diseases Group of the Spanish Neurological Society. Molecular signatures of vascular injury are associated with early growth of intracerebral hemorrhage. Stroke. 2005;36:86–91. doi: 10.1161/01.STR.0000149615.51204.0b. [DOI] [PubMed] [Google Scholar]


