Abstract
Background:
Nitric oxide (NO) has been shown to increase following hemorrhagic shock (HS). Peroxynitrite is produced by the reaction of NO with reactive oxygen species, leads to nitrosative stress mediated organ injury. We examined the protective effects of a potent inhibitor of NO synthase, aminoguanidine (AG), on myocardial and multiple organ structure in a rat model of HS.
Materials and Methods:
Male Sprague Dawley rats (300-350 g) were assigned to 3 experimental groups (n = 6 per group): (1) Normotensive rats (N), (2) HS rats and (3) HS rats treated with AG (HS-AG). Rats were hemorrhaged over 60 min to reach a mean arterial blood pressure of 40 mmHg. Rats were treated with 1 ml of 60 mg/kg AG intra-arterially after 60 min HS. Resuscitation was performed in vivo by the reinfusion of the shed blood for 30 min to restore normo-tension. Biopsy samples were taken for light and electron microscopy.
Results:
Histological examination of hemorrhagic shocked untreated rats revealed structural damage. Less histological damage was observed in multiple organs in AG-treated rats. AG-treatment decreased the number of inflammatory cells and mitochondrial swollen in myocardial cells.
Conclusion:
AG treatment reduced microscopic damage and injury in multiple organs in a HS model in rats.
Keywords: Aminoguanidine, hemorrhagic shock, rats, resuscitation
INTRODUCTION
Hemorrhagic shock (HS) is the major cause of death in trauma patients.[1,2,3,4,5] One of the causes of death is multiple organ dysfunction and failure.[4,6] The precise mechanisms of multiple organ injury and subsequent failure must be highlighted to improve the resuscitation strategies.
HS has been shown to increase the production of nitric oxide (NO).[7] Studies have shown that one mechanism of multiple organ damage is due to peroxynitrite, which is produced by the reaction of NO with reactive oxygen species that leads to nitrosative stress mediated organ injury.[7,8,9,10]
Previous studies have shown the beneficial effects of NO synthase (NOS) inhibitors following HS. L-arginine derivatives, such as L-NMMA and L-NAME, inhibit nitric oxide synthase and increase the survival rate following HS in rats.[11,12] aminoguanidine (AG) is a more potent NOS inhibitor than L-NMMA and L-NAME.[13,14,15] AG treatment delays the circulatory failure following septic shock in rats and improves survival in a murine model of septic shock.[15,16] The protective effects of AG on microscopic structures in multiple organs following HS have not been investigated.
The current study examined the protective effects of treatment with AG before resuscitation following HS against multiple organ injury and failure by examining the tissues from different organs under light microscopy. The study also examined the involvement of inflammatory pathways in the pathogenesis of multiple organ injury by measuring the levels of tumor necrosis factor-alpha after treatment with AG and comparing and comparing the results to the non-treated resuscitated group. The results of the current study showed that treatment with AG protects against multiple injuries and this may be a new therapeutic tool in the management of HS and resuscitation.
MATERIALS AND METHODS
Animal preparation
This study was approved by the National Plan for Sciences and Technologies, King Saud University. Male Sprague-Dawley weighing 300-350 g were used. Rats were injected intra-peritonealy (i.p.) with 2000 I.U of heparin sodium 15 min prior to anesthesia to prevent coagulation of the blood in the isolated hearts and vasculature. The rats were anesthetized using 125 mg/kg of urethane intra-peritoneally. The left carotid artery was cannulated and a three-way stopcock was attached in-line for monitoring the mean arterial blood pressure (MABP) using a blood pressure transducer. The animals were allowed to stabilize for a period of 30 min. The animals were assigned to the following experimental groups (n = 6 per group): (1) Normotensive rats (N), (2) HS rats (HS) and (3) HS rats treated with AG (HS-AG).
After 60 min HS, rats were treated or not by injection of 1 ml of 60 mg/kg AG dissolved in normal saline intra-arterially. A pilot experiment was performed without carotid artery cannulation to exclude any effects of carotid artery cannulation on contractility.
HS
After a stabilization period of 30 min, HS was induced. Rats were hemorrhaged using a technique similar to that described by Wiggers et al.[17,18] In this model, a reservoir (a 10 ml syringe) were connected to the arterial (carotid artery) a three-way stopcock (as described previously). Opening the stopcock and aspirating gently and gradually with the syringe will induce hemorrhage. Blood was aspirated 1 ml over 1 min time. Using this model, the animal blood pressure was observed by closing the stopcock to the syringe. When the stopcock was opened again to the syringe, hemorrhage was continued. Blood was continuously withdrawn or reinfused to the animal to maintain mean arterial pressure to 40 mmHg. The HS model with cannulation of one artery did not affect the recording of MABP. With the exception of inducing the hemorrhage, the surgical procedure was the same for the sham hemorrhage group. The HS model was used in our lab in several studies.[19,20,21]
The rats were resuscitated in vivo by reinfusion of the shed blood to restore normo-tension and the MABP was monitored for 30 min.
AG was obtained from Sigma (Sigma, St Louis, MO). The drug was dissolved in a 0.9% sodium chloride solution (Sigma).
MABP
The MABP was monitored for the 60-min duration of HS and for 30 min after resuscitation.
Experimental protocols
Three experimental groups (n = 6) were assigned for the study [Figure 1]:
Figure 1.
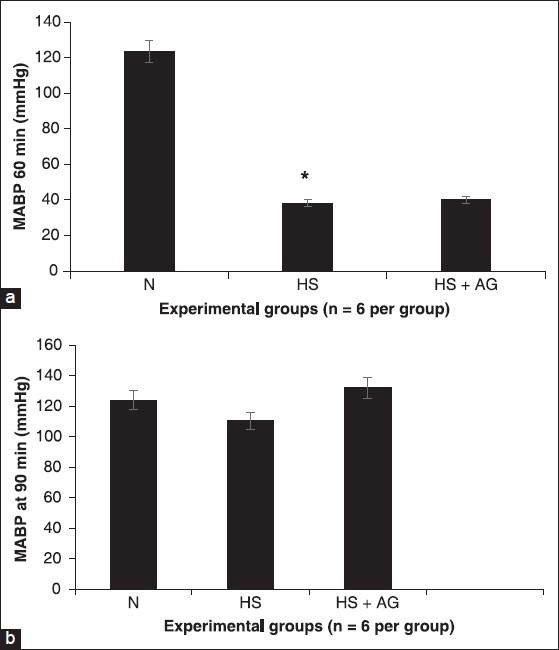
Effects of in vivo treatment with aminoguanidine (AG) on mean arterial blood pressure in rats (a and b). Recording of arterial blood pressure after 1 h hemorrhages (a) and 30 min of resuscitation (b) in the normotensive group (N), hemorrhage group hemorrhagic shock (HS), hemorrhage group treated with AG (HS-AG). *represents P < 0.05 versus hemorrhagic shock resuscitated group treated and not treated
Normotensive rats (N). The rats underwent the same surgical preparation and continuous blood pressure measurements were obtained for the 120-min experimental period. The control normotensive group was injected with the same volume (1 ml) normal saline.
HS rats. After a 30-min stabilization period, the rats were hemorrhaged to 40 mmHg for 60 min. The rats were then resuscitated and monitored for 30 min.
Effect of AG during (HS-AG). After a 30 min stabilization period, the rats were hemorrhaged to 40 mmHg for 60 min. AG was injected intra-arterially. The rats were then resuscitated and monitored for 30 min.
Light microscopy
To perform histological examination of multiple organs, rat heart, lung, intestine and kidney were harvested and stored in 10% formalin solution (n = 6 in each group). We obtained 2 transverse sections per organ for histopathological examination and sections were stained with H and E stain. The analysis was double blinded. The areas affected by HS and resuscitation consisting of inflammatory cells, necrosis and hemorrhage, were determined in the H and E staining. All data were analyzed in a blind fashion.
Electron microscopy
Myocardial biopsy samples were obtained by cutting 1 mm thick samples with a surgical blade from the left ventricles’ walls within 60 s of isolation of hearts. The tissue obtained at biopsy was cut into 1 mm3 pieces and fixed in 3% buffered glutaraldehyde for transmission electron microscopy. Tissue were fixed with 1% osmium tetraoxide in 0.1M cocodylate buffer, dehydrated and embedded in epon. Thin sections were stained with uranyl acetate and lead citrate and photographed with JEOL-JEM 1010 transmission electron microscopy.
Statistical analysis
Data were presented as means ± standard deviation. Data was analyzed with one way ANOVA. The post-hoc test used was Tukey's test. The values of P < 0.05 were considered as significant. The student t-test was used to compare mean values between the two experimental groups.
RESULTS
All animals underwent HS after the withdrawal of blood to reach a blood pressure of 35-40 mmHg. The total volume of blood withdrawn was 15 ± 2.3 ml blood/kg body weight.
MABP
Normotensive group rats maintained MABP at around 123.73 ± 4.84 mmHg at 60 min [Figure 1a]. Although an HS rats has a significantly lower levels of MABP at around 38.18 ± 1.27 mmHg. In hemorrhagic shocked animals, intra-arterial administration of AG (60 mg/kg) did not cause any significant difference in MABP at the end of the experimental period (90 min) compared with the normotensive group (N) and the HS group [Figure 1b].
Microscopic examination
Light microscopy
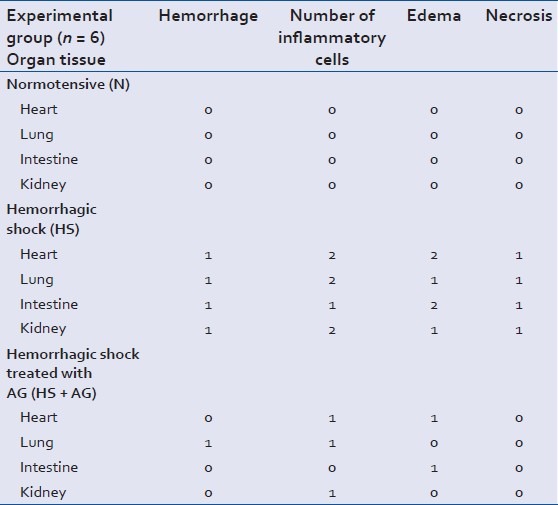
Figure 2 shows normal tissue sections of heart, lung, intestine and kidney from normotensive group. Multiple organs demonstrated microscopic injury following HS and resuscitation. The histological findings were quantified for index of organ injury in regards to hemorrhage, number of inflammatory cells, edema and necrosis in a scale from 0 to 1:0 is absent, 1 is mild and 3 is severe [Table 1].
Figure 2.
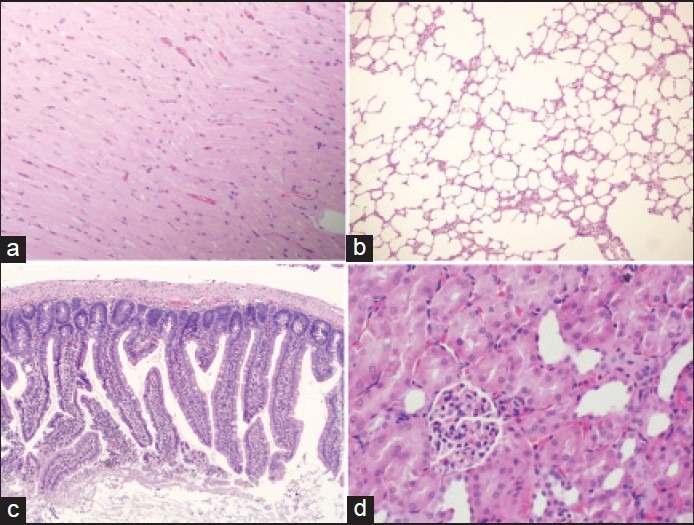
Light micrographs of multiple organs from normotensive rats. (a) Heart section showing, H and E, ×100. (b) Lung section, H and E, ×100. (c) Intestinal section, H and E, ×100. (d) Kidney section, H and E, ×400
Table 1.
Index of organ injury for organ from the experimental groups, 0 absent, 1 mild and 2 severe. Data is presented as mean (n = 6 per group)
Contraction bands were observed in the H and E-stained sections of the myocardium from hemorrhagic-shocked rats [Figure 3a–c]. This damage was less apparent in sections from hemorrhagic shocked hearts treated with AG [Figure 4a].
Marked cellular lung infiltration and evidence of intra-alveolar edema, congested capillaries and expansion of the interstitium was observed after HS [Figure 3d and e].
The intestinal mucosa revealed hemorrhagic necrosis and infiltration of mucosa by mononuclear cells and eosinophils with congestion of blood vessels [Figure 3f].
Kidney tissue sections exhibit glomerular edema and congestion of blood vessels [Figure 3g].
Figure 3.
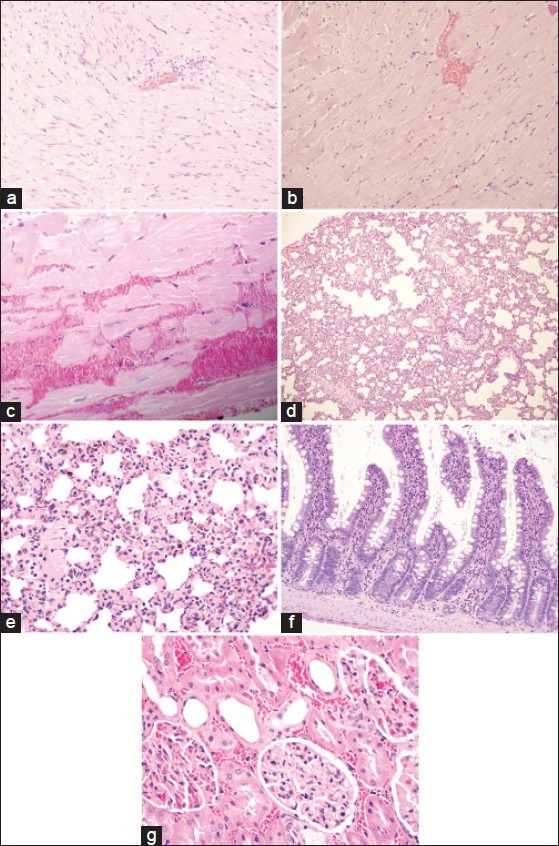
Light micrographs of multiple organs from hemorrhagic shocked rats. (a-c) Heart section showing foci of hemorrhage, esoinophilic contraction of some cardiac muscles and a few inflammatory cell infiltrations around blood vessels, H and E, ×200, ×200 and ×400. (d and e) Lung section with evidence of intra-alveolar edema, congested capillaries and expansion of the interstitium, H and E, ×100 and ×400. (f) Intestinal section showing intestinal mucosa revealing hemorrhagic necrosis and infiltration of mucosa by mononuclear cells and eosinophils with congestion of blood vessels, H and E, ×200. (g) Kidney section showing glomerular edema and congestion of blood vessels, H and E, ×400
Figure 4.
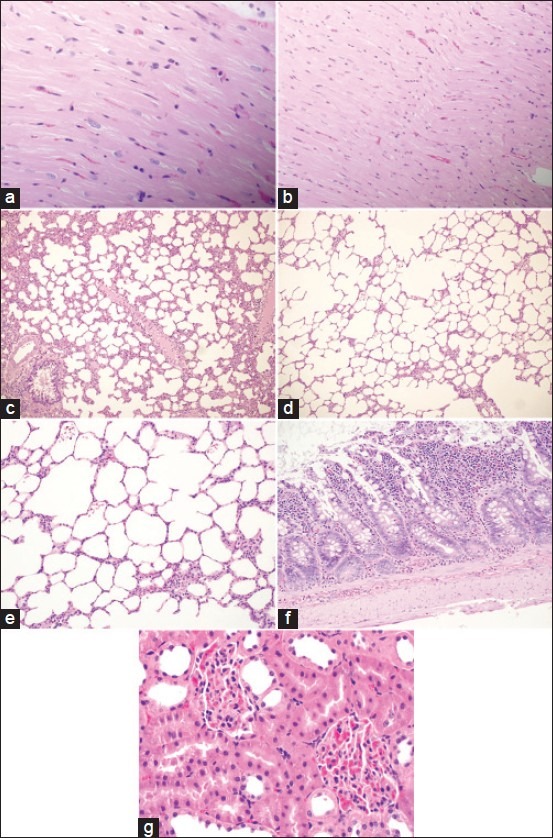
Light micrographs of multiple organs from hemorrhagic shocked rats treated with aminoguanidineshowing normal structure. (a and b) Heart section, H and E, ×200 and ×400. (c-e) Lung section, H and E, ×100, ×100 and ×400. (f) Intestinal section, H and E, ×200. (g) Kidney section,H and E, ×400
Sections from multiple organs of HS animals treated with AG exhibit less tissue injury. These data suggest that NOS inhibition is a novel therapy for protection against HS and resuscitation of multiple organ injury.
Electron microscopy
Electron microscopy was performed in randomly selected hearts from the in vivo hemorrhage treated and untreated groups [Figure 5]. The hemorrhage group showed prominent I bands and swollen mitochondria with disrupted cristae and amorphous matrix densities. However, no such changes were seen in the treated group. Significant inflammatory cell infiltrates around the coronary vessels was found in the untreated group.
Figure 5.
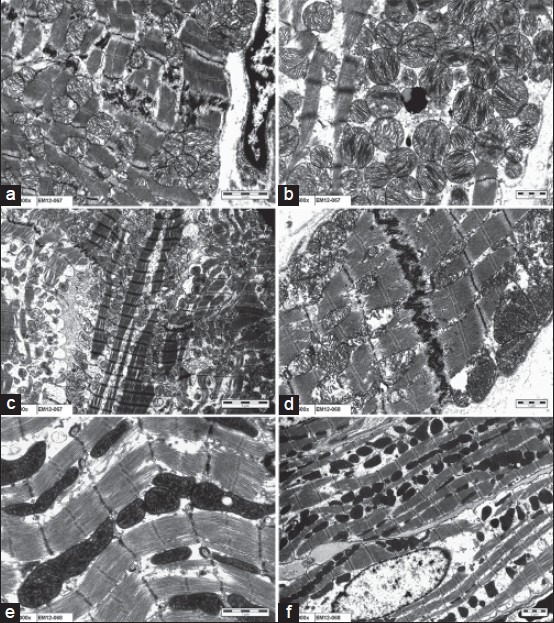
Electron microscopic analyses. (a-c) Hemorrhage untreated hearts showing mitochondrial swelling with disrupted cristae, ×2000, ×2500, ×800. (d-f) Aminoguanidine treated hearts showing mild mitochondrial swelling with clear cristae, ×2500, ×4000, ×1000
DISCUSSION
The present study demonstrated that HS produced multiple organ damage following HS and resuscitation, which suggested that organ injury, was due to, at least in part, to the increased production of NO. HS is associated with an increased production of NO.[22,23,24] NO contributes to the pathophysiology of multiple organ damage that is associated with HS.[8,24,25] An inducible isoform of NO synthase (iNOS) is expressed during HS, which results in the excessive formation of NO that contributes to multiple organ injury.[8] Regulation of NO production and the use of iNOS inhibitors might provide new aspect in the treatment of HS.
The present study also demonstrated that AG, a selective inhibitor of iNOS, protect against multiple organ injury and subsequent failure after resuscitation from HS in rats. The regulation of NO generation and the use of NOS inhibitors may produce novel treatments for HS.[23,26] A number of investigators have suggested that inhibition of NOS activity may be a useful therapeutic approach following low flow conditions. No derived from iNOS has been implicated in many pathological events[27,28] and in the development of tissue injury.[29]
NOS inhibitors have been shown to reduce the generation of peroxynitrite during reperfusion in an isolated rat heart model which led to protection.[30] Peroxynitrite generated during reperfusion mediates oxidative injury toward a membrane phospholipid,[31] damages mitochondrial enzymes and induces an increase in the intracellular Ca2+ concentration, resulting in impairment of the contractile protein (Ishida et al., 1994). In the present study, AG inhibited the production of NO by inhibition of iNOS, resulting in preventing organ injury and damage following HS.
AG has many pharmacological properties, including inhibition of advanced glycosylation end product formation, catalase, polyamine catabolism and histamine metabolism. Therefore, the beneficial effect of AG might not be only due to inhibition of iNOS activity (Hua and Moochhala 1999).
Our results demonstrated that a NOS inhibitor, AG, is a potential therapeutic agent for the treatment of HS in an experimental rat model. The route of administration is applied in animal experimental studies. The data suggest that NOS inhibition is a novel therapy for protection against multiple organ injury and failure following HS and resuscitation. Further studies are needed to investigate the proper route of administration in clinical conditions and to measure inflammatory mediators from ELISA.
In summary, the present study indicated that the administration of a potent NOS inhibitor, AG, reduced damage to cardiac myocytes and multiple organs after HS and resuscitation in rats. The protective effects of NOS inhibition by AG in the present study indicated that NO synthesis contributed to the multiple organ damage following HS and resuscitation.
NO per se, does not result in organ injury. Peroxynitrite which is produced by the reaction of NO with reactive oxygen species leads to nitrosative stress mediated organ injury.[15] However, the use of a potent NOS inhibitor, AG, decreased organ injury.
The present study also showed that HS resulted in microscopic multiple organ injuries in the rat. Our results demonstrated that a potent NOS inhibitor, AG, protected against multiple organ injury in rats following HS. AG (10 mg/kg) protected against microscopic injuries that are associated with HS.
Limitation of the study
The present study demonstrates that AG protect against organ injury following HS. The mechanism of protection is by inhibition of iNOS. However, the present study did not measure the levels of NO, which seems to be a limitation of the study. Further studies are needed to measure NO contents in plasma or tissue, NO expressions, messenger ribonucleic acid or protein levels. Furthermore, the measurement of organ functions would have supported the data.
ACKNOWLEDGMENT
This work was supported by a grant from the National Plan for Science, Technology and Innovation (08-MED560-02) at King Saud University, Riyadh, Saudi Arabia. Technical help from Mr. Sabirine is acknowledged.
Footnotes
Source of Support: The research has been supported by a grant from the National Plan for Science, Technology and Innovation at King Saud University, Riyadh, Saudi Arabia.
Conflict of Interest: None declared.
REFERENCES
- 1.Malinoski DJ, Patel MS, Lush S, Willis ML, Navarro S, Schulman D, et al. Impact of compliance with the American College of Surgeons trauma center verification requirements on organ donation-related outcomes. J Am Coll Surg. 2012;215:186–92. doi: 10.1016/j.jamcollsurg.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abjean J. Occlusal requirements in prosthetic reconstruction in view of deep periodontal lesions. J Parodontol. 1986;5:353–63. [PubMed] [Google Scholar]
- 3.Bellamy RF. The causes of death in conventional land warfare: Implications for combat casualty care research. Mil Med. 1984;149:55–62. [PubMed] [Google Scholar]
- 4.Cuschieri J, Johnson JL, Sperry J, West MA, Moore EE, Minei JP, et al. Benchmarking outcomes in the critically injured trauma patient and the effect of implementing standard operating procedures. Ann Surg. 2012;255:993–9. doi: 10.1097/SLA.0b013e31824f1ebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury multiple organ failure: A bimodal phenomenon. J Trauma. 1996;40:501–10. doi: 10.1097/00005373-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 6.DeCamp MM, Demling RH. Posttraumatic multisystem organ failure. JAMA. 1988;260:530–4. [PubMed] [Google Scholar]
- 7.Wang Y, Yan J, Xi L, Qian Z, Wang Z, Yang L. Protective effect of crocetin on hemorrhagic shock-induced acute renal failure in rats. Shock. 2012;38:63–7. doi: 10.1097/SHK.0b013e3182596ec4. [DOI] [PubMed] [Google Scholar]
- 8.Ochoa JB, Udekwu AO, Billiar TR, Curran RD, Cerra FB, Simmons RL, et al. Nitrogen oxide levels in patients after trauma and during sepsis. Ann Surg. 1991;214:621–6. doi: 10.1097/00000658-199111000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel NS, Nandra KK, Brines M, Collino M, Wong WF, Kapoor A, et al. A nonerythropoietic peptide that mimics the 3D structure of erythropoietin reduces organ injury/dysfunction and inflammation in experimental hemorrhagic shock. Mol Med. 2011;17:883–92. doi: 10.2119/molmed.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isayama K, Murao Y, Saito F, Hirakawa A, Nakatani T. Effects of hypertonic saline on CD4+CD25+Foxp3+ regulatory T cells after hemorrhagic shock in relation to iNOS and cytokines. J Surg Res. 2012;172:137–45. doi: 10.1016/j.jss.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 11.Calapai G, Squadrito F, Altavilla D, Zingarelli B, Campo GM, Cilia M, et al. Evidence that nitric oxide modulates drinking behaviour. Neuropharmacology. 1992;31:761–4. doi: 10.1016/0028-3908(92)90038-q. [DOI] [PubMed] [Google Scholar]
- 12.Balaszczuk AM, Arreche ND, Mc Laughlin M, Arranz C, Fellet AL. Nitric oxide synthases are involved in the modulation of cardiovascular adaptation in hemorrhaged rats. Vascul Pharmacol. 2006;44:417–26. doi: 10.1016/j.vph.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Moncada S. Adventures in pharmacology, aspirin, prostacyclin and nitric oxide. Methods Find Exp Clin Pharmacol. 1997;19(Suppl A):3–5. [PubMed] [Google Scholar]
- 14.James ML, Sullivan PM, Lascola CD, Vitek MP, Laskowitz DT. Pharmacogenomic effects of apolipoprotein e on intracerebral hemorrhage. Stroke. 2009;40:632–9. doi: 10.1161/STROKEAHA.108.530402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishikawa K, Calzavacca P, Bellomo R, Bailey M, May CN. Effect of selective inhibition of renal inducible nitric oxide synthase on renal blood flow and function in experimental hyperdynamic sepsis. Crit Care Med. 2012;40:2368–75. doi: 10.1097/CCM.0b013e3182514be9. [DOI] [PubMed] [Google Scholar]
- 16.Wu CC, Chen SJ, Szabó C, Thiemermann C, Vane JR. Aminoguanidine attenuates the delayed circulatory failure and improves survival in rodent models of endotoxic shock. Br J Pharmacol. 1995;114:1666–72. doi: 10.1111/j.1476-5381.1995.tb14955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans HM, Cannon WB, Fulton JF, Wiggers CJ. The Houssay journal fund. Science. 1945;102:161. doi: 10.1126/science.102.2641.161. [DOI] [PubMed] [Google Scholar]
- 18.Wiggers HC, Goldberg H, Roemhild F, Ingraham RC. Impending hemorrhagic shock and the course of events following administration of dibenamine. Circulation. 1950;2:179–85. doi: 10.1161/01.cir.2.2.179. [DOI] [PubMed] [Google Scholar]
- 19.Soliman MM. Na(+)-H(+) exchange blockade, using amiloride, decreases the inflammatory response following hemorrhagic shock and resuscitation in rats. Eur J Pharmacol. 2011;650:324–7. doi: 10.1016/j.ejphar.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 20.Soliman MM, Arafah MM. Treatment with dipyridamole improves cardiac function and prevent injury in a rat model of hemorrhage. Eur J Pharmacol. 2012;678:26–31. doi: 10.1016/j.ejphar.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 21.Soliman M. Nucleoside transport inhibitor, dipyridamole, induced myocardial protection following hemorrhagic shock in ex vivo perfused rat hearts. J Saudi Heart Assoc. 2011;23:75–80. doi: 10.1016/j.jsha.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chauhan A, Sharma U, Reeta KH, Jagannathan NR, Mehra RD, Gupta YK. Neuroimaging, biochemical and cellular evidence of protection by mycophenolate mofetil on middle cerebral artery occlusion induced injury in rats. Eur J Pharmacol. 2012;684:71–8. doi: 10.1016/j.ejphar.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 23.Arora TK, Malhotra AK, Ivatury R, Mangino MJ. L-arginine infusion during resuscitation for hemorrhagic shock: Impact and mechanism. J Trauma Acute Care Surg. 2012;72:397–402. doi: 10.1097/ta.0b013e3181d039fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly E, Mathew J, Kohler JE, Blass AL, Soybel AD. Hemorrhagic shock and surgical stress alter distribution of labile zinc within high-and low-molecular-weight plasma fractions. Shock. 2012;38:314–9. doi: 10.1097/SHK.0b013e3182627338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szabó C, Thiemermann C, Wu CC, Perretti M, Vane JR. Attenuation of the induction of nitric oxide synthase by endogenous glucocorticoids accounts for endotoxin tolerance in vivo. Proc Natl Acad Sci U S A. 1994;91:271–5. doi: 10.1073/pnas.91.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chong W, Li Y, Liu B, Liu Z, Zhao T, Wonsey DR, et al. Anti-inflammatory properties of histone deacetylase inhibitors: A mechanistic study. J Trauma Acute Care Surg. 2012;72:347–53. doi: 10.1097/TA.0b013e318243d8b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu T, Onuma T, Kawamori R, Makita Y, Tomino Y. Endothelial nitric oxide synthase gene and the development of diabetic nephropathy. Diabetes Res Clin Pract. 2002;58:179–85. doi: 10.1016/s0168-8227(02)00156-0. [DOI] [PubMed] [Google Scholar]
- 28.Kan W, Zhao KS, Jiang Y, Yan W, Huang Q, Wang J, et al. Lung, spleen, and kidney are the major places for inducible nitric oxide synthase expression in endotoxic shock: Role of p38 mitogen-activated protein kinase in signal transduction of inducible nitric oxide synthase expression. Shock. 2004;21:281–7. doi: 10.1097/01.shk.0000113314.37747.55. [DOI] [PubMed] [Google Scholar]
- 29.Menezes JM, Hierholzer C, Watkins SC, Billiar TR, Peitzman AB, Harbrecht BG. The modulation of hepatic injury and heat shock expression by inhibition of inducible nitric oxide synthase after hemorrhagic shock. Shock. 2002;17:13–8. doi: 10.1097/00024382-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Yasmin W, Strynadka KD, Schulz R. Generation of peroxynitrite contributes to ischemia-reperfusion injury in isolated rat hearts. Cardiovasc Res. 1997;33:422–32. doi: 10.1016/s0008-6363(96)00254-4. [DOI] [PubMed] [Google Scholar]
- 31.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288:481–7. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]


