Abstract
Magnetic resonance spectroscopy (MRS) is an imaging approach that allows for the noninvasive molecular characterization of a region of interest. By detecting signals of water, lipids, and other metabolites, MRS can provide metabolic information for lesion characterization and assessment of treatment response. Although MRS has been routinely used in the brain, clinical applications within the musculoskeletal system have only more recently emerged. The aim of this article is to review the technical considerations for performing MRS in the musculoskeletal system, focusing on proton MRS, and to discuss its potential roles in musculoskeletal tumor imaging and the assessment of muscle physiology and disease.
Keywords: Choline, MR spectroscopy, musculoskeletal
Introduction
Musculoskeletal pathology can be evaluated with a variety of radiologic modalities including radiography, computed tomography (CT), ultrasound (USG), and magnetic resonance imaging (MRI) for morphologic and functional features, as well as with positron emission tomography (PET) for metabolic features.[1] Unlike PET, conventional imaging techniques are limited in their ability to provide metabolic information for the analysis of musculoskeletal abnormalities, and unlike MRI, PET is limited as it requires an additional modality for anatomic imaging correlation. Recently, magnetic resonance spectroscopy (MRS) has emerged as a method for assessing biochemical processes within the musculoskeletal system. MRS is a noninvasive imaging technique that requires no intravenous contrast administration, and can be coupled with anatomic imaging provided by conventional MRI sequences.[2] By detecting and quantifying signals of water, lipid, and certain metabolites, MRS reflects the metabolism in the region of interest.
Different metabolites can be observed depending on the MRS technique [Figure 1]. For example, with phosophorus-31 (31P) MRS, signals from tissue metabolites containing phosphorus are examined, including phosphocreatine, inorganic phosphate, and ATP, which are all metabolites of interest in muscle physiology and disease.[1,3] In the research setting, MRS with 31P has also demonstrated potential for the evaluation of musculoskeletal masses and response to treatment.[4,5] However, phosophorus-31 MRS (similar to other heteronuclear MRS) requires specialized MRI hardware, which, along with its spatial resolution, limits its clinical utility.[5] Proton (1H) MRS is more easily integrated into clinical practice as it requires no specialized equipment and can be performed as part of a routine MRI examination; proton MRS is currently regularly utilized in the clinical setting for assessment of the brain.[1] More recently, improved spectral acquisition and analysis techniques, including both single-voxel and MR spectroscopic imaging (MRSI), along with enhanced gradient performance have led to the application of proton MRS for the evaluation of musculoskeletal pathology.[5]
Figure 1.
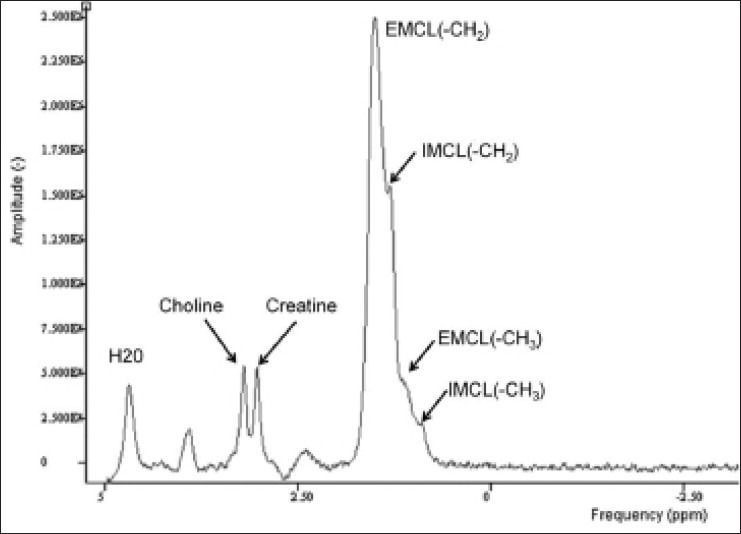
Graph demonstrates different molecular resonance frequencies for a variety of metabolites. The typical proton spectrum of normal muscle demonstrates frequencies corresponding to water, choline, creatine, and lipids. Total choline, a marker for malignancy, demonstrates a peak at 3.2 ppm. In addition, intramyocellular (IMCL) and extramyocellular (EMCL) lipid compartments are shown. Intramuscular metabolism, in particular, has broad potential clinical and research applications. (Reprinted with permission from AJR)[5]
An important and novel application of proton spectroscopy is the assessment of potential malignancy in suspected musculoskeletal tumors.[6,7,8,9,10] MRI is the preferred standard method for assessment of musculoskeletal tumors, primarily for defining tumor extent through the use of conventional pulse sequences. Other applications including tumor characterization and assessment of treatment response can be enhanced through techniques such as diffusion-weighted imaging, chemical shift imaging, and perfusion imaging.[2] A significant limitation of conventional anatomic MRI sequences and traditional imaging modalities, however, is the inability to reliably and consistently differentiate benign from malignant lesions.[5] Although certain benign entities such as a cyst, lipoma, or vascular malformation are often accurately diagnosed by conventional MRI imaging alone, there is overlap in the imaging appearance of many benign and malignant musculoskeletal tumors, even using multiple imaging modalities.[11] Consequently, biopsy of the lesion in question is needed to establish a diagnosis. In the post-treatment setting, this limitation manifests as difficulty distinguishing viable tumor from post-surgical or post-neoadjuvant chemotherapy or radiation-induced inflammation and granulation tissue.[12] Proton MRS, however, can provide important metabolic information for assessing a musculoskeletal lesion by measuring metabolites that are abundantly produced by malignant tumors, in particular, choline-containing compounds. Proton MRS, therefore, has the potential to differentiate benign from malignant lesions both in the pre-surgical and post-treatment settings.[5]
Technique
Proton MRS can be performed with either a single-voxel technique or a multi-voxel technique (MRSI). With the single-voxel method, only information from the selected region of interest within the voxel is obtained, although the voxel may be as large as desired.[12] Advantages include simplicity of the approach, a short acquisition time, and relatively easy maintenance of the homogeneity of the magnetic field within the volume of interest.[5] With the multi-voxel technique, on the other hand, information over a larger field of view is simultaneously obtained.[12] Advantages of MRSI include the ability to analyze the entire lesion and surrounding tissue, as well as the potential to analyze multiple lesions at once. In this way, heterogeneous tumors can be assessed for differences in tumor grade and viability.[5] The multi-voxel technique, however, necessitates a longer scan time and strict field homogeneity, and may be more technically demanding to perform, particularly when multislice multi-voxel technique is employed.[12]
Clinical MRS can be performed either at 1.5T or at 3T.[5,12] Higher field strengths provide a greater signal-to-noise ratio (SNR) and theoretically improve spectral resolution; however, potential limiting factors include the increased field inhomogeneity leading to increased metabolite line widths.[5,12,13] In addition, in the musculoskeletal system, the abundance of lipids (unlike in the brain) can result in contamination of other important metabolite peaks of interest. Hence, in the musculoskeletal system, the choice of echo time (TE) is critical; for applications in which the metabolites choline and creatine are sought (as is usually the case in tumor imaging), MRS is performed with an intermediate TE of 130-150 ms (to avoid contamination by lipids), but for applications in which lipids are the metabolites of interest, short TEs may be utilized. The MRS protocol utilized at our institution has been optimized for tumor assessment and was previously described by Fayad et al.[2] Preliminary anatomic images are acquired for localization of the voxel, prior to the administration of intravenous contrast. For quantification purposes, water-suppressed in addition to water-un-suppressed scans are performed for the determination of metabolite concentration using a point-resolved single-voxel spectroscopy sequence.
Several technical factors are important to note for optimization of musculoskeletal proton MRS. Volume selection is crucial since subtle metabolic peaks can be obscured by inclusion of excess subcutaneous fat and signal dropout from inclusion of vascular structures or cortical bone can contaminate the proton spectrum. Appropriate selection and use of coils is also important for optimizing a proton MRS exam. Surface coils are ideally used for evaluation of musculoskeletal system due to variation in size and shape of the regions of interest that are evaluated throughout the body. Lastly, shimming of the local magnetic field is necessary for optimizing a proton MRS exam in order to ensure high-quality spectra. In musculoskeletal imaging, the most effective technique is a semi-automated process including a manual shim, which requires approximately 5-10 min prior to the MRS acquisition.[5]
Limitations
The analysis of MRS data in musculoskeletal system may be either qualitative or quantitative. Reproducibility with either analytic approach has not been clearly established. Importantly, the qualitative approach (in which the presence or absence of a metabolite peak is used as a marker for disease) is hindered by false-positive results.[5] For this reason, increased emphasis on quantitative assessment of metabolite content by proton MRS has been advocated.
The relative quantification of a metabolite can be achieved by measuring the peak ratio between metabolites or between a metabolite and the background noise level (SNR). Multiple limitations inherent to the use of metabolite or SNR ratios, however, can lead to ambiguity in interpretation.[5] In particular, the SNR of a metabolite varies with the type of coil utilized, the distance between the voxel of interest and the coil, and the size of the lesion and tissues involved.[12] Absolute quantification is, therefore, more desirable,[5,12] and use of water as an internal reference has been demonstrated as a successful method for measuring metabolite concentration in the musculoskeletal system.[5] Nevertheless, the fact that total water content is not a constant variable in musculoskeletal tissues has spurred the development of alternative absolute quantitative techniques,[14] which have been recently utilized in vivo.[15]
In addition to the limitations of analysis, there are a number of factors that hinder the technical success of proton MRS in the musculoskeletal system specifically. Inherent heterogeneity of musculoskeletal tissues (compared to the brain) leads to increased variation in the local magnetic field inhomogeneity, which affects spectral quality. Heterogeneity of water, lipid, and tissue compartmentation is also greater in the musculoskeletal system and limits the accuracy of quantified measurements, and the abundance of lipids in musculoskeletal tissues may obscure nearby important metabolite peaks. Lastly, MRS of different body parts requires the use of different radiofrequency coils, which confounds the comparison of quantified findings.[5]
Clinical Applications
There are two main applications for proton MRS in the musculoskeletal system: the assessment of musculoskeletal tumors and the evaluation of muscle physiology and disease. Metabolic markers available by MRS are helpful for distinguishing normal or benign tissue from that which is diseased, and will be discussed below.
Choline Metabolism
Proton MRS detects signals of metabolites within a specified region of interest and provides metabolic information that can be used to evaluate suspected tumors. Since certain metabolites are distinctively increased in malignant lesions, MRS has the potential to noninvasively differentiate between malignant and benign lesions.[1,2,5,12] In particular, the presence of elevated levels of the total choline (Cho) peak (trimethylamine/choline-containing compounds including phosphocholine, glycerophosphocholine, and free choline) has been established as a marker of malignancy. Choline-containing compounds are a part of the phosopholipid metabolism of cell membranes; hence, the level of choline within a region of interest will mirror the degree of cell membrane turnover that is known to occur in malignancy due to specific tumor growth factors.[12,14] It follows that applications of choline measurement by MRS include the noninvasive characterization of newly detected masses [Figures 2 and 3] and the evaluation of treatment response.[12]
Figure 2 (A-D).
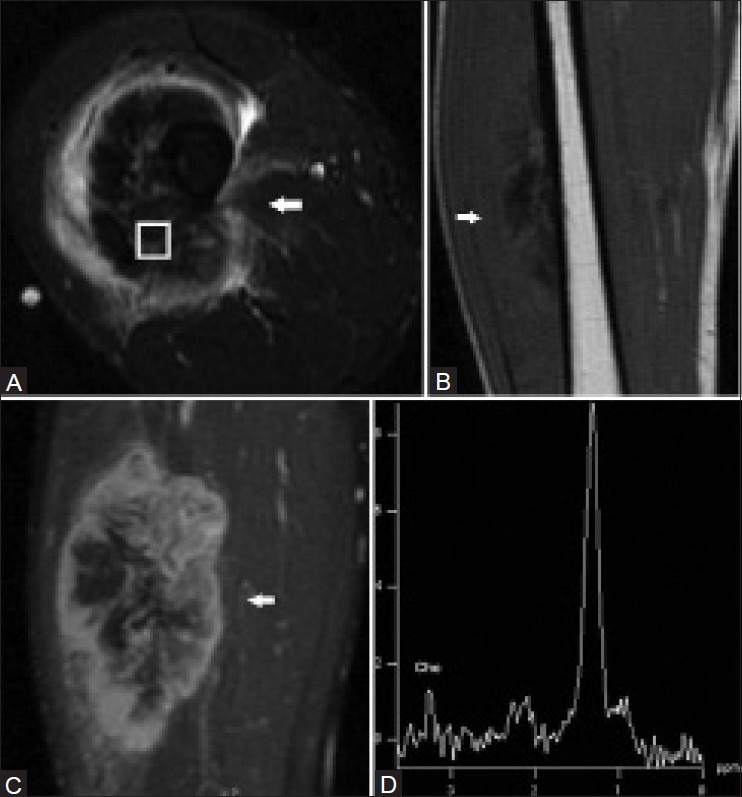
A 31 year old male with high-grade osteosarcoma of the right thigh. Axial short TI inversion recovery (STIR) fast spin-echo MRI image (A), coronal T1-weighted spin-echo MRI image (B), and coronal fat-saturated dynamic contrast-enhanced fast spin-echo MRI image acquired 40 s after contrast injection (C) demonstrate a heterogeneous enhancing mass in the anterolateral right thigh with central areas of necrosis and surrounding edema. A single-voxel MR spectroscopic map (D) demonstrates a discrete choline peak at 3.2 ppm. (Reprinted with permission from AJR)[5]
Figure 3 (A-B).
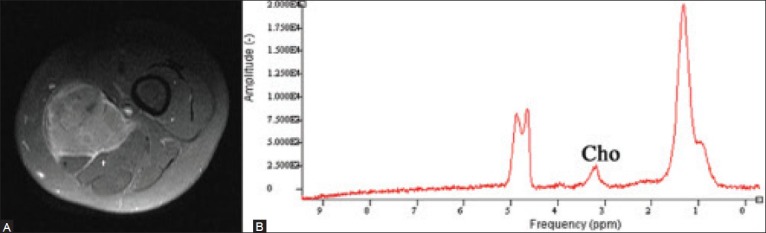
A 28 year old woman with a soft tissue mass of the thigh, shown to be a Ewing's sarcoma. Axial T1W image following contrast administration (A) the large enhancing soft tissue mass. Proton MRS (B) a discrete choline peak within the mass indicating malignant histology, concordant with the eventual biopsy results
Current literature investigating the use of choline as a marker for malignancy by musculoskeletal MRS has been largely qualitative.[2] A recent comprehensive pooled analysis of previously published literature on qualitative MRS assessment of de novo musculoskeletal lesions demonstrated a sensitivity of 88% and a specificity of 68% for the presence of detectable choline as a predictor of malignancy. The positive predictive value (PPV) and negative predictive value for malignancy in the presence of a discrete total choline peak were 73% and 86%, respectively. Interestingly, of the 20 benign entities with detectable choline peaks, 15 were either giant cell tumors or peripheral nerve sheath tumors which are lesions that may have aggressive biological behavior [Figure 4].[5] Using a quantitative approach, however, MRS measurements of choline concentration raised the negative predictive value for excluding malignancy to 100%(PPV remained at 73%) when a threshold of 0.3 IU was used. Therefore, at this time, proton MRS is most useful for its high negative predictive value in ruling out malignancy in a musculoskeletal lesion [Figure 5].
Figure 4 (A, B).
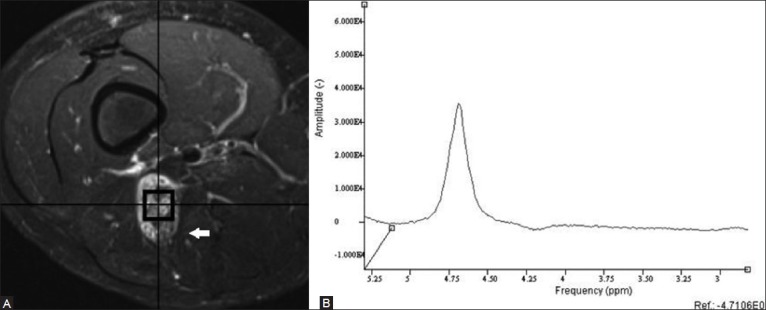
An 11 year old male with history of neurofibromatosis type 1 presents with marked enlargement of the right sciatic nerve. Axial fat-suppressed T2W fast spin-echo MRI image (A) demonstrates a mass that was found to be a benign neurofibroma. Single-voxel proton MR spectroscopic map (B) no discernible choline peak at 3.2 ppm, compatible with a benign lesion. (Reprinted with permission from AJR)[5]
Figure 5 (A, B).
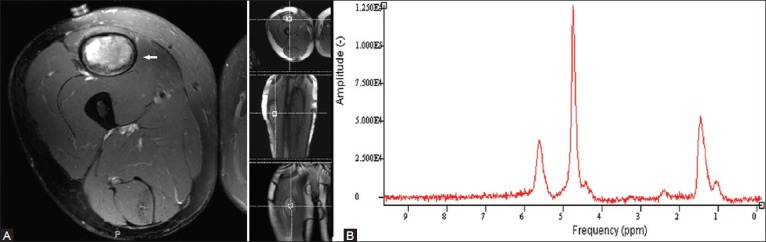
A 36 year old man who presented with a spontaneous soft tissue mass in the thigh. Although a hematoma was suspected, the lack of history of trauma raised the possibility of a tumor. Axial fat-suppressed T2W image (A) shows the hyperintense mass. Proton MRS (B) no evidence of a discrete choline peak, suggesting benign etiology. The patient underwent a biopsy which showed no evidence of malignant cells, and then a follow-up which showed resolution of the hematoma
In addition to tumor characterization, the MRS evaluation of choline content may be used to assess treatment response [Figure 6].[12] In an early study of three patients, Hsieh et al. demonstrated a decline of choline by MRS of malignant musculoskeletal lesions after chemotherapy.[16] Additional case reports have been discussed[2] and pre-clinical studies are being performed[14] that have yet to be translated into clinical work.
Figure 6 (A-D).
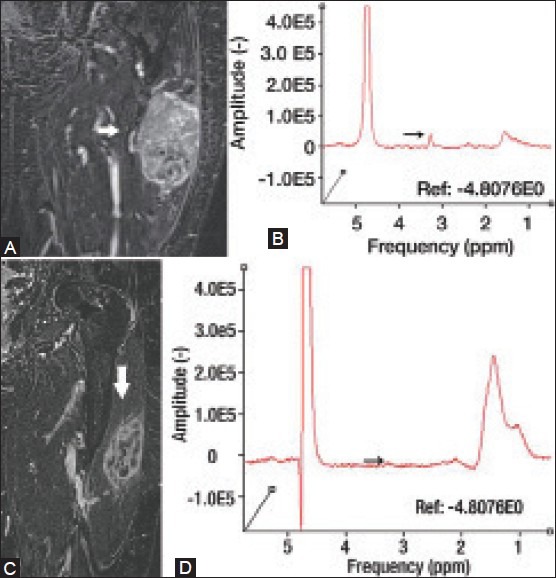
An 81 year old female with pleomorphic rhabdomyosarcoma. Coronal delayed contrast-enhanced MRI (A) and proton MRS (B) demonstrate an avidly enhancing mass with a discrete choline peak at 3.2 ppm. The patient was subsequently treated with chemotherapy. Post-treatment coronal delayed contrast-enhanced MRI (C) demonstrates substantially decreased enhancement within the lesion. Post-treatment proton MRS (D) demonstrates marked interval decrease of choline peak at 3.2 ppm, indicating chemotherapy-related tumor necrosis. (Reprinted with permission from Radiology)[2]
Proton MRS evaluation of choline content may have additional clinical applications in evaluating radiation injury and myopathy, as altered intramuscular choline content has been demonstrated in an early study of these conditions.[17]
Creatine metabolism
MRS serves as a tool for detecting and characterizing biochemical aberrations in muscle in vivo, particularly through the evaluation of creatine metabolism. By obtaining information on the cellular plane at the level of the myofibrils and mitochondria, MRS can be applied to observe variations and anomalies in muscle structure and function. A recent study demonstrated the potential role of proton MRS as a supplement to conventional MRI in the evaluation of patients with inflammatory myopathies, especially in cases where MRI showed no obvious muscle abnormalities.[15] In particular, there were substantial differences in creatine metabolism of muscles in patients with chronic myopathies compared with those of healthy volunteers.
Lipid Metabolism
A few studies in the literature have explored the role of MRS lipid content analysis in musculoskeletal imaging for bone marrow assessment as well as muscle physiology and disease. An early investigation of MRS in the study of bone marrow demonstrated higher lipid-to-water ratios in healthy subjects compared to patients with leukemia, probably reflecting the rise in water content within the bone marrow of leukemic patients that occurs with increased hematopoietic tissue formation.[18] MRS was further applied to a study of 21 patients with multiple myeloma, in which an increased lipid-to-water ratio within the bone marrow was noted only in patients responding to treatment.[19] Therefore, the MRS analysis of lipid-to-water ratios within the bone marrow may have important clinical roles in the diagnosis and evaluation of treatment response in bone marrow disorders.
For proton MRS applications in muscle, intracellular and extracellular lipid compartments can be assessed separately given the differences in the spatial arrangement of these two compartments, which allows them to be mapped to different locations along the spectrum [Figure 1]. Intramyocellular lipids (IMCL) are found in droplets and are located near the mitochondria, while extramyocellular lipids (EMCL) are in sheets of adipocytes scattered between muscle cells.[20,21] In particular, intramuscular lipid metabolism has a broad spectrum of potential clinical and research applications. Fatty acid metabolism is a part of energy production, and hence, lipid metabolism is important to the study of exercise physiology. In addition, the MRS evaluation of IMCL has been correlated with body composition and insulin resistance.[22] Studies have suggested the use of MRS measurement of IMCL as a noninvasive marker of insulin sensitivity, diabetes mellitns, and obesity:[23] IMCL causes increased fatty acyl-coenzyme A and other products that hamper signals from insulin that promote the transport of glucose into a cell.
Using more qualitative approaches to total muscle lipid content, MRS has been used to assess muscle function and the need for treatment. In patients with chronic back pain, MRS quantification of paraspinal muscle fat content may help clinical assessment and guide rehabilitation.[24,25] Similarly, a study assessing lipid content of the supraspinatus muscle demonstrated the utility of MRS in evaluating fat content of rotator cuff muscles in patients with rotator cuff tears. The degree of fatty atrophy of a torn rotator cuff muscles has important clinical implications because it serves as a predictor of surgical reconstruction. Fatty atrophy, which can occur as early as 6 weeks after the initial injury, is a relative contraindication for surgical reconstruction if more than half of the muscle is replaced with fat.[26] Finally, MRS has been recently shown to have good reproducibility across multiple centers and at multiple time points in the quantitative assessment of intramuscular lipid content in patients with Duchenne muscular dystrophy.[27]
Conclusion
Proton MRS is a promising imaging technique in the field of musculoskeletal imaging. A range of metabolites, in addition to lipid and water content, can be gauged and quantitatively measured to aid clinical decision making. The evaluation of total choline content, in particular, has important implications for differentiating malignant and benign musculoskeletal lesions. The assessment of creatine content is important in studying muscle disorders, and the assessment of lipid content has demonstrated potential in the evaluation of bone marrow pathology as well as muscle physiology and disease. However, further research is still needed to determine the reproducibility of MRS in the musculoskeletal system and definitively elucidate its clinical applications.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kransdorf MJ, Bridges MD. Current developments and recent advances in musculoskeletal tumor imaging. Semin Musculoskelet Radiol. 2013;17:145–55. doi: 10.1055/s-0033-1343070. [DOI] [PubMed] [Google Scholar]
- 2.Fayad LM, Jacobs MA, Wang X, Carrino JA, Bluemke DA. Musculoskeletal tumors: How to use anatomic, functional, and metabolic MR techniques. Radiology. 2012;265:340–56. doi: 10.1148/radiol.12111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee B, Sharma U, Balasubramanian K, Kalaivani M, Kalra V, Jagannathan NR. Effect of creatine monohydrate in improving cellular energetics and muscle strength in ambulatory Duchenne muscular dystrophy patients: A randomized, placebo-controlled 31P MRS study. Magn Reson Imaging. 2010;28:698–707. doi: 10.1016/j.mri.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Sijens PE. 31P (MRS) changes as a measure of therapy response in human osteosarcomas implanted into nude mice. Magn Reson Imaging. 1995;13:495–6. doi: 10.1016/0730-725x(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 5.Subhawong TK, Wang X, Durand DJ, Jacobs MA, Carrino JA, Machado AJ, et al. Proton MR spectroscopy in metabolic assessment of musculoskeletal lesions. AJR Am J Roentgenol. 2012;198:162–72. doi: 10.2214/AJR.11.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fayad LM, Salibi N, Wang X, Machado AJ, Jacobs MA, Bluemke DA, et al. Quantification of muscle choline concentrations by proton MR spectroscopy at 3 T: Technical feasibility. AJR Am J Roentgenol. 2010;194:W73–9. doi: 10.2214/AJR.09.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fayad LM, Wang X, Salibi N, Barker PB, Jacobs MA, Machado AJ, et al. A feasibility study of quantitative molecular characterization of musculoskeletal lesions by proton MR spectroscopy at 3 T. AJR Am J Roentgenol. 2010;195:W69–75. doi: 10.2214/AJR.09.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fayad LM, Barker PB, Jacobs MA, Eng J, Weber KL, Kulesza P, et al. Characterization of musculoskeletal lesions on 3-T proton MR spectroscopy. AJR Am J Roentgenol. 2007;188:1513–20. doi: 10.2214/AJR.06.0935. [DOI] [PubMed] [Google Scholar]
- 9.Fayad LM, Bluemke DA, McCarthy EF, Weber KL, Barker PB, Jacobs MA. Musculoskeletal tumors: Use of proton MR spectroscopic imaging for characterization. J Magn Reson Imaging. 2006;23:23–8. doi: 10.1002/jmri.20448. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Jacobs MA, Fayad LM. Therapeutic response in musculoskeletal soft tissue sarcomas: Evaluation by MRI. NMR Biomed. 2011;24:750–63. doi: 10.1002/nbm.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaman FD, Jelinek JS, Priebat DA. Current imaging and therapy of malignant soft tissue tumors and tumor-like lesions. Semin Musculoskelet Radiol. 2013;17:168–76. doi: 10.1055/s-0033-1343094. [DOI] [PubMed] [Google Scholar]
- 12.Fayad LM, Barker PB, Bluemke DA. Molecular characterization of musculoskeletal tumors by proton MR spectroscopy. Semin Musculoskelet Radiol. 2007;11:240–5. doi: 10.1055/s-2008-1038313. [DOI] [PubMed] [Google Scholar]
- 13.Barker PB, Hearshen DO, Boska MD. Singlevoxel proton MRS of the human brain at 1.5 T and 3.0 T. Magn Reson Med. 2001;45:765–9. doi: 10.1002/mrm.1104. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Fayad LM, Barker PB. Montréal, Québec, Canada: International Society for Magnetic Resonance in Medicine; 2011. Quantitative Musculoskeletal MRS Using the Phantom Replacement Method and Phased-Array Receiver Coils. Presented at. [Google Scholar]
- 15.Subhawong TK, Wang X, Machado AJ, Mammen AL, Christopher-Stine L, Barker PB, et al. 1H Magnetic resonance spectroscopy findings in idiopathic inflammatory myopathies at 3 T: Feasibility and first results. Invest Radiol. 2013;48:509–16. doi: 10.1097/RLI.0b013e3182823562. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh TJ, Li CW, Chuang HY, Liu GC, Wang CK. Longitudinally monitoring chemotherapy effect of malignant musculoskeletal tumors with in vivo proton magnetic resonance spectroscopy: An initial experience. J Comput Assist Tomogr. 2008;32:987–94. doi: 10.1097/RCT.0b013e31815b9ce9. [DOI] [PubMed] [Google Scholar]
- 17.Bongers H, Schick F, Skalej M, Jung WI, Stevens A. Localized in vivo 1H spectroscopy of human skeletal muscle: Normal and pathologic findings. Magn Reson Imaging. 1992;10:957–64. doi: 10.1016/0730-725x(92)90450-e. [DOI] [PubMed] [Google Scholar]
- 18.Jensen KE, Jensen M, Grundtvig P, Thomsen C, Karle H, Henriksen O. Localized in vivo proton spectroscopy of the bone marrow in patients with leukemia. Magn Reson Imaging. 1990;8:779–89. doi: 10.1016/0730-725x(90)90014-s. [DOI] [PubMed] [Google Scholar]
- 19.Oriol A, Valverde D, Capellades J, Cabañas ME, Ribera J, Arús C. In vivo quantification of response to treatment in patients with multiple myeloma by 1H magnetic resonance spectroscopy of bone marrow. MAGMA. 2007;20:93–101. doi: 10.1007/s10334-007-0072-4. [DOI] [PubMed] [Google Scholar]
- 20.Schick F, Eismann B, Jung WI, Bongers H, Bunse M, Lutz O. Comparison of localized proton NMR signals of skeletal muscle and fat tissue in vivo: Two lipid compartments in muscle tissue. Magn Reson Med. 1993;29:158–67. doi: 10.1002/mrm.1910290203. [DOI] [PubMed] [Google Scholar]
- 21.Boesch C, Slotboom J, Hoppeler H, Kreis R. In vivo determination of intra-myocellular lipids in human muscle by means of localized 1H-MR-spectroscopy. Magn Reson Med. 1997;37:484–93. doi: 10.1002/mrm.1910370403. [DOI] [PubMed] [Google Scholar]
- 22.Bredella MA, Ghomi RH, Thomas BJ, Miller KK, Torriani M. Comparison of 3.0 T proton magnetic resonance spectroscopy short and long echo-time measures of intramyocellular lipids in obese and normal-weight women. J Magn Reson Imaging. 2010;32:388–93. doi: 10.1002/jmri.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torriani M, Thomas BJ, Halpern EF, Jensen ME, Rosenthal DI, Palmer WE. Intramyocellular lipid quantification: Repeatability with 1H MR spectroscopy. Radiology. 2005;236:609–14. doi: 10.1148/radiol.2362041661. [DOI] [PubMed] [Google Scholar]
- 24.Fischer MA, Nanz D, Shimakawa A, Schirmer T, Guggenberger R, Chhabra A, et al. Quantification of muscle fat in patients with low back pain: Comparison of multi-echo MR imaging with single-voxel MR spectroscopy. Radiology. 2013;266:555–63. doi: 10.1148/radiol.12120399. [DOI] [PubMed] [Google Scholar]
- 25.Mengiardi B, Schmid MR, Boos N, Pfirrmann CW, Brunner F, Elfering A, et al. Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers: Quantification with MR spectroscopy. Radiology. 2006;240:786–92. doi: 10.1148/radiol.2403050820. [DOI] [PubMed] [Google Scholar]
- 26.Pfirrmann CW, Schmid MR, Zanetti M, Jost B, Gerber C, Hodler J. Assessment of fat content in supraspinatus muscle with proton MR spectroscopy in asymptomatic volunteers and patients with supraspinatus tendon lesions. Radiology. 2004;232:709–15. doi: 10.1148/radiol.2323030442. [DOI] [PubMed] [Google Scholar]
- 27.Forbes SC, Walter GA, Rooney WD, Wang DJ, Devos S, Pollaro J, et al. Skeletal Muscles of Ambulant Children with Duchenne Muscular Dystrophy: Validation of Multicenter Study of Evaluation with MR Imaging and MR Spectroscopy. Radiology. 2013;269:198–207. doi: 10.1148/radiol.13121948. [DOI] [PMC free article] [PubMed] [Google Scholar]


