Abstract
Focal lesions in bone are very common and many of these lesions are not bone tumors. These bone tumor mimickers can include numerous normal anatomic variants and non-neoplastic processes. Many of these tumor mimickers can be left alone, while others can be due to a significant disease process. It is important for the radiologist and clinician to be aware of these bone tumor mimickers and understand the characteristic features which allow discrimination between them and true neoplasms in order to avoid unnecessary additional workup. Knowing which lesions to leave alone or which ones require workup can prevent misdiagnosis and reduce patient anxiety.
Keywords: Bone tumors, MRI artifacts, osteomyelitis, traumatic lesions, tumor mimickers
Introduction
Focal lesions in bone are very common and are frequently encountered in routine imaging studies. While many lesions are true neoplasms, a number of these abnormalities in bone are not tumors. These lesions can include normal variants, congenital abnormalities, traumatic/iatrogenic lesions, metabolic and arthritic changes, infection, and artifacts related to imaging technique. It is important for the radiologist and clinician to be aware of this possibility and to identify the characteristic features which allow discrimination between bone tumors and bone tumor mimickers. Subjecting the patient to an inappropriate workup can lead to misdiagnosis, poor management, and anxiety for both the patient and physician. In many instances, these tumor mimickers can be left alone and no treatment is necessary; however, in other cases, they can indicate a significant disease process. Although there are innumerable processes that can lead to focal lesions in bone, we present here a review of commonly encountered bone lesions [Table 1] that can mimic bone tumors and discuss the key imaging and clinical features that can help distinguish these entities from neoplasms. For the purpose of this pictorial essay, we performed a systematic search of the electronic database PubMed to identify relevant studies published in the literature from 1991 to 2014 using the terms “bone tumor mimickers,” “bone tumor mimics,” and “pseudolesions of bone.” Additional targeted searches were performed for the specific disease conditions.
Table 1.
Common lesions mimicking bone tumors
Normal Variants
Red marrow
Erythropoietic or red marrow can be a common cause for concern on magnetic resonance imaging (MRI). This can be particularly problematic if the area of red marrow is mass-like in appearance. Red marrow should be hyperintense to fatty marrow on fat-suppressed T2-weighted (T2W) MRI sequences and hypointense on T1-weighted (T1W) MRI sequences.[1] The key feature is that the low signal intensity on T1W MRI sequences should be higher than that of skeletal muscle or the intervertebral discs.[2] In-phase and out-of-phase T1W MRI images can be helpful in equivocal cases as red marrow should have some intermixed fatty marrow and, consequently, should lose signal (become darker) on out-of-phase compared to in-phase MRI.[3] On the other hand, marrow-replacing tumors, such as many metastases, should replace all the fatty marrow and should not lose signal on out-of-phase T1W imaging [Figure 1]. Thus, when approaching marrow abnormalities on MRI, it is important to have T1W images that include skeletal muscle for comparison and in-phase and out-of-phase T1W images to show the presence or absence of fat. Yellow marrow can reconvert to red marrow with physiologic stressors such as anemia.[4] Moreover, red marrow should not extend past the physeal scar into the epiphysis and should not distort normal trabecular pattern.[5]
Figure 1 (A and B).
Island of red marrow in the sacrum. A 49 year old man with recurrent bloating underwent a MR enterography, which demonstrated an incidental lesion in the sacrum. He was recalled for in-phase and out-of-phase T1W MRI imaging. (A) In-phase T1W MRI image demonstrates the lesion (arrow) is slightly hyperintense to skeletal muscle (B) On the out-of-phase T1W MRI image, there is loss of signal due to the presence of intermixed fatty marrow (arrow)
Humeral pseudocyst
A radiolucent area in the humeral head may be seen due to a normal decrease in the trabeculae often associated with an increase in the amount of fat.[6] This radiolucency is seen in the superolateral humeral head and may be misdiagnosed as a chondroblastoma, giant cell tumor, Langerhans cell histiocytosis, or even an osteolytic metastasis on radiographs.[7] The increased fat in this region can be readily seen on MRI and helps make the diagnosis [Figure 2]. On radiographs, this pseudolesion will be seen on an external rotation view of the shoulder and there is usually a sharp line of demarcation inferiorly between the pseudolesion and adjacent marrow, which is due to the line of fusion between the epiphysis in the greater tuberosity and the shaft of the humerus. The remainder of the margin is usually ill-defined.[6]
Figure 2 (A-C).
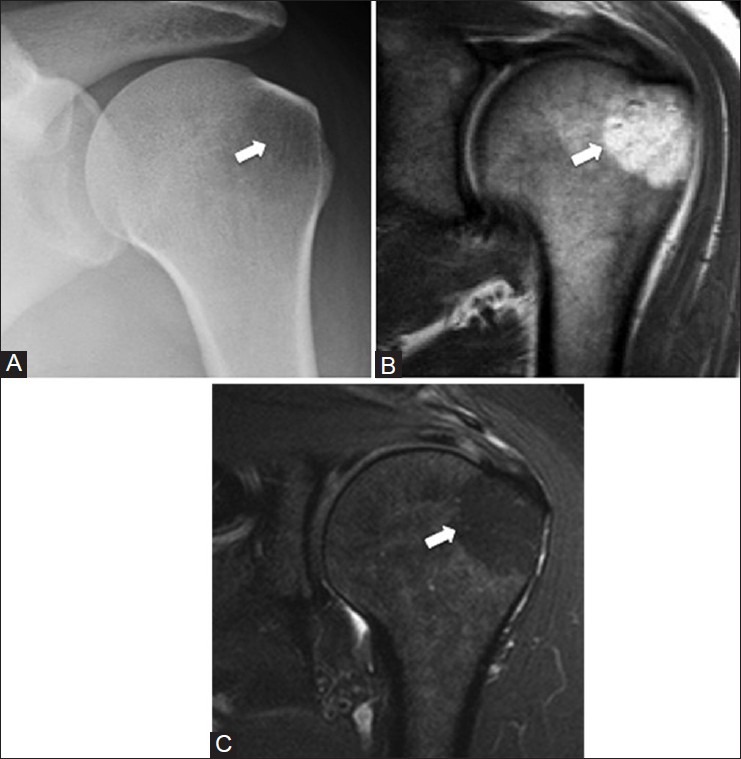
Humeral pseudocyst. A 47 year old female with left shoulder pain. A round radiolucency in the greater tuberosity (arrow) on the external rotation shoulder radiograph (A) corresponds to normal fatty marrow (arrow) which is hyperintense on the (B) T1W and hypointense on the (C) T2W fat-saturated coronal MRI images
Ward's triangle
A focal area of increased lucency is often seen in the femoral neck at the junction of the compressive and tensile trabeculae [Figure 3]. As with the humeral pseudocyst, this radiolucency can become less apparent if the patient is osteoporotic due to attenuation of the adjacent trabeculae.[7,8]
Figure 3 (A and B).
Ward's triangle. A 67 year old female with left hip pain. (A) Anteroposterior (AP) radiograph of the hip demonstrates a triangular radiolucency (arrows) in the femoral neck (B) The coronal CT image shows a paucity of trabecular lines in the femoral neck (arrows)
Calcaneal pseudocyst
In a similar pattern to Ward's triangle, a radiolucency in the anterior aspect of the calcaneus can be outlined by the major trabecular groups [Figure 4].[7] Although this is a normal appearance, a number of pathologic lesions can occur in this location and form a radiolucent region on radiographs. These tumors include simple bone cyst, giant cell tumor, chondroblastoma, and intraosseous lipoma. Intraosseous lipomas often develop central necrosis which can cause a central dystrophic calcification and tends to have well-defined sclerotic margins.[7]
Figure 4 (A and B).
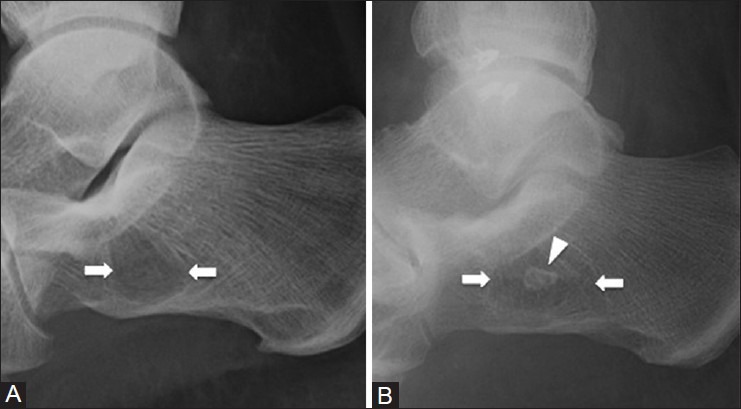
Calcaneal pseudocyst and intraosseous lipoma. (A) Lateral ankle radiograph of a 39 year old female with foot pain demonstrates a radiolucency (arrows) in the anterior calcaneus due to decrease in bony trabeculae (B) Lateral ankle radiograph of a 45 year old man with an intraosseous lipoma (arrows) shows a similar radiographic appearance to the calcaneal pseudocyst; however, there is focal central calcification (arrowhead) due to fat necrosis
Congenital and Developmental Abnormalities
Dorsal defect of the patella
A subarticular abnormality in the superolateral aspect of the patella is known as the dorsal defect of the patella. It is seen in approximately 1% of the population and can be bilateral.[9] The dorsal patellar defect can appear as a 1-2 cm rounded area of lucency in the same location as a bipartite patella and is believed to be due to incomplete fusion of the patellar ossification centers [Figure 5].[9] Another potential etiology is that it is due to traction at the insertion of vastus lateralis. Occasionally, this lesion may be symptomatic.[7]
Figure 5 (A and B).
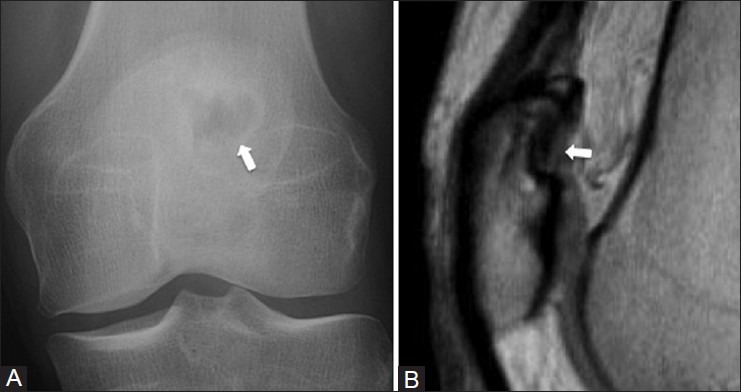
Dorsal defect of the patella. A 38 year old female with left knee pain. (A) AP radiograph of the knee demonstrates a focal radiolucency (arrow) in the superolateral aspect of patella (B) Sagittal PDW MRI image shows a focal area of cortical irregularity with intact overlying hyaline cartilage (arrow)
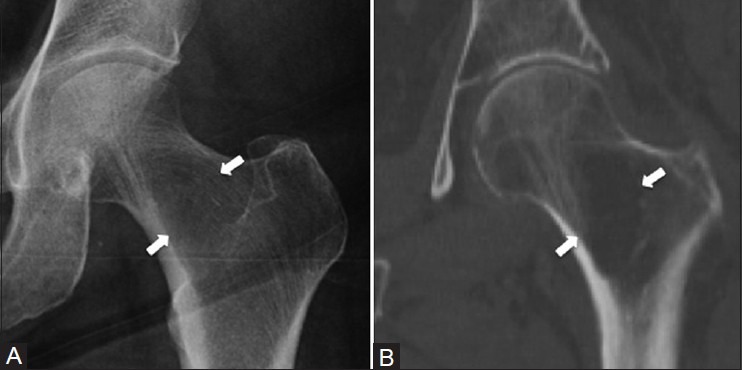
Synovial herniation pit in the proximal femur
A well-defined round or oval radiolucency in the proximal superior femoral neck is known as a synovial herniation pit or Pitt's pit.[10] It is thought to represent herniation of the synovium into cortical defects created by abrasion of the hip joint capsule against the femoral neck, although it may represent a normal variant [Figure 6].[11] Typically these lesions are less than 1 cm in size, but can grow up to 2-3 cm and may be lobulated.[12] Although these lesions have been considered asymptomatic, an association with femoracetabular impingement has been described.[11]
Figure 6 (A-C).
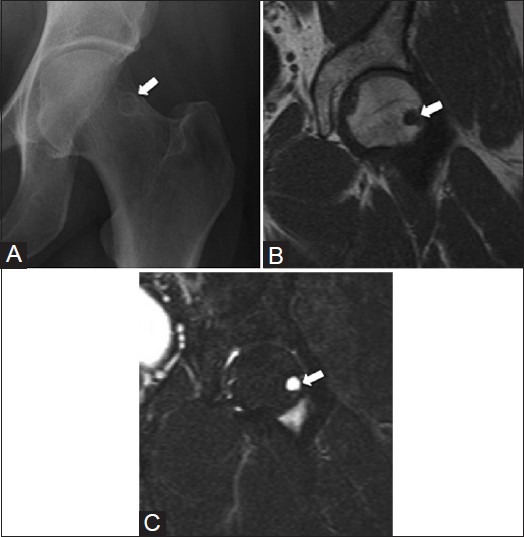
Synovial herniation pit in the proximal femur. A 60 year old female with suspected hip fracture after a fall. (A) AP radiograph shows a small round radiolucency (arrow) and sclerotic rim at the superior lateral aspect of the femoral neck. The lesion (arrow) is hypointense on the (B) coronal T1W MRI image and hyperintense on the (C) coronal T2W fat-saturated MRI image
Avulsive cortical irregularity of the posterior femur
An avulsive cortical irregularityof the posterior femur, known as a cortical desmoid, appears as an irregular focal radiolucent lesion along the posteromedial aspect of the distal femur in children [Figure 7].[13] Differential diagnosis for this appearance includes osteomyelitis and surface osteosarcoma, especially if the lesion has an aggressive appearance. It has been proposed that this lesion may be caused by traction due to the medial head of gastrocnemius or adductor magnus.[14] This lesion should not be seen in skeletally mature individuals.
Figure 7 (A and B).
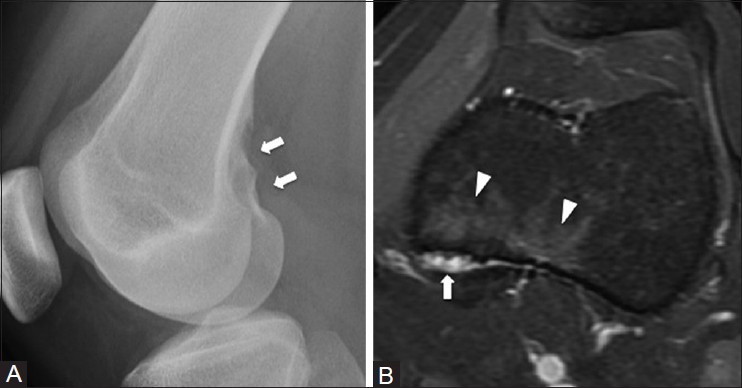
Avulsive cortical irregularity of the posterior femur. An 18 year old female with left knee pain. (A) Lateral radiograph of knee demonstrates an area of cortical irregularity at the medial aspect of the distal femoral metaphysis (arrow) (B) Corresponding axial T2W fat-saturated MRI image shows marrow edema (arrowhead) at the area of cortical irregularity (arrow)
Supracondylar process of the humerus
A supracondylar process in the humerus is a bony spur that arises from the anteromedial aspect of the humerus in about 1-3% of the population.[15] It is usually an incidental finding and should not be mistaken for an osteochondroma or surface osteosarcoma. Osteochondromas point away from the joint, whereas the supracondylar process points toward the elbow joint [Figure 8]. Occasionally, a ligament extends from the supracondylar process to the medial epicondyle (the ligament of Struthers), forming a tunnel that can entrap the median nerve and even the brachial artery, leading to symptoms.[16]
Figure 8 (A and B).
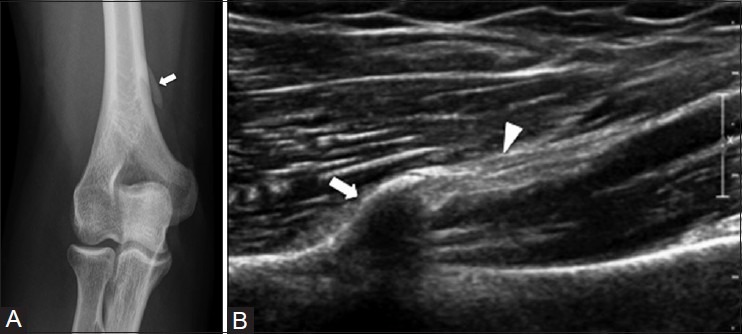
Supracondylar process of the humerus. A 45 year old male with right elbow pain. (A) AP radiograph of elbow shows an osseous process (arrow) arising from anteromedial aspect of the distal humerus. Corresponding ultrasound image (B) demonstrates an osseous excrescence (arrow) with a hyperintense ligament of Struthers (arrowhead) attached onto it
Soleal line
The soleal line is a bony “tug lesion” that can form on the tibia at the attachment of the soleus and mimics periostitis from a tumor, infection, or stress fracture [Figure 9].[17] The soleal line begins 1-2 cm below the fibular facet and may present as a line or a ridge.[18] This can arise from the tibial head of the soleus, with cortical thickening extending lateral to medial along the posterior upper one-third of the tibia. Similar bony changes can be seen at the fibular attachment of the soleus.
Figure 9 (A and B).
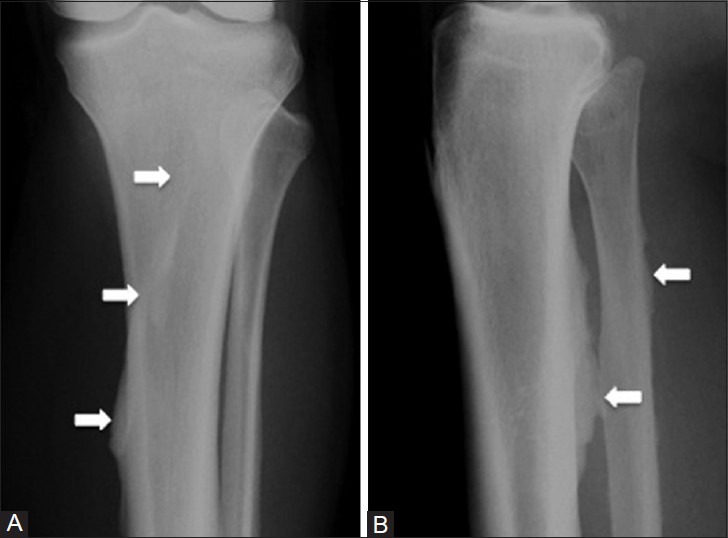
Soleal line. A 67 year old male with suspected right leg fracture after fall. (A) AP and (B) lateral radiographs of the proximal tibia demonstrate linear cortical thickening (arrows) along the proximal tibia and fibula, which corresponds to an enthesophyte from the attachment of the soleus. The tibial calcification extends lateral to medial along the posterior cortex
Trauma and Iatrogenic Lesions
Subperiosteal hematoma
The periosteum is a highly vascular thick fibrous membrane that is closely adherent to the bone.[19] Injury to the periosteum can result in a subperiosteal hematoma, which lifts the periosteum off the bone and can resemble a focal mass such as a parosteal osteosarcoma or osteochondroma [Figure 10]. Most often, they resolve without treatment; however, they may ossify and persist.[19] On imaging, these lesions have a non-aggressive appearance and are centered in the subperiosteum. If they ossify, they can contain fatty marrow.[20]
Figure 10 (A-C).
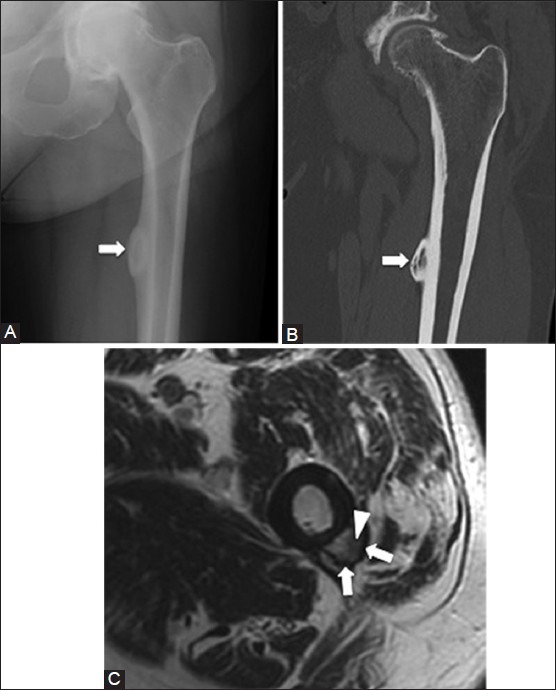
Subperiosteal hematoma. A 53 year old male with history of remote thigh trauma. (A) AP radiograph and (B) coronal CT image of left hip show a lesion (arrows) arising from the medial femoral cortex with central ossification (C) Axial T1W MRI image demonstrates a chronic subperiosteal hematoma that contains central fatty marrow (arrowhead) and cortical bone (arrow)
Stress fracture
Stress fractures may be related to fatigue, when excessive repetitive force is applied to a normal bone, or insufficiency, when normal stress is applied to abnormal bone such as in osteoporosis or Paget's disease. Common sites for stress fractures include the metatarsals, tarsals, and tibia.[21] Initially, stress fractures may not be visible on radiographs and are better detected on technetium-99 m pyrophosphate bone scintigraphy (bone scan) or MRI [Figure 11]. With time, periosteal reaction and cortical resorption may be seen. A fracture line may be visible on radiographs, but could be better seen on computed tomography (CT). The fracture line is usually perpendicular to the cortex, and vertically oriented fractures can be difficult to detect. Radiographic features of stress fracture in the tibia can resemble a soleal line or osteoid osteoma, but can be differentiated from one another on CT [Figure 12]. Moreover, if the periosteal reaction appears aggressive, it can mimic infection or an aggressive tumor.[22] The presence of a fracture line, lack of a soft tissue mass, and evidence of healing on follow-up studies should help distinguish stress fractures from other entities.
Figure 11 (A-C).
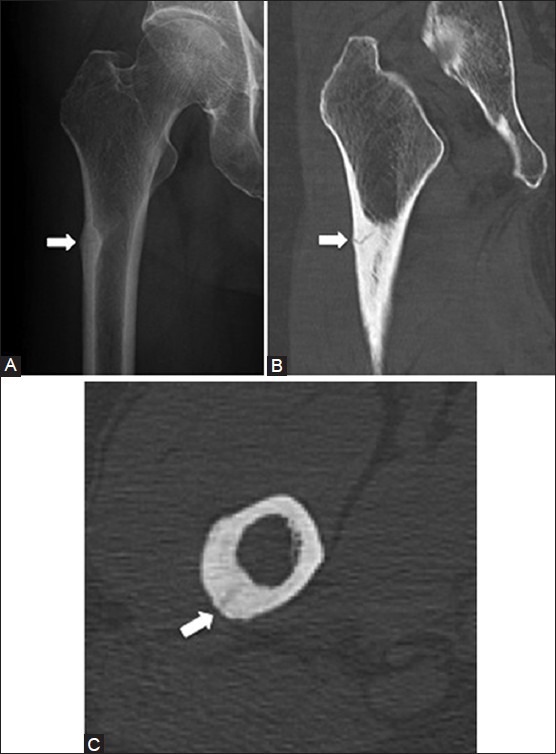
Stress fracture. A 68 year old female with right hip and thigh pain. (A) AP radiograph of the hip shows cortical thickening (arrow) in the lateral aspect of tibial shaft (B) Coronal and (C) axial CT images demonstrate a linear fracture line (arrows) within the cortical thickening
Figure 12 (A and B).
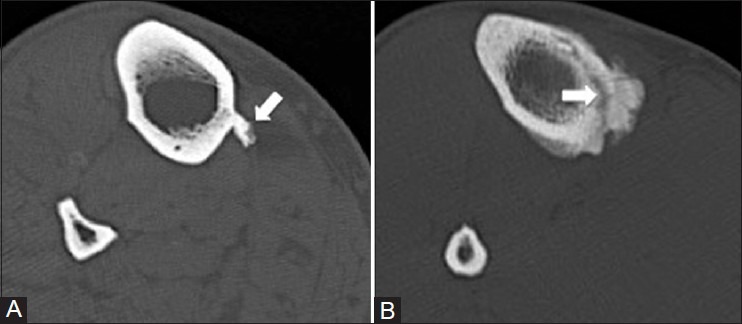
Axial CT images of the tibia show how CT can be helpful in distinguishing the soleal line (A) from a stress fracture (B) indicated by arrows
Myositis ossificans
Myositis ossificans is heterotopic ossification that occurs in muscle usually following trauma, although the patient may be unable to recall the precipitating trauma.[23] This commonly occurs in the upper and lower extremities, usually in the lateral muscles. Patients may be asymptomatic or present with pain, swelling, or an elevated erythrocyte sedimentation rate (ESR). Ossification develops 3-8 weeks after onset, beginning peripherally and progressing centrally. Initially, myositis ossificans forms faint irregular densities; but with time, a rim of mature lamellar bone and central osteoid matrix can develop [Figure 13]. The MRI appearance is variable depending on the stage of development, and earlier on, can mimic a sarcoma as there may be enhancement following contrast administration.[24] Differentiation from an osteochondroma or osteosarcoma may also be difficult if the area of ossification is adherent to the adjacent bone. CT can be helpful in demonstrating a plane of soft tissue between the mass and the bony cortex. Myositis ossificans may be difficult to distinguish from an osteosarcoma even on biopsy specimens.[25]
Figure 13 (A-C).
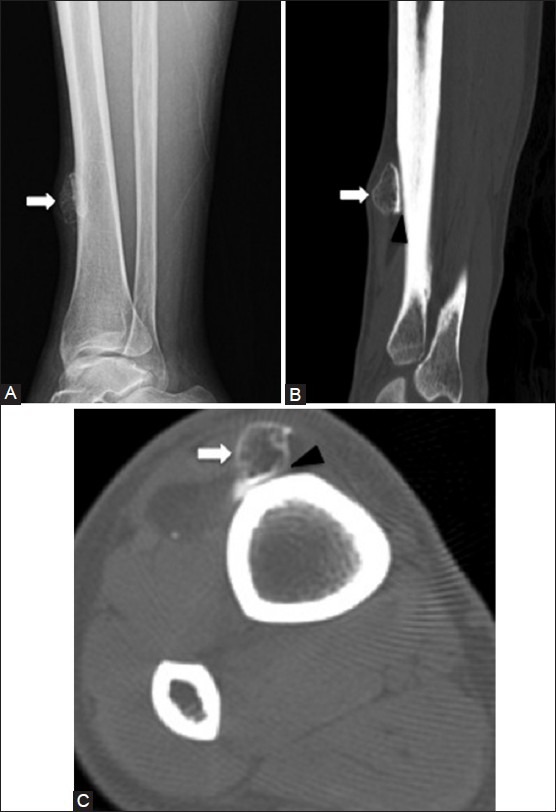
Myositis ossificans. A 53 year old female with a palpable mass along the right distal tibia. (A) Lateral radiograph shows an oval nodule (arrow) with dense periphery at the anterior aspect of tibial shaft (B) Sagittal and (C) axial CT images demonstrate a peripheral rim of calcification and central ossification in the lesion (arrows) and a small cleft (black arrowheads) between the mass and the tibial cortex
Biceps tenodesis
In biceps tenodesis, the intra-articular portion of the long head of the biceps tendon is cut and the proximal portion of the tendon is reattached to the proximal humeral diaphysis.[26] The site of attachment can mimic a radiolucent lesion with a sclerotic border [Figure 14]. This classic location along the proximal humerus should raise suspicion for this tumor mimicker, which can be confirmed by reviewing patients’ surgical notes.
Figure 14.
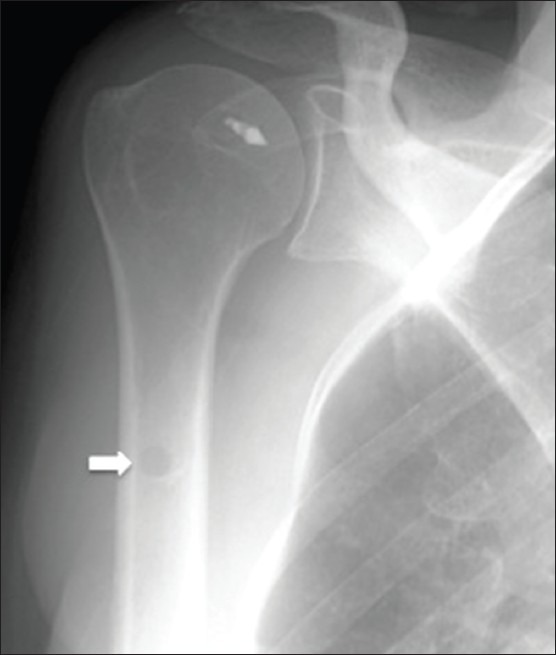
Biceps tenodesis. A 58 year old female with history of rotator cuff and labral tear. AP radiograph of right shoulder shows a focal lucent lesion (arrow) in the proximal humeral shaft from a biceps tenodesis. Suture anchor on the humeral head is also noted from rotator cuff surgery
Bone marrow biopsy and bone graft donor sites
Bone marrow aspiration and biopsy for hematological diagnosis is most often obtained from the iliac bone via a posterior approach.[27] If the biopsy has been recently performed, there may be marrow edema or cystic changes in the region, which can be mistaken for a focal lesion [Figure 15]. Similarly, bone graft donor sites can demonstrate edema in the early post-procedure period. In both cases, review of the patient's clinical history is essential to confirm that a procedure has been performed.
Figure 15.
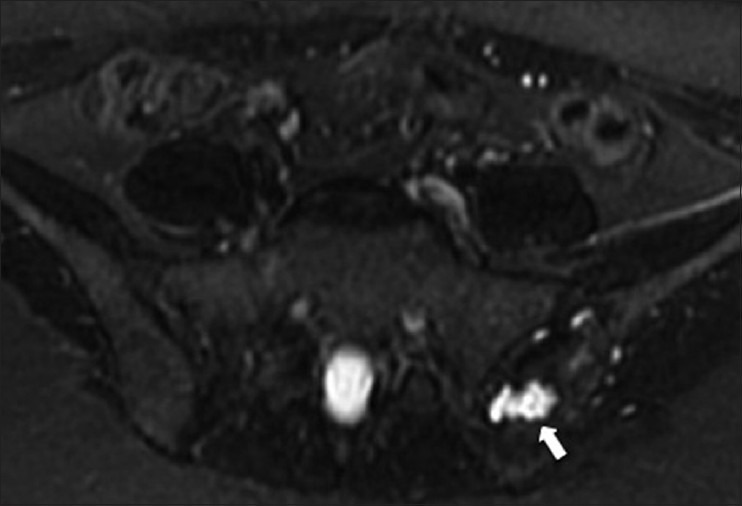
Bone marrow biopsy site. A 23 year old female with lymphoma and recent left iliac bone biopsy. Axial T2W fat-saturated MRI image shows a hyperintense lesion with irregular borders (arrow) in the left iliac bone, consistent with changes from a bone marrow biopsy
Particle disease
Particle disease can present as areas of radiolucency surrounding the hardware components, usually following arthroplasty.[28] However, unlike mechanical loosening, the lucent areas seen with particle disease typically do not follow the outline of the prosthesis [Figure 16].[29] The arthroplasty components can incite a macrophage-mediated granulomatous response, which then stimulates osteoclast activity.[28] Particle disease can mimic osteolytic tumors or infection; however, particle disease can be distinguished by the presence of hardware and the fact that abnormal lucencies are seen on both sides of a joint.
Figure 16 (A and B).
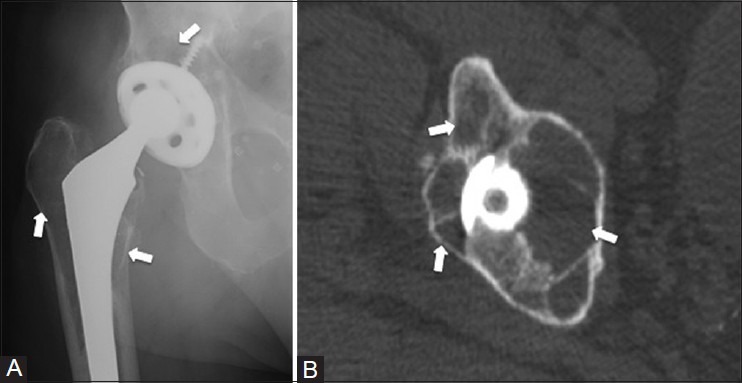
Particle disease. An 82 year old male who had undergone total hip replacement for hip pain. (A) AP radiograph of the right hip shows multiple lucent lesions (arrows) on both sides of the right hip joint, abutting both the femoral and acetabular components (B) Axial CT image demonstrates multiple cavities (arrows) around the prosthesis, which do not confine to the outline of the prosthesis
Radiation changes
Initially, radiotherapy causes vascular congestion, edema, and decreased cellularity in the bone marrow.[30] This will cause decreased signal on T1W sequences and increased signal on T2W sequences [Figure 17]. With time, the bone marrow will be replaced with fat and occasionally with fibrosis, with high signal on T1W and intermediate signal on T2W sequences.[30] There can be a clear line of demarcation along the borders of the radiation field. Irradiated bone can be at increased risk for insufficiency factures, osteonecrosis, and radiation-induced sarcomas.[31]
Figure 17.
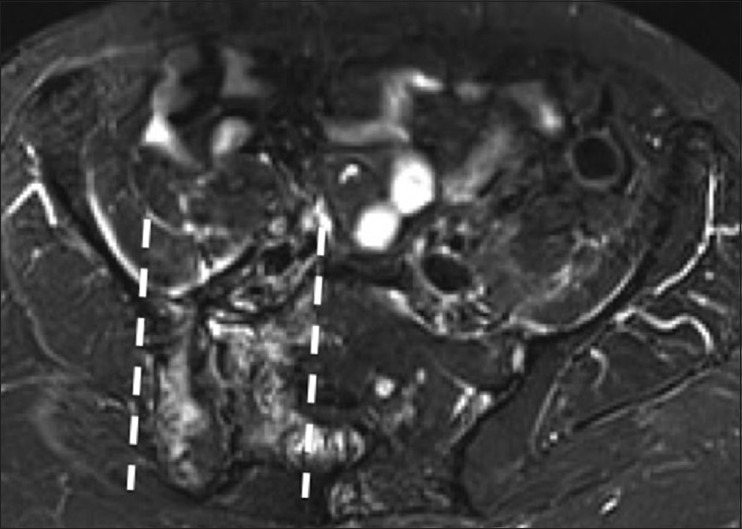
Radiation changes. A 59 year old female with history of endometrial cancer Salpingohysterectomy and radiation therapy. Axial short tau inversion recovery (STIR) MRI image shows a regional distribution of bony edema in the iliac bone and sacrum with demarcated borders (dotted lines), indicating the radiation field
Metabolic Disease and Arthritic Changes
Brown tumor of hyperparathyroidism
Longstanding untreated hyperparathyroidism can result in osteolytic lesions known as brown tumors (osteoclastomas) [Figure 18]. They can be seen in either primary or secondary hyperparathyroidism and are seen in 5% of patients with hyperparathyroidism.[32] However, the incidence has decreased with improved early diagnosis of the disease. The typical appearance of a brown tumor is a well-defined osteolytic lesion, which may have septations, be expansile, and can sometimes have aggressive features. Common sites include the long bones, ribs, pelvis, and facial bones.[33] The lesions improve with treatment, often becoming sclerotic. If lesions fail to improve in appearance with treatment, an alternative diagnosis should be considered.
Figure 18.
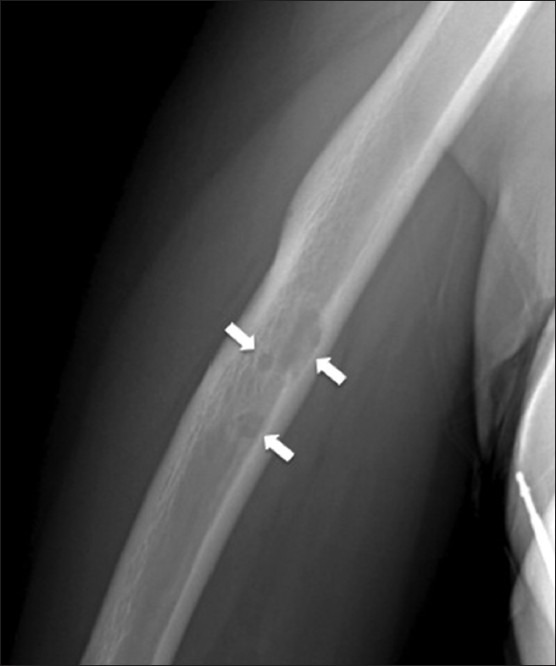
Brown tumor of hyperparathyroidism. A 42-year-old female with history of parathyroid adenoma. Focused AP radiograph of the humerus shows multiple well-defined lytic lesions (arrows) in the right humeral shaft
Melorheostosis
Melorheostosis is a benign bone dysplasia characterized by sclerotic bone lesions, often described as “dripping candle wax.”[34] Melorheostosis is not a hereditary disorder and is often asymptomatic; however, when symptoms do occur, they include pain, limb deformities and contractures related to muscle and tendon shortening, skin disorders, and poor circulation.[35] There is an association with soft tissue hemangiomas and neurofibromas.[36] The lesions can be mistaken for a surface osteosarcoma or osteochondroma. On imaging, there is characteristic flowing cortical hyperostosis [Figure 19] and can involve multiple contiguous bones in a sclerotomal distribution.[37] Low signal intensity is seen on all MRI sequences, but there may be surrounding soft tissue edema. The lesions may also be active on technetium-99 m pyrophosphate bone scintigraphy.[38]
Figure 19 (A and B).
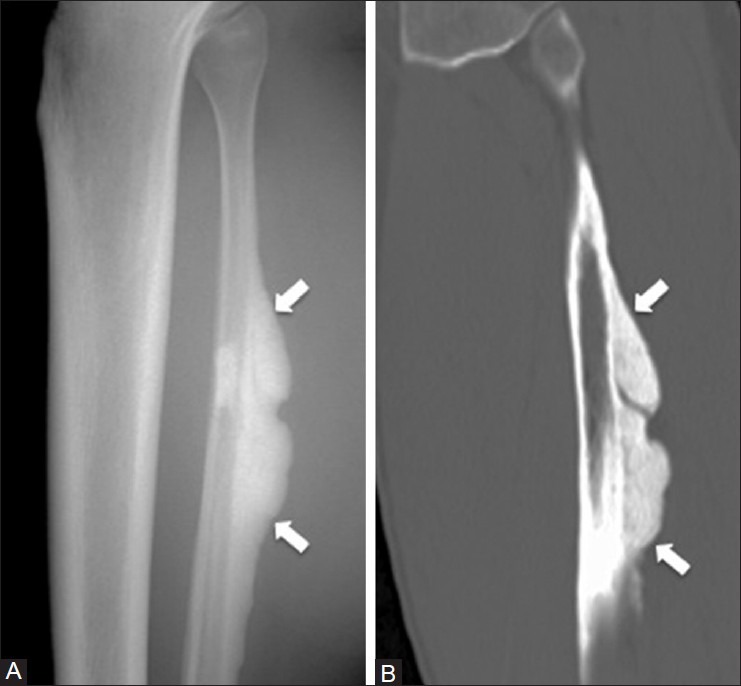
Melorheostosis. A 43 year old male with knee pain. (A) Lateral lower leg radiograph and (B) sagittal CT image of the tibia demonstrate dense cortical thickening (arrows) along the posterior fibula that simulates dripping candle wax
Osteonecrosis
Ischemic necrosis of the bone and marrow can be due to a variety of causes including trauma, steroids, hemoglobinopathies, alcoholism, radiotherapy, and chemotherapy.[39] When osteonecrosis involves the epiphysis (avascular necrosis), it can lead to subchondral bony collapse and osteoarthritis. Initially, osteonecrosis may be occult on radiography; but over time, it can manifest as a central radiolucency with a sclerotic margin. It may mimic enchondromas, but lacks central calcifications. MRI is sensitive for detection of bone infarcts. Initially, these areas appear as non-specific regions of marrow edema; but with time, the characteristic features of an outer band of low signal associated with an inner band of high signal on non-fat-saturated T2W images (double line sign) can develop.[22]
Calcific tendinitis (resorptive phase)
Calcific tendinitis is a common cause of joint pain and stiffness, and is caused by the deposition of calcium hydroxyapatite crystals in the tendons.[40] The tendons of the rotator cuff and around the hip [Figure 20] are most commonly involved; however, it can involve any tendon.[41] During the resorptive phase, calcific tendinitis can mimic an aggressive process such as infection or neoplasm.[42] Calcific tendinitis can be associated with erosions of the adjoining bone, mimicking a destructive bone lesion. This aggressive pattern is common along the posterior proximal femoral diaphysis. The process is typically self-limiting, but needle barbotage and steroid injection can provide symptomatic relief.[42]
Figure 20 (A and B).
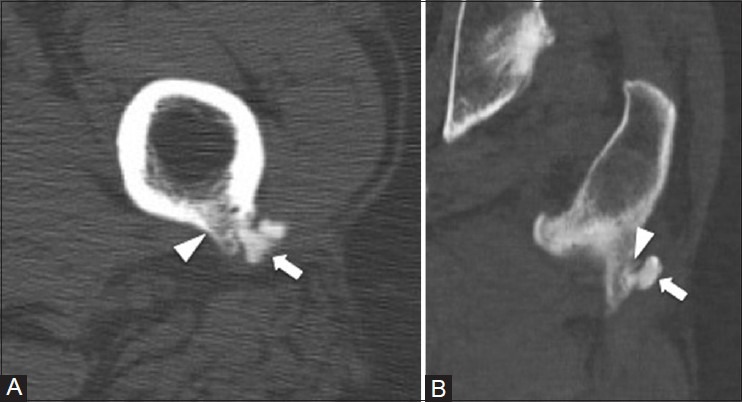
Calcific tendinitis (resorptive phase). A 47 year old male with left thigh pain. (A) Axial and (B) coronal CT images show calcifications at the insertion site of the gluteus maximus tendon to the posterior femur (arrow). Mild cortical erosion is also noted at the gluteal insertion site (arrowhead)
Subchondral cyst (geode)
In osteoarthritis, defects in the overlying cartilage can allow synovium and joint fluid to enter the subchondral bone causing subchondral cysts (geodes). They are typically small, about the articular surface, and have a sclerotic margin [Figure 21]. However, they can be large, but may extend down the shaft of a tubular bone mimicking a neoplasm.[22] CT can be helpful in demonstrating the sclerotic margin. On MRI, the lesion behaves like a cyst and is typically isointense to muscle on T1W images and hyperintense on T2W images. High T1 signal may occur in lesions that contain proteinaceous material, and internal enhancement may be seen if the lesions contain fibrous material. There should be evidence of osteoarthritis in the joint to support this diagnosis and changes are most often seen on both sides of the joint.[22]
Figure 21 (A and B).
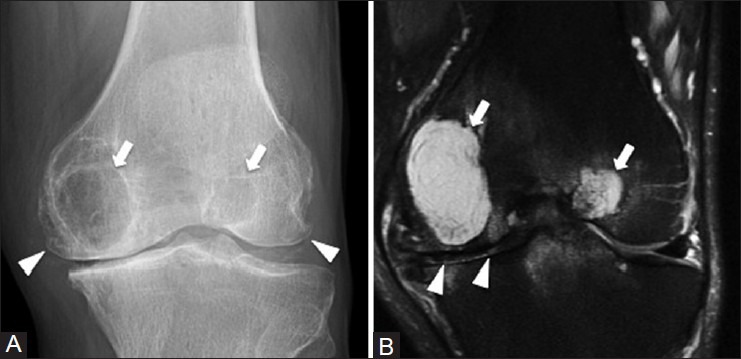
Subchondral cyst. A 61 year old male with left knee pain. (A) AP radiograph of knee shows large subarticular lucencies with sclerotic rims (arrows) in the medial and lateral femoral condyles. There is narrowing of the joint space and osteophytosis (arrowheads) consistent with degenerative osteoarthritis (B) Coronal T2W fat-saturated MRI image demonstrates cystic lesions (arrows) abutting the narrowed joint space (arrowheads)
Infection
Osteomyelitis/Brodie's abscess
In acute osteomyelitis, the radiographic findings include areas of aggressive periostitis, cortical destruction, endosteal scalloping, and intracortical tunneling. There may be soft tissue swelling or gas formation. However, the radiographic findings may not be present for 1-2 weeks. MRI and technetium-99 m pyrophosphate bone scintigraphy (bone scintigraphy) are more sensitive in the detection of early osteomyelitis.[43] Subacute or chronic osteomyelitis can cause an intraosseous abscess (Brodie's abscess), commonly in the metaphysis of tubular bones [Figure 22]. On radiographs, these lesions appear as single or multilobulated radiolucent lesions with surrounding sclerosis that fades toward the periphery. These lesions can mimic an osteoid osteoma or osteosarcoma.[44] Lesions without significant sclerosis can mimic Langerhans cell histiocytosis, chondroblastoma, giant cell tumor, and Ewing's sarcoma. CT can be helpful to delineate a sinus tract extending away from the central abscess, excluding other lesions.[45] Systemic signs of infection can be helpful; however, several of the lesions listed in the differential can also present with fever, pain, and other clinical signs of infection. Bone biopsy is often necessary for diagnosis and to identify an organism to guide appropriate antibiotic therapy.[43]
Figure 22 (A-C).
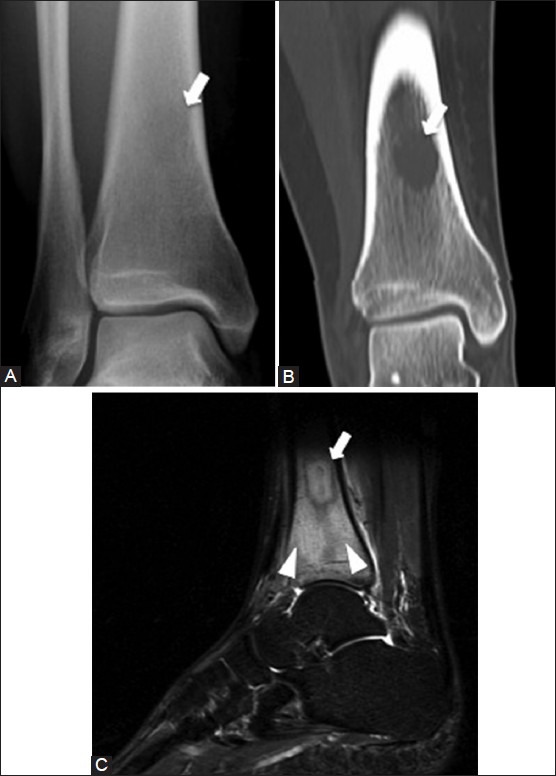
Osteomyelitis with Brodie's abscess. A 29 year old female with left lower leg pain. (A) AP radiograph of ankle demonstrates a faint radiolucency (arrow) in the distal tibial diaphysis (B) Coronal CT image shows that the lesion (arrow) is well demarcated with a non-sclerotic rim (C) Sagittal T2W fat-saturated MRI image shows the hyperintense intraosseous abscess (arrow) with surrounding marrow edema (arrowheads)
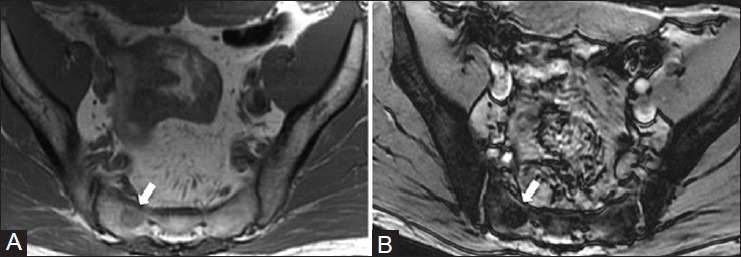
Tuberculosis infection of bone deserves special mention and has been called “the great mimicker.”[46] Most prevalent in underdeveloped countries, tuberculous osteomyelitis differs from pyogenic osteomyelitis as fever and pain can be absent and the symptoms are more insidious in onset.[47] Nearly any bone can be affected [Figure 23] and it is primarily caused by hematogenous spread from other sites, most commonly lung.[47] Bony destruction, loss of normal T1 marrow signal, marrow enhancement, and adjacent abscess or septic arthritis can occur. Spinal involvement by tuberculosis is not uncommon and can differ from bacterial spinal infection in that the disc spaces are preserved until late in the disease due to the lack of proteolytic destructive enzymes by Mycobacterium tuberculosis.[47,48] Finally, due to the hematogenous nature of spread, multifocal lesions can occur in the spine and appendicular skeleton, mimicking malignancy.[47,48]
Figure 23 (A-C).
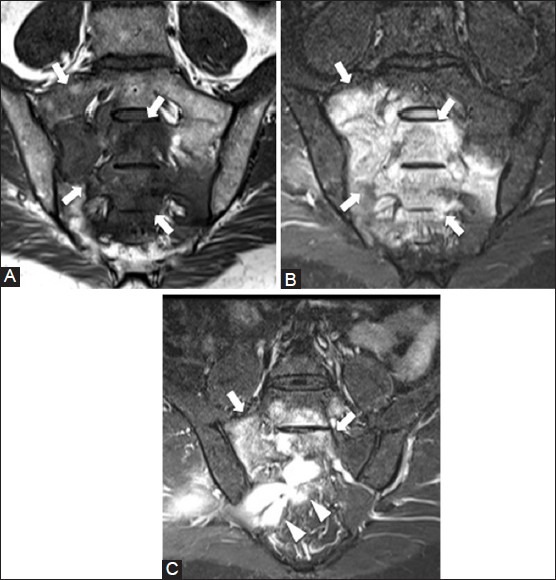
Tuberculous osteomyelitis. A 32 year old male with buttock pain. (A) Coronal T1W and (B) coronal T2W fat-saturated MRI images show marrow signal abnormality in the sacrum (arrows) (C) Coronal T2W fat-saturated MRI image shows hyperintense abscess (arrowheads) abutting the inferior border of sacral lesion (arrows). (Images courtesy of Dr. Aditya Daftary, MD)
Technical Artifacts
Humeral head - internal rotation view
On internal rotation radiographs of the shoulder, a pseudolesion with a sclerotic border and radiolucent center can appear in the humeral head [Figure 24]. A sharp sclerotic border is seen at the humeral neck as the diameter of the bone changes abruptly. The pseudolesion should not be seen on the external rotation or other views and should not be mistaken for an osteolytic lesion.
Figure 24 (A and B).
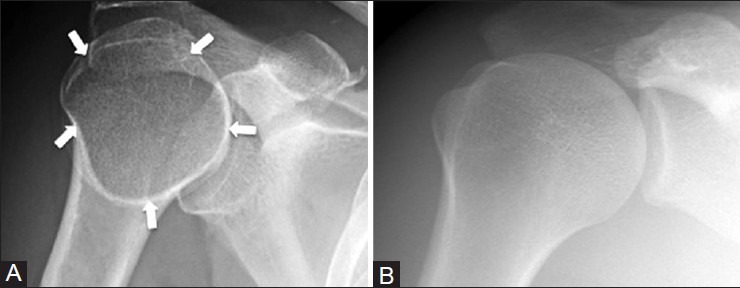
Humeral head pseudolesion. A 30 year old male with right shoulder pain. (A) Internal rotation view of right shoulder shows a lucent pseudolesion with a pseudosclerotic border (arrows) (B) This pseudolesion disappears on the external rotation view
Radial tuberosity - lateral view
The radial tuberosity is a normal anatomic structure in the proximal radius; however, on lateral projections, it is imaged en face and can appear as an ovoid radiolucent lesion [Figure 25]. On other projections, the tuberosity becomes clear and the artifactual radiolucency disappears. The bony protuberance of an osteochondroma can mimic a radiolucent lesion when seen en face as well. To avoid this pitfall, it is important to review additional projections.
Figure 25 (A and B).
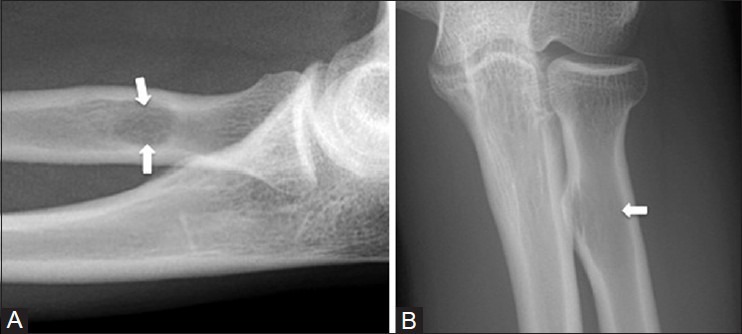
Radial tuberosity pseudolesion. A 33 year old female with medial elbow pain. (A) Lateral radiograph of elbow shows a lucent pseudolesion (arrows) in the radial tuberosity that disappears on the AP radiograph (B)
Wrap-around/aliasing in MRI
The field of view (FOV) in MRI refers to the anatomic region being imaged. Deciding on an appropriate FOV depends on the size of the structure being imaged and taking into account the trade-offs between spatial resolution and the signal-to-noise ratio. If a FOV is chosen which is smaller than the anatomy being imaged, wrap-around or aliasing artifacts can occur.[49] This can lead to image data that are outside the FOV being “wrapped around” and artifactually included within the image [Figure 26]. This can be corrected by using a large enough FOV in the phase-encoding direction to include the entire body part or by using phase oversampling techniques during imaging.
Figure 26.
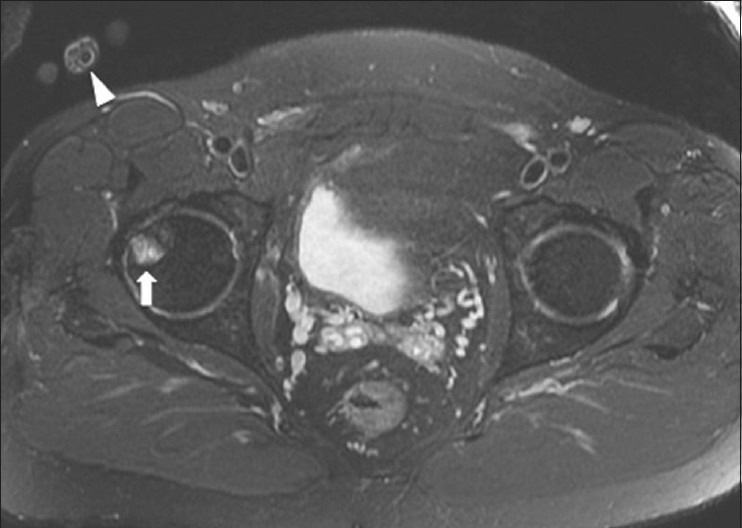
Wrap-around/aliasing in MRI. A 47 year old male with lower buttock pain. Axial STIR MRI image shows a wrap-around/aliasing artifact from right hand (arrowhead) and mimicking a focal lesion of right femoral head (arrow)
Pulsation artifact on MRI
Pulsation of vascular structures can cause “ghosting” on MRI.[49] This can mimic bone lesions as artifactual image data from the vessels are superimposed onto bone [Figure 27]. Repeating the imaging sequence after swapping the phase- and frequency-encoding directions can help to determine whether or not the lesion is real. To reduce pulsation artifact, one can place a saturation band over the vessels or not align the vessel and target lesion in the same phase-encoding direction.[50]
Figure 27.
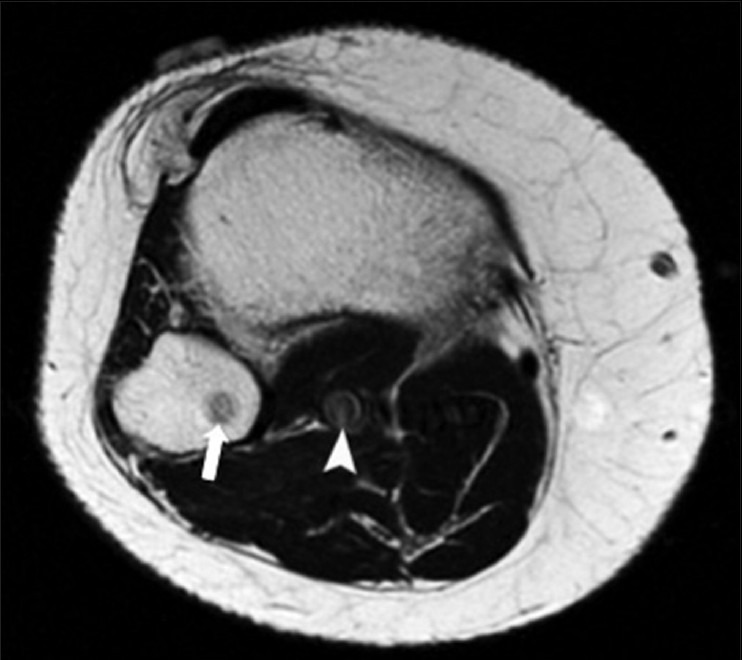
Pulsation artifact. A 31 year old male with right knee pain. Axial T1W MRI image shows a low signal rounded focus (arrow) in fibula, which is caused by pulsation artifact from popliteal artery (arrowhead) and mimics a tumor
External objects
External objects lying on a patient's skin can mimic bone lesions [Figure 28]. This commonly occurs in the acute trauma setting when urgent imaging is required and the technique may be suboptimal.
Figure 28 (A and B).
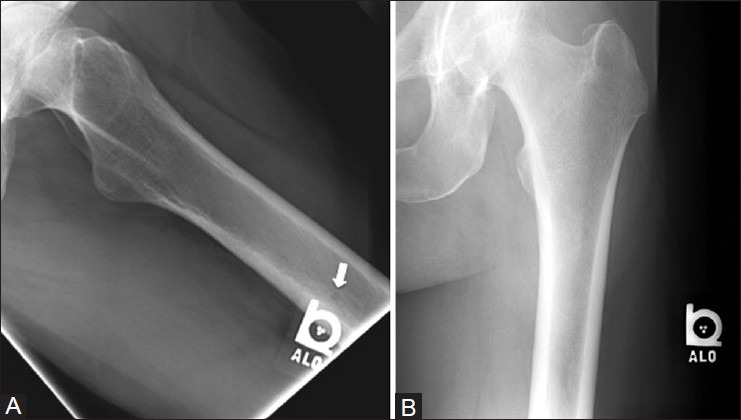
External object. A 60 year old female with left hip pain. (A) Frog view of left hip shows a tiny radiolucency (arrow) in proximal femoral shaft, which disappears on (B) AP radiograph of left hip. The radiolucency is caused by a small hole in the side locator tag
Conclusion
Numerous normal anatomic variants and non-neoplastic lesions can have an imaging appearance, which raises concern for a bone tumor. Awareness of these lesions and an understanding of their discriminating features are essential to avoid unnecessary additional imaging and procedures. Knowing which lesions to leave alone or which ones require workup can prevent misdiagnosis and reduce patient anxiety.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Boavida P, Muller LS, Rosendahl K. Magnetic resonance imaging of the immature skeleton. Acta Radiol. 2013;54:1007–14. doi: 10.1177/0284185113501945. [DOI] [PubMed] [Google Scholar]
- 2.Long SS, Yablon CM, Eisenberg RL. Bone marrow signal alteration in the spine and sacrum. AJR Am J Roentgenol. 2010;195:W178–200. doi: 10.2214/ajr.09.4134. [DOI] [PubMed] [Google Scholar]
- 3.Zajick DC, Jr, Morrison WB, Schweitzer ME, Parellada JA, Carrino JA. Benign and malignant processes: Normal values and differentiation with chemical shift MR imaging in vertebral marrow. Radiology. 2005;237:590–6. doi: 10.1148/radiol.2372040990. [DOI] [PubMed] [Google Scholar]
- 4.Foster K, Chapman S, Johnson K. MRI of the marrow in the paediatric skeleton. Clin Radiol. 2004;59:651–73. doi: 10.1016/j.crad.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Babyn PS, Ranson M, McCarville ME. Normal bone marrow: Signal characteristics and fatty conversion. Magn Reson Imaging Clin N Am. 1998;6:473–95. [PubMed] [Google Scholar]
- 6.Resnick D, Cone RO., 3rd The nature of humeral pseudocysts. Radiology. 1984;150:27–8. doi: 10.1148/radiology.150.1.6689775. [DOI] [PubMed] [Google Scholar]
- 7.De Wilde V, De Maeseneer M, Lenchik L, Van Roy P, Beeckman P, Osteaux M. Normal osseous variants presenting as cystic or lucent areas on radiography and CT imaging: A pictorial overview. Eur J Radiol. 2004;51:77–84. doi: 10.1016/S0720-048X(03)00180-3. [DOI] [PubMed] [Google Scholar]
- 8.Weissman BN, Sledge CB. Philadelphia, PA: Saunders; 1986. Orthopedic Radiology. [Google Scholar]
- 9.Johnson JF, Brogdon BG. Dorsal effect of the patella: Incidence and distribution. AJR Am J Roentgenol. 1982;139:339–40. doi: 10.2214/ajr.139.2.339. [DOI] [PubMed] [Google Scholar]
- 10.Pitt MJ, Graham AR, Shipman JH, Birkby W. Herniation pit of the femoral neck. AJR Am J Roentgenol. 1982;138:1115–21. doi: 10.2214/ajr.138.6.1115. [DOI] [PubMed] [Google Scholar]
- 11.Kavanagh EC, Read P, Carty F, Zoga AC, Parvizi J, Morrison WB. Three-dimensional magnetic resonance imaging analysis of hip morphology in the assessment of femoral acetabular impingement. Clin Radiol. 2011;66:742–7. doi: 10.1016/j.crad.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Nokes SR, Vogler JB, Spritzer CE, Martinez S, Herfkens RJ. Herniation pits of the femoral neck: Appearance at MR imaging. Radiology. 1989;172:231–4. doi: 10.1148/radiology.172.1.2740509. [DOI] [PubMed] [Google Scholar]
- 13.Suh JS, Cho JH, Shin KH, Won JH, Park SJ, Shin DH, et al. MR appearance of distal femoral cortical irregularity (cortical desmoid) J Comput Assist Tomogr. 1996;20:328–32. doi: 10.1097/00004728-199603000-00031. [DOI] [PubMed] [Google Scholar]
- 14.Resnick D, Greenway G. Distal femoral cortical defects, irregularities, and excavations. Radiology. 1982;143:345–54. doi: 10.1148/radiology.143.2.7041169. [DOI] [PubMed] [Google Scholar]
- 15.Natsis K. Supracondylar process of the humerus: Study on 375 caucasian subjects in Cologne, Germany. Clin Anat. 2008;21:138–41. doi: 10.1002/ca.20601. [DOI] [PubMed] [Google Scholar]
- 16.Camerlinck M, Vanhoenacker FM, Kiekens G. Ultrasound demonstration of Struthers’ ligament. J Clin Ultrasound. 2010;38:499–502. doi: 10.1002/jcu.20700. [DOI] [PubMed] [Google Scholar]
- 17.Levine AH, Pais MJ, Berinson H, Amenta PS. The soleal line: A cause of tibial pseudoperiostitis. Radiology. 1976;119:79–81. doi: 10.1148/119.1.79. [DOI] [PubMed] [Google Scholar]
- 18.Mysorekar VR, Nandedkar AN. The soleal line. Anat Rec. 1983;206:447–51. doi: 10.1002/ar.1092060410. [DOI] [PubMed] [Google Scholar]
- 19.Guillin R, Moser T, Koob M, Khoury V, Chapuis M, Ropars M, et al. Subperiosteal hematoma of the iliac bone: Imaging features of acute and chronic stages with emphasis on pathophysiology. Skeletal Radiol. 2012;41:667–75. doi: 10.1007/s00256-011-1267-3. [DOI] [PubMed] [Google Scholar]
- 20.Malghem J, Maldague B, Claus D, Clapuyt P. Transient cyst-like cortical defects following fractures in children. Medullary fat within the subperiosteal haematoma. J Bone Joint Surg Br. 1990;72:862–5. doi: 10.1302/0301-620X.72B5.2211773. [DOI] [PubMed] [Google Scholar]
- 21.Krestan CR, Nemec U, Nemec S. Imaging of insufficiency fractures. Semin Musculoskelet Radiol. 2011;15:198–207. doi: 10.1055/s-0031-1278420. [DOI] [PubMed] [Google Scholar]
- 22.Stacy GS, Kapur A. Mimics of bone and soft tissue neoplasms. Radiol Clin North Am. 2011;49:1261–86. doi: 10.1016/j.rcl.2011.07.009. vii. [DOI] [PubMed] [Google Scholar]
- 23.Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: A review. J Rehabil Med. 2005;37:129–36. doi: 10.1080/16501970510027628. [DOI] [PubMed] [Google Scholar]
- 24.Kransdorf MJ, Meis JM, Jelinek JS. Myositis ossificans: MR appearance with radiologic-pathologic correlation. AJR Am J Roentgenol. 1991;157:1243–8. doi: 10.2214/ajr.157.6.1950874. [DOI] [PubMed] [Google Scholar]
- 25.Klapsinou E, Despoina P, Dimitra D. Cytologic findings and potential pitfalls in proliferative myositis and myositis ossificans diagnosed by fine needle aspiration cytology: Report of four cases and review of the literature. Diagn Cytopathol. 2012;40:239–44. doi: 10.1002/dc.21549. [DOI] [PubMed] [Google Scholar]
- 26.Werner BC, Pehlivan HC, Hart JM, Lyons ML, Gilmore CJ, Garrett CB, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. (S1058-2746).J Shoulder Elbow Surg. 2013:ii. doi: 10.1016/j.jse.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Bain BJ. Bone marrow biopsy morbidity and mortality: 2002 data. Clin Lab Haematol. 2004;26:315–8. doi: 10.1111/j.1365-2257.2004.00630.x. [DOI] [PubMed] [Google Scholar]
- 28.Gallo J, Goodman SB, Konttinen YT, Raska M. Particle disease: Biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty. Innate Immun. 2013;19:213–24. doi: 10.1177/1753425912451779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beck RT, Illingworth KD, Saleh KJ. Review of periprosthetic osteolysis in total joint arthroplasty: An emphasis on host factors and future directions. J Orthop Res. 2012;30:541–6. doi: 10.1002/jor.21554. [DOI] [PubMed] [Google Scholar]
- 30.Stevens SK, Moore SG, Kaplan ID. Early and late bone-marrow changes after irradiation: MR evaluation. AJR Am J Roentgenol. 1990;154:745–50. doi: 10.2214/ajr.154.4.2107669. [DOI] [PubMed] [Google Scholar]
- 31.Kwon JW, Huh SJ, Yoon YC, Choi SH, Jung JY, Oh D, et al. Pelvic bone complications after radiation therapy of uterine cervical cancer: Evaluation with MRI. AJR Am J Roentgenol. 2008;191:987–94. doi: 10.2214/AJR.07.3634. [DOI] [PubMed] [Google Scholar]
- 32.Marcocci C, Cianferotti L, Cetani F. Bone disease in primary hyperparathyrodism. Ther Adv Musculoskelet Dis. 2012;4:357–68. doi: 10.1177/1759720X12441869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parisien M, Silverberg SJ, Shane E, Dempster DW, Bilezikian JP. Bone disease in primary hyperparathyroidism. Endocrinol Metab Clin North Am. 1990;19:19–34. [PubMed] [Google Scholar]
- 34.Jain VK, Arya RK, Bharadwaj M, Kumar S. Melorheostosis: Clinicopathological features, diagnosis, and management. Orthopedics. 2009;32:512. doi: 10.3928/01477447-20090527-20. [DOI] [PubMed] [Google Scholar]
- 35.Singh R, Singh Z, Bala R, Rana P, Sangwan SS. An unusual case of sciatic neuropraxia due to melorheostosis. Joint Bone Spine. 2010;77:614–5. doi: 10.1016/j.jbspin.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Roger D, Bonnetblanc JM, Leroux-Robert C. Melorheostosis with associated minimal change nephrotic syndrome, mesenteric fibromatosis and capillary haemangiomas. Dermatology. 1994;188:166–8. doi: 10.1159/000247127. [DOI] [PubMed] [Google Scholar]
- 37.Murray RO, McCredie J. Melorheostosis and the sclerotomes: A radiological correlation. Skeletal Radiol. 1979;4:57–71. doi: 10.1007/BF00349329. [DOI] [PubMed] [Google Scholar]
- 38.Sonoda LI, Halim MY, Balan KK. Detection of extensive melorheostosis on bone scintigram performed for suspected metastases. Clin Nucl Med. 2011;36:240–1. doi: 10.1097/RLU.0b013e318208f517. [DOI] [PubMed] [Google Scholar]
- 39.Janssens AM, Offner FC, Van Hove WZ. Bone marrow necrosis. Cancer. 2000;88:1769–80. [PubMed] [Google Scholar]
- 40.Garcia GM, McCord GC, Kumar R. Hydroxyapatite crystal deposition disease. Semin Musculoskelet Radiol. 2003;7:187–93. doi: 10.1055/s-2003-43229. [DOI] [PubMed] [Google Scholar]
- 41.Bancroft LW, Blankenbaker DG. Imaging of the tendons about the pelvis. AJR Am J Roentgenol. 2010;195:605–17. doi: 10.2214/AJR.10.4682. [DOI] [PubMed] [Google Scholar]
- 42.Siegal DS, Wu JS, Newman JS, Del Cura JL, Hochman MG. Calcific tendinitis: A pictorial review. Can Assoc Radiol J. 2009;60:263–72. doi: 10.1016/j.carj.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Hatzenbuehler J, Pulling TJ. Diagnosis and management of osteomyelitis. Am Fam Physician. 2011;84:1027–33. [PubMed] [Google Scholar]
- 44.Huang PY, Wu PK, Chen CF, Lee FT, Wu HT, Liu CL, et al. Osteomyelitis of the femur mimicking bone tumors: A review of 10 cases. World J Surg Oncol. 2013;11:283. doi: 10.1186/1477-7819-11-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hankin D, Bowling FL, Metcalfe SA, Whitehouse RA, Boulton AJ. Critically evaluating the role of diagnostic imaging in osteomyelitis. Foot Ankle Spec. 2011;4:100–5. doi: 10.1177/1938640010390934. [DOI] [PubMed] [Google Scholar]
- 46.De Backer AI, Mortelé KJ, Vanhoenacker FM, Parizel PM. Imaging of extraspinal musculoskeletal tuberculosis. Eur J Radiol. 2006;57:119–30. doi: 10.1016/j.ejrad.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 47.De Vuyst D, Vanhoenacker F, Gielen J, Bernaerts A, De Schepper AM. Imaging features of musculoskeletal tuberculosis. Eur Radiol. 2003;13:1809–19. doi: 10.1007/s00330-002-1609-6. [DOI] [PubMed] [Google Scholar]
- 48.Lebowitz D, Wolter L, Zenklusen C, Chouiter A, Malinverni R. TB determined: Tuberculous osteomyelitis. Am J Med. 2014;127:198–201. doi: 10.1016/j.amjmed.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Morelli JN, Runge VM, Ai F, Attenberger U, Vu L, Schmeets SH, et al. An image-based approach to understanding the physics of MR artifacts. Radiographics. 2011;31:849–66. doi: 10.1148/rg.313105115. [DOI] [PubMed] [Google Scholar]
- 50.Ojeda-Fournier H, Choe KA, Mahoney MC. Recognizing and interpreting artifacts and pitfalls in MR imaging of the breast. Radiographics. 2007;27(Suppl 1):S147–64. doi: 10.1148/rg.27si075516. [DOI] [PubMed] [Google Scholar]


