Abstract
Ultrasonography (USG) is an excellent cost-effective modality in imaging of peripheral nerves. With the newer high-frequency probes with different footprints which allow high-resolution imaging at relatively superficial location, USG can detect and evaluate traumatic, inflammatory, infective, neoplastic, and compressive pathologies of the peripheral nerves. This article describes the technique for evaluation of nerves by USG as well as the USG appearances of normal and diseased peripheral nerves.
Keywords: High-resolution, nerves, sonography, ultrasonography, ultrasound
Introduction
Peripheral nerves are affected by a number of disease processes like trauma, infection, inflammation, benign and malignant tumors, as well as entrapment neuropathies. USG with its high resolution, can detect and characterize these pathologies in a cost-effective manner. In our country, with the majority of population unable to afford costly diagnostic tests, USG remains an underutilized modality. The aim of this article is to familiarize readers with USG appearances of various nerve pathologies.
Technique
Almost all the nerves including digital nerves can be imaged by USG. Before starting the scan of a peripheral nerve in a particular region, one needs to know the detailed anatomy. A high-frequency linear array probe (8-15 MHz) is used. Small footprint probe like a hockey stick probe is used to image digital nerves. The examination is started from a known anatomic landmark near the nerve. Once the nerve is localized in the short axis, it is traced cranially and caudally to see for contour and architectural abnormality. If pathology is encountered, then the attention is focused on that particular segment. The probe is then turned in the long axis of the nerve and the pathology is evaluated. USG gel should be used liberally. This helps to avoid missing the nerve while tracing it. Use of focal zone and depth optimally dramatically improves the quality of the image.
Nerves generally run along borders of other structures, especially between different muscle groups. It is important to have a practiced survey pattern for each nerve using landmarks and borders that one can follow every time. If one loses the nerve while tracing it, the survey needs to be started again from the beginning. Movement of limb helps to differentiate nerve from tendons, whereas color Doppler helps to differentiate nerves from vessels. Lymph nodes are spherical and show a fatty hilum and can be easily differentiated from nerves by their shape and inability to trace them in longitudinal axis.
Normal Appearances
The normal nerve, in transverse section, reveals small hypoechoic areas separated by hyperechoic septae, giving a “honeycomb-like” appearance.[1] The hypoechoic areas represent nerve fascicles while the echogenic septae represent interfascicular perineurium [Figure 1]. The longitudinal sections also reveal the fascicular architecture, leading to a “bundle of straws” appearance [Figure 2]. The nerve is more echogenic as compared to the muscle which shows hypoechoic muscle fiber bundles with intervening echogenic perimysium. The tendon is more echogenic as compared to the nerve and shows a compact arrangement of echogenic fibrils. On dynamic examination, the nerves show sliding movement over the muscles and tendons. An altered movement or contour deformity during movement of the nerve gives us a clue to diagnose pathology.
Figure 1.

Axial USG image of normal nerve showing rounded hypoechoic areas separated by hyperechoic septae, giving a “honeycomb” appearance
Figure 2.
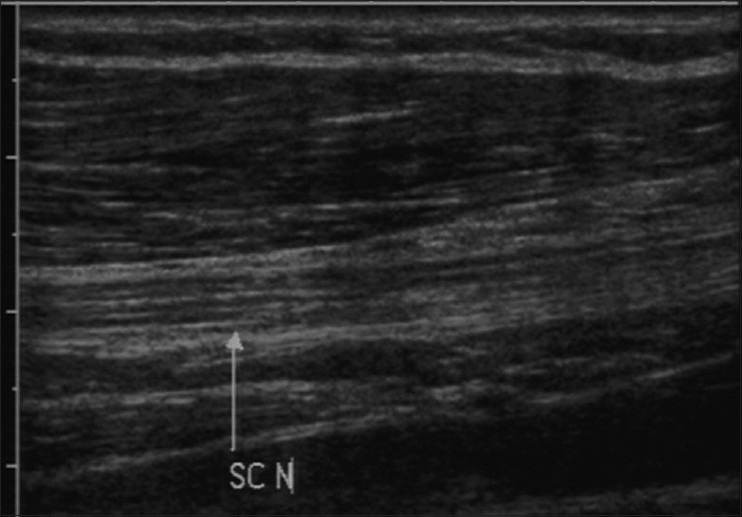
Longitudinal USG image of normal nerve depicting hypoechoic linear fascicles with intervening echogenic interfascicular perineurium i.e. “bundle of straws” appearance
Ultrasound Appearances of Various Pathologies
Trauma
Three peripheral nerve injury types have been described based on the mechanism of injury, i.e. stretch injuries, laceration, and compression injuries. Depending on the microscopic changes, nerve injuries are broadly classified as neurapraxia, axonotmesis, and neurotmesis. Neurapraxia is injury with maintenance of nerve continuity. Axonotmesis is disruption of axons and myelin with intact epi-and perineurium, while neurotmesis is complete disruption of the nerve. Neurapraxia and axonotmesis have good chances of recovery, while neurotmesis does not usually recover without surgery.[2]
USG can be used to detect and demonstrate the site of injury, differentiate nerve injury in continuity from nerve transection, evaluate the cause of compression, and detect foreign bodies as well as neuroma or scarring. USG is also useful in localizing iatrogenic nerve injury following limb lengthening procedures or due to orthopedic implants where magnetic resonance imaging (MRI) may be limited due to susceptibility artifacts [Figure 3A and B].[3]
Figure 3 (A and B).
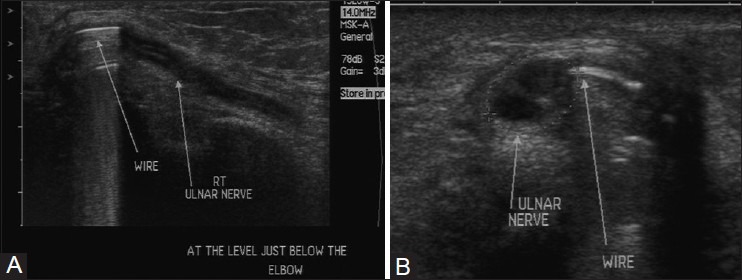
(A) Longitudinal and (B) axial USG images in a patient with previous history of fracture repair of humerus at the elbow. The K wire is impinging on the nerve, causing chronic nerve degeneration seen as hypoechoic appearance of nerve with loss of normal fascicular architecture
The high resolution of USG allows evaluation of small nerves like digital nerves which may be difficult with MRI. Also, MRI may not differentiate neural contusion from nerve disruption.[4]
Electrodiagnostic studies do not demonstrate morphologic information like site and degree of injury. Hence, USG has an important role to play in evaluation of patients with suspected nerve injury.
Neurapraxic injury is seen as swollen nerve with hypoechoic appearance.
Complete and partial transection of nerves can be differentiated by USG. In cases with transection, it is important to provide the distance between the stumps as it helps in deciding surgical management [Figure 4]. Stump or amputation neuromas may be seen as focal thickening or mass-like lesions at the nerve ends [Figure 5].[5] These are reactive thickening of the nerves and are not true tumors.
Figure 4.
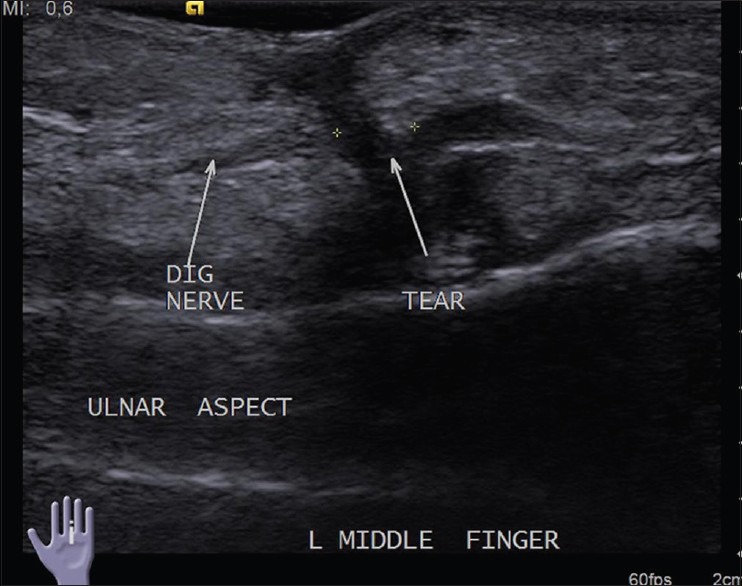
Longitudinal USG image reveals complete transection of the volar digital nerve of middle finger following penetrating injury
Figure 5.

Longitudinal USG image shows complete transection of the radial nerve following old penetrating trauma. Note the amputation neuromas at both the cut ends seen as bulbous lesions
Tumors
The most common nerve tumors are nerve sheath tumors which include schwannomas and neurofibromas. It may not always be possible to differentiate between them on USG. They are seen as well-defined ovoid homogeneous hypoechoic lesions with nerve entering and exiting from them. Schwannomas are eccentric along the long axis of nerve, with nerve fascicles seen separately [Figure 6A–C]. Neurofibromas are spindle-shaped with loss of normal fascicular architecture.[6]
Figure 6 (A-C).
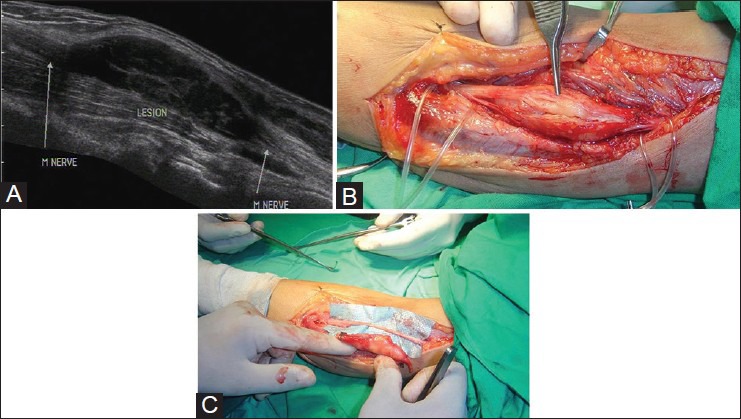
(A) Longitudinal USG image showing a fusiform predominantly hypoechoic mass lesion along the median nerve in forearm. The nerve can be located eccentrically along the ventral aspect of the mass lesion, suggesting the diagnosis of schwannoma (B) Intraoperative image of the lesion confirming the ultrasound findings (C) Intraoperative image after excision of the mass lesion with preservation of the nerve
Malignant peripheral nerve sheath tumors may be seen in neurofibromatosis. They have ill-defined indistinct margins, but USG may not be always able to differentiate them from their benign counterparts. Any sudden increase in size or pain warrants exclusion of malignant transformation.
Lipofibromatous hamartoma is benign proliferation of mature adipocytes in peripheral nerves separating the axons. The median nerve is most commonly affected. USG shows fusiform enlargement of the nerve with a characteristic appearance with cable-like hypoechoic nerve fascicles separated by hyperechoic fat [Figure 7A and B].[7]
Figure 7 (A and B).

(A) Longitudinal and (B) axial USG images in a patient with fibrolipomatous hamartoma of the median nerve in mid palm. There is fusiform enlargement of the nerve with hyperechoic fat interspersed between hypoechoic nerve fascicles
USG also helps in evaluating the status of nerve in relation to mass lesions like soft tissue tumors, i.e. whether the nerve is displaced or involved by the lesion. This information is essential in surgical management.
Morton's neuromas are not true tumors. They are formed due to perineural fibrosis and thickening of plantar digital nerves. They occur due to chronic microtrauma, especially in women who wear high heels. The usual location is in second or third intermetatarsal space at the level of heads of metatarsals, the former being more common. They are seen as hypoechoic mass replacing the normal hyperechoic fat in intermetatarsal space [Figure 8]. Scan in longitudinal axis demonstrates the plantar digital nerve in continuity with the mass.[8,9]
Figure 8.

Morton's neuroma in second intermetatarsal space seen as a hypoechoic lesion replacing the normal hyperechoic fat
Infective Lesions
In India, leprosy is a common treatable condition whose hallmark is nerve enlargement and inflammation. Clinical examination in cases with leprosy may be subjective and inaccurate. Also, many nerves may not be amenable to palpation. Early detection of nerve impairment can help in preventing disability.[10]
USG can provide objective evidence of nerve enlargement and also evaluate its internal architecture. In leprosy, the nerves may show enlargement as well as edema, loss of fascicular architecture, and increased peri- and endoneurium vascularity on Doppler [Figure 9A and B]. Jain et al. have demonstrated these changes in ulnar, median, lateral peroneal, and popliteal nerves.[11]
Figure 9 (A and B).
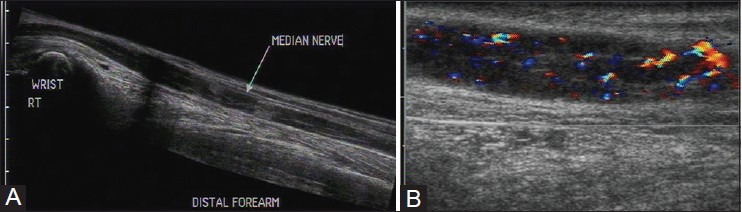
(A) Longitudinal USG image and (B) color Doppler image of median nerve in a patient with leprosy. The entire nerve is thickened with loss of fascicular architecture and hypoechoic appearance. There is increased endoneurium and perineurium vascularity on color Doppler
Entrapment Neuropathies
Entrapment or compression neuropathies are often unrecognized cause of pain and neural impairment.
The nerves are more prone to compression in specific locations where they course through osteofibrous tunnels. The median nerve in carpal tunnel and the ulnar nerve in Guyon's canal and cubital tunnel are the common sites of entrapment in the upper limb and can be evaluated with USG [Figure 10]. Common peroneal nerve near fibular neck and posterior tibial nerve in tarsal tunnel are commonly involved in the lower limb.[12]
Figure 10.
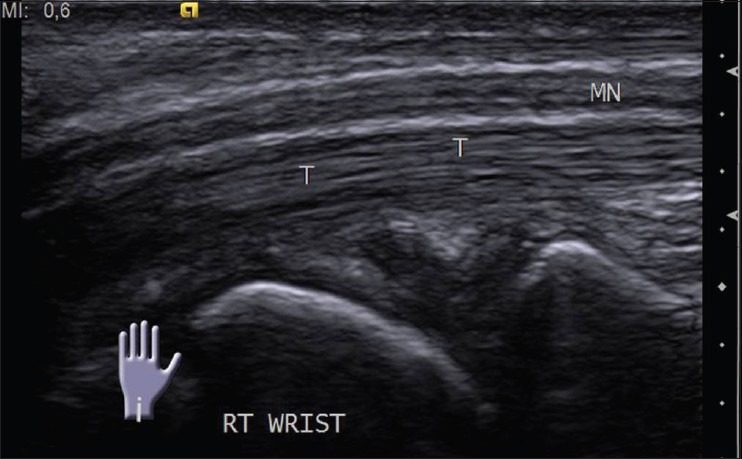
Longitudinal USG image of normal median nerve proximal to and in the carpal tunnel. T: Flexor tendons in the carpal tunnel; MN: Median nerve
Carpal tunnel syndrome is the most common entrapment neuropathy. It occurs due to compression of the median nerve in the carpal tunnel bounded by the carpal bones and the flexor retinaculum. The diagnosis is based on the patient's history of sensory and motor symptoms in median nerve distribution and clinical examination findings. USG is comparable with nerve conduction studies in the diagnosis of carpal tunnel syndrome. It shows the classic triad of enlargement of the nerve at the level of distal radius and proximal carpal tunnel, flattening of the nerve in distal carpal tunnel, and palmar bowing of the flexor retinaculum.
A cross-sectional area of the median nerve proximal to the tunnel inlet more than 10 mm2 is abnormal. This site is identified by the “rising sun” appearance of the proximal lunate.[13] The abrupt change in nerve caliber at the entrance of carpal tunnel is called “notch sign” [Figure 11A and B].[14] The nerve may show a homogeneous hypoechoic appearance with loss of fascicular echopattern [Figure 12A and B]. On dynamic examination, there is reduced transverse sliding movement in some cases. A contralateral comparison usually helps in detecting subtle signs to reach the diagnosis. However, one should remember that a normal nerve does not exclude a diagnosis of neuropathy.
Figure 11 (A and B).
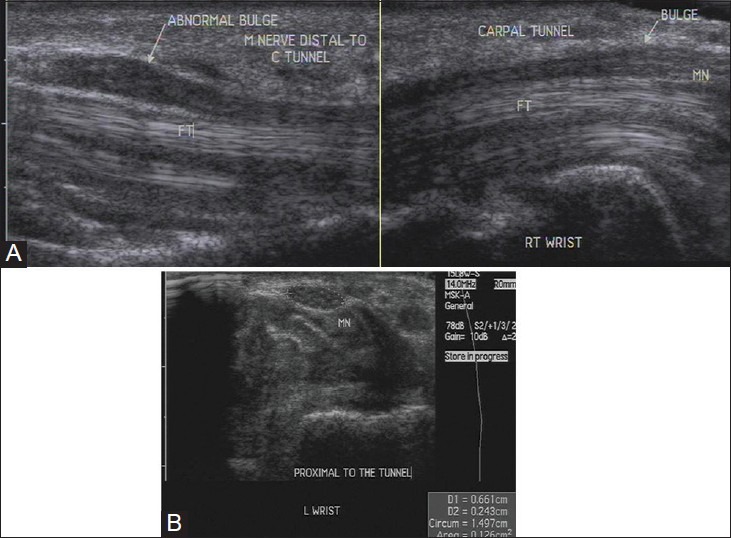
(A) Longitudinal USG image shows enlargement of the median nerve at carpal tunnel inlet and outlet in carpal tunnel syndrome. Also seen is hypoechoic synovium encasing the flexor tendons in the tunnel, suggestive of tenosynovitis (B) Axial USG image shows increase in cross-sectional area of the median nerve proximal to the tunnel. It is 12.6 mm2 (normal being less than 10 mm2
Figure 12 (A and B).
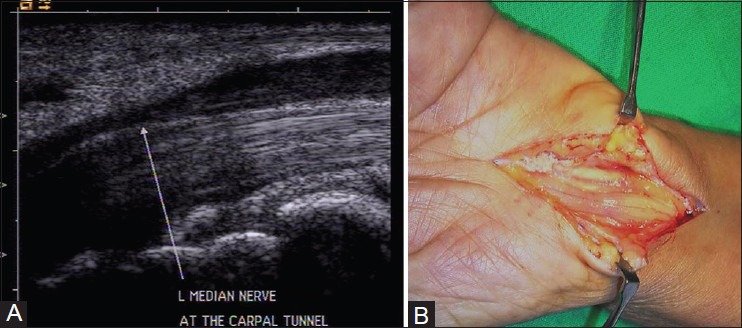
(A) Longitudinal USG image reveals abrupt change in the caliber of median nerve at the entrance of carpal tunnel (notch sign) in carpal tunnel syndrome. The nerve also shows hypoechoic appearance (B) Intraoperative image confirming the ultrasound findings
USG can detect extrinsic causes of entrapment neuropathy like tenosynovitis or space-occupying lesions like ganglion or tumors.
Thus, USG is a useful modality in the evaluation of almost all the pathologies of the peripheral nerves. Lack of awareness about the potential of USG images in a form that the referring clinicians do not understand easily, and lack of expertise have led to MRI being favored over USG in imaging of nerves.
Zaidman et al. have reported that USG has a higher sensitivity and equivalent specificity as compared to MRI, and also detects multifocal nerve lesions better than MRI. They go on to say that USG is the preferred initial modality in the anatomic evaluation of suspected peripheral nerve lesions in USG accessible regions.[15]
Making the referring clinicians aware of its possibilities, proper labeling of images along with panoramic views, and adequate training of radiologists are steps in the right direction to overcome these drawbacks of USG.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Martinoli C, Bianchi S, Derchi LE. Ultrasonography of peripheral nerves. Semin Ultrasound CT MR. 2000;21:205–13. doi: 10.1016/s0887-2171(00)90043-x. [DOI] [PubMed] [Google Scholar]
- 2.Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: A brief review. Neurosurg Focus. 2004;16:E1. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 3.Rozbruch SR, Fryman C, Bigman D, Adler R. Use of ultrasound in detection and treatment of nerve compromise in a case of humeral lengthening. HSS J. 2011;7:80–4. doi: 10.1007/s11420-010-9182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umans H, Kessler J, de la Lama M, Magge K, Liebling R, Negron J. Sonographic assessment of volar digital nerve injury in the context of penetrating trauma. AJR Am J Roentgenol. 2010;194:1310–3. doi: 10.2214/AJR.09.3884. [DOI] [PubMed] [Google Scholar]
- 5.Kele H. Ultrasonography of the peripheral nervous system. Perspectives in Medicine. 2012;1:417–21. [Google Scholar]
- 6.Peer S, Kovacs P, Harpf C, Bodner G. High-resolution sonography of lower extremity peripheral nerves: Anatomic correlation and spectrum of disease. J Ultrasound Med. 2002;21:315–22. doi: 10.7863/jum.2002.21.3.315. [DOI] [PubMed] [Google Scholar]
- 7.Toms AP, Anastakis D, Bleakney RR, Marshall TJ. Lipofibromatous hamartoma of the upper extremity: A review of the radiologic findings for 15 patients. AJR Am J Roentgenol. 2006;186:805–11. doi: 10.2214/AJR.04.1717. [DOI] [PubMed] [Google Scholar]
- 8.Soo MJ, Perera SD, Payne S. The use of ultrasound in diagnosing Morton's neuroma and histological correlation. Ultrasound. 2010;18:14–7. [Google Scholar]
- 9.Quinn TJ, Jacobson JA, Craig JG, van Holsbeeck MT. Sonography of Morton's neuromas. AJR Am J Roentgenol. 2000;174:1723–8. doi: 10.2214/ajr.174.6.1741723. [DOI] [PubMed] [Google Scholar]
- 10.Wilder-Smith EP, van Brakel WH. Nerve damage in leprosy and its management. Nat Clin Pract Neurol. 2008;4:656–63. doi: 10.1038/ncpneuro0941. [DOI] [PubMed] [Google Scholar]
- 11.Jain S, Visser LH, Praveen TL, Rao PN, Surekha T, Ellanti R, et al. High-resolution sonography: A new technique to detect nerve damage in leprosy. PLoS Negl Trop Dis. 2009;3:e498. doi: 10.1371/journal.pntd.0000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinoli C, Bianchi S, Gandolfo N, Valle M, Simonetti S, Derchi LE. US of nerve entrapments in osteofibrous tunnels of the upper and lower limbs. Radiographics. 2000;20:S199–217. doi: 10.1148/radiographics.20.suppl_1.g00oc08s199. [DOI] [PubMed] [Google Scholar]
- 13.Chan K, George J, Goh K, Ahmad TS. Ultrasonography in the evaluation of carpal tunnel syndrome: Diagnostic criteria and comparison with nerve conduction studies. Neurology Asia. 2011;16:57–64. [Google Scholar]
- 14.Lee D, van Holsbeeck MT, Janevski PK, Ganos DL, Ditmars DM, Darian VB. Diagnosis of carpal tunnel syndrome. Ultrasound versus electromyography. Radiol Clin North Am. 1999;37:859–72. doi: 10.1016/s0033-8389(05)70132-9. x. [DOI] [PubMed] [Google Scholar]
- 15.Zaidman CM, Seelig MJ, Baker JC, Mackinnon SE, Pestronk A. Detection of peripheral nerve pathology: Comparison of ultrasound and MRI. Neurology. 2013;80:1634–40. doi: 10.1212/WNL.0b013e3182904f3f. [DOI] [PMC free article] [PubMed] [Google Scholar]


