Abstract
The knowledge that excitatory synapses on aspiny hippocampal interneurons can develop genuine forms of activity-dependent remodeling, independently from the surrounding network of principal cells, is a relatively new concept. Cumulative evidence has now unequivocally demonstrated that, despite the absence of specialized postsynaptic spines that serve as compartmentalized structure for intracellular signaling in principal cell plasticity, excitatory inputs onto interneurons can undergo forms of synaptic plasticity that are induced and expressed autonomously from principal cells. Yet, the rules for induction and expression of interneuron plasticity are much more heterogeneous than in principal neurons. Long-term plasticity in interneurons is not necessarily dependent upon postsynaptic activation of NMDA receptors nor relies on the same postsynaptic membrane potential requirements as principal cells. Plasticity in interneurons rather requires activation of Ca2+-permeable AMPA receptors and/or metabotropic glutamate receptors and is triggered by postsynaptic hyperpolarization. In this review we will outline these distinct features of interneuron plasticity and identify potential critical candidate molecules that might be important for sustaining long-lasting changes in synaptic strength at excitatory inputs onto interneurons.
This article is part of a Special Issue entitled ‘Synaptic Plasticity & Interneurons’.
Keywords: Interneurons, Synapses, GABA, Plasticity, Hippocampus
1. Introduction
That synaptic spines are specialized compartments critical for long-lasting activity-dependent remodeling in principal cells is well known (Newpher and Ehlers, 2009). Postsynaptic spines in principal cells serve as an organized compartment for Ca2+ influx sensing and provide a structural substrate for the cell signaling machinery that sustains long-lasting synaptic plasticity. Interestingly, despite the fact that excitatory presynaptic terminals contact both principal neurons and GABAergic interneurons, the micro-anatomy of these two connections is strikingly different. Most excitatory synapses on principal neurons are spiny, whereas most interneuron dendrites are free of spines and receive excitatory inputs directly on shafts (Freund and Buzsaki, 1996). For years the absence of synaptic spines had been considered an argument against the ability of interneurons to undergo synaptic plasticity independently from surrounding principal cells (McBain et al., 1999). Lacking visible postsynaptic microdomains, excitatory inputs on interneurons were believed to be deprived of the molecular postsynaptic machinery needed for long-lasting synaptic remodeling. This issue was disputed for a considerable time, but a growing body of evidence indicates that interneurons are equipped with plastic synapses that adapt to activity (Kullmann and Lamsa, 2007; Pelletier and Lacaille, 2008). However, the rules of induction and expression of plasticity appear different and possibly more heterogeneous for interneurons in comparison to principal neurons (Kullmann and Lamsa, 2008). Long-term plasticity in interneurons is not necessarily dependent upon post-synaptic activation of N-methyl-D-aspartic acid (NMDA) receptors nor relies on the same postsynaptic membrane potential requirements as principal cells and, even though spines are infrequent, organized postsynaptic molecular domains exist in interneurons that can serve as compartmentalized structures for spatially restricted intracellular signaling. While revealing a fundamental difference between synaptic structures in interneurons versus principal cells, the lack of anatomical spines does not preclude interneurons developing genuine forms of synaptic plasticity. An open challenge will be to determine the structure of pre and postsynaptic compartments at excitatory inputs on interneurons and unravel the functional rules for induction and expression of synaptic plasticity. The goals of this review are to: i) outline the forms of plasticity found in interneurons; ii) identify pre and post-synaptic receptors and/or molecules that have been implicated in induction and/or expression of plasticity in interneurons; iii) introduce novel targets with potential roles in interneuron plasticity. Our hope is to create a template that might serve as guidance and intellectual stimulus for innovative scientific ventures in this growing field of neuroscience.
2. Mechanisms of induction of synaptic plasticity in interneurons
2.1. AMPA receptor-dependent plasticity
One of the most striking differences between synaptic plasticity in hippocampal interneurons and pyramidal cells is the differential roles of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors in the induction of synaptic remodeling. AMPA receptors are heterotetramers formed by the heterologous combination of GluA1-GluA4 subunits; these subunits determine ionic permeability and contribute to the biophysical properties of the channel (Traynelis et al., 2010). AMPA receptors mediate fast excitatory synaptic transmission in both principal cells and interneurons. Once activated by presynaptic glutamate release, they elicit excitatory postsynaptic currents (EPSCs), which are notably different in the two cell populations. Typically, interneurons express a considerable lower level of the GluA2 subunit compared to pyramidal cells (Geiger et al., 1995; Washburn et al., 1997). GluA2-deficient AMPA receptors are highly permeable to Ca2+ and inwardly rectifying due to a voltage-dependent channel block by cytoplasmic polyamines, which are positively charged molecules abundant in the cytoplasm (Rozov and Burnashev, 1999). By occluding the AMPA receptor pore at positive membrane potentials, polyamines allow cation influx only at hyperpolarizing potentials with high channel conductivity and Ca2+ permeability at membrane resting potentials (Bowie and Mayer, 1995; Koh, 1995 #11790; Donevan and Rogawski, 1995). In principal neurons, by contrast, the major route for intracellular Ca2+ flux is through NMDA receptor channels and is the basis for long-term potentiation (LTP) or long-term depression (LTD) induction at positive membrane potentials (Bliss and Collingridge, 1993; Lisman and Spruston, 2005). Activation of NMDA receptors during LTP induction is considered the basis of Hebbian learning, as these receptors can act as coincidence detectors for postsynaptic membrane depolarization and presynaptic high-frequency release of glutamate.
The role of Ca2+-permeable (CP) AMPA receptors in synaptic plasticity in hippocampal interneurons was first reported at CA3 inputs onto CA3 stratum radiatum interneurons. High-frequency release of glutamate from CA3 afferent fibers onto radiatum inter-neurons induced LTD of synaptic transmission in the presence of NMDA receptor blockers, and interestingly, only if the postsynaptic membrane potential was hyperpolarized (Laezza et al., 1999). LTD induction depended upon Ca2+ entry through CP-AMPA receptors and was prevented by postsynaptic membrane depolarization. This report demonstrated for the first time a genuine form of plasticity in hippocampal interneurons, induced postsynaptically and convincingly independent of any other principal cell network modification (discussed by Maccaferri and McBain, 1995); this study defined new induction rules in interneuron plasticity. At the same inputs, synaptic activation of CP-AMPA receptors and NMDA receptors resulted in LTP and/or LTD depending on the postsynaptic membrane potential, suggesting that CP-AMPA receptors might act as switch for voltage-dependent bi-directional plasticity in concert with NMDA receptors (Laezza and Dingledine, 2004). Subsequent studies have extended these findings to other excitatory synapses onto interneurons expressing CP-AMPA receptors, such as the mossy fiber synapses onto CA3 stratum lucidum interneurons (Ho et al., 2009; Lei and McBain, 2004; Pelkey et al., 2006; Toth et al., 2000). Furthermore, at excitatory synaptic inputs onto the CA1 stratum oriens-lacunosum moleculare interneurons, high-frequency release of glutamate induced LTP in the presence of NMDA receptor blockers by activation of CP-AMPA receptors (Oren et al., 2009). LTP was prevented by postsynaptic depolarization, so CP-AMPA receptor activation at these interneuron synapses triggers LTP with the opposite dependence on membrane potential of LTP mediated by NMDA receptor activation in principal cells (Lamsa et al., 2007b). Additional analysis of synaptic plasticity in CA1 oriens-lacunosum moleculare interneurons showed an intriguing correlation between CP-AMPA receptor-dependent plasticity and the cell type-dependent expression of parvalbumin or CB1 cannabinoid receptors (Nissen et al., 2010). Thus, CP-AMPA receptors appear to be a permissive element for long-term synaptic plasticity in interneurons, with the direction of plasticity dependent upon additional factors. Altogether these studies demonstrated unequivocally the specialized role of CP-AMPA receptors in the induction of synaptic plasticity in interneurons, introduced a new concept in the induction rules, and revealed an entire novel layer of complexity and computational roles of these synaptic receptors in hippocampal interneurons (Kullmann and Lamsa, 2007; Laezza and Dingledine, 2004; Lamsa et al., 2007b).
Given the opposite membrane potential requirement than traditional NMDA receptor-dependent Hebbian synaptic plasticity, CP-AMPA receptor-dependent plasticity has been characterized as anti-Hebbian in the sense that these CP-AMPA receptors would serve as coincident detectors of excitatory inputs and postsynaptically inactive, hyperpolarized synapses (Kullmann and Lamsa, 2007; Laezza and Dingledine, 2004; Lamsa et al., 2007b). Oncoming GABAergic inputs or other non-glutamatergic intra or extra hippo-campal fibers terminating on interneurons could provide a source of postsynaptic hyperpolarization and/or depolarization and thereby modulate plasticity induction (Isaac et al., 2009; Laezza and Dingledine, 2004; Mok and Kew, 2006; Varga et al., 2009; Wanaverbecq et al., 2007). The presence of CP-AMPA receptors thus defines a unique computational capability of interneurons versus pyramidal cells. The next challenge will be to understand how “anti-Hebbian” interneuron plasticity collaborates with Hebbian plasticity in principal neurons to modify network properties. Additionally, it will be important to identify the structural and functional differences in the pre and post synaptic sides that are needed for plastic remodeling at excitatory inputs to interneurons.
2.2. NMDA receptor-dependent plasticity
NMDA receptors mediate glutamatergic excitation in both principal cells and GABAergic interneurons. Although their role in the induction of synaptic plasticity in hippocampal principal cells has been established in detail, NMDA receptor-dependent synaptic plasticity in interneurons has been controversial (Christie et al., 2000; Maccaferri and McBain, 1996; McMahon and Kauer, 1997). A major concern when studying NMDA receptor-dependent plasticity in interneurons is how to dissect the influence of the surrounding network of principal cells. Why? Upon high-frequency stimulation of excitatory inputs, the whole network of pyramidal cells becomes potentiated and the risk of passive propagation or heterosynaptic diffusion is considerable (Maccaferri and McBain, 1995). Pairing protocols, which consist of low frequency presynaptic stimulation temporally linked to postsynaptic depolarization, are often used to induce single neuron potentiation in principal neurons. This protocol allows activation of NMDA receptors at a level that produces sufficient Ca2+ entry to induce LTP in the postsynaptic cell (Gustafsson et al., 1987; Malinow, 1991), but not in the surrounding network, which can instead develop LTD if the stimulation frequency is low enough (Maccaferri and McBain, 1995, 1996). Both LTP and LTD have been elicited in hippocampal interneurons with such a pairing protocol (Cowan et al., 1998; Laezza and Dingledine, 2004; Lamsa et al., 2007a; Wang and Kelly, 2001). Other studies using high-frequency stimulation or pairing protocols, however, reported long-lasting depression (McMahon and Kauer, 1997) or potentiation (Christie et al., 2000; Maccaferri and McBain, 1996; Ouardouz and Lacaille, 1995), depending upon activation of NMDA receptors. That suggests that either NMDA receptors on interneurons do not operate as in principal cells or that the postsynaptic signaling necessary for the expression of NMDA receptor-dependent plasticity in interneurons differs from principal cells. Interestingly, synapses on interneurons that contain CP-AMPA receptors often have a small NMDA receptor-mediated component (Lamsa et al., 2007b; Lei and McBain, 2004), whereas interneuron synapses that express Ca2+-impermeable (CI)-AMPA receptors possess a larger NMDA receptor-mediated component. However, in CA3 stratum radiatum interneurons the pattern of postsynaptic NMDA receptor abundance is the opposite, with a more prominent NMDA receptor component at synapses expressing CP-AMPA receptors (Laezza and Dingledine, 2004). At these synapses expressing both CP-AMPA and NMDA receptors, either LTP or LTD can be induced depending on the level of the postsynaptic membrane potential (Laezza and Dingledine, 2004). Interneuron heterogeneity plays a major role in plasticity in excitatory interneuron synapses dominated by CI-AMPA receptors. For example, NMDA receptor-dependent plasticity cannot be induced in CA3 stratum radiatum interneurons but does occur at CA3 stratum lucidum interneurons (Lei and McBain, 2004). Taken together, these findings suggest a complex interaction among GluA2 expression, NMDA receptor expression, and postsynaptic membrane potential. Thus, excitatory synapses onto interneurons display remarkably versatile signal integration if postsynaptic NMDA and AMPA receptors are activated concomitantly.
2.3. mGlu receptor dependent plasticity
Metabotropic glutamate (mGlu) receptors are G-protein coupled receptors expressed both pre- and postsynaptically (Niswender and Conn, 2010). In principal cells mGlu receptors have been implicated in synaptic plasticity and metaplasticity (Anwyl, 2009; Bortolotto et al., 1994; Gladding et al., 2009; Luscher et al., 2000). For example, in principal neurons, agonist stimulation of mGlu receptors triggers LTD independently of NMDA receptors, appears to dampen LTP mediated by NMDA receptor activation, and can thereby shift the threshold for induction of LTP and LTD (Mockett and Hulme, 2008). The role of group I and group III mGlu receptors in the induction of plasticity in interneurons is well documented. A study combining immunohistochemical techniques, single cell RT-PCR and whole-cell patch clamp recording has reported heterogeneous expression of the group I mGlu receptors, mGlu1 and mGlu5, in oriens-lacunosum moleculare interneurons of the CA1 region (van Hooft et al., 2000). In the same neurons, mGlu1 receptor activation, produced either by high-frequency stimulation or by application of group I mGlu agonists in the presence of NMDA receptor blockers, induced a Hebbian form of LTP (Lapointe et al., 2004; Le Vasseur et al., 2008; Perez et al., 2001; Topolnik et al., 2009). The same synapses feature mGlu5-dependent LTP that is also independent of NMDA receptor activation (Topolnik et al., 2009). Both mGlu1-and mGlu5-induced LTP are expressed at synapses containing CP-AMPAR (Le Vasseur et al., 2008). At hippocampal mossy fiber synapses on CA3 lacunosum moleculare interneurons, which express CI-AMPA receptors, a Hebbian form of NMDA receptor-independent LTP can be induced by high-frequency stimulation (Galvan et al., 2008) and requires post-synaptic activation of protein kinase A (PKA) and protein kinase C (PKC) (Galvan et al., 2010). This form of LTP is prevented by voltage clamping the postsynaptic cell soma at a hyperpolarized level during high-frequency stimulation, by chelating intracellular Ca2+, or by blocking L-type Ca2+ channels, revealing yet another route of Ca2+ entry that can trigger plasticity. Intriguingly, in the presence of mGlu1 antagonists, the same high-frequency stimulation of mossy fibers that induces LTP in naïve slices triggered NMDA receptor-independent LTD. This form of LTD, like LTP, required activation of L-type Ca2+ channels and was prevented by block of inositol triphosphate (IP3) receptors or depletion of intracellular Ca2+ stores, suggesting that mGlu-dependent Ca2+ signaling at CI-AMPA synapses controls the polarity of mossy fibers long-term plasticity induced by joint pre- and postsynaptic activities (Galvan et al., 2008).
A series of studies have implicated group III mGlu receptors in synaptic transmission and plasticity in interneurons of the CA3 region. Of these receptors, mGlu7 is particularly enriched at excitatory terminals impinging onto interneurons (Ferraguti et al., 2005; Shigemoto et al., 1997). The first evidence of a role for mGlu7 in synaptic transmission and plasticity in interneurons was shown at CA3 inputs onto CA3 stratum radiatum interneurons (Laezza et al., 1999). At these terminals the group III-selective agonist L-(1)-2-amino-4-phosphonobutyric acid (L-AP4), used at concentrations consistent with low-affinity mGlu7 activation (Conn and Pin, 1997), effectively suppressed excitatory synaptic transmission at CA3 stratum radiatum interneurons expressing either CI-AMPA or CP-AMPA receptors. Importantly, a group III mGlu antagonist prevented the induction of CP-AMPA receptor dependent LTD by high-frequency stimulation (Laezza et al., 1999). Thus, postsynaptic CP-AMPA receptors and presynaptic mGlu7 are functionally coupled in a form of NMDA receptor-independent LTD expressed in CA3 interneurons. Subsequent studies investigated in more detail the role of mGlu7 in synaptic plasticity at mossy fiber terminals innervating CA3 stratum lucidum interneurons. Similarly to CA3 stratum radiatum interneurons, mGlu7 is activated during CP-AMPA receptor-dependent LTD at these synapses (Pelkey et al., 2006). Furthermore, at these synapses mGlu7 has been proposed to serve as a metaplastic switch controlling bidirectional plasticity (Pelkey et al., 2005). At naïve synapses, surface expressed mGlu7 receptors are activated and internalized during high-frequency stimulation of mossy fiber terminals, causing a persistent inhibition of presynaptic P/Q-type Ca2+ channels (Pelkey et al., 2005), a process that requires PKC phosphorylation-dependent binding of the mGlu7 receptor to the intracellular scaffolding protein PICK1 (Suh et al., 2008). However, following mGlu7 internalization, high-frequency afferent stimulation produces presynaptic LTP by a mechanism that is mediated by cAMP increase and requires the integrity of the mGlu7-RIM1α complex, suggesting a model in which mGlu7 activation and internalization controls plasticity polarity by gating cAMP sensitivity of release (Pelkey et al., 2008).
Collectively these results indicate that excitatory inputs onto interneurons are equipped with specific and unique pre and post-synaptic glutamate receptor subtypes that confer on hippocampal interneurons a distinctive means for differential decoding of high-frequency inputs. This arrangement results in enhanced or depressed transmission depending on the functional state of the interneuron (membrane potential, availability of dendritic L-type Ca2+ channels, activity of protein kinases). It remains to be determined, however, whether other structural and functional differences between excitatory synapses in interneurons versus pyramidal cells also participate in determining the difference in the induction and expression rules of plasticity.
3. Mechanisms of synaptic plasticity expression in interneurons
A puzzling aspect of how synaptic plasticity can be expressed in interneurons remains the observation of lack of postsynaptic microanatomical constraints in these cells. The microanatomy and the molecular architecture of postsynaptic spines in principal cells provides a structural mechanism for localized increase in Ca2+ and localized activation of intracellular signaling cascades critical for induction and expression of synaptic plasticity. What are the sources of postsynaptic Ca2+ entry and intracellular signaling mechanisms in aspiny interneurons compared to principal cells? We will first present a summary of the most relevant steps underlying the expression mechanisms of NMDA receptor-dependent plasticity in principal cells and use it as a point of discussion for interneuron plasticity.
3.1. Expression of NMDA receptor-dependent plasticity in principal cells
The postsynaptic triggering event for LTP or LTD induction in principal cells is a rise in Ca2+ entering through NMDA receptor channels, followed by activation of the Ca2+-sensor protein calmodulin (CaM). Depending on the magnitude and spatio-temporal profile of Ca2+ rise, CaM induces either autophosphorylation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Lisman et al., 2002; Wayman et al., 2008) or activation of the phosphatases PP1 and calcineurin (PP2B) (Mulkey et al., 1994; Mulkey and Malenka, 1992), leading respectively to potentiation or depression of synaptic strength. The mechanisms of expression of LTP involve changes in AMPA receptor trafficking and spine structure remodeling. In addition to CaMKII and phosphoinositide 3-kinase (PI3K), which are considered core kinases for LTP expression, evidence exists for roles of PKA, PKC and the extracellular signal-regulated protein kinase (ERK-MAPK) (English and Sweatt, 1997; Esteban et al., 2003; Hayashi et al., 2000; Malinow et al., 1989; Man et al., 2003; Silva et al., 1992). A proposed mechanism for LTP expression consists of: i) insertion of AMPA receptors induced by kinases; ii) diffusion of pre-existing or newly inserted AMPA receptors to synaptic sites; and iii) retention of AMPA receptors at synaptic sites by CaMKII and PI3K activity (Opazo and Choquet, 2010). These processes are regulated by the interaction of AMPA receptors with PDZ (named after Post synaptic density protein 95, Drosophila disc large tumor suppressor DlgA, and Zonula occludens-1 protein zo-1) scaffolding proteins such as PSD-95 and PICK1 (Kim and Sheng, 2004) and with the transmembrane AMPA receptor regulatory proteins (TARPs) (Bats et al., 2007; Chen et al., 2000; Ehrlich et al., 2007; Ehrlich and Malinow, 2004; Elias et al., 2006; Elias and Nicoll, 2007; Schnell et al., 2002; Stein et al., 2003; Tomita et al., 2004, 2005; Xu et al., 2008). Stability, retention and recruitment of AMPA receptors at synapses all require direct binding of the receptors to PSD-95 or indirect binding to TARP proteins. Furthermore, knock-down of PICK1 or inhibition of PICK1 binding to AMPA receptors attenuates NMDA receptor-dependent LTP (Terashima et al., 2008; Thorsen et al., 2010). Mechanisms opposite to LTP have been proposed to underlie LTD expression by activation of phosphatases. Upon LTD induction, synaptic AMPA receptors are destabilized (step 1), diffuse out of synapses (step 2) and are then internalized through a clathrin-mediated endocytosis (step 3) (Opazo and Choquet, 2010).
In addition to causing increased number of functional AMPA receptors located in the synapse, activation of CaMKII induces remodeling of the actin cytoskeleton of spines through pathways converging on the Rho family of small GTPases (Okamoto et al., 2009). These small GTPases are controlled bidirectionally by the guanine-nucleotide-exchange factors (GEFs) and GTPase activating proteins (GAPs) pathways, which regulate polymerization and depolymerization of actin filament through a kinase-dependent action on the actin-binding proteins cofilin and profilin (Meng et al., 2003, 2002). For example, phosphorylation of the GEF kalirin-7 by CaMKII activation induces spine enlargement through an action on cofilin and actin (Xie et al., 2007). Conversely, a cofilin-dependent spine shrinkage is observed during LTD (Zhou et al., 2004).
Are any of the mechanisms described for LTP/LTD expression in principal cells common to interneuron plasticity? Are any molecular components involved in LTP/LTD expression present in interneurons? We will discuss these points below.
3.2. Sources of Ca2+-entry in interneurons
Although NMDA receptors are expressed in interneurons and participate in the induction of synaptic plasticity in these cells, only a few studies have evaluated the degree to which Ca2+ entry through NMDA receptors contributes to interneuron plasticity. In one study high-frequency stimulation of mossy fibers to CA3 stratum lucidum interneurons has been shown to induce NMDA receptor-dependent LTD at synapses that co-express CI-AMPA receptors and NMDA receptors. At these synapses NMDA receptors are characterized by a lower NR2B subunit content, higher open probability and larger single channel conductance than their CP-AMPA counterparts, and as such are likely to be the primary source for Ca2+ entry during high-frequency stimulation in these cells (Lei and McBain, 2004). A small NMDA receptor component has also been found in CA1 stratum oriens-alveus interneurons expressing CP-AMPA receptors (Lamsa et al., 2007b). Conversely, at CA3 inputs to CA3 stratum radiatum interneurons, NMDA receptors are co-expressed with CP-AMPA receptors and, depending on the postsynaptic membrane potential, Ca2+ flux might arise from either CP-AMPA receptors alone, or from a combination of CP-AMPA and NMDA receptor activation (Laezza and Dingledine, 2004). Another source of postsynaptic Ca2+ relevant for interneuron plasticity are the L-type Ca2+ channels, as blockade of these channels prevents mossy fiber long-term plasticity in CA3 lacunosum moleculare interneurons (Galvan et al., 2008).
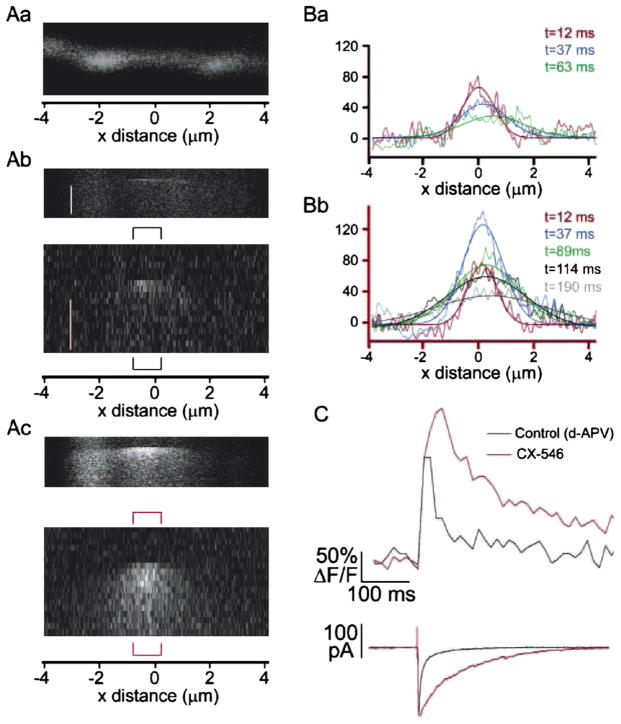
The dynamics of postsynaptic Ca2+ entry in cortical aspiny interneurons have been studied elegantly by Goldberg et al. (2003), who showed that the combination of the fast kinetics of CP-AMPA receptors and the activity of the Na+/Ca2+ exchanger is sufficient to generate dendritic Ca2+ compartmentalization in cortical aspiny interneurons. Two-photon images of CP-AMPA receptor mediated Ca2+ flux from this study are illustrated in Fig. 1. Notably, in these neurons Ca2+ transients depend upon the fast kinetics of CP-AMPA receptors and the Na+/Ca2+ exchange pump limits spatially the spread of Ca2+ away from the activated synapse (Goldberg et al., 2003). It is tempting to speculate that CP-AMPA receptors at aspiny excitatory synapses onto hippocampal interneurons could operate similarly and create functional domains within dendrites that could compensate for the lack of spines and provide the structural basis of a spine-free mechanism for input-specific Ca2+ compartmentalization and signal integration. Of particular importance for future studies would be to determine the spatio-temporal profile of Ca2+ entry and the relative contribution of NMDA receptors, CP-AMPA receptors, and postsynaptic Ca2+ channels as multiple routes of Ca2+ entry during interneuron plasticity induction and expression.
Fig. 1.
Two-photon images of Ca2+ transients mediated by CP-AMPA receptors in a cortical interneuron. (Aa) A horizontal dendrite filled with the Ca2+ dye Fluo-4. (Ab) Top, control line scan through dendrite in (Aa), average of three successes. Vertical scale, 400 ms. Bottom, ΔF/F image from line scan above. Vertical scale bar, 100 ms. (Ac) Images are presented as in (Ab), after addition of 1 mM CX-546, an inhibitor of AMPA receptor deactivation. Note how the microdomain spread to adjacent dendritic segment. Average of four successes. (Ba) ΔF/F versus dendritic space plot for calcium transient in (Ab). (Bb) Data plotted as in (Ba) for the transient in the presence of CX-546. Note that panels in (A)–(B) have the same x axis. (C) Top, calcium transients from bracketed regions of line scans in (A), before, black trace, and after, red, addition of CX-546. Bottom, EPSC time locked to the calcium transients above. Note how CX-546 dramatically prolonged the AMPA receptor-mediated currents. All the experiments were performed in the presence of the NMDA receptor blocker d-APV (2-amino-5-phosphonovaleric acid). Modified from Goldberg et al., Neuron 2003.
3.3. Role of kinases in interneuron plasticity
The CaM/CaMKII complex is expressed at low abundance in hippocampal interneurons (Liu and Jones,1996; Sik et al., 1998) and its role in synaptic plasticity is debatable. Whereas a direct role of CaM/CaMKII in mediating NMDA receptor-dependent interneuron plasticity has been demonstrated at glutamatergic inputs onto CA1 interneurons (Wang and Kelly, 2001), CA1 stratum radiatum interneurons of αCaMKII T286A mutant mice show intact NMDA receptor dependent LTP (Lamsa et al., 2007a) suggesting that the CaM/αCaMKII complex is not a requirement for interneuron plasticity. Possibly, βCAMKII, an isoform that is more prominent in some interneurons (Wang and Kelly, 2001), or other kinases such as CaMKI and CaMKIV (Soderling, 1999), might play redundant roles and compensate for the lack of functional αCAMKII. Notably, emerging evidence indicates that Ca2+ binding proteins enriched in interneurons, such as calbindin, calretinin and parvalbumin, in addition to operating as Ca2+ buffers, might serve as Ca2+ sensors that provide an alternative Ca2+ route to CaM (Burgoyne et al., 2004).
Another possibility to consider is that CP-AMPA receptor plasticity might rely on a different class of kinases. For example, recent evidence has revealed a novel form of LTP triggered by CP-AMPA in CA1 pyramidal neurons that engages a PI3-kinase/MAPK cascade rather than αCAMKII (Asrar et al., 2009). This pathway might be the default pathway linked to CP-AMPA receptors, at both interneurons and principal cells, as an alternative to αCaMKII.
More evidence is available for the role of PKA and PKC in interneuron plasticity. A form of NMDA receptor-independent LTP at mossy fibers to CA3 lacunosum moleculare interneuron synapses expressing CI-AMPA requires postsynaptic activation of PKA (Galvan et al., 2010), similar to the LTP produced by these same inputs onto CA3 pyramidal neurons (Duffy and Nguyen, 2003; Huang and Kandel, 1994). However, extracellular application of a PKA inhibitor is not sufficient to prevent a form of LTP at the synapse made by mossy fibers onto inhibitory basket cells in the dentate gyrus (Alle et al., 2001), indicating that PKA activation is not required for all forms of interneuron plasticity. Presynaptic activation of PKA is required for internalization of presynaptic mGlu7 in a form of LTP at mossy fibers to CA3 stratum lucidum interneurons (Pelkey et al., 2008) further supporting a role of this kinase in interneuron plasticity, both pre and postsynaptically. PKC activation in conjunction with PKA is required for LTP at mossy fibers to CA3 lacunosum moleculare interneuron synapses (Galvan et al., 2010). PKC engagement has also been described in conjunction with dendritic Ca2+ signaling at inputs to CA1 stratum alveus-oriens (Topolnik et al., 2009) and during mGlu7-dependent LTD at the mossy fiber inputs to CA3 stratum lucidum interneurons (Pelkey et al., 2005). Thus, although there are some likely similar signaling cascades underlying expression of plasticity in interneurons and pyramidal cells, plasticity in interneurons does not exclusively depend upon CaMKII activation as in principal cells, and none of the kinases that appear to mediate interneuron plasticity appears to be universally required for plasticity. One issue that should be addressed is whether the differences in Ca2+-induced kinase activity requirement are specific for interneurons or are dictated by phenotypic expression of postsynaptic receptors (for example, NMDA vs CP-AMPA receptors).
3.4. PDZ domain binding proteins and other postsynaptic molecules
The role of scaffolding proteins in the expression of synaptic plasticity in interneurons is largely unknown and the repertoire of studies on the expression and distribution of these molecules in hippocampal interneurons is limited (Craig et al., 2006; Kim and Sheng, 2004; Penzes et al., 2000; Tomita et al., 2001). The PDZ domain is a common structural domain found in a number of proteins. Within the PDZ domain family of proteins localized in synaptic spines, (Craig et al., 2006; Ma, 2010; Ma et al., 2010, 2008; Zhang et al., 1999), PSD-95 has been found enriched postsynaptically in hippocampal interneurons; its overexpression increased the number of GluA1 clusters in dendritic shafts and the frequency of miniature EPSCs (El-Husseini et al., 2000), suggesting a potential role of PSD-95 in stabilizing or inserting AMPA receptors in interneurons. Other PDZ domain proteins, such as the GRIPa/b isoforms, are also expressed in interneurons, although at low levels (Charych et al., 2006, 2004); these could also be critical for AMPA receptor stability and trafficking in interneurons, but there are no available studies on this matter. Interestingly, in stellate inhibitory neurons of the cerebellum, the interaction of PICK1 and GluA2 controls mobility of AMPA receptors in a form of synaptic plasticity expressed as a switch in AMPA receptor composition, consisting in increase of synaptic GluA2 content and decrease in AMPA receptor Ca2+ permeability (Liu and Cull-Candy, 2002, 2005, 2000). The same mechanism might underlie CP-AMPA receptor-dependent plasticity in hippocampal interneurons and should be examined.
3.5. AMPA receptor trafficking
There has been only one report of a potential involvement of AMPA receptor trafficking as a mechanism for expression of interneuron plasticity. Similar to reports in principal cells (Kessels and Malinow, 2009), a peptide inhibiting the activity of the AP2-clathrin adaptor protein 2 occludes LTD expression at mossy fibers to CI-AMPA receptor synapses but not at CP-AMPA receptor synapses of stratum lucidum interneurons, suggesting that a clathrin-induced endocytosis of AMPA receptors might underlie the reduction in postsynaptic AMPA receptor trafficking (Lei and McBain, 2004) and serve as a mechanism of LTD expression. However, this mechanism appears to be specific for synapses expressing CI-AMPA receptors, and as such, more similar to excitatory synapses in principal cells.
3.6. Structural plasticity
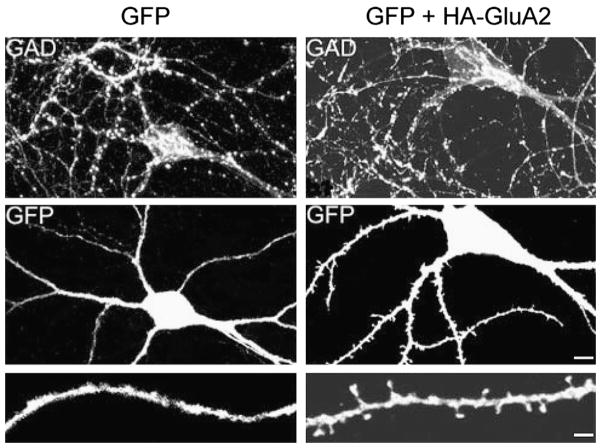
Although structural plasticity in interneurons has not yet been demonstrated, compelling evidence indicates that synaptic spines can be induced in aspiny interneurons upon overexpression of selected synaptic components, suggesting that the cytoskeleton of interneurons is dynamic and might therefore provide a structural basis for dynamic remodeling underlying synaptic plasticity. One of the first lines of evidence for spine-induction in interneurons came from Passafaro et al. (2003). In this milestone study, the authors reported that spines were induced on interneuron dendritic shafts by overexpression of the GluA2 subunit (He et al., 1998; Passafaro et al., 2003) as illustrated in Fig. 2. In more recent studies, kalirin-7, a multifunctional Rho GEF exchange protein that acts as a post-synaptic scaffold and co-localizes with PSD-95 at excitatory synapses onto interneuron dendritic shafts (Ma, 2010; Ma et al., 2010, 2008), has been shown to play similar roles. Whereas reduction of kalirin-7 levels reduces the size of PSD-95-positive puncta, overexpression of kalirin-7 increases dendritic branching and induces the formation of spine-like structures along the dendrites of aspiny hippocampal interneurons by a PDZ-mediated mechanism (Ma, 2010; Ma et al., 2010, 2008). Collectively the role of GluA2 and of kalirin-7 demonstrates an important concept, namely that spines can be induced in aspiny interneurons and that these aspiny shafts are dynamic molecular structures. Of obvious importance would be to determine whether upon synaptic remodeling, changes in the level of GluA2 expression converts CP-AMPA receptor aspiny synapses into GluA2 containing CI-AMPA receptors and thus switches aspiny interneurons into spiny neurons. Furthermore, the composition of the cytoskeleton in dendritic shafts or in induced synaptic spines in interneurons has not been studied yet.
Fig. 2.
GluA2 induces dendritic spines in GABA-releasing interneurons. Hippocampal neurons at DIV14 were transfected with enhanced green fluorescent protein (EGFP) alone, or with EGFP plus a haemagglutinin (HA)-tagged GluA2 construct, and stained for GFP, glutamic acid decarboxylase (GAD) and HA at DIV22. Paired panels show GFP and GAD staining in the same neuron, as indicated. Interneurons (defined by typical dendritic morphology and GAD6 immunoreactivity in cell soma) have no dendritic spines when transfected with EGFP (top panel and middle panels, left images), but display protrusions with a clear head and neck when transfected with HA–GluA2 (top and middle panels, right images). Bottom panels show higher magnification of the dendrite (GFP image). Scale bar, 10 μm (low magnification) and 2.5 μm (high magnification). Modified from Passafaro et al. Nature 2003.
Given the role of the Rho pathway in mediating structural plasticity in principal neurons, other molecules of the Rho family found in interneurons, such as Citron-N (citron) (Zhang and Benson, 2006), might also be considered as candidate substrates underlying structural remodeling of interneuron synapses. Furthermore, other cytoskeleton-associated molecules such as α-actinin-2, a protein that provides a scaffolding bridge between NMDA receptors and membrane lipids (Michailidis et al., 2007) have been identified in interneurons (Ratzliff and Soltesz, 2001). Given that the interaction between α-actinin-2 and NMDA receptors is regulated by CaM in principal neurons (Merrill et al., 2007), this molecule might also be a candidate for structural plasticity in interneurons.
4. Conclusion
Collectively these results indicate that excitatory inputs onto interneurons are equipped with specific and unique combinations of pre and postsynaptic glutamate receptor subtypes that confer distinctive means for differential decoding of high-frequency inputs, resulting in enhanced or depressed transmission depending on the functional state of the interneuron. Compared to plasticity mechanisms operating in principal neurons, interneuron plasticity is different in the following ways as discussed above. First, although the dendritic structure of interneurons is dynamic and spines can be induced, aspiny synapses do not prevent signaling compartmentalization. Second, postsynaptic Ca2+ entry during the induction phase of plasticity in interneurons might be even faster than in principal cells as dictated by the kinetics of CP-AMPA receptors. Third, in contrast to principal cells, the postsynaptic signaling cascade that triggers interneuron plasticity does not necessarily involve CaMKII (at least for LTP); nor appear to present a universal requirement for any single kinase, although PKA and PKC have been found critical for some forms of interneuron plasticity. Fourth, CP-AMPA receptors play a much more prominent role in the induction of synaptic plasticity in interneurons compared to principal cells.
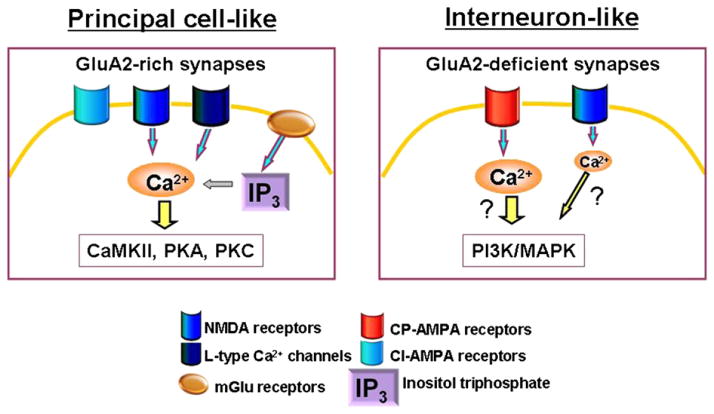
Although a universal scheme to describe the mechanisms of induction and expression of synaptic plasticity in interneurons is still lacking, the level of GluA2 content in interneuron synapses appears to be the critical determinant of whether an interneuron synapse will phenotypically resemble principal cell synapses. Based on the fragmentary and heterogeneous data on this topic, we propose a tentative working model in which we envision two extreme forms of interneuron plasticity based on the content of synaptic GluA2 (Fig. 3). In principal cell-like forms of plasticity, high levels of synaptic GluA2 might be typically associated with Hebbian forms of plasticity induced by activation of NMDA receptors (Lamsa et al., 2005; Maccaferri and McBain, 1996; McMahon and Kauer, 1997; Ouardouz and Lacaille, 1995), L-type Ca2+ channels (Galvan et al., 2008) and/or mGlu receptors (Galvan et al., 2008; Ouardouz and Lacaille, 1995); this form of plasticity could depend upon activation of CaMKII (Wang and Kelly, 2001), PKA and/or PKC (Galvan et al., 2008, 2010). Alternatively, synapses with low GluA2 synaptic content might be primarily, but not exclusively (Le Vasseur et al., 2008; Laezza and Dingledine, 2004), associated with anti-Hebbian, NMDA receptor-independent forms of plasticity that require postsynaptic hyperpolarization during induction (Laezza and Dingledine, 2004; Laezza et al., 1999; Lamsa et al., 2007b). Whether this form of plasticity requires a PI3K/MAPK-dependent intracellular signaling cascade (Asrar et al., 2009), or is expressed through a PICK1-dependent trafficking of GluA2 containing AMPA receptors (Liu and Cull-Candy, 2002, 2005, 2000) that drives new spine formation (Passafaro et al., 2003), should be of immediate interest. Furthermore, identifying the physiological or pathophysiological conditions that convert a GluA2-deficient into a GluA2-rich synapse, as part of an interneuron-like plastic remodeling, would be another challenging and fascinating issue to address.
Fig. 3.
Working model of interneuron plasticity. At interneuron synapses containing CI-AMPA receptors (GluA2-rich), the mechanisms of induction and expression of synaptic plasticity might follow Hebbian rules and be more similar to principal cells. Conversely, at interneuron synapses containing CP-AMPA receptors (GluA2-deficient), the mechanisms of induction and expression of synaptic plasticity follows anti-Hebbian rules and might require different intracellular signaling cascades.
Acknowledgments
This work was supported by NINDS grant R01 NS36604 (RD) and by a grant from the Institute for Translational Sciences at UTMB (FL).
References
- Alle H, Jonas P, Geiger JR. PTP and LTP at a hippocampal mossy fiber-interneuron synapse. Proc Natl Acad Sci USA. 2001;98:14708–14713. doi: 10.1073/pnas.251610898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56:735–740. doi: 10.1016/j.neuropharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Asrar S, Zhou Z, Ren W, Jia Z. Ca(2+) permeable AMPA receptor induced long-term potentiation requires PI3/MAP kinases but not Ca/CaM-dependent kinase II. PLoS One. 2009;4:e4339. doi: 10.1371/journal.pone.0004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Bashir ZI, Davies CH, Collingridge GL. A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. Nature. 1994;368:740–743. doi: 10.1038/368740a0. [DOI] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, O’Callaghan DW, Hasdemir B, Haynes LP, Tepikin AV. Neuronal Ca2+-sensor proteins: multitalented regulators of neuronal function. Trends Neurosci. 2004;27:203–209. doi: 10.1016/j.tins.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Charych EI, Li R, Serwanski DR, Li X, Miralles CP, Pinal N, De Blas AL. Identification and characterization of two novel splice forms of GRIP1 in the rat brain. J Neurochem. 2006;97:884–898. doi: 10.1111/j.1471-4159.2006.03795.x. [DOI] [PubMed] [Google Scholar]
- Charych EI, Yu W, Li R, Serwanski DR, Miralles CP, Li X, Yang BY, Pinal N, Walikonis R, De Blas AL. A four PDZ domain-containing splice variant form of GRIP1 is localized in GABAergic and glutamatergic synapses in the brain. J Biol Chem. 2004;279:38978–38990. doi: 10.1074/jbc.M405786200. [DOI] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Christie BR, Franks KM, Seamans JK, Saga K, Sejnowski TJ. Synaptic plasticity in morphologically identified CA1 stratum radiatum interneurons and giant projection cells. Hippocampus. 2000;10:673–683. doi: 10.1002/1098-1063(2000)10:6<673::AID-HIPO1005>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Cowan AI, Stricker C, Reece LJ, Redman SJ. Long-term plasticity at excitatory synapses on aspinous interneurons in area CA1 lacks synaptic specificity. J Neurophysiol. 1998;79:13–20. doi: 10.1152/jn.1998.79.1.13. [DOI] [PubMed] [Google Scholar]
- Craig AM, Graf ER, Linhoff MW. How to build a central synapse: clues from cell culture. Trends Neurosci. 2006;29:8–20. doi: 10.1016/j.tins.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donevan SD, Rogawski MA. Intracellular polyamines mediate inward rectification of Ca(2+)-permeable alpha-amino-3-hydroxy-5-methyl-4-iso-xazolepropionic acid receptors. Proc Natl Acad Sci USA. 1995;92:9298–9302. doi: 10.1073/pnas.92.20.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SN, Nguyen PV. Postsynaptic application of a peptide inhibitor of cAMP-dependent protein kinase blocks expression of long-lasting synaptic potentiation in hippocampal neurons. J Neurosci. 2003;23:1142–1150. doi: 10.1523/JNEUROSCI.23-04-01142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci USA. 2007;104:4176–4181. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Elias GM, Nicoll RA. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007;17:343–352. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem. 1997;272:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Klausberger T, Cobden P, Baude A, Roberts JD, Szucs P, Kinoshita A, Shigemoto R, Somogyi P, Dalezios Y. Metabotropic glutamate receptor 8-expressing nerve terminals target subsets of GABAergic neurons in the hippocampus. J Neurosci. 2005;25:10520–10536. doi: 10.1523/JNEUROSCI.2547-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Galvan EJ, Calixto E, Barrionuevo G. Bidirectional Hebbian plasticity at hippocampal mossy fiber synapses on CA3 interneurons. J Neurosci. 2008;28:14042–14055. doi: 10.1523/JNEUROSCI.4848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan EJ, Cosgrove KE, Mauna JC, Card JP, Thiels E, Meriney SD, Barrionuevo G. Critical involvement of postsynaptic protein kinase activation in long-term potentiation at hippocampal mossy fiber synapses on CA3 interneurons. J Neurosci. 2010;30:2844–2855. doi: 10.1523/JNEUROSCI.5269-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Gladding CM, Fitzjohn SM, Molnar E. Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacol Rev. 2009;61:395–412. doi: 10.1124/pr.109.001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JH, Tamas G, Aronov D, Yuste R. Calcium microdomains in aspiny dendrites. Neuron. 2003;40:807–821. doi: 10.1016/s0896-6273(03)00714-1. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Wigstrom H, Abraham WC, Huang YY. Long-term potentiation in the hippocampus using depolarizing current pulses as the conditioning stimulus to single volley synaptic potentials. J Neurosci. 1987;7:774–780. doi: 10.1523/JNEUROSCI.07-03-00774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- He Y, Janssen WG, Vissavajjhala P, Morrison JH. Synaptic distribution of GluR2 in hippocampal GABAergic interneurons and pyramidal cells: a double-label immunogold analysis. Exp Neurol. 1998;150:1–13. doi: 10.1006/exnr.1997.6720. [DOI] [PubMed] [Google Scholar]
- Ho MT, Pelkey KA, Pelletier JG, Huganir RL, Lacaille JC, McBain CJ. Burst firing induces postsynaptic LTD at developing mossy fibre-CA3 pyramid synapses. J Physiol. 2009;587:4441–4454. doi: 10.1113/jphysiol.2009.173880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- Isaac JT, Buchanan KA, Muller RU, Mellor JR. Hippocampal place cell firing patterns can induce long-term synaptic plasticity in vitro. J Neurosci. 2009;29:6840–6850. doi: 10.1523/JNEUROSCI.0731-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Lamsa K. Roles of distinct glutamate receptors in induction of anti-Hebbian long-term potentiation. J Physiol. 2008;586:1481–1486. doi: 10.1113/jphysiol.2007.148064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Lamsa KP. Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci. 2007;8:687–699. doi: 10.1038/nrn2207. [DOI] [PubMed] [Google Scholar]
- Laezza F, Dingledine R. Voltage-controlled plasticity at GluR2-deficient synapses onto hippocampal interneurons. J Neurophysiol. 2004;92:3575– 3581. doi: 10.1152/jn.00425.2004. [DOI] [PubMed] [Google Scholar]
- Laezza F, Doherty JJ, Dingledine R. Long-term depression in hippocampal interneurons: joint requirement for pre- and postsynaptic events. Science. 1999;285:1411–1414. doi: 10.1126/science.285.5432.1411. [DOI] [PubMed] [Google Scholar]
- Lamsa KP, Heeroma JH, Kullmann DM. Hebbian LTP in feed-forward inhibitory interneurons and the temporal fidelity of input discrimination. Nat Neurosci. 2005;7:916–924. doi: 10.1038/nn1486. [DOI] [PubMed] [Google Scholar]
- Lamsa K, Irvine EE, Giese KP, Kullmann DM. NMDA receptor-dependent long-term potentiation in mouse hippocampal interneurons shows a unique dependence on Ca(2+)/calmodulin-dependent kinases. J Physiol. 2007a;584:885–894. doi: 10.1113/jphysiol.2007.137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsa KP, Heeroma JH, Somogyi P, Rusakov DA, Kullmann DM. Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science. 2007b;315:1262–1266. doi: 10.1126/science.1137450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe V, Morin F, Ratte S, Croce A, Conquet F, Lacaille JC. Synapse-specific mGluR1-dependent long-term potentiation in interneurones regulates mouse hippocampal inhibition. J Physiol. 2004;555:125–135. doi: 10.1113/jphysiol.2003.053603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Vasseur M, Ran I, Lacaille JC. Selective induction of metabotropic glutamate receptor 1- and metabotropic glutamate receptor 5-dependent chemical long-term potentiation at oriens/alveus interneuron synapses of mouse hippocampus. Neuroscience. 2008;151:28–42. doi: 10.1016/j.neuroscience.2007.09.071. [DOI] [PubMed] [Google Scholar]
- Lei S, McBain CJ. Two Loci of expression for long-term depression at hippocampal mossy fiber-interneuron synapses. J Neurosci. 2004;24:2112– 2121. doi: 10.1523/JNEUROSCI.4645-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lisman J, Spruston N. Postsynaptic depolarization requirements for LTP and LTD: a critique of spike timing-dependent plasticity. Nat Neurosci. 2005;8:839–841. doi: 10.1038/nn0705-839. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Cull-Candy SG. Activity-dependent change in AMPA receptor properties in cerebellar stellate cells. J Neurosci. 2002;22:3881–3889. doi: 10.1523/JNEUROSCI.22-10-03881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Cull-Candy SG. Subunit interaction with PICK and GRIP controls Ca2+ permeability of AMPARs at cerebellar synapses. Nat Neurosci. 2005;8:768–775. doi: 10.1038/nn1468. [DOI] [PubMed] [Google Scholar]
- Liu SQ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 2000;405:454–458. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- Liu XB, Jones EG. Localization of alpha type II calcium calmodulin-dependent protein kinase at glutamatergic but not gamma-aminobutyric acid (GABAergic) synapses in thalamus and cerebral cortex. Proc Natl Acad Sci USA. 1996;93:7332–7336. doi: 10.1073/pnas.93.14.7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- Ma XM. Kalirin-7 is a key player in the formation of excitatory synapses in hippocampal neurons. Sci World J. 2010;10:1655–1666. doi: 10.1100/tsw.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Huang JP, Kim EJ, Zhu Q, Kuchel GA, Mains RE, Eipper BA. Kalirin-7, an important component of excitatory synapses, is regulated by estradiol in hippocampal neurons. Hippocampus. 2010 doi: 10.1002/hipo.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Kiraly DD, Gaier ED, Wang Y, Kim EJ, Levine ES, Eipper BA, Mains RE. Kalirin-7 is required for synaptic structure and function. J Neurosci. 2008;28:12368–12382. doi: 10.1523/JNEUROSCI.4269-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. Passive propagation of LTD to stratum oriens-alveus inhibitory neurons modulates the temporoammonic input to the hippocampal CA1 region. Neuron. 1995;15:137–145. doi: 10.1016/0896-6273(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. Long-term potentiation in distinct subtypes of hippocampal nonpyramidal neurons. J Neurosci. 1996;16:5334–5343. doi: 10.1523/JNEUROSCI.16-17-05334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R. Transmission between pairs of hippocampal slice neurons: quantal levels, oscillations, and LTP. Science. 1991;252:722–724. doi: 10.1126/science.1850871. [DOI] [PubMed] [Google Scholar]
- Malinow R, Schulman H, Tsien RW. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989;245:862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Man HY, Wang Q, Lu WY, Ju W, Ahmadian G, Liu L, D’Souza S, Wong TP, Taghibiglou C, Lu J, et al. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron. 2003;38:611–624. doi: 10.1016/s0896-6273(03)00228-9. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Freund TF, Mody I. Glutamatergic synapses onto hippocampal interneurons: precision timing without lasting plasticity. Trends Neurosci. 1999;22:228–235. doi: 10.1016/s0166-2236(98)01347-2. [DOI] [PubMed] [Google Scholar]
- McMahon LL, Kauer JA. Hippocampal interneurons express a novel form of synaptic plasticity. Neuron. 1997;18:295–305. doi: 10.1016/s0896-6273(00)80269-x. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Tregoubov V, Falls DL, Jia Z. Regulation of spine morphology and synaptic function by LIMK and the actin cytoskeleton. Rev Neurosci. 2003;14:233–240. doi: 10.1515/revneuro.2003.14.3.233. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Tregoubov V, Janus C, Cruz L, Jackson M, Lu WY, MacDonald JF, Wang JY, Falls DL, Jia Z. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35:121–133. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- Merrill MA, Malik Z, Akyol Z, Bartos JA, Leonard AS, Hudmon A, Shea MA, Hell JW. Displacement of alpha-actinin from the NMDA receptor NR1 C0 domain By Ca2+/calmodulin promotes CaMKII binding. Biochemistry. 2007;46:8485–8497. doi: 10.1021/bi0623025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailidis IE, Helton TD, Petrou VI, Mirshahi T, Ehlers MD, Logothetis DE. Phosphatidylinositol-4,5-bisphosphate regulates NMDA receptor activity through alpha-actinin. J Neurosci. 2007;27:5523–5532. doi: 10.1523/JNEUROSCI.4378-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockett BG, Hulme SR. Metaplasticity: new insights through electro-physiological investigations. J Integr Neurosci. 2008;7:315–336. doi: 10.1142/s0219635208001782. [DOI] [PubMed] [Google Scholar]
- Mok MHS, Kew JN. Excitation of rat hippocampal interneurons via modulation of endogenous agonist activity at the alpha7 nicotinic ACh receptor. J Physiol. 2006;574:699–710. doi: 10.1113/jphysiol.2006.104794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homo-synaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Spine microdomains for postsynaptic signaling and plasticity. Trends Cell Biol. 2009;19:218–227. doi: 10.1016/j.tcb.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Nissen W, Szabo A, Somogyi J, Somogyi P, Lamsa KP. Cell type-specific long-term plasticity at glutamatergic synapses onto hippocampal interneurons expressing either parvalbumin or CB1 cannabinoid receptor. J Neurosci. 2010;30:1337–1347. doi: 10.1523/JNEUROSCI.3481-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Bosch M, Hayashi Y. The roles of CaMKII and F-actin in the structural plasticity of dendritic spines: a potential molecular identity of a synaptic tag? Physiology (Bethesda) 2009;24:357–366. doi: 10.1152/physiol.00029.2009. [DOI] [PubMed] [Google Scholar]
- Opazo P, Choquet D. A three-step model for the synaptic recruitment of AMPA receptors. Mol Cell Neurosci. 2010 doi: 10.1016/j.mcn.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Oren I, Nissen W, Kullmann DM, Somogyi P, Lamsa KP. Role of iono-tropic glutamate receptors in long-term potentiation in rat hippocampal CA1 oriens-lacunosum moleculare interneurons. J Neurosci. 2009;29:939–950. doi: 10.1523/JNEUROSCI.3251-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M, Lacaille JC. Mechanisms of selective long-term potentiation of excitatory synapses in stratum oriens/alveus interneurons of rat hippocampal slices. J Neurophysiol. 1995;73:810–819. doi: 10.1152/jn.1995.73.2.810. [DOI] [PubMed] [Google Scholar]
- Passafaro M, Nakagawa T, Sala C, Sheng M. Induction of dendritic spines by an extracellular domain of AMPA receptor subunit GluR2. Nature. 2003;424:677–681. doi: 10.1038/nature01781. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Lavezzari G, Racca C, Roche KW, McBain CJ. mGluR7 is a metaplastic switch controlling bidirectional plasticity of feedforward inhibition. Neuron. 2005;46:89–102. doi: 10.1016/j.neuron.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Topolnik L, Lacaille JC, McBain CJ. Compartmentalized Ca(2+) channel regulation at divergent mossy-fiber release sites underlies target cell-dependent plasticity. Neuron. 2006;52:497–510. doi: 10.1016/j.neuron.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Topolnik L, Yuan XQ, Lacaille JC, McBain CJ. State-dependent cAMP sensitivity of presynaptic function underlies metaplasticity in a hippocampal feedforward inhibitory circuit. Neuron. 2008;60:980–987. doi: 10.1016/j.neuron.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier JG, Lacaille JC. Long-term synaptic plasticity in hippocampal feedback inhibitory networks. Prog Brain Res. 2008;169:241–250. doi: 10.1016/S0079-6123(07)00014-3. [DOI] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Alam MR, Kambampati V, Mains RE, Eipper BA. An isoform of kalirin, a brain-specific GDP/GTP exchange factor, is enriched in the postsynaptic density fraction. J Biol Chem. 2000;275:6395–6403. doi: 10.1074/jbc.275.9.6395. [DOI] [PubMed] [Google Scholar]
- Perez Y, Morin F, Lacaille JC. A hebbian form of long-term potentiation dependent on mGluR1a in hippocampal inhibitory interneurons. Proc Natl Acad Sci USA. 2001;98:9401–9406. doi: 10.1073/pnas.161493498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzliff AD, Soltesz I. Differential immunoreactivity for alpha-actinin-2, an N-methyl-D-aspartate-receptor/actin binding protein, in hippocampal inter-neurons. Neuroscience. 2001;103:337–349. doi: 10.1016/s0306-4522(01)00013-6. [DOI] [PubMed] [Google Scholar]
- Rozov A, Burnashev N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature. 1999;401:594–598. doi: 10.1038/44151. [DOI] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci USA. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Hajos N, Gulacsi A, Mody I, Freund TF. The absence of a major Ca2+ signaling pathway in GABAergic neurons of the hippocampus. Proc Natl Acad Sci USA. 1998;95:3245–3250. doi: 10.1073/pnas.95.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Soderling TR. The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem Sci. 1999;24:232–236. doi: 10.1016/s0968-0004(99)01383-3. [DOI] [PubMed] [Google Scholar]
- Stein V, House DR, Bredt DS, Nicoll RA. Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression. J Neurosci. 2003;23:5503–5506. doi: 10.1523/JNEUROSCI.23-13-05503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YH, Pelkey KA, Lavezzari G, Roche PA, Huganir RL, McBain CJ, Roche KW. Corequirement of PICK1 binding and PKC phosphorylation for stable surface expression of the metabotropic glutamate receptor mGluR7. Neuron. 2008;58:736–748. doi: 10.1016/j.neuron.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima A, Pelkey KA, Rah JC, Suh YH, Roche KW, Collingridge GL, McBain CJ, Isaac JT. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron. 2008;57:872–882. doi: 10.1016/j.neuron.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen TS, Madsen KL, Rebola N, Rathje M, Anggono V, Bach A, Moreira IS, Stuhr-Hansen N, Dyhring T, Peters D, et al. Identification of a small-molecule inhibitor of the PICK1 PDZ domain that inhibits hippocampal LTP and LTD. Proc Natl Acad Sci USA. 2010;107:413–418. doi: 10.1073/pnas.0902225107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Fukata M, Nicoll RA, Bredt DS. Dynamic interaction of star-gazin-like TARPs with cycling AMPA receptors at synapses. Science. 2004;303:1508–1511. doi: 10.1126/science.1090262. [DOI] [PubMed] [Google Scholar]
- Tomita S, Nicoll RA, Bredt DS. PDZ protein interactions regulating glutamate receptor function and plasticity. J Cell Biol. 2001;153:F19–F24. doi: 10.1083/jcb.153.5.f19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Topolnik L, Chamberland S, Pelletier JG, Ran I, Lacaille JC. Activity-dependent compartmentalized regulation of dendritic Ca2+ signaling in hippocampal interneurons. J Neurosci. 2009;29:4658–4663. doi: 10.1523/JNEUROSCI.0493-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ. Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci. 2000;20:8279–8289. doi: 10.1523/JNEUROSCI.20-22-08279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R, Sibley D. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hooft JA, Giuffrida R, Blatow M, Monyer H. Differential expression of group I metabotropic glutamate receptors in functionally distinct hippocampal interneurons. J Neurosci. 2000;20:3544–3551. doi: 10.1523/JNEUROSCI.20-10-03544.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga V, Losonczy A, Zemelman BV, Borhegyi Z, Nyiri G, Domonkos A, Hangya B, Holderith N, Magee JC, Freund TF. Fast synaptic subcortical control of hippocampal circuits. Science. 2009;326:449–453. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]
- Wanaverbecq N, Semyanov A, Pavlov I, Walker MC, Kullmann DM. Cholinergic axons modulate GABAergic signaling among hippocampal interneurons via postsynaptic alpha 7 nicotinic receptors. J Neurosci. 2007;27:5683–5693. doi: 10.1523/JNEUROSCI.1732-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Kelly P. Calcium-calmodulin signalling pathway up-regulates glutamatergic synaptic function in non-pyramidal, fast spiking rat hippocampal CA1 neurons. J Physiol. 2001;533:407–422. doi: 10.1111/j.1469-7793.2001.0407a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, Numberger M, Zhang S, Dingledine R. Differential dependence on GluR2 expression of three characteristic features of AMPA receptors. J Neurosci. 1997;17:9393–9406. doi: 10.1523/JNEUROSCI.17-24-09393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Lee YS, Tokumitsu H, Silva AJ, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Schluter OM, Steiner P, Czervionke BL, Sabatini B, Malenka RC. Molecular dissociation of the role of PSD-95 in regulating synaptic strength and LTD. Neuron. 2008;57:248–262. doi: 10.1016/j.neuron.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Benson DL. Targeting and clustering citron to synapses. Mol Cell Neurosci. 2006;31:26–36. doi: 10.1016/j.mcn.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Vazquez L, Apperson M, Kennedy MB. Citron binds to PSD-95 at glutamatergic synapses on inhibitory neurons in the hippocampus. J Neurosci. 1999;19:96–108. doi: 10.1523/JNEUROSCI.19-01-00096.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]