Abstract
Welders in this study were selected from a vehicle manufacturer; control subjects were from a nearby food factory. Airborne manganese levels in the breathing zones of welders and controls were 1.45 ± SD1.08 mg/m3 and 0.11 ± 0.07 μg/m3, respectively. Serum levels of manganese and iron in welders were 4.3-fold and 1.9-fold, respectively, higher than those of controls. Blood lead concentrations in welders increased 2.5-fold, whereas serum zinc levels decreased 1.2-fold, in comparison with controls. Linear regression revealed the lack of associations between blood levels of five metals and welder’s age. Furthermore, welders had erythrocytic superoxide dismutase activity and serum malondialdehyde levels 24% less and 78% higher, respectively, than those of controls. These findings suggest that occupational exposure to welding fumes among welders disturbs the homeostasis of trace elements in systemic circulation and induces oxidative stress.
The welding fume generated during the welding process possesses at least 13 metals, including manganese (Mn), beryllium (Be), cadmium (Cd), chromium (Cr), cobalt (Co), copper (Cu), iron (Fe), lead (Pb), mercury (Hg), molybdenum (Mo), nickel (Ni), zinc (Zn), antimony (Sb), and vanadium (V).1 Welders are known to be at risk, particularly for chronic exposure to airborne manganese, which is one of the major coating materials in welding products (eg, bars and wires).2–6 A recent study even suggests that exposure to manganese during welding may be a risk factor for the etiology of Parkinson’s disease among the career welders.7 Although limited studies have documented airborne manganese levels during welding, the question as to how low-level, long-term exposure to manganese or welding fume may affect blood levels of trace metals is unanswered. However, altered systemic homeostasis of iron and manganese is known to be associated with neurodegenerative disorders.8–10
Manganese-induced neurological lesions are reportedly located in the globus pallidus and striatum of the basal ganglia. The pallidus and striatum display a marked decrease in myelinated nerve fibers, accompanied by depletion of striatal dopamine.11–14 The mechanism whereby manganese induces neurodegenerative damage remains elusive. Nonetheless, several recent reports have suggested that manganese neurotoxicity may be associated with its interaction with other essential trace elements, including iron,10,13,15,16 zinc,17 copper,17 and aluminum.13,15,18 Particularly regarding manganese-induced neurotoxicity, studies have shown that chronic exposure to manganese appears to be associated with altered iron concentrations in blood as well as in the cerebrospinal fluid, presumably the result of Mn–Fe interaction at certain [Fe–S]-containing proteins, which regulate the homeostasis of iron.10,16,19–21 The excess accumulation of iron in neurons may consequently produce cellular oxidative stress, leading to neuronal damage.
Superoxide dismutase (SOD), a cytoplasmic enzyme, catalyzes the reaction to decompose superoxide free radicals generated due to the cellular oxidative stress and thus has been described as a specific superoxide radical scavenger.22 The activity of SOD in erythrocytes is directly associated with the oxidative status and has been used as the marker for systemic oxidative stress.23,24 Malondialdehyde (MDA), on the other hand, is a product of lipid peroxidation.24,25 An elevated level of MDA reflects, to a certain degree, tissue injury resulting from oxidative damage. Some researchers have reported an alteration of MDA level in animals exposed to manganese.26 Misiewicz and his colleagues27 also demonstrate an increased serum concentration of MDA in workers engaged in the production of iron-manganese alloys. Therefore, the levels of either SOD or MDA, or both, in the systemic circulation may serve as the useful biomarker(s) for oxidative status after long-term, low-level exposure to the welding fume among career welders.
The purposes of this study were to use a human population known to be chronically exposed to welding fume to investigate 1) airborne concentrations of manganese during the welding process, 2) whether or not occupational exposure to welding fume altered the homeostasis of essential trace elements, such as manganese, iron, copper, and zinc, in biological fluids, and 3) the status of the oxidative stress among career welders following chronic exposure by using oxidative biomarkers. Because lead is a frequently encountered industrial metal and is present in welding fume, the study was also extended to determine if exposure to welding fume leads to an elevated blood lead concentration.
Subjects and Methods
Study Population
Selection and recruiting of the subjects for this study were based on routine surveillance data obtained by Beijing Fengtai Center for Disease Control and Prevention. A Beijing vehicle factory was chosen for the case study for its intensive, day-to-day indoor welding practice in the manufacture of vehicles. The factory is located in the southwest region of Beijing metropolitan area and is not adjacent to any other metal industries. Thirty-seven welders who have been regularly engaged in the electric arc weld with a potentially high level of exposure were selected among about 500 welders in that factory as the exposed group. They included 22 males and 15 females with an average age and SD of 38.1 ± 1.5 years. The welders worked 7–8 hours per day and had been employed with the factory for 2–36 years.
The control subjects consisted of 50 workers, who were recruited from a nearby Beijing food factory with no history of occupational exposure to manganese and other metals. They included 31 males and 19 females who were frequency-matched to the welder group by sex, age, and work shift distribution. Their average age was 34.2 ± 1.5 years and was not statistically significantly different from the welders. The demographic data of the study population are summarized in Table 1.
TABLE 1.
Summary of Demographic Data in Fume-Exposed Welders and Control Subjects
| Welders (n = 37) | Nonwelders (n = 52) | |
|---|---|---|
| Age (years) | 38.1 ± 1.54 | 34.8 ± 1.46 |
| Sex | 24(M) /13(F) | 30(M) /22(F) |
| Years in employment | 2–36 | 1–38 |
| Initial age at employment (years) | 22.0 ± 3.23 | 21.3 ± 3.02 |
| Smoking (pack-years) (consumptive ratio) | 81.8 ± 106.3 (54.0%) | 70.2 ± 90.01 (52.0%) |
| Alcohol (mL-days) (consumptive ratio) | 35.1 ± 52.53 (35.1%) | 40.0 ± 58.90 (40.0%) |
| Airborne Mn in working place (MnD2) | 1.45 ± 1.08 (mg/m3) | 0.11 ± 0.07* (μg/m3) |
Data represent mean ± standard deviation. No significant statistical difference existed between the 2 groups unless otherwise stated.
P < 0.001.
Subjects in both groups had no reported exposure to other toxins, radiation therapy, or substance abuse at the time of interview. Subjects were excluded from the study if they were subjected to any dietary restrictions, or diagnosed with cardiovascular disorders, renal dysfunctions, or respiratory diseases.
Information on occupational history, job description, socioeconomic status (salary, education), and lifestyle information (smoking, alcohol consumption, drug uses, and dietary habits) were obtained from questionnaires and interviews completed by each worker with a trained interviewer.
Collection of Biological and Ambient Samples
Blood and urine samples were collected at start of the study. A 24-hour urine specimen was collected from each study participant in a plastic container, from which an aliquot of 250 mL was mixed with concentrated ammonia and stored at 4°C. Blood samples (10 mL) were drawn from a cubital vein of the participant after being fasted overnight. An aliquot (1 mL) of blood samples was placed in heparin-containing vials for the whole blood sample analyses and the rest for separation of serum. The whole blood and serum samples were frozen at –20°C until analysis. Containers for collection and storage of biological samples were acid-washed and distilled, deionized, ultrapure water (>18 ohm-cm2) was used in the process.
Air samples surrounding the welders’ breathing zones inside the vehicles were monitored by a station air sampler. Air samples were collected by a Model BFC-35 pump equipped with a filter that has a diameter of 40 mm with particulate matter below a cut size of 0.8 μm. Flows were checked before and after sampling. Airflow was pumped at a flow rate of 5 L/minute for 4 minutes and 1 hour after the welding started. The samples were collected in duplicates every other hour two more times in the same day and the procedure was repeated once more within the month of August. The mean values of all samples are presented in this report. For control sites, a similar surveillance procedure was performed at randomly chosen sites. Air manganese levels as determined represented the typical exposure scenery when the welding practice was routinely conducted during that season.
The filters were digested with 5 mL of HClO4–HNO3 mixture (1:9 vol/vol) at 200°C. The dry residues were dissolved in 10 mL of 1% HCl. The solutions were diluted 20- to 50-fold before atomic absorption spectrophotometry. Air manganese concentrations were measured by a model HITACHI Z-5000 flame atomic absorption spectrophotometer according to a China National Standard Operation Protocol (GB/ T16018 –1995) for occupational safety surveillance.
Determination of Metal Content in Biological Samples
Concentrations of manganese in urine, manganese, iron, zinc, and copper in serum and lead in whole blood were determined by a Finnigan multi-element analysis technique using magnetic sector high-resolution inductively coupled-plasma mass-spectrometry (HR-ICP-MS). Before analyses, samples were microwave-digested with HNO3. The masses of 65Cu, 56Fe, 55Mn, and 64Zn were determined using 45Sc as an internal standard; for lead measurement, the masses of 204Pb, 206Pb, 207Pb, 208Pb and 201Hg (used to correct the interference to 204Pb) were determined using 209Bi as an internal standard.28 The external certified standards for all five metals were purchased from SPEX CertiPrep, Inc. (Metuchen, NJ) and pretreated in the same way as the samples. All assays were performed in duplicate.
Determination of Oxidative Stress
The activity of SOD in erythrocytes was determined by a xanthine–xanthine oxidase method.29,30 The assay was conducted using a commercially available assay kit and according to the instruction by the manufacturer (Nanjing Institute of Jiancheng Biological Engineering, Nanjing, PRC). In principle, the xanthine- xanthine oxidase system produces superoxygen free radicals, which oxidizes hydroxyl amine to produce nitrite. The latter reacts with a developer to produce a purple color product with a maximal absorbance at 550 nm. The enzyme activity was expressed as international units per gram of hemoglobin (U/g Hb).
The concentration of MDA in serum, which reflects the status of lipid peroxidation, was determined by a thiobarbituric acid reactive substance method.30 The assay was conducted using a commercially available kit and according to the instruction by the manufacturer (Nanjing Institute of Jiancheng Biological Engineering, Nanjing, PRC). In the reaction, serum MDA reacts with thiobarbituric acid to produce a red color product, which possesses the maximal absorbance at 532 nm. The concentrations of MDA were calculated from a standard curve and expressed as μmol/ mL.
Statistics
Records of interviews and other reports were reviewed and abstracted for demographic data. All data are expressed as the mean ± SD unless otherwise stated. Associations between serum trace elements and the markers for oxidative stress as the function of welder’s age were analyzed by a linear regression, following the data transformation to logarithm. This transformation is valid with regards to the symmetric distribution and the linearity of variables following the logarithm transformation. The differences between two means were analyzed by a standard, parametric analysis of variance. A statistics software SPSS/PC+ for Windows was used in data analysis.
Materials
Chemicals were obtained from the following sources: Pb acetate, nitric acid (HNO3), hydrochloric acid (HCl), and perchloric acid (HClO4) from Sigma Chemical Co (St. Louis, MO); Na acetate from Fisher Scientific Co (Fair Lawn, NJ); NH4H2PO4 from Aldrich Chemical Co. (Milwaukee, WI); AA standards of Mn from Alfa Products (Danvers, MA); the assay kits for serum MDA and erythrocytic Mn-SOD from Nanjing Institute of Jiancheng Biological Engineering; the internal standard and external certified standard for HR-ICP-MS analysis from SPEX Certiprep, Inc. (Metuchen, NJ). All reagents were of analytical grade, high-performance liquid chromatography grade or the best-available pharmaceutical grade.
Results
Comparison of Demographic Data
Among the welder group, the average professional years as a welder were about 15 (range, 2 to 36 years). The working conditions for these welders in the same factory remained fairly stable without significant changes over the past several decades. The working years in the control group averaged about 25 (range, 1 to 38 years). The age and sex ratio between the case and control groups were comparable, and the parameters of smoking and alcohol consumption did not differ significantly between the two groups (Table 1).
Airborne Concentration of Manganese During Welding Process
The concentration of ambient manganese in welders’ breathing zone inside the vehicles had a geometric mean of 1.45 mg/m3, whereas it was 0.11 μg/m3 in the breathing zone of the control subjects (Table 1). These two geometric means were statistically significantly different from each other with the former on the order of ten thousand-fold magnitude higher than the latter. The threshold limit value (TLV) for ambient manganese in the workplace established by the American Conference of Governmental Industrial Hygienists is 0.20 mg/m3,31 and the World Health Organization (WHO) standard value is 0.3 mg/m3.32 Thus, the airborne manganese levels measured during welding were more than 7-fold higher than these recommended workplace exposure values. The airborne levels of other metals were not determined.
Trace Metals in Biological Samples
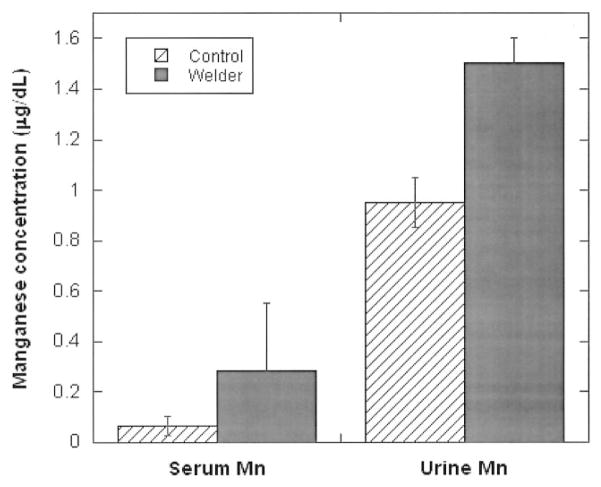
Table 2 summarizes the concentrations of five tested metals in blood samples collected from the study populations. In general, serum concentrations of manganese and iron as well as the blood lead concentration were significantly higher in welders than in control subjects, while zinc concentrations were significantly lower in welders than in controls. Serum levels of copper did not differ significantly between these two groups. Noticeably, serum manganese concentrations in welders showed approximately 4-fold increases as compared with those of control subjects, whereas the increases in levels of serum iron and blood lead were 1.9-fold and 2.6-fold, respectively. Urinary manganese levels appeared to be higher in welders than in controls; however, this difference did not achieve a statistically significant difference (Fig. 1).
TABLE 2.
Concentrations of Trace Elements in Sera or Whole Blood of Welders or Control Subjects
| Group | No. | Mn | Fe | Zn | Cu | BPb |
|---|---|---|---|---|---|---|
| (μg/dL) | ||||||
| Controls | 50 | 0.066 ± 0.038 | 159.9 ± 78.85 | 73.45 ± 15.00 | 74.64 ± 16.13 | 1.219 ± 0.827 |
| Welders | 37 | 0.286 ± 0.264* | 300.2 ± 137.3* | 59.37 ± 8.196* | 75.26 ± 19.14 | 3.074 ± 0.244* |
Data represent mean ± standard deviation.
P < 0.01 as compared with the controls. Concentrations of manganese, iron, zinc, and copper were determined in sera, whereas lead concentrations were measured in the whole blood.
Fig. 1.
Concentrations of manganese in serum and urine among career welders as compared with control subjects. Data represent mean ± SD, n = 37 for welders and n = 50 for controls. **P < 0.01 compared with controls.
To analyze the age- or employment duration-associated variation of metals among welders, the age and employment duration were divided into three groups. Serum manganese concentrations were the highest in the youngest age group (≤30 years) and thus were in the group with the least employment years as career welders (<10 years; Table 3). Urinary manganese concentrations appeared to increase with increasing age as well as in welder’s professional years (Table 3). Serum concentrations of iron and zinc also showed the same trend, ie, the highest in the youngest age group (Table 3). When the serum concentrations of manganese, iron, zinc, and copper, and blood lead were analyzed as a function of welders’ age by linear regression analyses, there were no age-associated changes in all these trace metals (data not shown).
TABLE 3.
Variations of Serum or Urine Concentrations (μg/dL) of Manganese (Mn) as a Function of Age or Employment Duration in Career Welders
| Age at the Study (years)
|
Time as career welders (years)
|
|||||
|---|---|---|---|---|---|---|
| ≤30 | 31~ | 41~ | <10 | 10~ | 20~ | |
| (n) | (9) | (12) | (16) | (12) | (10) | (15) |
| Serum Mn | 0.39 ± 0.26 | 0.28 ± 0.27 | 0.24 ± 0.22 | 0.36 ± 0.30 | 0.16 ± 0.14 | 0.31 ± 0.29 |
| Urine Mn | 1.39 ± 0.38 | 1.27 ± 0.27 | 2.39 ± 0.39 | 1.16 ± 0.25 | 1.37 ± 0.37 | 1.44 ± 0.52 |
| Serum Fe | 399 ± 204 | 277 ± 94.2 | 266 ± 103 | 312 ± 139 | 288 ± 178 | 298 ± 120 |
| Serum Zn | 64.6 ± 10.7 | 57.6 ± 8.47 | 58.0 ± 5.38 | 61.8 ± 7.94 | 60.9 ± 9.70 | 56.3 ± 6.82 |
| Serum Cu | 67.8 ± 29.4 | 79.8 ± 13.5 | 76.0 ± 30.1 | 77.2 ± 14.2 | 74.4 ± 29.4 | 74.1 ± 15.8 |
| BPb | 0.36 ± 0.22 | 0.35 ± 0.29 | 0.23 ± 0.21 | 0.32 ± 0.25 | 0.18 ± 0.19 | 0.38 ± 0.24 |
Data represent mean ± standard deviation.
No., the numbers of welders in designated groups.
Systemic Status of Oxidative Stress
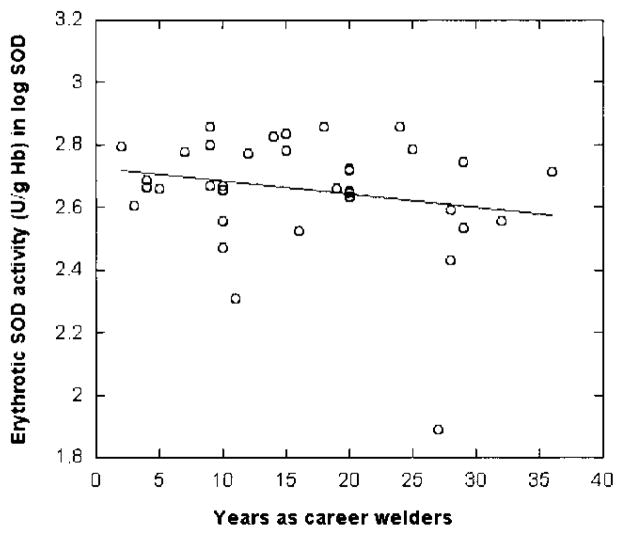
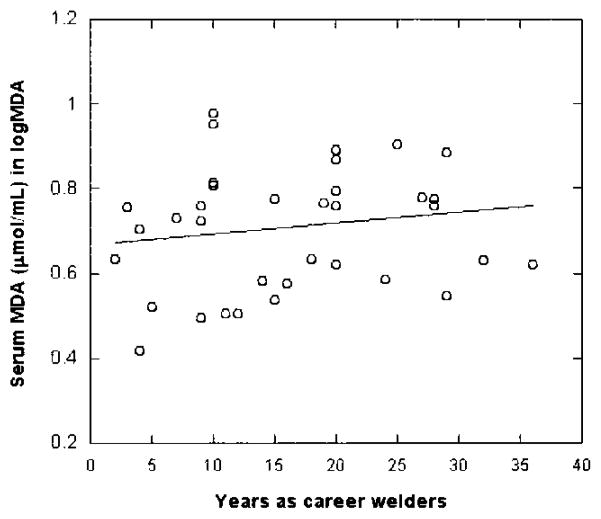
Because in vivo exposure to manganese has been shown to induce the oxidative stress,26,27 the activities of SOD and levels of MDA were assayed to test the hypothesis that altered SOD and/or MDA among welders may be used as the biomarker(s) for manganese exposure in humans. Table 4 summarizes the comparisons made between welders and control subjects. Erythrocytic SOD activities in welders were significantly reduced by 24% as compared to control subjects (P < 0.05), whereas the levels of serum MDA were significantly increased by 78%. More interestingly, there appeared to be a welder-year–associated decrease in SOD activities among the welders, although this relationship did not reach statistical significance (r = 0.211, P = 0.105; Fig. 2). The level of MDA, on the other hand, was increased as the duration of employment increases (r = 0.161, P = 0.171; Fig. 3), suggesting an elevated systemic oxidative stress among these welders.
TABLE 4.
SOD Activities in Erythrocytes and MDA Levels in Sera of Welders and Control Subjects
| Group | No. | SOD (U/g of Hb) | MDA (μmol/mL) |
|---|---|---|---|
| Control | 50 | 636.86 ± 100.86 | 3.06 ± 1.93 |
| Welder (% change) | 37 | 483.65 ± 146.83* (−24%) | 5.46 ± 1.74* (+78%) |
Data represent mean ± standard deviation
P < 0.05 as compared with the control.
Fig. 2.
Relationship between erythrocytic SOD activity and welder’s professional years. SOD activity was determined using a commercially available assay kit. Data were analyzed by a linear regression (r = −0.211, P = 0.105, n = 37).
Fig. 3.
Relationship between serum MDA concentration and welder’s professional years. Serum MDA concentrations were determined by using a commercially available assay kit. Data were analyzed by a linear regression (r = 0.161, P = 0.171, n = 37).
Discussion
The data presented in this report clearly show that manganese was emitted in the fume produced during the welding practice. The concentration of airborne manganese surrounding the welders’ breathing zones inside the vehicles exceeded the TLV by more than seven times. Zayed’s group in Canada6 reported a similar finding. By using an air sampler with a pore size cut-off of 4 μm, they found that the airborne manganese level was about 0.24 mg/m3 during the assembly of large pieces of heavy excavation machinery, while it was below the TLV, about 0.06 mg/m3, in construction of smaller pieces. Sinczuk-Walczak et al33 have also reported that air manganese concentrations varied from 0.004 to 2.67 mg/m3 during the welding process. The values in our study appeared to be higher than that detected by Zayed’s team. However, in the current study, the air sampler was stationed inside rather than outside the vehicles, a setting similar to that used in the study by Sinczuk-Walczak et al,33 where the air was sampled in a ship. The relatively closed air ventilation within the vehicle may result in a high accumulation of manganese-containing particles in the ambient air. Based on the EPA document, the particles generated by the arc possess range in diameter between 0.4 and 0.8 μm.34 Because the filter used in our investigation had a diameter of 0.8 μm, it is conceivable that the actual concentrations of manganese in the ambient air during the welding may be much higher than those observed in this study.
The average ambient air level of manganese near industrial sources ranges from 0.22~0.3 μg/m3.35 The United States Environmental Protection Agency36 has posted a reference concentration value for manganese exposure that was largely based on changes in neuropsychological tests (such as finger tapping, hand steadiness) detected in an occupational study by Roels et al.37 The estimated lowest observed adverse effect level was 150 μg Mn/m3. By adjusting for nonoccupational lifetime exposure and some uncertainty factors, the recommended safe level of ambient manganese is 0.05 μg Mn/m3. In our control study, the ambient concentration of manganese appears to be higher than this value, but it was considerably below the TLV (0.20 mg/m3).31
The adverse health effect of exposure to welding fume has been recognized.5,7,33,38,39 Results by Sinczuk-Walczak et al.33 suggest that airborne manganese concentrations within the range of 0.004 to 2.67 mg/m3 could induce subclinical effects on the nervous system. Barceloux35 has summarized that workers exposed to manganese levels near 1–5 mg Mn /m3 for 20 years could develop early subclinical changes in neuropsychological parameters, such as hand tremor, reduced memory, and prolonged reaction times. A recent epidemiological study including 15 cases of career welders further suggests that the welders may have a younger age of onset of Parkinson’s disease compared to sequentially ascertained Parkinson’s patients.7 A parallel study to assess neurobehavioral functions of the welders involved in this study, by using a WHO-recommended Neurobehavioral Core Test Battery, is currently in progress. The data presented in this report nonetheless suggest that exposure to the welding fume could lead to an altered level of at least four trace metals in sera or in whole blood.
The results from this study showed that career welders had a significantly higher level of serum manganese in comparison to control subjects. Serum concentrations of manganese among welders were about 4.3-fold greater than those detected in controls. More surprising is the fact that the welders with younger ages appeared to bear a higher serum manganese level, although they had less professional years as welders. The same is true for serum concentrations of iron and zinc. Currently, there is no satisfactory explanation to this observation; it is possible that the physiological factors (such as faster breath rate and more vigorous cardiac function), the physical activities (such as longer working hours typically among the younger welders), or inexperience (such as unsafe work practice) may cause a higher exposure to manganese and other metals in the welding fume. It is also possible that variations in biochemical parameters (such as plasma protein binding) and differences in toxicokinetics among different age groups may contribute to higher serum levels of these metals. It remains unclear, however, whether the higher exposure in the younger welders may render this group of welders more vulnerable to metal toxicities, although some have suggested an earlier onset of Parkinson’s disease among welders.7
Whether or not the blood concentration of manganese can be used as a biological indicator or biomarker for manganese exposure remains a topic of dispute. Some investigators suggest that manganese concentrations in blood seem to be fairly stable over long periods of time in humans exposed to this metal in mining and industrial environments, and thus can be used to reflect the manganese body burden.40,41 Others, mainly based on the animal studies,42–44 point out that manganese is rapidly eliminated from the blood circulation and possesses a rather short blood t1/2, but a prolonged tissue t1/2, after exposure. The discrepancy between blood and tissue t1/2 and possibly a predominant tissue accumulation of manganese may render the blood manganese level less relevant as an indicator of total body burden of manganese. In the current human study, the career welders did show a significantly higher manganese level in sera as compared to control subjects. Because the elevated serum manganese concentrations among the welders were not associated with welder’s age, it seems inappropriate to use serum manganese concentrations as a sign of total body burden of manganese. However, serum manganese levels may reasonably indicate the status of systemic manganese following recent exposure among the active career welders.
Although the precise mechanism by which manganese induces neurodegenerative toxicity is poorly understood, evidence suggests that manganese toxicity may be partly the result of its action on iron homeostasis.10,16,20,45 A predominant intracellular overload of iron and subsequent iron-mediated oxidative stress may lead to damage of neurons. Results in this study showed that exposure to the welding fume significantly increased the concentration of iron in serum. This increase could partly be attributed to the possible action of manganese on iron metabolism. However, the fact that the welding fume contains more than a dozen metals including iron, copper, and lead34 suggests that the elevated serum concentrations of iron and lead may be a direct result of the overexposure to these metals in the fume. It should be pointed out that the excessive iron in the systemic circulation could promote the brain overload of iron, leading to a catastrophic oxidative injury in neurons.
Trace metals play an important role in biological processes by coordinating enzymatic reactions or by affecting the permeability of cell membranes, among others. For example, zinc is an element required for SOD, which is located in the cytoplasm of eukaryotic cells. The enzyme dismutates the superoxide radical and therefore prevents the cellular damage resulting from reactive oxygen species.46 Copper is an essential metal that, by virtue of its ability to undergo reversible redox reactions, acts as a cofactor for enzymes involved in respiration and DNA synthesis.47 Since exposure to the welding fume apparently alters the homeostasis of these trace elements, future studies should investigate the importance of these trace metals in welding-associated neurotoxicity.
Idiopathic Parkinson’s disease and MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-induced Parkinsonism have been shown to be associated with the excess oxidative stress.48,49 Animal studies have also shown that manganese exposure may produce cellular oxidative damage.21 The levels of SOD and MDA have been used as the indicators of the disease status associated with oxidative injury. For example, serum levels of SOD and MDA are significantly changed in patients with chronic hepatitis,50 aluminum poisoning,51 cardiovascular disease,23 and Alzheimer’s disease.24 Some environmental or other factors such as acute exercise, smoking, and aging could lead to a significant increase in lipid peroxidation (MDA as an indicator) and a significant decrease in antioxidant enzyme activity (SOD as an indicator).52 A marked reduction of SOD activity with an increased MDA in this study clearly suggests an elevated oxidative stress level among the career welders. It remains unclear, though, whether the undue oxidative stress among welders is due to exposure only to manganese or due to a combined effect of exposure to mixed metals in the welding fume.
In summary, the results of this study indicate that the welding fume generated during the welding process possesses a higher-than-normal level of manganese. Long-term, low-level exposure to the welding fume increases serum concentrations of manganese and iron and the blood concentration of lead, while it decreases serum zinc level among the career welders. Occupational exposure to the welding fume also appears to induce oxidative stress among the career welders.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Guo Fenying, Guan Dongzhu, Gao Xiaofeng, and Tian Qingmin in Fengtai Center for Disease Control and Prevention. Special appreciation is also extended to the managers of local factories for their kind assistance.
This project was partly supported by a pilot fund by US-NIH/NIEHS Environmental Health Sciences Center in Northern Manhattan at Columbia University (Grant ES-09,089). This work was also supported by US-National Institute of Environmental Health Sciences Grant ES-08,146, National Natural Science Foundation of China grant 30000140, Beijing Science Committee Grant No. 9558102800, and China Ministry of Education Foundation Grant No. 52.
References
- 1.OSHA. Welding Fumes (Total Particulate). Chemical Sampling Information. 1995 http://www.osha-slc.gov/dts/chemicalsampling/data/CH276100.html.
- 2.Chandra SV, Shukla GS, Srivastawa RS, Singh H, Gupta VP. An exploratory study of manganese exposure to welders. Clin Toxicol. 1981;18:407–418. doi: 10.3109/15563658108990264. [DOI] [PubMed] [Google Scholar]
- 3.Mergler D, Huel G, Bowler R, et al. Nervous system dysfunction among workers with long-term exposure to manganese. Environ Res. 1994;64:151–180. doi: 10.1006/enrs.1994.1013. [DOI] [PubMed] [Google Scholar]
- 4.Roels H, Lauwerys R, Buchet JP, et al. Epidemiological survey among workers exposed to manganese: effects on lung, central nervous system, and some biological indices. Am J Ind Med. 1987;11:307–327. doi: 10.1002/ajim.4700110308. [DOI] [PubMed] [Google Scholar]
- 5.Sjogren B, Iregren A, Frech W, et al. Effects on the nervous system among welders exposed to aluminium and manganese. Occup Environ Med. 1996;53:32–40. doi: 10.1136/oem.53.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smargiassi A, Baldwin M, Savard S, et al. Assessment of exposure to manganese in welding operations during the assembly of heavy excavation machinery accessories. Appl Occup Environ Hyg. 2000;15:746–750. doi: 10.1080/10473220050129383. [DOI] [PubMed] [Google Scholar]
- 7.Racette BA, McGee-Minnich L, Moerlein SM, et al. Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology. 2001;56:8–13. doi: 10.1212/wnl.56.1.8. [DOI] [PubMed] [Google Scholar]
- 8.Dexter DT, Carayon A, Javoy-Agid F, et al. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114:1953–1975. doi: 10.1093/brain/114.4.1953. [DOI] [PubMed] [Google Scholar]
- 9.Loeffler DA, Connor JR, Juneau PL, et al. Transferrin and iron in normal, Alzheimer’s disease, and Parkinson’s disease brain regions. J Neurochem. 1995;65:710–724. doi: 10.1046/j.1471-4159.1995.65020710.x. [DOI] [PubMed] [Google Scholar]
- 10.Zheng W, Zhao Q, Slavkovich V, Aschner M, Graziano JH. Alteration of iron homeostasis following chronic exposure to manganese in rats. Brain Res. 1999;833:125–132. doi: 10.1016/s0006-8993(99)01558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mena I, Marin O, Fuenzalida S, Cotzias GC. Chronic manganese poisoning: clinical picture and manganese turnover. Neurology. 1967;17:128–136. doi: 10.1212/wnl.17.2.128. [DOI] [PubMed] [Google Scholar]
- 12.Neff NH, Barrett RE, Costa E. Selective depletion of caudate nucleus dopamine and serotonin during chronic manganese dioxide administration to squirrel monkeys. Experientia. 1969;25:1140–1141. doi: 10.1007/BF01900234. [DOI] [PubMed] [Google Scholar]
- 13.Olanow CW, Good PF, Shinotoh H, et al. Manganese intoxication in the rhesus monkey: a clinical, imaging, pathologic, and biochemical study. Neurology. 1996;46:492–498. doi: 10.1212/wnl.46.2.492. [DOI] [PubMed] [Google Scholar]
- 14.Yamada M, Ohno S, Okayasu I, et al. Chronic manganese poisoning: a neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol (Berl) 1986;70:273–278. doi: 10.1007/BF00686083. [DOI] [PubMed] [Google Scholar]
- 15.Verity MA. Manganese neurotoxicity: a mechanistic hypothesis. Neurotoxicology. 1999;20:489–497. [PubMed] [Google Scholar]
- 16.Zheng W, Zhao Q. Cellular overload of iron following manganese exposure in cultured neuronal, but not neuroglial cells. Brain Res. 2001;897:175–179. doi: 10.1016/s0006-8993(01)02049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai JC, Minski MJ, Chan AW, Leung TK, Lim L. Manganese mineral interactions in brain. Neurotoxicology. 1999;20:433–444. [PubMed] [Google Scholar]
- 18.Kao HJ, Chen WH, Liu JS. Rapid progression of Parkinsonism associated with an increase of blood manganese. Kaohsiung J Med Sci. 1999;15:297–301. [PubMed] [Google Scholar]
- 19.Zheng W, Ren S, Graziano JH. Manganese inhibits mitochondrial aconitase: a mechanism of manganese neurotoxicity. Brain Res. 1998;799:334–342. doi: 10.1016/s0006-8993(98)00481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng W. Neurotoxicology of the brain barrier system: new implications. J Toxicol Clin Toxicol. 2001;39:711–719. doi: 10.1081/clt-100108512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen JY, Tsao GC, Zhao QQ, Zheng W. Differential cytotoxicity of Mn (II) and Mn (III): special reference to mitochondrial [Fe-S] containing enzymes. Toxicol Appl Pharmacol. 2001;175:160–168. doi: 10.1006/taap.2001.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oda T, Akaike T, Hamamoto T, et al. Oxygen radicals in influenza-induced pathogenesis and treatment with pyran polymer-conjugated SOD. Science. 1989;244:974–976. doi: 10.1126/science.2543070. [DOI] [PubMed] [Google Scholar]
- 23.Sabuncu T, Vural H, Harma M, Harma M. Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease. Clin Biochem. 2001;34:407–413. doi: 10.1016/s0009-9120(01)00245-4. [DOI] [PubMed] [Google Scholar]
- 24.Ozcankaya R, Delibas N. Malondialdehyde, superoxide dismutase, melatonin, iron, copper, and zinc blood concentrations in patients with Alzheimer disease: cross-sectional study. Croat Med J. 2002;43:28–32. [PubMed] [Google Scholar]
- 25.Reimund JM, Arondel Y, Duclos B, Baumann R. Vitamins and trace elements in home parenteral nutrition patients. J Nutr Health Aging. 2000;4:13–18. [PubMed] [Google Scholar]
- 26.Chen MT, Yiin SJ, Sheu JY, Huang YL. Brain lipid peroxidation and changes of trace metals in rats following chronic manganese chloride exposure. J Toxicol Environ Health A. 2002;65:305–316. doi: 10.1080/15287390252800882. [DOI] [PubMed] [Google Scholar]
- 27.Misiewicz A, Radwan K, Misiewicz A, Dziewit T. Malonyl dialdehyde concentration in red blood cells of workers engaged in the production of iron-manganese alloys [in Polish] Med Pr. 1999;50:277–281. [PubMed] [Google Scholar]
- 28.Smith DR, Calacsan C, Woolard D, et al. Succimer and the urinary excretion of essential elements in a primate model of childhood lead exposure. Toxicol Sci. 2000;54:473–480. doi: 10.1093/toxsci/54.2.473. [DOI] [PubMed] [Google Scholar]
- 29.Minami M, Yoshikawa H. A simplified assay method of superoxide dismutase activity for clinical use. Clin Chim Acta. 1979;92:337–342. doi: 10.1016/0009-8981(79)90211-0. [DOI] [PubMed] [Google Scholar]
- 30.Shang YZ, Ye JW, Tang XC. Improving effects of huperzine A on abnormal lipid peroxidation and superoxide dismutase in aged rats. Zhongguo Yao Li Xue Bao. 1999;20:824–828. [PubMed] [Google Scholar]
- 31.American Conference of Governmental Industrial Hygienists. ABBBE-93: Threshold Limit Values for Chemical Substances and Physical Agents Biological Exposure Indices. Cincinnati, OH: American Conference of Governmental Industrial Hygienists; 1992. [Google Scholar]
- 32.World Health Organization. Air Quality Guidelines for Europe. Copenhagen: WHO, Regional Office for Europe; 1987. Manganese; pp. 262–271. European Series No. 23. [Google Scholar]
- 33.Sinczuk-Walczak H, Jakubowski M, Matczak W. Neurological and neuro-physiological examinations of workers occupationally exposed to manganese. Int J Occup Med Environ Health. 2001;14:329–337. [PubMed] [Google Scholar]
- 34.US Environmental Protection Agency: Office of Air Quality Planning and Standards Emission Inventory Branch. Development of Particulate and Hazardous Emission Factors for Electric Arc Welding. Washington, DC: DPA; 1994. pp. 2.1–2.23.pp. 4.1–4.6. EPA Contract No. 68-D2–0159. [Google Scholar]
- 35.Barceloux DG. Mangenese. Clin Toxicol. 1999;37:293–307. doi: 10.1081/clt-100102427. [DOI] [PubMed] [Google Scholar]
- 36.US Environmental Protection Agency. Integrated Risk Information System (IRIS) on Manganese. National Center for Environmental Assessment, Office of Research and Development; Washington, DC: EPA; 1999. Available at: http://www.epa.gov/iris/subst/0373.htm#refinhal. [Google Scholar]
- 37.Roels HA, Ghyselen P, Buchet JP, Ceulemans E, Lauwerys RR. Assessment of the permissible exposure level to manganese in workers exposed to manganese dioxide dust. Br J Ind Med. 1992;49:25–34. doi: 10.1136/oem.49.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becker N, Claude J, Frentzel-Beyme R. Cancer risk of arc welders exposed to fumes containing chromium and nickel. Scand J Work Env Health. 1985;11:75–82. doi: 10.5271/sjweh.2242. [DOI] [PubMed] [Google Scholar]
- 39.Korczynski RE. Occupational health concerns in the welding industry. Appl Occup Env Hyg. 2000;15:936–945. doi: 10.1080/104732200750051175. [DOI] [PubMed] [Google Scholar]
- 40.Dietz MC, Ihrig A, Wrazidlo W, et al. Results of magnetic resonance imaging in long-term manganese dioxide-exposed workers. Environ Res. 2001;85:37–40. doi: 10.1006/enrs.2000.4068. [DOI] [PubMed] [Google Scholar]
- 41.Boojar MM, Goodarzi F. A longitudinal follow-up of pulmonary function and respiratory symptoms in workers exposed to manganese. J Occup Environ Med. 2002;44:282–290. doi: 10.1097/00043764-200203000-00016. [DOI] [PubMed] [Google Scholar]
- 42.Newland MC, Cox C, Hamada R, Oberdorster G, Weiss B. The clearance of manganese chloride in the primates. Fundam Appl Toxicol. 1987;9:314–328. doi: 10.1016/0272-0590(87)90054-6. [DOI] [PubMed] [Google Scholar]
- 43.Takeda A, Sawashita J, Okada S. Biological half-lives of zinc and manganese in rat brain. Brain Res. 1995;695:53–58. doi: 10.1016/0006-8993(95)00916-e. [DOI] [PubMed] [Google Scholar]
- 44.Zheng W, Kim H, Zhao Q. Comparative toxicokinetics of manganese chloride and methylcyclo-pentadienyl Mn tricarbonyl in male Sprague-Dawley rats. Toxico Sci. 2000;54:295–301. doi: 10.1093/toxsci/54.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aschner M, Vrana KE, Zheng W. Manganese uptake and distribution in the central nervous system (CNS) Neurotoxicology. 1999;20:173–180. [PubMed] [Google Scholar]
- 46.Abdel-Mageed AB, Oehme FW. A review of the biochemical roles, toxicity and interactions of zinc, copper and iron: I. Zinc. Vet Hum Toxicol. 1990;32:34–39. [PubMed] [Google Scholar]
- 47.Chang A, Fink GR. Metal ion metabolism. The copper-iron connection. Curr Biol. 1994;4:532–533. doi: 10.1016/s0960-9822(00)00116-0. [DOI] [PubMed] [Google Scholar]
- 48.Ebadi M, Govitrapong P, Sharma S, et al. Ubiquinone (coenzyme q10) and mitochondria in oxidative stress of Parkinson’s disease. Biol Signals Recept. 2001;10:224–253. doi: 10.1159/000046889. [DOI] [PubMed] [Google Scholar]
- 49.Wu DC, Teismann P, Tieu K, et al. NADPH oxidase mediates oxidative stress in the1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc Natl Acad Sci USA. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li W, Dai QT, Liu ZE. Preliminary study on early fibrosis of chronic hepatitis B treated with Ginkgo biloba Composita [in Chinese] Zhongguo Zhong Xi Yi Jie He Za Zhi. 1995;15:593–595. [PubMed] [Google Scholar]
- 51.Chugh SN, Arora V, Sharma A, Chugh K. Free radical scavengers & lipid peroxidation in acute aluminium phosphide poisoning. Indian J Med Res. 1996;104:190–193. [PubMed] [Google Scholar]
- 52.Ozbay B, Dulger H. Lipid peroxidation and antioxidant enzymes in Turkish population: relation to age, gender, exercise, and smoking. Tohoku J Exp Med. 2002;197:119–124. doi: 10.1620/tjem.197.119. [DOI] [PubMed] [Google Scholar]