Abstract
Purpose
To evaluate the effects of central vision loss (CVL) on mutual gaze perception (knowing whether somebody else is looking at you), an important nonverbal visual cue in social interactions.
Methods
23 persons with CVL (visual acuity 20/50 to 20/200), 16 with a bilateral central scotoma and 7 without, and 23 age-matched controls completed a gaze perception task, and a brief questionnaire. They adjusted the eyes of a life-size virtual head on a monitor at a 1-m distance until they appeared either to be looking straight at them, or were at the extreme left/right or up/down positions at which the eyes still appeared to be looking toward them (defining the range of mutual gaze in the horizontal and vertical planes).
Results
The nonscotoma group did not differ from the controls in any gaze task measure. However, the gaze direction judgments of the scotoma group had significantly greater variability than those of the nonscotoma and control groups (p < 0.001). In addition, their mutual gaze range tended to be wider (p = 0.15), suggesting a more liberal judgment criterion. Contrast sensitivity was the strongest predictor of variability in gaze direction judgments followed by self-reported difficulties.
Conclusions
Our results suggest that mutual gaze perception is relatively robust to CVL. However, a follow-up study that simulates less-than-optimal viewing conditions of everyday social interactions is needed. The gaze perception task holds promise as a research tool for investigating the effects of vision impairment on mutual gaze judgments. Self-reported difficulty and contrast sensitivity were both independent predictors of gaze perception performance suggesting that the task captured higher-order as well as low-level visual abilities.
Keywords: mutual gaze, social interactions, macular degeneration, central scotoma, central field loss, questionnaire
People with central vision loss (CVL) often report difficulties with social interactions and reduced social functioning.1, 2 A limited access to the nonverbal visual cues inherent to effective communication can be a disadvantage in social interactions. Wang and Boerner3 reported that for people with vision impairment, difficulty in social situations was due either to the individual’s own lack of ability to perceive visual cues or other people’s lack of understanding.
The facial region has been noted as an important source of nonverbal visual information relevant to social situations.4 Prior studies of individuals with CVL due to age-related macular degeneration have focused primarily on difficulty with recognizing and discriminating faces and facial expressions.5–8 However, the ability to perceive mutual gaze [knowing whether somebody else is looking at you] also provides important nonverbal social cues.9, 10 For example, a gaze in your direction may be a cue signaling turn-taking in a conversation, or can indicate certain interpersonal attitudes, such as hostility or attraction. Therefore, an inability to perceive mutual gaze could potentially be detrimental to an individual’s social relationships and interactions. However, difficulty with gaze perception has, to our knowledge, never been evaluated in a low vision population. Here we report a study in which we evaluated mutual gaze judgments of individuals with CVL compared to normally sighted controls.
Although gaze direction can in principle be judged on the basis of the position of the iris relative to the visible portion of the surrounding sclera,11, 12 other cues, such as head orientation are known to be factored into gaze direction judgments.13 Detecting mutual gaze is a higher-order visual function based on a complex interaction between these visual cues.12, 14, 15 Therefore, it is hard to predict exactly how gaze perception of individuals with CVL might be affected. Reduction of low-level visual functions, including visual acuity and contrast sensitivity, may or may not translate into difficulties in gaze perception.
To quantify the functional ability of participants with CVL to judge mutual gaze, we used an interactive gaze perception task developed by Gamer and Hecht.16 The task involves adjustment of the gaze direction of a computer-generated virtual head, which was previously validated against a real person.16 Mutual gaze with another person is perceived to occur over a range of gaze directions, which Gamer and Hecht referred to as the cone of gaze.16 The gaze perception task quantifies both the direction of this cone of gaze and the width (the range of gaze directions that are perceived as ‘being looked at’). The task has been able to measure differences in gaze perception between controls and people with social phobias,17 but, so far, has never been used to evaluate mutual gaze performance of visually-impaired observers.
We hypothesized that participants with CVL would have greater difficulty perceiving mutual gaze than normally-sighted controls as they would have greater difficulties seeing the visual cues used to make judgments about the virtual head’s gaze direction. We predicted that these gaze perception difficulties would manifest as greater judgment variability on the gaze perception task. Furthermore, depending on how they might respond to uncertainties about mutual gaze, their judgments might become either more conservative or more liberal, resulting in either a narrower or a wider gaze cone width, respectively, when compared to peers with unimpaired vision. Finally, we compared gaze perception performance of CVL participants with a bilateral central scotoma (a scotoma that encompasses the fovea) to CVL participants without a bilateral central scotoma.
METHODS
Participants
Twenty-three individuals with congenital or acquired CVL with visual acuity in the range 20/50 to 20/200 and 23 normally sighted (visual acuity of 20/30 or better) age-similar controls participated. The duration of the CVL ranged from 7 to 60 years. Sixteen of the CVL participants had an absolute bilateral central scotoma (0.7° target at 1 m). CVL in the scotoma group was caused by age-related macular degeneration (AMD) (n = 6), juvenile macular dystrophy (JMD) (n = 5), myopic degeneration (n = 2), Doyne honeycomb retinal dystrophy (n = 1), optic neuropathy (n = 1), and diabetic maculopathy (n = 1). The median radius of the binocular scotoma was 6.2° (range 3.0 to 21.5°). CVL in the nonscotoma group was caused by albinism (n = 3), infantile nystagmus (n = 2), retinopathy of prematurity (n = 1) and multifocal choroiditis (n = 1).
Design and Procedure
For all the participants, the study consisted of one session and took approximately one to two hours. All vision measures and gaze perception tasks were undertaken binocularly with the habitual spectacle or contact lens correction used by the participant in social situations. Additional lenses were not used to provide an optimal refractive correction for the distance at which a specific test/task was performed. The study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) at Schepens Eye Research Institute. All participants were given a full explanation of the experimental procedures, and written informed consent was obtained with the option to withdraw from the study at any time.
Vision Measures
The visual status of the participants was examined through an assessment of binocular visual acuity, binocular contrast sensitivity, and mapping of any binocular central scotoma(s) (visually impaired group only). Visual acuity (VA) measurements were taken at a 1 m test distance (as this was the distance at which the gaze task was performed) using the ‘Tumbling E’ option of the Freiburg Acuity Test (FrACT, http://michaelbach.de/fract/).18 Contrast sensitivity was assessed at a 0.45 m testing distance using a custom computerized program, equivalent to the Pelli-Robson chart, for letters subtending 2.5°visual angle presented on a luminance-calibrated display.19 Visual fields were assessed with kinetic perimetry using a computerized central visual fields program20 at a 1 m test distance with a white square stimulus (0.7° visual angle) and a white fixation cross (0.7° visual angle) presented against a black background. Participants were encouraged to keep the fixation cross as clear as possible during the test (i.e., to use the preferred retinal locus for fixation). Binocular scotoma location was categorized as predominantly horizontal (left or right of the binocular preferred retinal locus in visual field space) or predominantly vertical (above or below the binocular preferred retinal locus in visual field space).
Gaze Perception Task
Participants were seated in front of a computer monitor displaying a 2D life-size virtual head (Figure 1). The test distance was 1 m, which represents a comfortable distance for personal interactions without an intrusion into personal space,21, 22 and is typical of the distances used in prior gaze perception studies (which have ranged from 0.5 m23, 24 to 2 m).25 A chin-rest was used to align the participant to the eye-level of the virtual head and maintain the 1 m test distance. Participants could rotate the eyes of the virtual head without constraint in either the horizontal or the vertical plane (but never both planes together) using a mouse.
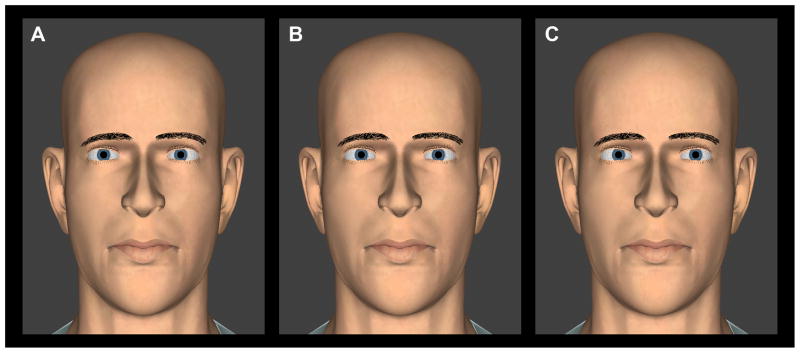
Figure 1.
Screen shots of direct and averted gaze of the virtual head for the gaze perception task in the horizontal plane. (A) Head straight, eyes looking to the left of the observer; (B) Head straight, eyes looking directly toward the observer; and (C) Head straight, eyes looking to the right of the observer. A color version of this figure is available online at www.optvissci.com.
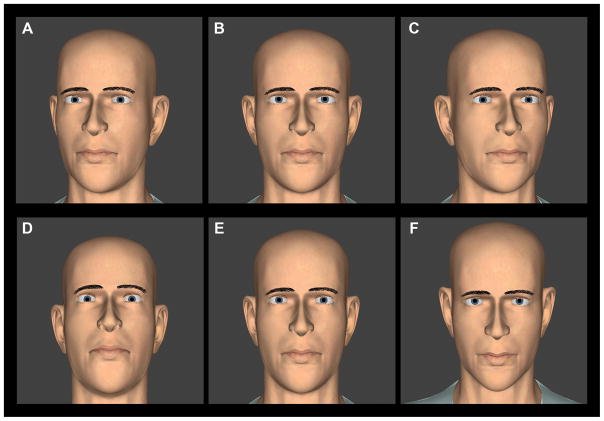
To enable comparison with prior studies, we used the same virtual head as in the original study by Gamer and Hecht.16 The eyes were modeled in 3D with appropriate convergence settings such that they moved in synchrony and remained converged at the subject’s eye plane at all times (this did result in slightly different angular rotations of the two eyes). Shadows were not cast, simulating the situation of frontal and ambient illumination. The orientation of the virtual head could either be directed straight toward the subject, or turned away by 8° vertically (up or down), or 8° horizontally (left or right) (Figure 2), thus simulating social situations in which the direction of gaze has to be judged when the other person’s head orientation is not directly aligned with the gaze. We chose not to exceed 8° because in the case of vertical eye rotations, too much of the iris would have been occluded in the initial settings for the centering task (making for rather a grotesque appearance).
Figure 2.
Screen shots of direct, straight-ahead gaze for each of the horizontal (top) and vertical (bottom) orientations of the virtual head in the gaze perception task. A color version of this figure is available online at www.optvissci.com.
In the centering task, participants moved the eyes in a specified direction until the virtual head seemed to be gazing straight toward them. The eyes were either moved horizontally right or left from an initial random leftward or rightward gaze offset, or vertically down or up from an initial random upward or downward gaze offset. For each trial, the angular deviation of the final position of the virtual eyes from the true straight ahead gaze position was computed. There were a total of 24 trials with four for each of the three head orientations (−8°, 0°, 8°) in the vertical and horizontal planes. The mean and standard deviation of the final eye positions for the four trials in each condition were calculated for each participant, providing an assessment of their ability to judge straight ahead gaze.
In the decentering task, the initial direction of the virtual eyes was always centered. Participants then moved the eyes in a specified direction, left or right in the horizontal plane and up or down in the vertical plane, until the point where they felt that the eyes were just about to stop looking at them. The eyes could be rotated without constraint, theoretically allowing for impossible rotations. However, none of our subjects ever chose such extreme settings (> 40° in the horizontal plane). There were a total of 24 trials with two for each of the two directions and three head orientations in the vertical and horizontal planes. The horizontal (vertical) width of the range of mutual gaze, the gaze cone, was then computed as the difference between the corresponding values of the six pairs of decentering values to the left and to the right (upward and downward). The mean and standard deviation of these six horizontal (vertical) gaze cone widths were calculated for each participant. (There were insufficient trials to calculate means and standard deviations of the gaze cone widths for each head orientation).
Thus there were a total of 48 trials, 24 of the centering task (12 horizontal and 12 vertical) and 24 of the decentering task (12 horizontal and 12 vertical). The trials were randomly interleaved and took about 20–30 minutes to complete. Participants were given as much time as needed to practice the tasks and become familiar with operating the mouse before experimental data collection commenced.
Questionnaire
Participants were administered a short, 6-item questionnaire (see Appendix, available online at http://links.lww.com/OPX/A180) to quantify opinions about the importance of eye contact in social interactions, difficulties with gaze perception due to their vision impairment, and difficulties seeing how people react during a conversation. With the exception of one question from the National Eye Institute Visual Function Questionnaire,26 questions were devised for the study based on informal discussions with visually impaired individuals. The opinion questions were rated on a six-point scale ranging from ‘definitely true’ (1) to ‘definitely false’ (6), and the difficulties questions were rated a six-point scale from ‘no difficulty’ (1) to ‘stopped doing this because of your eyesight’ (6). A seventh possible response on the difficulties questions was ‘stopped doing this for other reasons or not interested in doing this,’ which was scored as missing data.
Appendix.
Questions and Response Options on the Questionnaire
| Item # | Category | Question | Response Options
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| 1 | Opinion | I believe that maintaining eye contact is important for social communication | Definitely true | Mostly true | Somewhat true | Somewhat false | Mostly false | Definitely false | - |
| 2 | Opinion | I look people in the eye and maintain eye contact when speaking with them | Definitely true | Mostly true | Somewhat true | Somewhat false | Mostly false | Definitely false | - |
| 3 | Opinion | My eyesight has no impact on my ability to communicate with others | Definitely true | Mostly true | Somewhat true | Somewhat false | Mostly false | Definitely false | - |
| 4 | Social interaction difficulties | Because of your eyesight, how much difficulty do you have in knowing whether a person is looking at you during social interactions (e.g., conversations) | No difficulty | A little difficulty | Some difficulty | Moderate difficulty | Extreme difficulty | Stopped doing this because of your eyesight | Stopped doing this for other reasons or not interested in doing this |
| 5 | Social interaction difficulties | Because of your eyesight, how much difficulty do you have in knowing whether a person is looking at somebody else during social interactions (e.g., conversations) | No difficulty | A little difficulty | Some difficulty | Moderate difficulty | Extreme difficulty | Stopped doing this because of your eyesight | Stopped doing this for other reasons or not interested in doing this |
| 6 | Social interaction difficulties | Because of your eyesight, how much difficulty do you have seeing how people react to things you say? | No difficulty | A little difficulty | Some difficulty | Moderate difficulty | Extreme difficulty | Stopped doing this because of your eyesight | Stopped doing this for other reasons or not interested in doing this |
Data Analyses
All statistical analyses were conducted using SPSS 11.5.0 (Chicago, IL) and an alpha level of 0.05 was taken to indicate statistical significance.
For the centering gaze perception task, the dependent variables were the mean and standard deviation (i.e., variability within the final ‘straight ahead’ gaze positions of the virtual eyes) of the straight ahead gaze judgments. Data for the horizontal and vertical planes were analyzed separately using a two-way repeated measures ANOVA with the factors Vision Group (scotoma vs. nonscotoma vs. normally-sighted) and Virtual Head Orientation [horizontal plane: left (−8°) vs. centered (0°) vs. right (8°); vertical plane: down (−8°) vs. centered (0°) vs. up (8°)]. Data were analyzed separately for each plane because we did not want to make any assumptions about an 8° and −8° rotation of the virtual head in the horizontal plane equating to an 8° and −8° rotation of the virtual head in the vertical plane with regards to the perception of the straight ahead gaze position.
For the decentering gaze perception task, the dependent variables were the mean and standard deviation (variability) of the gaze cone width in the horizontal and vertical planes. Data were analyzed with a two-way repeated measures ANOVA with the factors Vision Group (scotoma vs. non-scotoma vs. normally-sighted) and Spatial Plane (horizontal vs. vertical). Due to a technical recording error, only twelve CVL participants with a scotoma, and six without a scotoma had data for the vertical gaze cone width. One normally-sighted participant was found to be an extreme outlier (three times the interquartile range) on the decentering task and was excluded from the analysis.
Pearson’s correlation coefficients and Spearman’s rho were used to quantify the relationships between the questionnaire responses, vision measures, and gaze task measures. Forward and backward stepwise multiple linear regression analysis was used to determine the best predictors of judgment variability (difficulty) in gaze task performance.
RESULTS
Sample Characteristics
The demographics and vision characteristics of the three vision groups (normally-sighted, scotoma CVL, and non-scotoma CVL) are presented in Table 1. The groups did not differ in gender distribution (χ2 (2, N = 46) = 0.09, p = 0.96), but there was a trend for the scotoma CVL group to be older (F(2, 45) = 2.71, p = 0.08). The duration of the CVL was greater for the participants in the non-scotoma than the scotoma group (t(21) = 2.40, p < 0.05). The three groups did differ significantly in their visual acuity (F(2, 45) = 91.32, p < 0.001) and contrast sensitivity (F(2, 44) = 34.70, p < 0.001). As expected, the normally-sighted group had significantly better visual acuity and contrast sensitivity than the scotoma CVL group (p < 0.001 and p < 0.001, respectively) and the non-scotoma CVL group (p < 0.001 and p < 0.05 respectively). The scotoma and non-scotoma groups, on the other hand, did not differ in their visual acuity (p = 0.31), but the scotoma group did have poorer contrast sensitivity (p < 0.01).
Table 1.
Participant demographics and vision characteristics.
| Central Vision Loss | |||
|---|---|---|---|
|
| |||
| Normally-Sighted (n = 23) | Scotoma (n = 16) | Nonscotoma (n = 7) | |
|
| |||
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Demographics | |||
| Age (years) | 62.5 (16.5) | 65.4 (15.0) | 48.8 (16.8) |
| Gender, Male (%) | 52% | 56% | 57% |
| Length of Vision Impairment (years) | - | 22.8 (14.9) | 39.3 (15.8) |
|
| |||
| Vision Measures | |||
| Visual Acuity (logMAR)↓ | 0.05 (0.09) | 0.72 (0.21) | 0.60 (0.21) |
| Contrast Sensitivity (log units)↑ | 1.87 (0.15)a | 1.28 (0.20) | 1.63 (0.37) |
Note. All tests were performed binocularly.
n = 22
↑Higher scores indicate better performance
↓Lower scores indicate better performance
Gaze Perception Task
Straight Ahead Gaze Direction
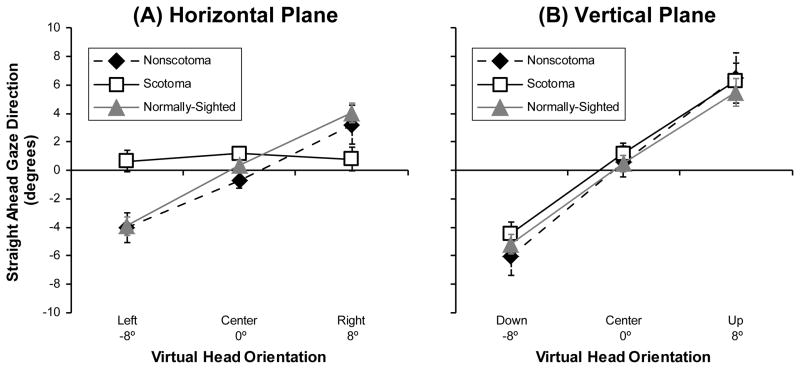
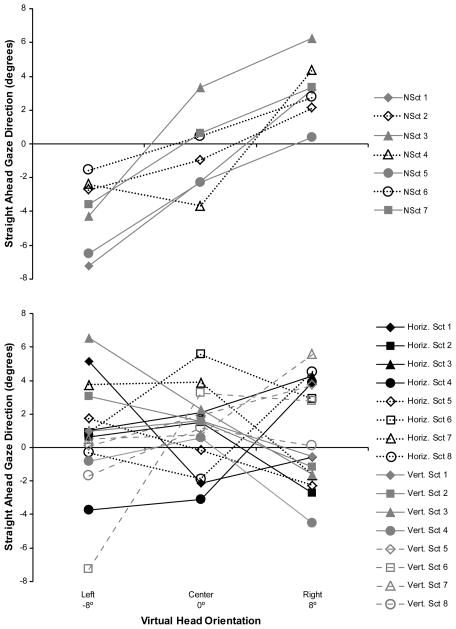
Surprisingly, there was no main effect of vision group on the perception of straight ahead gaze in either the horizontal or vertical planes. The mean judgments of the non-scotoma CVL participants were similar to those of the controls when centering the gaze in both the vertical and horizontal planes, and the mean judgments of the scotoma CVL participants were also similar to those of the controls in the vertical plane (Figure 3). However, there was an interesting interaction between vision group and head orientation in the horizontal plane (F(4,86) = 8.64, p < 0.001). On average, the scotoma CVL participants had mean judgments of straight ahead that were closer to 0° for the right and left head rotations than the judgments of the non-scotoma and control participants, which were shifted in the direction of the virtual head rotation (Figure 3a). Overall, this “attractor” effect of the virtual head rotation was significant in both the horizontal (F(2,86) = 26.59, p < 0.001) and vertical (F(2,86) = 94.70, p < 0.001) planes. It should be noted that were large between-subject differences in the patterns of the individual responses of the scotoma CVL participants in the horizontal plane (Figure 4 bottom), which were not related to scotoma size or position. In contrast, the individuals in the non-scotoma group all behaved in a reasonably similar manner (Figure 4 top) with head orientation effects that were comparable to those observed in the control group (Figure 3A).
Figure 3.
Mean group values for the non-scotoma CVL, scotoma CVL, and normally-sighted participants of the direction of straight ahead gaze in the (A) horizontal plane when the virtual head was orientated left, center, and right, and (B) the vertical plane when the virtual head was orientated down, center, and up. The perceived direction of straight ahead gaze shifted in the direction of the head rotation; the only exception was the scotoma group in the horizontal plane. Error bars indicate the standard error of the mean. Please see Figure 4 for individual data showing wide between-subject variability in the scotoma group.
Figure 4.
Mean values for each participant of the direction of straight ahead gaze in the horizontal plane when the virtual head was orientated left, center, and right. Top: Each participant in the non-scotoma CVL group. Bottom: Each participant in the scotoma CVL group by whether it was a horizontal or a vertical scotoma. There was wide between-subject variability in the response patterns of participants in the scotoma group. By comparison, the head orientation effects were more consistent in the non-scotoma group.
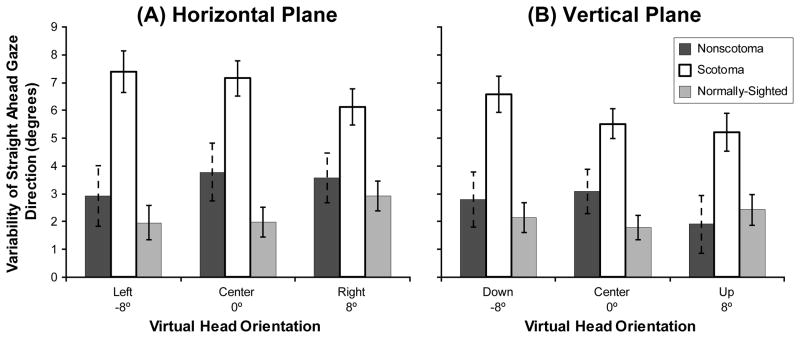
We also examined the variability (SD) of the straight ahead gaze judgments and found a highly significant main effect of vision group in both the horizontal (F(2,43) = 18.71, p < 0.001) and vertical (F(2,43) = 14.83, p < 0.001) planes (Figure 5). Post hoc analyses using the Bonferroni post hoc criterion for significance indicated that the scotoma group had significantly greater variability in their horizontal and vertical judgments than both the non-scotoma group and the normally-sighted group (all p < 0.01).
Figure 5.
Mean group values for the non-scotoma CVL, scotoma CVL, and normally-sighted participants of the standard deviation (judgment uncertainty) of the direction of straight ahead gaze in (A) the horizontal plane when the virtual head was orientated left, center, and right; and (B) the vertical plane when the virtual head was orientated down, center, and up. Scotoma participants had more variability in their straight ahead gaze judgments than the other participants. Error bars indicate the standard error of the mean.
Gaze Cone Width
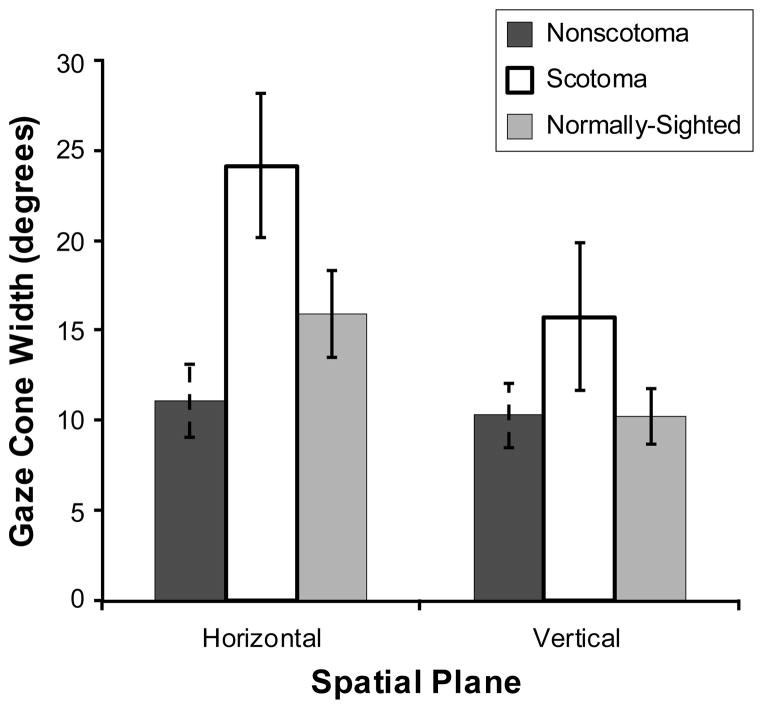
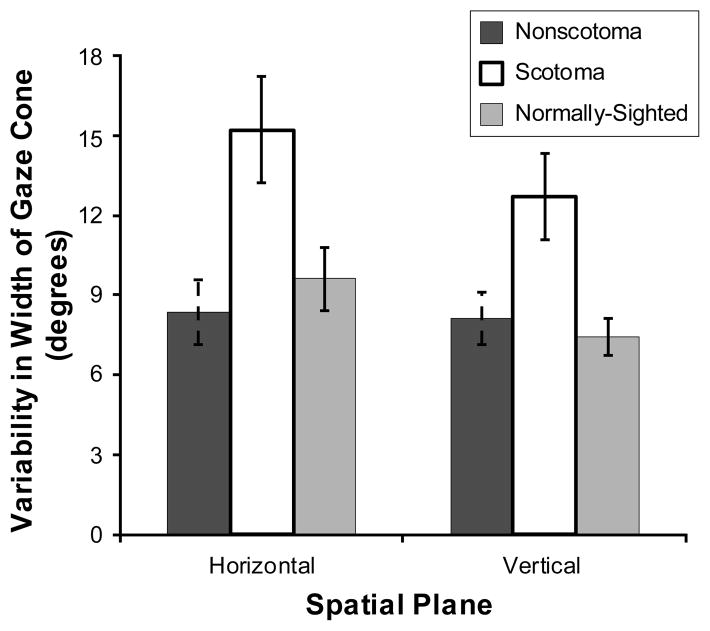
The scotoma group tended to have a wider gaze cone than the normally-sighted group and the non-scotoma group (F(2,37) = 1.99, p = 0.15) (Figure 6), as well as more variability in their gaze cone width (F(2,37) = 4.78, p < 0.05) (Figure 7). Overall, the horizontal gaze cone was significantly wider (F(1,37) = 6.66, p < 0.05) with more variability in the width (F(1,37) = 3.04, p = 0.09) than the vertical gaze cone.
Figure 6.
Mean group values for the non-scotoma CVL, scotoma CVL, and normally-sighted participants of their gaze cone width in the horizontal plane and the vertical plane. Scotoma participants had a larger gaze cone than the other participants. Error bars indicate the standard error of the mean.
Figure 7.
Mean group values for the non-scotoma CVL, scotoma CVL, and normally-sighted participants of the standard deviation (judgment uncertainty) of the gaze cone width in the horizontal plane and the vertical plane. Scotoma participants had more gaze cone width variability than the other participants. Error bars indicate the standard error of the mean.
Differences between the Two CVL Groups
As the scotoma group tended to be older, but to have had CVL for a shorter time than the non-scotoma group (Table 1), the gaze measure ANOVAs were repeated including seven scotoma participants who were matched in both age and CVL duration to the seven participants in the nonscotoma group, and 14 age-matched controls (see SDC Table 1, available online at http://links.lww.com/OPX/A183). The matched scotoma and nonscotoma groups did not differ significantly for visual acuity; however, there was a trend for the scotoma group to have poorer contrast sensitivity (see SDC Table 2, available online at http://links.lww.com/OPX/A183). Unfortunately we were unable to match scotoma participants to nonscotoma participants for age, CVL duration and contrast sensitivity. The ANOVA results for the age-matched groups (see SDC Tables 3 and 4, available online at http://links.lww.com/OPX/A183) did not differ from the analyses with the age-dissimilar scotoma and nonscotoma groups suggesting that neither age nor CVL duration were significant factors accounting for the differences between the two CVL groups.
Questionnaire Responses
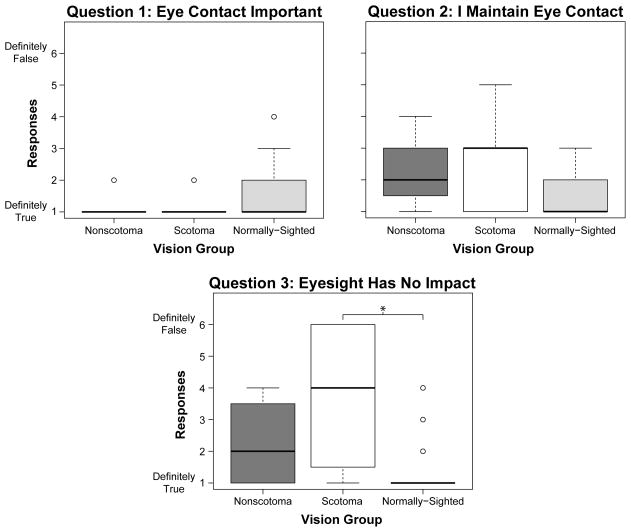
Most participants with CVL thought that it was definitely important to maintain eye contact (87% scotoma group and 86% nonscotoma), more so than the normally-sighted group (61%) (Figure 8, Question 1). However, only 33% of the scotoma group, and 29% of the nonscotoma group, said that they definitely did so, as compared to 68% of the normally-sighted group (Figure 8, Question 2). Furthermore, 53% of the scotoma group and 29% of the nonscotoma group disagreed with the statement that their eyesight had no impact on their ability to communicate, compared to only 6% of the normally-sighted participants (Figure 8, Question 3). A Kruskal-Wallis test confirmed that the median responses of the three groups were significantly different for the latter two questions [Question 2: χ2(2, N = 40) = 6.14, p < 0.05; Question 3: χ2(2, N = 40) = 11.92, p < 0.01].
Figure 8.
Boxplots of responses for each of the opinion questions by the non-scotoma CVL, scotoma CVL, and normally-sighted participants. Open circles are outliers. *p < 0.0167 (Bonferroni adjusted alpha level).
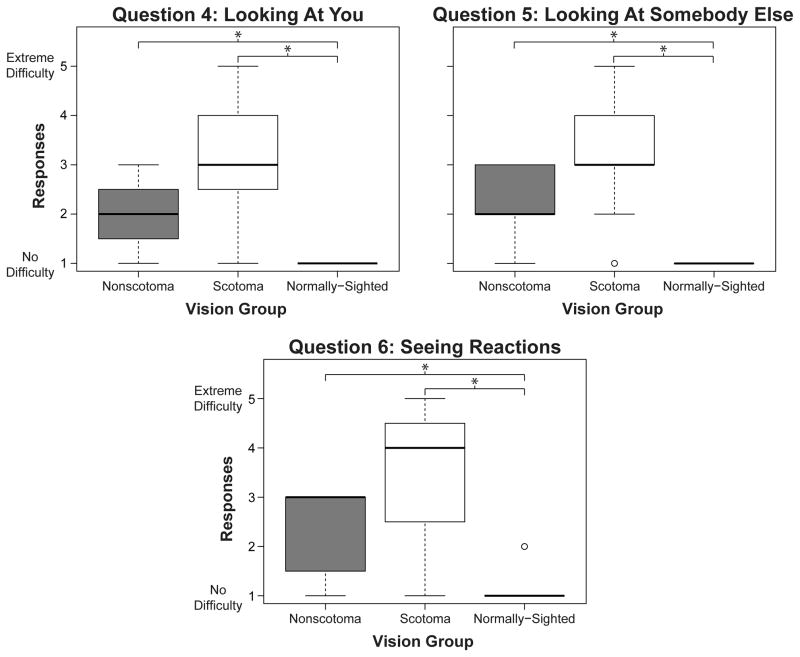
When asked about perceiving the gaze of others, 40% of the scotoma group reported moderate to extreme difficulty, whereas the majority of the nonscotoma group reported little to some difficulty (Figure 9, Questions 4 and 5). Similarly, more than half of the scotoma group (54%) reported moderate to extreme difficulties in seeing reactions during a conversation, whereas the majority of the nonscotoma group (71%) reported little to some difficulty (Figure 9, Question 6). Not surprisingly, almost all of the normally-sighted participants reported no difficulties on these three questions. A Kruskal-Wallis test confirmed that the median responses of the three groups were significantly different for these questions [Question 4: χ2(2, N = 39) = 24.79, p < 0.001; Question 5: χ2(2, N = 40) = 27.73, p < 0.001; Question 6: χ2(2, N = 40) = 23.81, p < 0.001].
Figure 9.
Boxplots of responses for each of the questions addressing difficulties with gaze perception and seeing reactions by the non-scotoma CVL, scotoma CVL, and normally-sighted participants. Response options six and seven are not shown because option six was never chosen and option seven was coded as missing data. Open circles are outliers. *p < 0.0167 (Bonferroni adjusted alpha level)
Relationships between Vision Measures, Self-Reported Difficulties and Gaze Measures
Only gaze task measures in the horizontal plane were included in this analysis, as this is the plane in which gaze judgments are most frequently made. Age did not have a significant correlation with any measure except for presence of a scotoma and contrast sensitivity (Table 2). The mean direction of straight ahead gaze did not correlate with vision measures or self-reported difficulty. However, gaze cone width was weakly correlated with visual acuity (as visual acuity decreased, gaze cone width increased). Furthermore, the variability in both the straight ahead direction and the gaze cone width were significantly related to acuity and contrast sensitivity; judgment variability increased as the level of vision impairment increased. There was a very strong correlation between gaze cone width and variability: as gaze cone width increased, judgment variability also increased (the same was true for the control participants).
Table 2.
Correlations between age, presence of a scotoma, vision measures, measures from the gaze perception task in the horizontal plane, and self-reported difficulty with gaze perception for participants with CVL.
| Vision Measures | Gaze Perception Task: Horizontal Plane | Difficulty Questiona | ||||||
|---|---|---|---|---|---|---|---|---|
| Age | Scotoma Presenceb | Visual Acuity↓ | Contrast Sensitivity↑ | Straight Ahead Direction | SD in Straight Ahead Direction | Gaze Cone Width | SD in Gaze Cone Width | Q4: If someone looks at youb |
| Age | 0.46* | 0.12 | −0.50* | −0.14 | 0.28 | 0.17 | 0.23 | −0.18 |
| Scotoma Presenceb | Scotoma Presence | 0.31 | −0.52* | −0.40 | 0.37 | 0.41 | 0.41 | 0.45* |
| Visual Acuity↓ | Visual Acuity | −0.64** | 0.22 | 0.65** | 0.42* | 0.56** | 0.52* | |
| Contrast Sensitivity↑ | Contrast Sensitivity | −0.04 | −0.68** | −0.23 | −0.44* | −0.23 | ||
| Straight Ahead Direction | Straight Ahead Direction | 0.15 | −0.06 | −0.03 | −0.07 | |||
| SD in Straight Ahead Direction | SD in Straight Ahead Direction | 0.45** | 0.59** | 0.55** | ||||
| Gaze Cone Width | Gaze Cone Width | 0.95** | 0.40 | |||||
| SD in Cone Width | SD in Gaze Cone Width | 0.37 | ||||||
| Q4: If someone looks at you | Q4: If someone looks at you | |||||||
Note. All values are Pearson correlation coefficients unless otherwise noted. SD = variability (i.e., judgment uncertainty) in measure from the gaze perception task. n = 23 unless otherwise noted.
n = 22
Spearman correlation coefficient values
p < 0.05
p < 0.01
↑Higher scores indicate better performance
↓Lower scores indicate better performance
The responses to the main difficulty question (Question 4: difficulty in knowing whether someone was looking at you) were significantly related to the variability in the straight ahead direction, but not the variability in the gaze cone width; those who reported greater difficulty in knowing whether someone was looking at them had more uncertainty in the judgment of straight ahead gaze. Finally, responses to difficulty Question 4 were also moderately related to visual acuity and the presence of a scotoma (participants with greater vision impairment tended to report greater difficulty). Although not shown in Table 2, correlation coefficients of a similar magnitude were also observed for the other two difficulty questions (Questions 5 and 6).
Predictors of Measured Difficulty/Uncertainty on Gaze Perception Task
To identify the best predictors of measured difficulty/uncertainty (i.e., variability) on the mutual gaze perception task, we performed a stepwise multiple linear regression analysis (Table 3) where the independent variables were the three vision measures (visual acuity, contrast sensitivity and presence of a scotoma), self-reported difficulty with gaze perception (Question 4), and gaze cone width (as this correlated with variability in both the centering and decentering task, Table 2). Question 4 was the only difficulty question included as it was the most relevant to the gaze perception task participants performed in the study. Again, only measures in the horizontal plane were considered.
Table 3.
Results from stepwise multiple linear regression analysis: Vision measures, self-reported difficulty with gaze perception, and (horizontal) gaze cone width as predictors of judgment variability on the mutual gaze perception task in the horizontal plane (n = 22).
| Prediction Model | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Unstandarized | |||||||
|
| |||||||
| Dependent Variable | Independent Variable | B | SE | β | t | p-value | sr2(unique)a |
| SD in Straight Ahead Gaze Direction | (Constant) | 11.70 | 2.52 | 4.64 | 0.00 | ||
| Contrast Sensitivity | −5.86 | 1.48 | −0.58 | −3.95 | 0.00 | 0.31 | |
| Difficulty Q4: If someone looks at you | 1.00 | 0.37 | 0.42 | 2.88 | 0.01 | 0.17 | |
|
| |||||||
| SD in Gaze Cone Width | (Constant) | 12.00 | 2.14 | 5.60 | 0.00 | ||
| Gaze Cone Width | 0.46 | 0.03 | 0.89 | 16.23 | 0.00 | 0.76 | |
| Contrast Sensitivity | −5.81 | 1.37 | −0.23 | −4.24 | 0.00 | 0.08 | |
Note. SD = variability (i.e., judgment uncertainty) in measure from the gaze perception task. Excludes all non-significant variables. Both backward and forward stepping models yielded identical results.
sr2 = squared semipartial correlation = proportion of the unique variance contributed by each independent variable
Contrast sensitivity and self-reported difficulty were significant predictors of the variability in judgments of straight ahead gaze direction (r2 = 0.62, r2[adj] = 0.58, F(2, 21) = 15.56, p < 0.001), with 31% of the unique variance explained by contrast sensitivity and 17% by self-reported difficulty (Table 3). Presence of a scotoma was not a significant predictor. When forced into the regression model it only accounted for 2% of the unique variance. The significant predictors of gaze cone width variability were contrast sensitivity and gaze cone width (r2 = 0.95, r2[adj] = 0.94, F(2, 21) = 165.30, p < 0.001). Much of the predictive ability was accounted for by gaze cone width (76%), with only 8% contributed by contrast sensitivity. Again, presence of a scotoma was not a significant predictor.
DISCUSSION
Given the loss in visual acuity and contrast sensitivity of the CVL participants in this study, we had anticipated that mutual gaze perception would be affected to a greater extent than was measured in the psychophysical task. Surprisingly, CVL had only modest negative effects. The CVL participants without a scotoma did not differ significantly from the normally-sighted controls in any of the gaze task measures, including their perception and variability of the straight ahead gaze direction, and their perception and variability of the range of mutual gaze (gaze cone width). The CVL participants with a scotoma did differ significantly from the controls and the non-scotoma CVL group, but not as much as might have been expected. They exhibited greater variability in their judgment of straight ahead gaze and tended to have a wider gaze cone in both the horizontal and vertical planes. These results suggest that they had greater difficulty performing mutual gaze judgments than the other two groups and adopted a more liberal criterion i.e., they were more likely to accept a gaze as being directed toward them than the non-scotoma and control groups.
In multiple regression analyses, the main predictors of variability in the judgment of the straight ahead gaze direction were contrast sensitivity followed by self-reported difficulty. By comparison, the main predictor of variability in the horizontal range of mutual gaze was the actual gaze cone width rather than vision measures or self-reported difficulty. These findings suggest that gaze perception difficulties of people with CVL manifest to a greater extent as variability in judging the straight ahead gaze direction than the range of mutual gaze perception.
Consistent with prior studies,11, 16, 25 we found that the orientation of the virtual head affected the perception of straight ahead gaze. Specifically, there was a shift of direct gaze toward the direction in which the head was rotated - an “attractor” effect - for both the horizontal and vertical planes. The non-scotoma and control groups exhibited similar behaviors in both planes (Figure 3), with an attractor effect of similar magnitude to that reported by Hecht and colleagues in prior studies of young normally-sighted observers.16, 27 By comparison, the scotoma participants demonstrated an attractor effect of similar magnitude to the other two groups only in the vertical plane, but no overall attractor effect in the horizontal plane where the mean group straight ahead gaze direction was close to 0° for both leftward and rightward rotations of the virtual head. One possible interpretation is that the CVL participants might have been employing a complex compensatory strategy such that they were less biased by head orientation cues than the other participants. However, it seems more likely that the widely differing individual response patterns of the scotoma CVL participants to the horizontal head rotations (Figure 4) just happened by chance to average out at about 0°.
There are several possible reasons for the differences in gaze perception performance between the scotoma and non-scotoma groups. Participants in the scotoma group were older, had acquired rather than congenital vision loss, a shorter duration of vision impairment, greater impairment in contrast sensitivity (though similar impairment in visual acuity) and used a peripheral rather than a foveal location for fixation. Our analyses clearly suggest that contrast sensitivity was the most important visual factor contributing to the differences. Age was not a significant factor and duration of vision impairment was unlikely to be a significant factor because all participants had CVL for at least 7 years. One aspect not addressed in this study was whether differences in fixation patterns between participants in the scotoma and non-scotoma group were related to differences in gaze perception and whether scotoma participants used any compensatory gaze strategies. For example, in a recent study28 people with central scotomas (AMD) had a significantly greater proportion of fixations on external facial features (internal features included eyes, nose and mouth) when viewing the image of a face in a scanning laser ophthalmoscope, as compared to control participants with or without simulated visual acuity loss.
How can our findings be interpreted? There can be no doubt that the CVL was a substantial impairment in our participants, as evidenced by the decrements in visual acuity and contrast sensitivity. Yet, in spite of this low-level impairment, the higher-level task of judging gaze direction appeared to have remained substantially intact. We used a task previously validated as a research tool for observers with normal vision16 which simulated a gaze perception situation with good ambient lighting. The task used a single head without any other distracting stimuli and specified the direction in which the eyes had to be moved to achieve straight ahead gaze. Thus, we may have overestimated the gaze perception abilities of participants with CVL relative to the unconstrained situations and less-than-optimal viewing conditions encountered in everyday social interactions. We selected to use a 1 m viewing distance as a comfortable and realistic distance at which gaze perception judgments are typically made. We did not, however, evaluate whether reducing the viewing distance (e.g., to 0.5 m) might have improved performance or whether people with CVL might compensate for their vision loss by making even more use of head orientation cues or other cues that are typically related to gaze perception. To address these questions, a follow up study is clearly warranted with a larger sample, less optimal viewing conditions, a larger range of head rotations than we used (e.g. up to 30°11, 25) and more than one viewing distance.
To our knowledge, this is the first study to evaluate mutual gaze perception of people with vision impairment. Although our sample size was relatively small and heterogeneous, our results demonstrate that our gaze perception task holds promise as a research tool for investigating the effects of vision impairment on mutual gaze judgments and the compensatory strategies that may be adopted. Contrast sensitivity was the strongest predictor of gaze perception performance. However, self-reported difficulty was also a significant independent predictor suggesting that the gaze task captured higher-order skills as well as the lower-level visual abilities measured by traditional vision tests.
Supplementary Material
Acknowledgments
Supported in part by NIH grant T35EY007149 and the Alice J. Adler Fellowship of the Schepens Eye Research Institute. Agnes Münch programmed the gaze cone task. Anna Sterczek assisted with data collection.
APPENDIX
The appendix, a 6-item questionnaire to quantify opinions about the importance of eye contact in social interactions, difficulties with gaze perception due to their vision impairment, and difficulties seeing how people react during a conversation, is available online at http://links.lww.com/OPX/A180.
Footnotes
The data were presented in part at the annual meeting of the Association for Research in Vision and Ophthalmology, Seattle, WA, May 2013.
References
- 1.Alma MA, van der Mei SF, Melis-Dankers BJ, van Tilburg TG, Groothoff JW, Suurmeijer TP. Participation of the elderly after vision loss. Disabil Rehabil. 2011;33:63–72. doi: 10.3109/09638288.2010.488711. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell J, Bradley C. Quality of life in age-related macular degeneration: a review of the literature. Health Qual Life Outcomes. 2006;4:97. doi: 10.1186/1477-7525-4-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang SW, Boerner K. Staying connected: re-establishing social relationships following vision loss. Clin Rehabil. 2008;22:816–24. doi: 10.1177/0269215508091435. [DOI] [PubMed] [Google Scholar]
- 4.App B, Reed CL, McIntosh DN. Relative contributions of face and body configurations: perceiving emotional state and motion intention. Cogn Emot. 2012;26:690–8. doi: 10.1080/02699931.2011.588688. [DOI] [PubMed] [Google Scholar]
- 5.Boucart M, Dinon JF, Despretz P, Desmettre T, Hladiuk K, Oliva A. Recognition of facial emotion in low vision: a flexible usage of facial features. Vis Neurosci. 2008;25:603–9. doi: 10.1017/S0952523808080656. [DOI] [PubMed] [Google Scholar]
- 6.Barnes CS, De L’Aune W, Schuchard RA. A test of face discrimination ability in aging and vision loss. Optom Vis Sci. 2011;88:188–99. doi: 10.1097/OPX.0b013e318205a17c. [DOI] [PubMed] [Google Scholar]
- 7.Bullimore MA, Bailey IL, Wacker RT. Face recognition in age-related maculopathy. Invest Ophthalmol Vis Sci. 1991;32:2020–9. [PubMed] [Google Scholar]
- 8.Tejeria L, Harper RA, Artes PH, Dickinson CM. Face recognition in age related macular degeneration: perceived disability, measured disability, and performance with a bioptic device. Br J Ophthalmol. 2002;86:1019–26. doi: 10.1136/bjo.86.9.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emery NJ. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci Biobehav Rev. 2000;24:581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- 10.Langton SR. The mutual influence of gaze and head orientation in the analysis of social attention direction. Q J Exp Psychol A. 2000;53:825–45. doi: 10.1080/713755908. [DOI] [PubMed] [Google Scholar]
- 11.Anstis SM, Mayhew JW, Morley T. The perception of where a face or television “portrait” is looking. Am J Psychol. 1969;82:474–89. [PubMed] [Google Scholar]
- 12.Todorovic D. Geometrical basis of perception of gaze direction. Vision Res. 2006;46:3549–62. doi: 10.1016/j.visres.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Wollaston WH. On the apparent direction of eyes in a portrait. Phil Trans R Soc Lond. 1824;114:247–56. [Google Scholar]
- 14.Todorovic D. The effect of face eccentricity on the perception of gaze direction. Perception. 2009;38:109–32. doi: 10.1068/p5930. [DOI] [PubMed] [Google Scholar]
- 15.Wilson HR, Wilkinson F, Lin LM, Castillo M. Perception of head orientation. Vision Res. 2000;40:459–72. doi: 10.1016/s0042-6989(99)00195-9. [DOI] [PubMed] [Google Scholar]
- 16.Gamer M, Hecht H. Are you looking at me? Measuring the cone of gaze. J Exp Psychol Hum Percept Perform. 2007;33:705–15. doi: 10.1037/0096-1523.33.3.705. [DOI] [PubMed] [Google Scholar]
- 17.Harbort J, Witthoft M, Spiegel J, Nick K, Hecht H. The widening of the gaze cone in patients with social anxiety disorder and its normalization after CBT. Behav Res Ther. 2013;51:359–67. doi: 10.1016/j.brat.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Bach M. The Freiburg Visual Acuity test--automatic measurement of visual acuity. Optom Vis Sci. 1996;73:49–53. doi: 10.1097/00006324-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 19.To L, Woods RL, Goldstein RB, Peli E. Psychophysical contrast calibration. Vision Res. 2013;90:15–24. doi: 10.1016/j.visres.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woods RL, Apfelbaum HL, Peli E. DLP-based dichoptic vision test system. J Biomed Opt. 2010;15:016011. doi: 10.1117/1.3292015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailenson JN, Blascovich J, Beall AC, Loomis JM. Equilibrium theory revisited: mutual gaze and personal space in virtual environments. Presence. 2001;10:583–98. [Google Scholar]
- 22.Lloyd DM. The space between us: A neurophilosophical framework for the investigation of human interpersonal space. Neurosci Biobehavl Rev. 2009;33:297–304. doi: 10.1016/j.neubiorev.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Tipples J. Orienting to eye gaze and face processing. J Exper Psychol Hum Percept Perform. 2005;31:843–56. doi: 10.1037/0096-1523.31.5.843. [DOI] [PubMed] [Google Scholar]
- 24.Ewbank MP, Jennings C, Calder AJ. Why are you angry with me? Facial expressions of threat influence perception of gaze direction. J Vis. 2009;9:16, 1–7. doi: 10.1167/9.12.16. [DOI] [PubMed] [Google Scholar]
- 25.Gibson JJ, Pick AD. Perception of another person’s looking behavior. Am J Psychol. 1963;76:386–94. [PubMed] [Google Scholar]
- 26.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–8. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 27.Stevanov J, Uesaki M, Kitaoka A, Ashida H, Hecht H. ‘Face inversion effect’ on perception of the vertical gaze direction. Perception. 2013;42:199. ECVP Abstract Suppl. [Google Scholar]
- 28.Seiple W, Rosen RB, Garcia PM. Abnormal fixation in individuals with age-related macular degeneration when viewing an image of a face. Optom Vis Sci. 2013;90:45–56. doi: 10.1097/OPX.0b013e3182794775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.