Abstract
The early proximal tubule preferentially reabsorbs organic solutes and bicarbonate over chloride ions, resulting in a luminal fluid with a higher chloride concentration than that in blood. From this late proximal tubular fluid, one-half of NaCl reabsorption by the adult proximal tubule is active and transcellular and one-half is passive and paracellular. The purpose of the present in vitro microperfusion study was to determine the characteristics of passive chloride transport and permeability properties of the adult and neonatal proximal straight tubules (PST). In tubules perfused with a late proximal tubular fluid, net passive chloride flux was 131.7 ± 37.7 pmol·mm−1·min−1 in adult tubules and −17.1 ± 23.3 pmol·mm−1·min−1 in neonatal proximal tubules (P < 0.01). Chloride permeability was 10.94 ± 5.21 × 10−5 cm/s in adult proximal tubules and −1.26 ± 1.84 × 10−5 cm/s in neonatal proximal tubules (P < 0.05). Thus neonatal PST have a chloride permeability not different from zero and have no net passive chloride transport. Bicarbonate permeability is also less in neonates than adults in this segment (−0.07 ± 0.03 × 10−5 vs. 0.93 ± 0.27 × 10−5 cm/s, P < 0.01). Neonatal PST have higher sodium-to chloride and bicarbonate-to-chloride permeability ratios than adult PST. However, mannitol and sucrose permeabilities were not different in adult proximal tubules and neonatal PST. Transepithelial resistance was measured using current injection and cable analysis. The resistance was 6.7 ± 0.7 Ω·cm2 in adult tubules and 11.3 ± 1.4 Ω·cm2 in neonatal PST (P < 0.01). In conclusion, there are significant maturational changes in the characteristics of the PST paracellular pathway affecting transport in this nephron segment.
Keywords: chloride transport, renal development, passive transport, tight junction, chloride permeability, bicarbonate permeability
The early proximal tubule preferentially reabsorbs glucose, amino acids, and bicarbonate over chloride ions (18, 25). This leaves the luminal fluid in the late proximal tubule with a lower bicarbonate concentration and higher chloride concentration than that in the peritubular plasma (18, 25). In proximal tubules perfused with a high-chloride-low-bicarbonate solution without organic solutes simulating late proximal tubular fluid, approximately one-half of NaCl transport is active and transcellular and one-half is passive and paracellular (3, 4, 28, 29). Active NaCl transport is mediated by the parallel operation of the sodium/hydrogen exchanger and a chloride/base exchanger (3, 28).
Passive chloride transport is mediated by passive diffusion of chloride down its electrochemical gradient across the paracellular pathway (1, 4, 16). The magnitude of passive chloride transport is not only dependent on the chloride concentration gradient that develops by the late proximal tubule but also the permeability properties of the paracellular pathway. We have recently compared the rates of volume absorption in neonatal and adult proximal straight tubules (PST) perfused with a high-chloride solution simulating late proximal tubular fluid and bathed in a serumlike albumin solution (28, 29). The rate of volume absorption was higher in adult PST than in neonatal tubules (28, 29). The rate of volume absorption in adult PST was also higher when active transport was inhibited with bath ouabain (28, 29). The higher rate of passive volume absorption in adult PST than in neonatal tubules was consistent with a maturational change in the para-cellular pathway that may affect net chloride transport in this segment. The purpose of the present study was to directly determine whether there are developmental changes in the PST paracellular pathway that impact passive chloride transport.
METHODS
Animals
Pregnant New Zealand White rabbits arrived at our institution at least 4 days before their expected date of delivery. Neonatal rabbits were cared for by their mothers and were studied between 17 and 22 days of age. Adult animals were at least 8 wk of age.
In vitro microperfusion flux studies
Isolated segments of superficial PST were perfused as previously described (4, 5, 28, 29). Briefly, tubules were dissected in Hanks’ balanced salt solution containing (in mM) 137 NaCl, 5 KCl, 0.8 MgSO4, 0.33 Na2HPO4, 0.44 KH2PO4, 1 MgCl2, 10 Tris, 0.25 CaCl2, 2 glutamine, and 2 L-lactate at 4°C. Tubules were transferred to a 1.2-ml temperature-controlled bath. The tubules were perfused using concentric glass pipettes.
PST were perfused at ~10 nl/min. For flux studies, the bathing solution was a serumlike albumin solution containing (in mM) 115 NaCl, 25 NaHCO3, 2.3 Na2HPO4, 10 Na acetate, 1.8 mM CaCl2, 1 MgSO4, 5 KCl, 8.3 glucose, and 5 alanine as well as 6 g/dl bovine serum albumin. The osmolality of these solutions were adjusted to 295 mosmol/kgH2O. The pH and osmolality of the bathing solution were maintained constant by continuously changing the bath at a rate of 0.5 ml/min. Net volume absorption (JV; in nl·mm−1 · min−1) was measured as the difference between the perfusion (V0) and collection (VL) rates (nl/min) normalized per millimeter of tubular length (L). Exhaustively dialyzed [methoxy-3H]inulin was added to the perfusate at a concentration of 75 μCi/ml so that the perfusion rate could be calculated. The collection rate was measured with a 50-nl constant-volume pipette. The length (in mm) and internal diameter (in μm) were measured with an eyepiece micrometer. Tubules were incubated for at least 30 min before initiation of the control period.
The transepithelial potential difference (PD; in mV) was measured using the perfusion pipette as the bridge into the tubular lumen. The perfusion and bath solutions were connected to the recording and reference calomel half-cells via bridges containing perfusion and an ultrafiltrate of the bathing solution, respectively, in series with 3.6 M KCl/0.9 M KNO3-agarose bridges. This arrangement avoided direct contact of KCl/KNO3-agarose bridges with the solution that bathed the tubule. The recording and reference calomel half-cells were connected to the high- and low-impedance side, respectively, of an electrometer (model 602; Keithley Instruments, Cleveland, OH). The reported transepithelial PD values were corrected for the measured liquid junction potentials.
Measurement of neonatal superficial proximal convoluted tubule transport
In the first series of experiments, we examined whether the superficial proximal convoluted tubule (PCT) could generate a higher luminal chloride than the glomerular ultrafiltrate or the blood as in the adult (18, 25). We perfused outer superficial and surface neonatal PCT with an ultrafiltrate-like solution containing (in mM) 115 NaCl, 25 NaHCO3, 2.3 Na2HPO4, 10 Na acetate, 1.8 mM CaCl2, 1 MgSO4, 5 KCl, 8.3 glucose, and 5 alanine at 5 nl/min. The chloride concentration in the initial luminal and collected fluid were measured using the microtitration method of Ramsay (24), and net chloride flux was determined.
Measurement of neonatal and adult chloride permeability and transport in PST
In the second series of experiments, we examined net chloride flux and chloride permeability in PST from neonatal and adult rabbits. Tubules were perfused with a high-chloride solution simulating late proximal tubular fluid containing (in mM) 140 NaCl, 5 NaHCO3, 5 KCl, 4 Na2HPO4, 1 CaCl2, and 1 MgSO4. The bath chamber was cooled to 20°C to inhibit active transport (5).
The chloride concentrations were measured to determine the net passive chloride flux and chloride permeability. Net passive chloride flux was determined using the following equation
where V0 and VL are perfusion and collection rates, respectively; C0 and CL are perfusion and collection concentrations; and L is tubular length.
From the passive chloride flux, the chloride permeability was calculated using the following equation (1)
where DFCl, the driving force for Cl, is defined as
C̄L = (C0 + CL)/2 is the mean luminal chloride concentration, and C̄ = (CL + CB)/2 is the mean chloride concentration, where CB is the bath chloride concentration. ΔV is the transepithelial PD, F is the Faraday constant, T is the temperature (in Kelvin), and R is the gas constant. Active transport (Jact) was zero in these studies because tubules were cooled to 20°C.
There were at least three measurements of each parameter in each period. The mean rate of volume absorption or measurement of solute flux was used as the rate for that tubule. Student’s t-test was used to determine statistical significance.
Measurement of mannitol and sucrose permeability in neonatal and adult PST
In the next series of experiments, we measured mannitol and sucrose permeabilities (Pmann) and (Psucrose) in neonatal and adult PST. PST were perfused with a solution containing (in mM) 10 mannitol (or 10 sucrose), 110 NaCl, 30 Na gluconate, 5 NaHCO3, 5 KCl, 1 Na2HPO4, 1.8 CaCl2, 1 MgSO4, 1 acetazolamide, and 25 μCi/ml [14C]mannitol (or [14C]sucrose). Tubules were bathed in a serumlike albumin solution at 37°C. We previously showed that under these conditions the rate of volume absorption was not different from zero (23, 27).
Pmann was calculated using the equation below (23, 27)
where M0 is the concentration of mannitol in the perfusate, and and are the concentration of mannitol (in counts·min−1 ·nl−1) in the perfusate and collected fluid, respectively. Pmann was normalized per millimeter tubule length and for the tubular radius. Psucrose was measured in adult and neonatal PST in the exact same fashion as was Pmann. In the experiments where Psucrose was determined, perfused and collected bicarbonate were measured so that the bicarbonate permeability could be determined using the same formulas as that for chloride. Bicarbonate was measured using a picapnotherm (WPI, New Haven, CT).
Measurement of sodium-to-chloride and bicarbonate-to-chloride permeability ratios
In the next series of experiments, we measured the relative sodium-to-chloride permeability (PNa/PCl) and bicarbonate-to-chloride permeability ratios (PHCO3/PCl) in neonatal and adult PST. Tubules were perfused with the ultrafiltrate-like solution shown in Table 1 and bathed in an ultrafiltrate-like solution at 37°C. PNa/PCl and PHCO3/PCl were calculated from the passive transepithelial PD due to ion concentration gradients as previously described (6, 13, 31). After incubation with the ultrafiltrate-like solution, the bathing solution was changed to the NaCl dilution solution, and the transepithelial PD was measured. The bathing solution was then changed to a sodium bicarbonate dilution solution, and the transepithelial PD was measured. All bathing solutions contained 10−5 M ouabain to inhibit any transepithelial PD generated from active transport. The composition of the bathing and luminal solutions are shown in Table 1. The transepithelial PD was corrected for the small liquid junction potentials due to asymmetrical luminal and bath solutions as described by Berry and Rector (7).
Table 1.
Solutions used to measure PNa/PCl and PHCO3/PCl
| Ultrafiltrate-Like Solution | NaCl Dilution, mM | NaHCO3 Dilution, mM | |
|---|---|---|---|
| NaCl | 104 | 54 | 104 |
| NaHCO3 | 25 | 25 | 5 |
| Na2HPO4 | 4 | 4 | 4 |
| Na acetate | 7.5 | 7.5 | 7.5 |
| CaCl2 | 1 | 1 | 1 |
| MgSO4 | 1 | 1 | 1 |
| KCl | 5 | 5 | 5 |
| Glucose | 5 | 5 | 5 |
| Alanine | 5 | 5 | 5 |
| Urea | 5 | 5 | 5 |
| Mannitol | * | * |
All solutions were bubbled with a CO2–O2 gas mixture to give an average bath PCO2 of 40 mmHg; the osmolality was 295 mosmol/ kgH2O in all solutions. *Mannitol was added to increase the osmolality to 295 mosmol/kgH2O. PNa/PCl and PHCO3/PCl, sodium-to-chloride and bicarbonate-to-chloride permeability ratio, respectively.
PNa/PCl and PHCO3/PCl were calculated from the transepithelial PD values generated from the imposed ion concentration gradients using the Goldman equation (12)
In the above equation, l and b refer to the luminal and bath ion concentrations, respectively.
Measurement of transepithelial resistance
The specific resistance (Rm) was measured as described by Berry (6). Briefly, PST from adult and neonatal New Zealand White rabbits were perfused and bathed with Hanks’ solution at 37°C. Tubules were perfused with a double-barreled pipette made from theta glass (Hilgenberg Glass, Hilgenberg, Germany). One barrel of the pipette was used to measure the PD at the perfusion end (PD0), while the other barrel was used to pass current (30–60 nA) using a Grass S44 stimulator (Grass Instruments, Quincy, MA) via silver wire. The current injected was measured with a Keithley 617 programmable electrometer (Keithley Instruments, Cleveland, OH). A KCl/ KNO3-agarose bridge was also placed in the collecting end to measure the voltage deflection at the distal end of the tubule (PDL). The potential difference measurements were recorded on a Linseis, L6512B (Linseis, Princeton, NJ) two-channel chart recorder. The coupling resistance was determined by measuring the voltage deflections at the perfusion and collection ends with no tubule present. These voltage deflections were subtracted from the readings with the perfused tubule in place and are represented in the equations below by ΔPD0 and ΔPDL for the corrected voltage deflection at the perfusion and collection ends, respectively.
Cable analysis (6, 17, 19) was used to calculate the length constant (λ; μm), input resistance (Ri; Ω), transepithelial resistance (RT; Ω·cm), and Rm (Ω·cm2) according to the following equations
where L is the tubule length, ΔPD0 and ΔPDL are the corrected voltage deflections at the perfusion and collection ends, respectively; I0 is the input current; and ρ is the solution resistivity. Measured with a YSI conductance meter, ρ was found to be 63.3 Ω·cm at 37°C (Yellow Springs Instruments, Yellow Springs, OH).
RESULTS
The first series of experiments was designed to determine whether the neonatal superficial PCT could generate a chloride gradient by preferentially absorbing solutes over chloride ions, as has been determined in the adult nephron (18, 25). The average tubule length was 1.1 ± 0.1 mm. Neonatal superficial PCT (n = 5) perfused with an ultrafiltrate-like solution absorbed fluid at a rate of 0.48 ± 0.08 nl/mm and had a transepithelial PD of −2.0 ± 0.6 mV. The initial chloride concentration in the perfusate was 111.4 ± 1.3 meq/l and rose to 122.0 ± 2.2 meq/l in the collected fluid (P < 0.01). Despite the high rate of volume absorption, there was virtually no chloride transport (5.9 ± 8.3 pmol·mm−1 ·min−1). Thus the superficial proximal tubule has preferential absorption of solutes over chloride and delivers a solution with a higher luminal chloride than the ultrafiltrate to the PST.
We have previously shown that the rate of volume absorption in neonatal PST perfused with a high chloride solution simulating late proximal tubule fluid was less than that in the adult (28, 29). We also perfused PST with an ultrafiltrate-like solution to determine whether there was a maturational change in transport from an ultrafiltrate-like solution. Adult PST (n = 6) had a rate of volume absorption of 0.44 ± 0.13 nl·mm−1 ·min−1 compared with −0.02 ± 0.03 nl·mm−1 ·min−1 in neonatal PST (P < 0.05). Thus the rate of volume absorption in the neonatal PST is less than that of the adult from an ultrafiltrate and late proximal tubule solution.
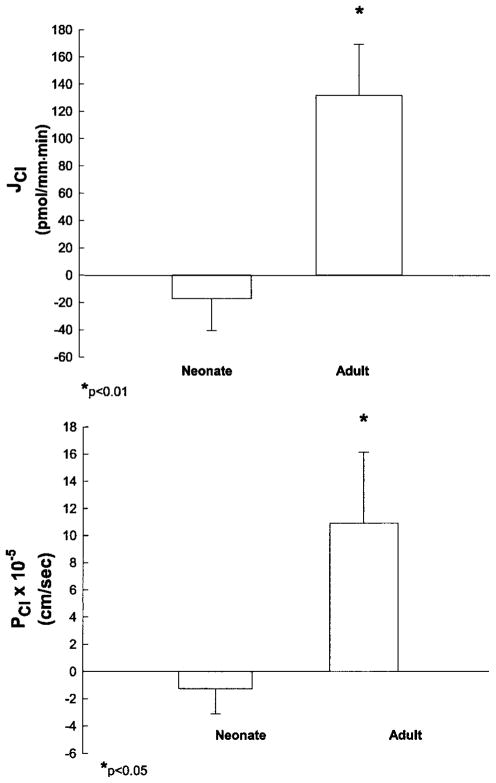
The next series of experiments was designed to examine whether there was a maturational change in chloride permeability and passive chloride flux in the PST. PST were perfused with a high chloride solution simulating late proximal tubular fluid and bathed in a serumlike albumin solution at 20°C to inhibit active transport. The rate of volume absorption in neonatal PST was 0.08 ± 0.05 nl·mm−1 ·min−1, which was less than the 0.39 ± 0.09 nl·mm−1 ·min−1 measured in adult proximal tubules (P = 0.01). The transepithelial PD was 0.7 ± 0.5 mV in neonatal PST and 3.7 ± 0.3 mV in adult tubules (P < 0.001). The developmental changes in chloride flux and chloride permeability are shown in Fig. 1. Net chloride flux was −17.1 ± 23.3 pmol·mm−1 ·min−1 in neonatal proximal tubules and 131.7 ± 37.7 pmol·mm−1 ·min−1 in adult tubules (P < 0.01). Chloride permeability was −1.26 ± 1.84 × 10−5 cm/s in neonatal proximal tubules and 10.94 ± 5.21 × 10−5 cm/s in adult proximal tubules (P < 0.05). Thus neonatal PST have a chloride permeability not different from zero and have no net passive chloride transport.
Fig. 1.
Passive chloride transport in isolated perfused adult and neonatal proximal straight tubules (PST). PST were perfused in vitro at 20°C to inhibit active transport. Net chloride transport (JCl; top) was measured using the microtitration technique of Ramsay et al. (24). Inulin was used as a volume marker (n = 7 for both groups). PCl, chloride permeability.
We also compared bicarbonate permeability in neonatal and adult PST. Tubules were perfused with a solution containing a bicarbonate concentration of 5 meq/l while there was 25 meq/l bicarbonate in the bathing solution. Bicarbonate permeability was 0.93 ± 0.27 × 10−5 cm/s in adult PST (n = 6) and −0.07 × 10−5 in neonatal PST (n = 5; P < 0.01). Thus bicarbonate permeability is also higher in the adult than in the neonatal PST.
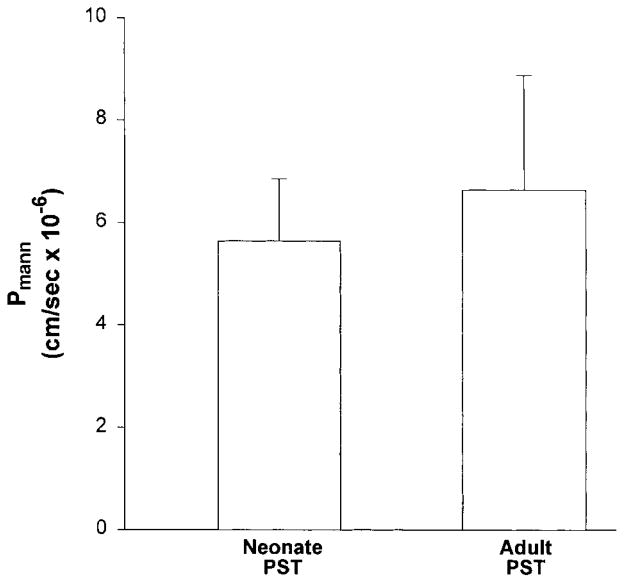
In the next series of experiments, we examined whether there was a developmental change in Pmann. In these experiments, tubules were perfused with a solution that results in no net volume flux (23, 27). The rate of volume absorption in neonatal and adult proximal tubules was −0.12 ± 0.05 and −0.03 ± 0.05 nl·mm−1 ·min−1, respectively (P = NS). Mannitol is a sugar that is not actively transported and is not metabolized. The results of these experiments are shown in Fig. 2. Pmann was 6.63 ± 2.23 × 10−6 in adult proximal tubules and 5.64 ± 1.21 × 10−6 cm/s in neonatal proximal tubules. Similarly, Psucrose was 4.22 ± 1.11 × 10−6 in adult PST and 4.20 ± 1.50 × 10−6 cm/s in neonatal PST (n = 6 for both). Thus there were no differences in Pmann and Psucrose between neonatal and adult PST.
Fig. 2.
Mannitol permeability (Pmann) measured in neonatal and adult isolated perfused PST using luminal [14C]mannitol (n = 9 for both groups).
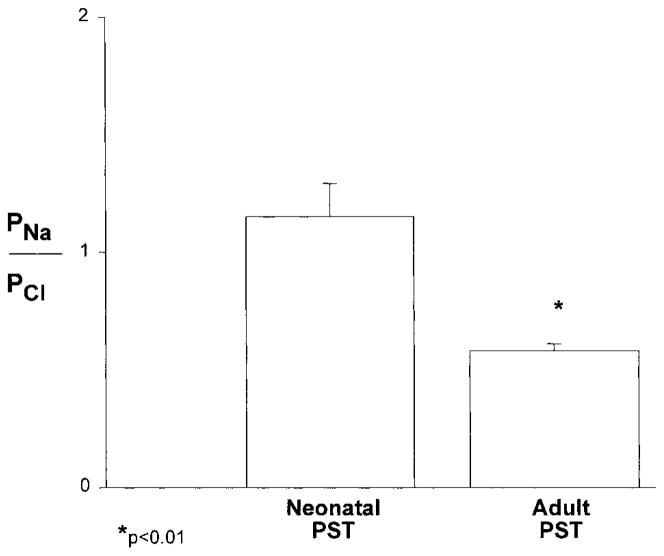
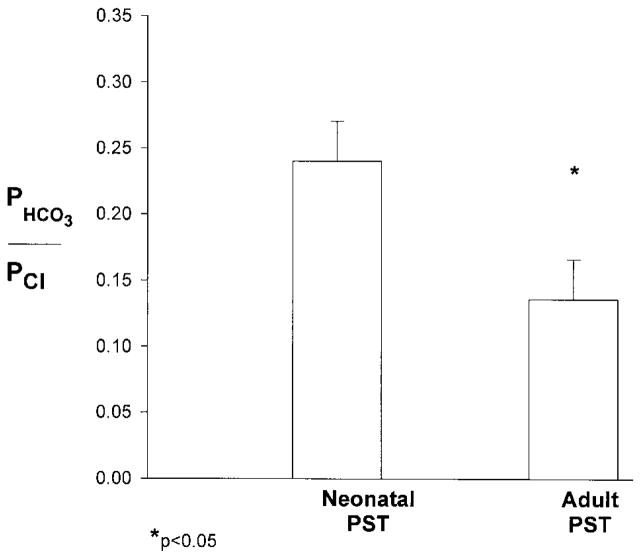
The next series of experiments were designed to determine the relative PST passive permeability of sodium and bicarbonate compared with chloride ions. The results of these experiments are shown in Figs. 3 and 4. As can be seen, both PNa/PCl and PHCO3/PCl were significantly lower in adult than in neonatal PST. Adult proximal tubules were more permeable to chloride than to sodium ions, whereas the relative permeability of the neonatal PST was not different from one. In both the neonatal and adult PST, chloride was more permeable than bicarbonate ions. However, PHCO3/PCl was greater in the neonate tubules.
Fig. 3.
Relative sodium-to-chloride permeability ratio (PNa/PCl) measured at 37°C using dilution potentials in neonatal (n = 6) and adult (n = 5) isolated perfused PST.
Fig. 4.
Relative bicarbonate-to-chloride permeability ratio (PHCO3/PCl) measured at 37°C using dilution potentials in neonatal (n = 6) and adult (n = 5) isolated perfused PST.
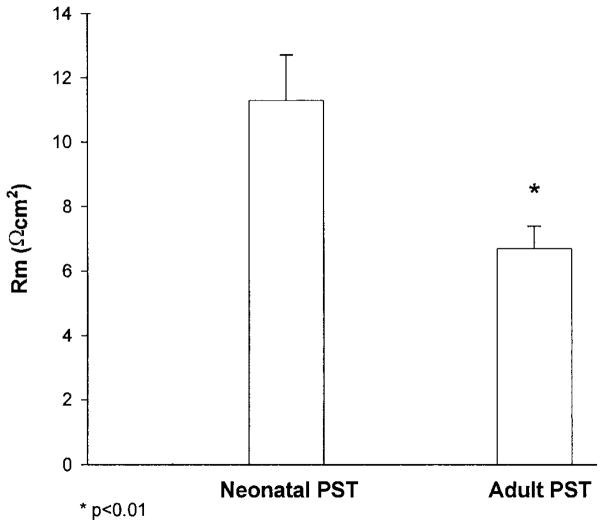
In the final series of experiments, we examined whether there was a maturational change in transepithelial resistance. These results are shown in Fig. 5. The transepithelial resistance was 6.7 ± 0.7 Ω·cm2 in adult tubules and 11.3 ± 1.4 Ω·cm2 in neonatal PST, respectively (P < 0.01). Thus there is a maturational decrease in transepithelial resistance in this segment.
Fig. 5.
Neonatal and adult PST specific resistance (Rm) measured at 37°C using current injection and cable analysis (n = 8 neonatal PST and 7 adult PST).
DISCUSSION
The present study examined the passive permeability properties of the neonatal PST. Compared with adult PST, neonatal PST have a lower rate of passive chloride transport due to a lower permeability of the PST to chloride ions. Bicarbonate permeability was also less in the neonatal PST than the adult PST. PNa/PCl and PHCO3/PCl were both greater in neonatal than in adult PST. The transepithelial resistance was significantly higher in neonatal PST than in adult PST. However, there were no differences in the Pmann and Psucrose between neonatal and adult PST.
As with the mature kidney, the neonatal kidney must reclaim the vast majority of the filtered chloride. We have recently examined the relative rates of volume absorption from a high-chloride perfusate simulating late proximal tubular fluid (28, 29). The rate of volume absorption was greater in adult tubules in the presence and absence of active transport (28, 29). Active NaCl transport in this segment is mediated by the parallel operations of luminal sodium/hydrogen and chloride/base exchangers in both the adult and neonatal segments (28). There was an approximately fivefold increase in the rate of both sodium/hydrogen and chloride/ base exchanger activity during postnatal development of the rabbit PST, which accounts for the maturational increase in active NaCl transport in this segment. The present study directly demonstrates that the lower rate of passive volume absorption in neonatal tubules was due to lower chloride permeability, resulting in a lower rate of passive paracellular chloride transport.
Maturational changes in passive transport have received little attention. In a previous study, Kaskel et al. (15) injected sucrose, creatinine, and mannitol into early proximal tubules of neonatal and adult guinea pigs and measured the urinary recovery from the ipsilateral kidney. While urinary recovery of sucrose and creatinine did not vary with age and was comparable to inulin, mannitol recovery increased from 92% at 1 day of age to 100% at 49 days of age. While these studies were indirect and assessed mannitol absorption along the entire nephron, these data were consistent with a maturational decrease in paracellular pathway permeability to small molecules such as mannitol. In addition, Horster and Larsson (14) found that there was passage of microperoxidase into the intercellular spaces of rabbit proximal tubules perfused under a transtubular hydrostatic pressure of 20 cmH2O. However, this study did not find differences in the appearance in the lateral cellular spaces and tight junctions between neonatal and adult rabbit proximal tubules when they were examined using electron microscopy.
Our data seem to be at variance with these previous findings. We found that the neonate had a lower permeability to chloride and bicarbonate and a higher transepithelial resistance. There was no difference in PST Pmann and Psucrose in our study. The difference between our findings and previous studies may reflect a difference in techniques used to assess paracellular permeability. It is also possible that the maturational changes in permeability predominantly affect anions such as chloride and bicarbonate. However, it should be noted that the present study directly measured chloride and bicarbonate permeabilities and PST resistance showing a maturational increase in chloride and bicarbonate permeabilities and decrease in resistance in the PST.
Compared with the distal nephron, the proximal tubule is a low-resistance epithelium. In this study, we used current injection and cable analysis to determine the relative resistance of the neonatal and adult proximal tubule using methods comparable to those previously described (6, 17, 19). We found that the resistance of the PST was higher in neonatal PST than in adult PST, consistent with a maturational change in the properties of the paracellular pathway. The reason there was not an inverse change in the Pmann and Psucrose and resistance during development is unclear.
The preferential reabsorption of bicarbonate over chloride ions by the early proximal tubule results in an ion gradient favoring chloride absorption and bicarbonate secretion. The relative rates of chloride absorption to bicarbonate secretion are dependent on the PHCO3/ PCl of the PST. The lumen positive PD in tubules perfused with a high-chloride-low-bicarbonate perfusate simulating late proximal tubular fluid is consistent with a PHCO3/PCl of less than one, which was measured in both adult and neonatal PST. The magnitude of the lumen positive transepithelial PD was greater in adult tubules and was consistent with a lower PHCO3/PCl in the mature tubule than in that of the neonate. There was a maturational decrease in PHCO3/PCl that was a reflection of the change in properties of the paracellular pathway. The increase in chloride permeability results in a developmental increase in passive chloride transport.
The dilution potential measurement of PHCO3/PCl measures the relative permeabilities and not their actual value. The measured chloride permeability in the neonate was slightly negative, and it might be questioned how a permeability could be negative. Direct measurement of chloride permeability, while negative, was not different from zero in the neonatal PST, which was very different from the high permeability in the adult PST.
We also examined whether there was a maturational change in the relative PNa/PCl during development. While adult proximal tubules have a greater chloride than sodium permeability, this was not true of neonatal tubules. As speculation, these data suggest that there may be maturational changes in the paracellular pathway that result in greater anion/cation permeability as the tubule matures.
The changes in the permeability properties of the paracellular pathway are likely mediated by a change in the composition of tight junctional proteins. The tight junction creates the primary permeability barrier to diffusion of solutes across the paracellular pathway. Recently, several protein components of the tight junction have been identified and characterized (2, 8, 21). Of potential relevance are occludin and claudin proteins, which are localized to junctional fibrils and are the transmembrane component of tight junctions (9–11, 20, 26, 30). Both occludin and claudins have four transmembrane domains and two extracellular loops, which form a seal at the tight junction between neighboring cells (9–11, 20, 26, 30). Unlike occludin, there are several claudin isoforms (9, 11, 22, 30). Different claudin isoforms have been found to have different tissue distributions (22). It is possible that the permeability changes seen during postnatal maturation are due to developmental changes in the abundance of occludin or claudin or a maturational change in the claudin isoforms that constitute the tight junctional strands. As speculation, the higher resistance and lower permeability of chloride ions in neonatal PST compared with adults could be due to a maturational decrease in a claudin with net negative charges on the extracellular loops.
Acknowledgments
We are grateful for the secretarial assistance of Janell McQuinn. We also acknowledge the assistance of Drs. Al Rouch and Jim Shafer in establishing the methodology to measure transepithelial resistance in our laboratory.
This work was supported by National Institute of Diabetes and Digestive and Kidney Disease Grant DK-41612 (to M. Baum).
References
- 1.Alpern RJ, Howlin KJ, Preisig PA. Active and passive components of chloride transport in the rat proximal convoluted tubule. J Clin Invest. 1985;76:1360–1366. doi: 10.1172/JCI112111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol Gastrointest Liver Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 3.Aronson PS, Giebisch G. Mechanisms of chloride transport in the proximal tubule. Am J Physiol Renal Physiol. 1997;273:F179–F192. doi: 10.1152/ajprenal.1997.273.2.F179. [DOI] [PubMed] [Google Scholar]
- 4.Baum M, Berry CA. Evidence for neutral transcellular NaCl transport and neutral basolateral chloride exit in the rabbit proximal tubule. J Clin Invest. 1984;74:205–211. doi: 10.1172/JCI111403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baum M, Berry CA. Peritubular protein modulates neutral active NaCl transport in the rabbit proximal convoluted tubule. Am J Physiol Renal Fluid Electrolyte Physiol. 1985;248:F790–F795. doi: 10.1152/ajprenal.1985.248.6.F790. [DOI] [PubMed] [Google Scholar]
- 6.Berry CA. Lack of effect of peritubular protein on passive NaCl transport in the rabbit proximal tubule. J Clin Invest. 1983;71:268–281. doi: 10.1172/JCI110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry CA, Rector FC. Relative sodium-to-chloride permeability in the proximal convoluted tubule. Am J Physiol Renal Fluid Electrolyte Physiol. 1978;235:F592–F604. doi: 10.1152/ajprenal.1978.235.6.F592. [DOI] [PubMed] [Google Scholar]
- 8.Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol Renal Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 9.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and −2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or −2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldman DE. Potential, impedance and rectification in membranes. J Gen Physiol. 1943;27:37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmberg C, Kokko JP, Jacobson HR. Determination of chloride and bicarbonate permeabilities in proximal convoluted tubules. Am J Physiol Renal Fluid Electrolyte Physiol. 1981;241:F386–F394. doi: 10.1152/ajprenal.1981.241.4.F386. [DOI] [PubMed] [Google Scholar]
- 14.Horster M, Larsson L. Mechanisms of fluid absorption during proximal tubule development. Kidney Int. 1976;10:348–363. doi: 10.1038/ki.1976.121. [DOI] [PubMed] [Google Scholar]
- 15.Kaskel FJ, Kumar AM, Lockhart EA, Evan A, Spitzer A. Factors affecting proximal tubular reabsorption during development. Am J Physiol Renal Fluid Electrolyte Physiol. 1987;252:F188–F197. doi: 10.1152/ajprenal.1987.252.1.F188. [DOI] [PubMed] [Google Scholar]
- 16.Kokko JP, Baum M. Handbook of Physiology. I. Bethesda, MD: Am Physiol Soc; 1992. Chloride transport; pp. 739–766. sect. 8. chapt. 17. [Google Scholar]
- 17.Lapointe JY, Laprade R, Cardinal J. Transepithelial and cell membrane electrical resistances of the rabbit proximal convoluted tubule. Am J Physiol Renal Fluid Electrolyte Physiol. 1984;247:F637–F649. doi: 10.1152/ajprenal.1984.247.4.F637. [DOI] [PubMed] [Google Scholar]
- 18.Liu FY, Cogan MG. Axial heterogeneity in the rat proximal convoluted tubule. I. Bicarbonate, chloride, and water transport. Am J Physiol Renal Fluid Electrolyte Physiol. 1984;247:F816–F821. doi: 10.1152/ajprenal.1984.247.5.F816. [DOI] [PubMed] [Google Scholar]
- 19.Lutz MD, Cardinal J, Burg MB. Electrical resistance of renal proximal tubule perfused in vitro. Am J Physiol. 1973;225:729–734. doi: 10.1152/ajplegacy.1973.225.3.729. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Science. 1996;109:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- 21.Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- 22.Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA. 1999;96:511–515. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quigley R, Baum M. Effects of epidermal growth factor and transforming growth factor-α on rabbit proximal tubule solute transport. Am J Physiol Renal Fluid Electrolyte Physiol. 1994;266:F459–F465. doi: 10.1152/ajprenal.1994.266.3.F459. [DOI] [PubMed] [Google Scholar]
- 24.Ramsay JA, Brown RH, Croghan PC. Electrometric titration of chloride in small volumes. J Exp Biol. 1955;32:822–829. [Google Scholar]
- 25.Rector FC., Jr Sodium, bicarbonate, and chloride absorption by the proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol. 1983;244:F461–F471. doi: 10.1152/ajprenal.1983.244.5.F461. [DOI] [PubMed] [Google Scholar]
- 26.Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto R, Furuse M, Takano H, Noda T, Tsukita S. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salmon RF, Baum M. Intracellular cystine loading inhibits transport in the rabbit proximal convoluted tubule. J Clin Invest. 1990;85:340–344. doi: 10.1172/JCI114443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah M, Quigley R, Baum M. Maturation of rabbit proximal straight tubule chloride/base exchange. Am J Physiol Renal Physiol. 1998;274:F883–F888. doi: 10.1152/ajprenal.1998.274.5.F883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah M, Quigley R, Baum M. Maturation of proximal straight tubule NaCl transport: role of thyroid hormone. Am J Physiol Renal Physiol. 2000;278:F596–F602. doi: 10.1152/ajprenal.2000.278.4.F596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 31.Warnock DG, Yee VJ. Anion permeabilities of the isolated perfused rabbit proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol. 1982;242:F395–F405. doi: 10.1152/ajprenal.1982.242.4.F395. [DOI] [PubMed] [Google Scholar]