Abstract
The aim of the present study was at evaluating the effects of oxidative stress in blood and placenta of mild diabetic Wistar rats. At birth, Wistar rats received citrate buffer (nondiabetic group, n = 15) and another group received streptozotocin (100 mg/kg, subcutaneous) to induce mild diabetes (diabetic, n = 15). The glycemia of these pregnant adult female rats were evaluated at days 0, 7, 14, and 21 of pregnancy, and at term pregnancy, the blood and placental samples were collected for oxidative stress measurements. The mild diabetes caused glycemia superior to 120 mg/dL during pregnancy, increased superoxide dismutase, glutathione peroxidase, glutathione reductase activities, and malondialdehyde levels in the blood, and catalase activity in the placenta. Thus, mild diabetes increased activities of antioxidant substances aiming at defending against the exacerbated oxidative stress but were not enough. The placenta also answered to diabetic milieu and increased antioxidant defense, showing that even a mild hyperglycemia was enough to cause placental and maternal blood changes.
Keywords: streptozotocin, rats, diabetes, oxidative stress, placenta
Introduction
Diabetes mellitus (DM) is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion and action, or both. The chronic hyperglycemia of diabetes is associated with long-term damage, dysfunction, and failure of different organs.1 There is strong evidence that diabetes/hyperglycemia results a state of oxidative stress and that reactive oxygen species (ROS) contribute to the production of insulin resistance, beta-cell dysfunction,2 and both the microvascular and macrovascular complications.3 Literature has showed that there is a change in the pro-oxidant and antioxidant defenses associated with body and circulation changes that are inherent to the pregnancy process.4 In physiologic state, the gestation increases peroxide products in a proportional way to the mechanisms of antioxidant defense.5 But, in the end, the antioxidant agents overcome the oxidative processes. In the hyperglycemic environment, glycated proteins show necessarily a different functionality, and consequently their glycation level can give account for the long-term diabetic complications.6
The placenta is a complex fetal organ that fulfills pleiotropic roles during fetal growth. It separates the maternal and fetal circulation, and it plays an important role in the protection of the fetus from the adverse effects of diabetic maternal environment.7 The placenta generates ROS that may contribute to the oxidative stress seen even in normal pregnancy, but this is increased in pregnancies complicated by preeclampsia, intrauterine growth restriction and pregestational diabetes where oxidative and nitrative stress have been clearly documented.8 Previous studies in women with gestational diabetes show the presence of oxidative stress as well as in their respective placentas as a result of the reduction in the antioxidant defense mechanism and increased ROS production.9 Besides, the placenta seems particularly vulnerable to the oxidative stress due to its extensive cellular division and high metabolic activity10 and is thought to play an important role in the development of many pregnancy complications.11
Our research group has developed several investigations using laboratory animals to demonstrate the repercussions of the severe diabetes (glycemia superior to 300 mg/dL) on the pregnancy and fetal development.12–15 In contrast, there are no studies evaluating mild diabetes-induced placental effects. Therefore, the aim of this study was at evaluating the effects of oxidative stress status in maternal blood and in the placentas of mild diabetic rats and investigating superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), glutathione reductase (GSH-Rd) activities, and malondialdehyde (MDA) levels at term pregnancy.
Materials and Methods
Animals and Experimental Groups
Female and male Wistar rats weighing approximately 190 and 220 g, respectively, were obtained from Univ Estadual Paulista_UNESP, São Paulo State, Brazil. These animals were adapted and maintained in Vivarium of Laboratory of Experimental Research on Gynecology and Obstetrics, Unesp, and were maintained under standard laboratory conditions (22°C ± 2°C, 12-hour light–dark cycle), with pelleted food (Purina rat chow, Purina, São Paulo State, Brazil) and tap water ad libitum. The local Committee of Ethics in Animal Experimentation approved all experimental procedures of this study.
Diabetes Induction
Nondiabetic female rats were mated with nondiabetic males. To induce mild diabetes, at birth (day 0), their female offspring were subcutaneously injected with 100 mg/kg streptozotocin (STZ; Sigma Chemical Company, Millstone, St Louis, Missouri) dissolved in citrate buffer (0.1 mol/L, pH 4.5).16 The nondiabetic group received only subcutaneous citrate buffer in the similar period. All pups remained with their mothers until reaching adulthood (day 21 postnatal).
Mating Period
All adult females (nondiabetic and diabetic) were mated overnight with nondiabetic males. The morning when sperm was found in the vaginal smear was designated as day 0 of pregnancy. The mating protocol was followed for 15 consecutive days (approximately 3 estrous cycles). The females that failed to become pregnant during this period were considered infertile and excluded from the study.17 At day 0 of pregnancy, the rats from the diabetic group presenting glycemia from 120 to 300 mg/dL were included in the mild diabetic group and for the nondiabetic group, the rats presenting glycemia <120 mg/dL were included.16
Pregnancy Period
Glycemia
During pregnancy, all female rats were maintained in individual cages. In the mornings of days 0, 7, 14, and 21, glycemia was determined with a specific glucosemeter (One Touch Ultra, Johnson & Johnson Medical, Brasil), and values were expressed in milligrams per deciliter (mg/dL).
Measurements of maternal blood and placental samples
At day 21 of pregnancy, the dams (n = 15/group) were anesthetized with sodium pentobarbital (Hypnol 3%) to collect blood and placental samples for oxidative stress evaluation.
Assay for oxidative stress status in maternal blood samples
Maternal blood samples collected in tubes with anticoagulant were centrifuged at 90g for 10 minutes at 4°C. The supernatant was discarded and the erythrocytes were washed with phosphate buffer saline (0.01 mol/L, pH 7.4) followed by centrifugation at 263g for 1 minute at 4°C. This procedure was repeated 3 times and final infranatants were used for the measurement of GSH-Rd, SOD, and GSH-Px activities, and MDA concentration. The GSH-Px and SOD activities and MDA levels were determined by de Souza et al (but modified).14 The GSH-Rd activity was performed by monitoring the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) oxidation. The mixture consisted of the addition of 780 µL of distilled water, 50 µL of Tris-HCl buffer (1 mol/L; EDTA 5 mmol/L; pH 8.0), 100 µL of GSSH (33 mmol/L, Sigma Chemical Company, Millstone, St Louis, Missouri) and 20 µL of the hemolysate. The mixture was agitated in a vortex mixer for 10 seconds. Next, 50 µL of NADPH (2 mmol/L) was added and maintained at 37°C for 10 minutes. Absorbance was determined by a spectrophotometer with a wavelength of 340 nm. The GSH-Rd activity was expressed in enzymatic activity units per gram of hemoglobin (IU/g Hb).
Assay for oxidative stress status in placental samples
The placentas were collected, weighed, and washed in physiological buffer solution (PBS 0.1 mol/L) to reduce the presence of blood, and they were maintained in freezer at −80°C. At the moment of analysis, placentas were thawed, perforated, and triturated in a homogenizer (MPV 306; Marconi, Piracicaba, São Paulo - Brasil) with PBS (0.1 mol/L, pH 7.4) for 1 minute. The mixture was centrifuged at 9000g for 10 minutes at 4°C for obtaining the supernatant and were used to measure MDA concentration18 and SOD19,20 and catalase enzymatic activities.20
Statistics
Data were expressed as mean and standard error of the mean (SEM). Statistical comparisons were made using Student t test. P < .05 was considered as statistical significance limit.
Results
The diabetic group presented blood glucose levels superior to 120 mg/dL on day 0 of pregnancy (P < .05). At days 7 and 21 of pregnancy, the diabetic dams presented no glycemic changes. It was observed an increased blood glucose level at day 14 of pregnancy in diabetic dams (P < .05) as compared to nondiabetic group (Figure 1).
Figure 1.
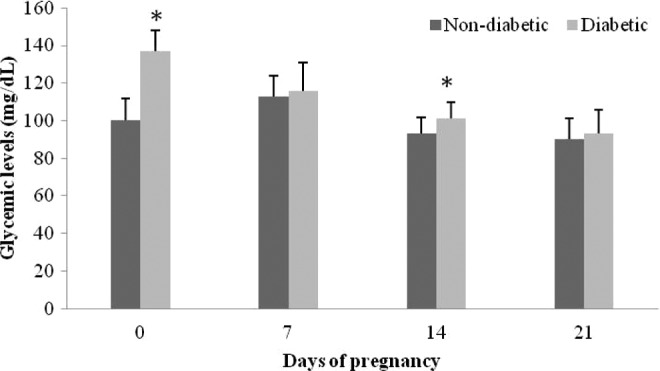
Blood glucose levels at days 0, 7, 14, and 21 of pregnancy in mild diabetic and nondiabetic rats. Data are reported as mean ± standard error of the mean (SEM). *P < .05 statistically significant difference (Student t test).
The SOD, GSH-Px, and GSH-Rd activities as well as MDA concentrations were increased in diabetic dams as compared to nondiabetic group (P < .05; Table 1).
Table 1.
Oxidative Stress Status in Blood Samples From Nondiabetic and Mild Diabetic Rats.a
| Nondiabetic | Diabetic | |
|---|---|---|
| (n = 15) | (n = 15) | |
| Malondialdehyde (MDA), nmol/g Hb | 103.36 ± 11.80 | 205.73 ± 43.73* |
| Superoxide dismutase (SOD), IU/mg Hb | 4.52 ± 1.19 | 9.23 ± 3.84* |
| Glutathione peroxidase (GSH-Px), IU/mg Hb | 0.64 ± 0.17 | 1.18 ± 0.54* |
| Glutathione reductase (GHS-Rd), IU/mg Hb | 2.79 ± 0.29 | 6.57 ± 1.50* |
a Data are reported as mean + standard error of the mean (SEM). * P < .05 statistically significant difference (Student t test).
In placental tissue, the mild diabetes caused no alteration in the MDA concentrations and SOD activity (P > .05). The catalase activity was statistically higher (P < .05) in placental tissue of mild diabetic group as compared to the nondiabetic group (Table 2).
Table 2.
Oxidative Stress Status in Placental Samples From Nondiabetic and Mild Diabetic Rats.
| Nondiabetic | Diabetic | |
|---|---|---|
| (n = 15) | (n = 15) | |
| Malondialdehyde (MDA), mol/L | 0.02 ± 0.00 | 0.01 ± 0.00 |
| Superoxide dismutase (SOD), IU/mg protein | 0.73 ± 0.05 | 0.70 ± 0.06 |
| Catalase, IU/mg protein | 0.34 ± 0.03 | 0.48 ± 0.04* |
* P < .05 statistically significant difference (Student t test).
Discussion
In the present study, the rats that received STZ in neonatal period presented increased glycemia on days 0 and 14 of pregnancy. These results corroborate with another experimental study performed by Sinzato et al,16 showing that this glycemia is very similar to that found in diabetes of the pregnant women. Similarly Desoye and Mouzon7 verified that pregnant woman with gestational diabetes presents hyperglycemia during 24 to 26 weeks of pregnancy, which is equivalent to day 14 of pregnancy in rats.
It was observed significant increases in SOD, GSH-Px, and GSH-Rd activities in mild diabetic dams. However, increased antioxidant defenses were not enough to control the elevated oxidative stress, as demonstrated by increased concentrations of MDA. It is known that the classic pathophysiological features of diabetes present a hyperglycemic state, causing a lipoperoxidation profile. Hyperglycemia causes tissue damaging and activation of protein kinase C isoforms through de novo synthesis of the lipid second messenger diacylglycerol end product formation, and also polyol pathway flux contributes for ROS concentration.21–23 Therefore, experimental mild diabetes may present metabolic alterations about oxidative stress increasing.24 In contrast, it already demonstrated no changes in oxidative stress profile from mild hyperglycemia.25 It have been suggested that there may be temporal changes in enzyme activity that are both transitory and biphasic in nature. For instance, after prolonged hyperglycemia in severe diabetes (glycemia superior to 300 mg/dL), the induction of certain antioxidant enzymes or a return to normal values from previously decreased values may occur as a compensatory mechanism to respond to the constant exposure to increased oxidative stress.26 This could explain the decrease, increase, or no alteration in the antioxidant enzymatic activities in different studies.
The placenta plays crucial role in pregnancy as the interface between mother and fetus. It anchors the conceptus, provides an interface for the exchange and modification of nutrients and gases, synthesizes and secretes a range of steroid and peptide hormones, and provided an immune barrier between the mother and the semi-allogeneic fetus.27 Measurements of oxidative stress markers in maternal blood and urine28 show that pregnancy per se is a state of oxidative stress due to the high metabolic activity of the placenta and maternal metabolism in pregnancy.27 Our results showed that mild diabetes caused no change in the SOD activity and MDA concentration in the placental tissue samples, however, the catalase activity was increased. Jones et al29 demonstrated that the catalase and SOD expressions were consistently higher in the labyrinth zone at term pregnancy of normoglycemic rat placenta. These authors suggested that this higher antioxidant capacity might cope with a higher metabolic activity in this zone at late gestation. Another study verified that placenta from gestational DM (GDM) is associated with increased messenger RNA expression of catalase and glutathione reductase.30 These results suggest a protective or adaptive mechanism to prevent hyperglycemic damage from further oxidative insult as indicated by increased placental antioxidant activity, such as in mild diabetic rats and GDM.
In contrast, some studies has shown lower levels of catalase activity in plasma and placental tissue during diabetic complicated pregnancy, this reduction is achieved by the inability of scavenge enzymes in lipoperoxidation processes.4 According to Choi et al,31 the divergence observed shows that an increased oxidative stress have no direct relation with acute hyperglycemia. Besides, this increase develops only in those individuals with compromised antioxidant defense capacity. Droge32 suggests that this is caused by the cellular adaptation mechanism due to glycemic imbalance or hyperglycemia associated with poor antioxidant status. ROS have been implicated in the activation of a variety of transcription factors. Among the various transcription factors, nuclear factor-κB (NF-κB) is one of the most important, whose activity can be influenced by oxidative stress and is recognized as central to regulating the expression of oxidative stress-responsive genes.33 NF-kB regulates the expression of multiple immune and inflammatory genes, including proinflammatory cytokines and the proteins and enzymes involved in ROS generation.34 Differently from the other studies that show in vivo studies and measuring oxidative stress markers, Coughlan et al35 observed that ex situ placental tissue from GDM in presence of superoxide generating xanthine/xanthine oxidase (X/XO) system) presented a blunted oxidative stress with no change in 8-isoprostane release, an increase in tumor necrosis factor-α (TNF-α) release, and a reduction in NF-κB activity. These data suggest that placenta from women with GDM display an attenuated response to an in vitro oxidative challenge with respect to the release of the proinflammatory cytokine, suggesting that GDM placenta may be preconditioned by transient intracellular oxidative stress that attenuates responsiveness to a further oxidative insult.35
The present study is described in animals but allows the scientific community to extrapolate some of these findings for possible effects of oxidative stress also in human placentas, especially in diabetic environment. This investigation opens possibilities for important research in the area. Thus, an increased activity of the maternal antioxidant defense system intending to eliminate peroxides and to act against oxidative stress generated by hyperglycemia was demonstrated. In contrast, the increased antioxidant activity is not effective in decreasing lipid peroxidation in blood tissue. In the placental tissue, the scenario reflects changes in the antioxidant defense as demonstrated by the increased catalase activity, showing ability of placental antioxidant defense system to prevent mild diabetes-induced oxidative stress. Thus, this reinforces the need to advance the research on pharmacological interventions to minimize the repercussions resulting from diabetes.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors grateful to Fernanda Pereira Lima for technical assistance, to students Isabela Lovizutto Iessi and Aline Bueno by animal care, to Research Support Group (GAP) of Botucatu Medical School_Univ Estadual Paulista, Unesp for help with the statistical analysis and to Foundation Research Support of São Paulo State (FAPESP) for fellowship to APM Spada.
References
- 1. American Diabetes Association. Diagnosis and classification of Diabetes mellitus. Diabetes Care. 2013;36(suppl 1):S67–S75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moreira RO, Campos SC, Soldera AL. Type 2 Diabetes Mellitus and Alzheimer's Disease: from physiopathology to treatment implications [published online July 18, 2013]. Diabetes Metab Res Rev. 2013. doi: 10.1002/dmrr.2442 [DOI] [PubMed] [Google Scholar]
- 3. Fatehi Hassanabad Z, Chan CB, Furman BL. Reactive oxygen species and endothelial function in diabetes. Eur J Pharmacol. 2010;636(1-3):8–17 [DOI] [PubMed] [Google Scholar]
- 4. Leal CA, Schetinger MR, Leal DB, et al. Oxidative stress and antioxidant defenses in pregnant women. Redox Rep. 2011;16(6):230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122(4):369–382 [DOI] [PubMed] [Google Scholar]
- 6. Lapolla A, Molin L, Traldi P. Protein glycation in diabetes as determined by mass spectrometry. Int J Endocrinol. 2013;2013:412103. doi: 10.1155/2013/412103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Desoye G, Mouzon SH. The human placenta in gestational Diabetes mellitus: the insulin and cytokine network. Diabetes Care. 2007;30 suppl 2:S120–S126 [DOI] [PubMed] [Google Scholar]
- 8. Myatt L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010;31:S66–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coughlan MT, Oliva K, Georgiou HM, Permezel JM, Rice GE. Glucose-induced release of tumor necrosis factor-alpha from human placental and adipose tissues in gestational diabetes mellitus. Diabetic Med. 2001;18(11):921–927 [DOI] [PubMed] [Google Scholar]
- 10. Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12(6):747–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond). 2007;113(1):1–13 [DOI] [PubMed] [Google Scholar]
- 12. Damasceno DC, Silva HP, Vaz GF, et al. Diabetic rats exercised prior to and during pregnancy: maternal reproductive outcome, biochemical profile, and frequency of fetal anomalies. Reprod Sci. 2013;20(7):730–738 [DOI] [PubMed] [Google Scholar]
- 13. Lima PH, Sinzato YK, Gelaleti RB, Calderon IM, Rudge MV, Damasceno DC. Genotoxicity evaluation in severe or mild diabetic pregnancy in laboratory animals. Exp Clin Endocrinol Diabetes. 2012;120(5):303–307 [DOI] [PubMed] [Google Scholar]
- 14. de Souza MD, Sinzato YK, Lima PH, Calderon IM, Rudge MV, Damasceno DC. Oxidative stress status and lipid profiles of diabetic pregnant rats exposed to cigarette smoke. Reprod Biomed Online. 2010;20(4):547–552 [DOI] [PubMed] [Google Scholar]
- 15. Damasceno DC, Sinzato YK, Lima PH, et al. Effects of exposure to cigarette smoke prior to pregnancy in diabetic rats. Diabetol Metab Syndr. 2011;3:20. doi: 10.1186/1758-5996-3-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sinzato YK, Volpato GT, Iessi IL, et al. Neonatally induced mild diabetes in rats and its effect on maternal, placental, and fetal parameters. Exp Diabetes Res. 2012;2012:108163. doi: 10.1155/2012/108163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Damasceno DC, Kempinas W, Volpato GT, Consonni M, Rudge MVC, Paumgartten FJR. Anomalias congênitas: estudos experimentais. 1st ed. Coopmed, Belo Horizonte. 2008 [Google Scholar]
- 18. Jocelyn PC. Spectrophotometric assay of thiols. Methods Enzymol. 1987;143:44–67 [DOI] [PubMed] [Google Scholar]
- 19. Kitani K, Kanai S, Ivy GO, Carrillo MC. Assessing the effects of deprenyl on longevity and antioxidant defenses in different animal models. Ann N Y Acad Sci. 1998;854:291–306 [DOI] [PubMed] [Google Scholar]
- 20. McCord JM, Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem. 1969;244(22):6056–6063 [PubMed] [Google Scholar]
- 21. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23(5):599–622 [DOI] [PubMed] [Google Scholar]
- 22. Maritim AC, Sanders RA, Watkins III JB. Diabetes, oxidative stress and antioxidants: a review. J Biochem Mol Toxicol. 2003;17(1):24–38 [DOI] [PubMed] [Google Scholar]
- 23. Ceriello A. Oxidative stress and diabetes-associated complications. Endocr Prat. 2006;12 suppl 1:60–62 [DOI] [PubMed] [Google Scholar]
- 24. Damasceno DC, Sinzato YK, Bueno A, et al. Mild diabetes models and their maternal-fetal repercussions. J Diabetes Res. 2013;2013:473575. doi: 10.1155/2013/473575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sinzato YK, Lima PHO, Campos KE, Kiss AC, Rudge MV, Damasceno DC. Neonatally-induced diabetes: lipid profile outcomes and oxidative stress status in adult rats. Rev Ass Med Bras. 2009;55(4):384–388 [DOI] [PubMed] [Google Scholar]
- 26. Pieper GM, Jordan M, DondLinger LA, Adams MB, Roza AM. Peroxidative stress in diabetic blood vessels. Reversal by pancreatic islet transplantation. Diabetes. 1995;44:884–889 [DOI] [PubMed] [Google Scholar]
- 27. Myatt L. Review: reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010;31 suppl:S66–S69. doi: 10.1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Palm M, Axelsson O, Wernroth L, Basu S. F(2)-isoprostanes, tocopherols and normal pregnancy. Free Radic Res. 2009;43(6):546–552 [DOI] [PubMed] [Google Scholar]
- 29. Jones ML, Mark PJ, Lewis JL, Mori TA, Keelan JA, Waddell BJ. Antioxidant defenses in the rat placenta in late gestation: increased labyrinthine expression of superoxide dismutases, glutathione peroxidase 3, and uncoupling protein 2. Biol Reprod. 2010;83(2):254–260 [DOI] [PubMed] [Google Scholar]
- 30. Lappas M, Mitton A, Permezel M. In response to oxidative stress, the expression of inflammatory cytokines and antioxidant enzymes are impaired in placenta, but not adipose tissue, of women with gestational diabetes. J Endocrinol. 2010;204(1):75–84 [DOI] [PubMed] [Google Scholar]
- 31. Choi SW, Benzie IFF, Ma SW, Strain JJ, Hannigan BM. Acute hyperglycemia and oxidative stress: direct cause and effect? Free Radic Biol Med. 2008;44(7):1217–1231 [DOI] [PubMed] [Google Scholar]
- 32. Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95 [DOI] [PubMed] [Google Scholar]
- 33. Haddad JJ. Oxygen homeostasis, thiol equilibrium and redox regulation of signalling transcription factors in the alveolar epithelium. Cell Signal. 2002;14(10):799–810 [DOI] [PubMed] [Google Scholar]
- 34. Barnes PJ, Karin M. Nuclear factor- B: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336(15):1066–1071 [DOI] [PubMed] [Google Scholar]
- 35. Coughlan MT, Permezel M, Georgiou HM, Rice GE. Repression of oxidant-induced nuclear factor-kappaB activity mediates placental cytokine responses in gestational diabetes. J Clin Endocrinol Metab. 2004;89(7):3585–3594 [DOI] [PubMed] [Google Scholar]


