Abstract
The prevalence of polycystic ovary syndrome (PCOS) and its distinct clinical phenotypes were assessed using 3 sets of international diagnostic criteria in women self-reporting concerns over outward features of PCOS. Revised ultrasonographic criteria for polycystic ovaries (PCO) based on modern ultrasound technology were used. Of the participants, 53%, 62%, and 70% were diagnosed with PCOS using National Institutes of Health, Androgen Excess and PCOS Society, and Rotterdam criteria, respectively. Prevalence of Frank, Ovulatory, Normoandrogenic, and Non-PCO PCOS were 66%, 13%, 11%, and 9%, respectively. Frank PCOS was associated with the severest metabolic disturbances whereas metabolic profiles in Normoandrogenic PCOS did not differ from controls, supporting reduced health risks in women without androgen excess. Metabolic disturbances and hyperandrogenism were linked to excess adiposity across all the groups. Using updated criteria for PCO, the prevalence of Non-PCO PCOS and PCO alone in healthy women recruited from the general population was reduced compared to the previous reports.
Keywords: polycystic ovary syndrome, phenotypes, hyperandrogenism, hirsutism, ultrasonography
Introduction
Polycystic ovary syndrome (PCOS) is a heterogeneous disorder characterized by a diverse collection of reproductive and metabolic abnormalities. Defining symptoms of PCOS have evolved since first described1 in 1935 and remain a topic of debate among clinicians and scientists worldwide.2,3 In 1990, the National Institutes of Health (NIH) generated consensus diagnostic criteria for PCOS, defining the condition as the combined presence of hyperandrogenism and chronic anovulation in the absence of other causes of anovulatory infertility.4 The NIH criteria were criticized for reflecting majority opinion rather than clinical trial data2 and for their omission of ultrasonographic evidence of polycystic ovaries (PCO) that many considered a definitive marker of PCOS. In 2003, the European Society for Human Reproduction and Embryology and the American Society for Reproductive Medicine redefined PCOS as the presence of 2 or more features: (1) oligo- or chronic anovulation, (2) biochemical and/or clinical hyperandrogenism, and (3) ultrasonographic evidence of PCO.5,6 These “Rotterdam criteria” were intended to reflect the clinical heterogeneity of PCOS. However, they have since been criticized for being too expansive.2,7 More recently, the Androgen Excess and PCOS (AE-PCOS) Society has proposed a set of criteria defining PCOS foremost as a condition of abnormal androgen production wherein PCOS is diagnosed as the combined presence of androgen excess (hirsutism and/or hyperandrogenemia) and evidence of ovarian dysfunction (oligoanovulation and/or PCO).7
The Rotterdam and AE-PCOS Society criteria recognize at least 3 unique clinical phenotypes: (1) Frank PCOS (oligomenorrhea, hyperandrogenism, and PCO), (2) Ovulatory PCOS (hyperandrogenism, PCO, and regular menstrual cycles), and (3) Non-PCO PCOS (oligomenorrhea, hyperandrogenism, and normal ovaries). The Rotterdam criteria also recognize a fourth phenotype, Mild8 or Normoandrogenic PCOS,9 which is defined by oligomenorrhea, PCO, and normal androgens. Whether these 4 phenotypes represent a spectrum of the same condition is currently an area of debate.2,3,7 To date, most studies investigating the pathogenesis and long-term health risks in PCOS have used the NIH criteria. As such, there is significant evidence that women with oligomenorrhea having hyperandrogenism have heightened risks of insulin resistance, diabetes, cardiovascular disease, endometrial dysfunction, and pregnancy complications.5,6 By contrast, emerging data on “newer” forms of PCOS support the conclusion that women with Normoandrogenic and Ovulatory PCOS have lower risk of chronic diseases as judged by lower incidences of abdominal obesity, insulin resistance, and inflammation.7,9,10 Moreover, women with Ovulatory PCOS appear to have lower risks of endometrial carcinoma.11 Although both the Rotterdam and the AE-PCOS Society criteria currently reflect the majority opinion that PCOS is a broad-spectrum disorder, paucity of data investigating PCOS phenotypes limits knowledge of the actual prevalence and long-term health outcomes for this condition.
Compounding the debate over the actual phenotypic spectrum of PCOS is the controversy over the accurate definition of PCO. Numerous reports have recently questioned the specificity of PCO to detect PCOS, given that an unusually high proportion of healthy women demonstrates an average of 12 follicles per ovary—the threshold recognized by the 2003 Rotterdam consensus.12 We recently reported that a threshold of 26 follicles throughout the entire ovary had better diagnostic potential to distinguish between women with PCOS and controls compared to follicle counts made in a single plane or ovarian volume.13 Although more than double the threshold proposed by the Rotterdam consensus, our findings are consistent with other groups showing that revised criteria for PCO are needed in light of advances in imaging technology, which have occurred in 10 years since the Rotterdam consensus.14,15 Revised sonographic criteria with improved accuracy for discriminating PCO may be important for unraveling phenotypic variations in PCOS. To that end, the goal of the current study was to explore the prevalence of PCOS as defined by the NIH, Rotterdam, and AE-PCOS Society criteria in consecutive women self-reporting PCOS features using updated sonographic thresholds for PCO. In addition, we compared the severity of diagnostic and metabolic features among women with distinct PCOS phenotypes, those with isolated symptoms of PCOS, and healthy controls. We hypothesized that an updated definition of PCO using modern ultrasound equipment would reveal evidence for a reduced prevalence of Non-PCO PCOS as well as a lower prevalence of PCO in healthy women recruited from the general population.
Materials and Methods
Participants
Advertisements seeking women of reproductive age (18-36 years), not taking hormonal contraception, with any 1 or a combination of the following concerns were circulated in local newspapers, university buildings, electronic newsletters, hospital elevators, and in our clinical practice waiting rooms: (1) unpredictable periods, (2) unwanted facial/body hair growth, (3) infertility, and/or (4) a family history of type 2 diabetes. The advertisements seeking reproductive-age women that considered themselves to have regular periods, not taking hormonal contraception, and otherwise being healthy were used to recruit controls. Between March 2006 and June 2008, 126 women were evaluated for self-reported features of PCOS and 42 women were evaluated as controls. Participants should not have used fertility medications, antiepileptic drugs, insulin sensitizers, or drugs known to alter lipid metabolism in the 3 months prior to enrollment. A subset of participants evaluated in this study was used to develop revised sonographic criteria for polycystic ovarian morphology as described previously.13
Study Procedures
All participants underwent clinical evaluation for PCOS which included (1) evaluation of menstrual cycle history to determine the extent of menstrual cycle disturbance and/or duration of infertility; (2) a physical examination to assess height, weight, waist and hip circumference, blood pressure, and terminal hair growth on 9 regions of the body using the modified Ferriman-Gallwey scoring system16; (3) a transvaginal ultrasound scan of the ovaries using a 9-MHz transducer and an UltraSonix RP Scanner (Version 2.3.5, Vancouver, British Columbia, Canada); and (4) fasting blood tests. Ultrasound scans were performed by 2 experienced sonographers using a standardized protocol for collecting sonographic images and cineloops of the ovaries. Images were collected in a deidentified manner so that offline evaluation of sonographic end points was conducted in a blinded manner (described subsequently). The transvaginal ultrasound scan and fasting blood tests were performed on days 2 to 6 in women reporting regular menstrual cycles or at a time when no dominant follicle or ovulation gland was sonographically detected in women reporting irregular or absent cycles. Fasting blood tests were performed to assess levels of gonadotropins, total and free androgens as well as serum markers of the metabolic syndrome including lipids (total cholesterol, triglycerides, high-density lipoprotein [HDL], and low-density lipoprotein [LDL]), C-reactive protein (CRP), and hemoglobin A1C (HbA1C). Fasting tests included serum assessments for cortisol, prolactin, thyroid hormones, dehydroepiandrosterone sulfate, and 17-hydroxyprogesterone to exclude for other conditions that may cause symptoms similar to PCOS. A 75 g, 2-hour oral glucose tolerance test was also performed according to the World Health Organization protocol, with a minor modification being an additional blood sample collected at 1 hour postglucose ingestion.
This study was approved by the University of Saskatchewan Biomedical Research Ethics Review Board. Interactions with human participants occurred at the Royal University Hospital within the Department of Obstetrics, Gynecology and Reproductive Sciences (Saskatoon, Saskatchewan, Canada). Deidentified clinical records, ultrasound images, and biological samples were analyzed by the research team in the Division of Nutritional Sciences at Cornell University (Ithaca, New York).
Clinical Definitions of PCOS
Oligo- or Frank amenorrhea was defined as a history of menstrual cycles of >38 days.17 Hyperandrogenism was defined as a modified hirsutism score of ≥7 and/or an elevated total testosterone value of ≥3.96 nmol/L as described previously.13 PCO on ultrasonography was defined as the presence of ≥26 follicles measuring 2 to 9 mm throughout the entire ovary and/or an ovarian volume (OV) greater than 10 cm3.13 All sonographic measurements were made offline by a single observer using Santesoft DICOM Editor software (Emmanouil Kanellopoulus, Athens, Greece). OV was estimated using the equation for a prolate ellipsoid. Follicle counts were performed using a reliable grid system approach for counting follicles throughout the entire ovary as described previously.18 Values reported for follicle count and OV represent the mean recorded values of the left and right ovaries rounded to the nearest whole number or 10th decimal place, respectively.
Biochemical Assays
Total testosterone was measured by isotope dilution liquid chromatography tandem mass spectrometry as described previously.19 Intra- and interassay coefficients of variation (CV) were <5% consistent with good assay performance in both PCOS and control populations despite absolute values reading higher compared to those reported by others.20 Glucose, total cholesterol, triglycerides, HDL, LDL, and CRP were measured by colorimetry or nephelometry using the Beckman Coulter DxC 600i chemistry analyzer (Fullerton, California). Intra-assay CV for these assays ranged from 2% to 5% and interassay CV ranged from 3% to 7.5%. Insulin, sex hormone-binding globulin (SHBG), and androstenedione were measured by a commercially available 2-site chemiluminescent immunogenic assay and the immunoassay analyzer Immulite 2500 (Siemens Medical Solutions Diagnostics, Malvern, PA). Free androgen index (FAI) was calculated by dividing total testosterone by SHBG and multiplying by 100. Hemoglobin A1C was measured by high-performance liquid chromatography using the VARIANT II Hemoglobin Testing System (BioRad Laboratories, Hercules, California). Intra- and interassay CV for SHBG, insulin, androstenedione, and HbA1C were <7.3%.
Statistical Analysis
JMP 9 statistical software (SAS Institute Inc, Cary, North Carolina) was used to carry out the analyses. Descriptive statistics for clinical, hormonal, and ultrasonographic features were tabulated. Data not normally distributed were transformed using a Box-Cox best transformation test. Student t tests or Tukey-Kramer multiple comparison tests were used to assess differences in parameters among the groups. A Bonferroni correction for multiple comparisons within a table was applied to assess any significant overall group effect.
Results
Patient Classification
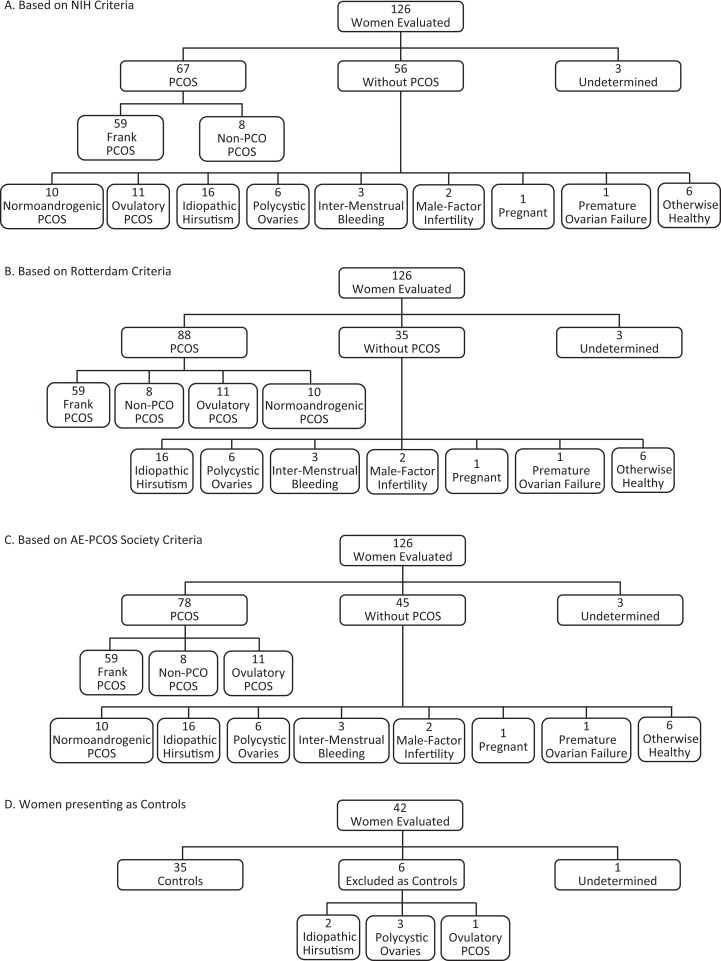
Of the 126 women self-reporting concerns over PCOS features, 67 (53%), 88 (70%), and 78 (62%) met the criteria for PCOS based on the NIH, Rotterdam, and AE-PCOS Society guidelines, respectively (Figure 1A-C). Three women were designated as “undetermined” because their ovaries could not be optimally visualized or they were lost to follow-up. Those classified as not having PCOS generally had only 1 outward symptom or alternate condition, including idiopathic hirsutism, PCO alone, mild spotting between periods, pregnancy, or premature ovarian failure. Two healthy women who presented with concerns over infertility were confirmed to have male factor infertility at a follow-up clinical consultation at our center, and 6 asymptomatic women with concerns over a family history of diabetes were confirmed to be otherwise healthy. Of the 42 women who presented as healthy controls, 35 were included (Figure 1D). Seven women were excluded from the control cohort because of idiopathic hirsutism, PCO alone, Ovulatory PCOS, or failure to complete the study (undetermined). The population studied was multiethnic and representative of the local demographic. The ethnicity of the participants was as follows: 91% European, 4.7% First Nations, 2.6% Asian, 1% black/African, and 0.5% Hispanic/Latino.
Figure 1.
Classification of participants using NIH, Rotterdam, and AE-PCOS Society guidelines for PCOS. One hundred and twenty-six women self-reporting features of PCOS were evaluated. According to the NIH criteria, 67 (53%) women met the criteria for PCOS and 2 PCOS phenotypes were recognized (A). Eighty-eight (70%) women met the Rotterdam criteria for PCOS and 4 PCOS phenotypes were recognized (B). The AE-PCOS Soceity criteria identified 78 (62%) women meeting the criteria for PCOS and 3 phenotypes were recognized (C). Of the 42 women who presented as healthy controls, 35 met the inclusion criteria for controls while the remaining participants had idiopathic hirsutism, PCO alone, Ovulatory PCOS, or failed to complete the study (D). AE-PCOS indicates Androgen Excess and PCOS; NIH, National Institute of Health; PCOS, polycystic ovary syndrome.
Clinical and Metabolic Features of Women With PCOS
Clinical and metabolic features of women meeting the diagnosis of PCOS based on NIH, Rotterdam, and AE-PCOS Society guidelines are compared to controls in Table 1. As a group, women with PCOS as defined by any of the 3 guidelines had higher body mass index (BMI), longer menstrual cycles and higher indices of androgen excess and ovarian dysmorphology compared to controls (Table 1 panel A; P < .0002). In addition to lower HDL and SHBG levels (P < .0001), women with PCOS had higher cholesterol–HDL ratios, CRP, HbA1C, fasting insulin, homeostatic model assessment of insulin resistance (HOMA-IR), and integrated insulin and glucose secretion following a glucose challenge (P < .002). Following correction for BMI, differences in SHBG persisted in women diagnosed using NIH and Rotterdam criteria, and differences in fasting insulin and HOMA-IR persisted in women diagnosed with PCOS by any of the 3 guidelines (Table 1 panel B).
Table 1.
Comparison of Diagnostic Criteria and Metabolic Features Among Women Diagnosed With PCOS Using the NIH, Rotterdam, and AE-PCOS Society Criteria and Controls.a
| NIH (n = 67) | Rotterdam (n = 89)c | AE-PCOS (n = 79) | Controls (n = 43)d | |
|---|---|---|---|---|
| (A) Diagnostic features | ||||
| Age (y) | 27.7 ± 4.5 | 27.8 ± 4.4 | 27.8 ± 4.5 | 26.9 ± 4.0 |
| BMI, kg/m2 | 33.3 ± 8.9b | 32 ± 8.6b | 32.8 ± 8.5b | 24.8 ± 5.0 |
| Menstrual cycle length, d | 129 ± 98b | 118 ± 100b | 116 ± 97b | 30 ± 6 |
| Modified hirsutism score | 12.2 ± 6.5b | 10.7 ± 6.6b | 11.9 ± 6.1b | 3.1 ± 3.2 |
| Total testosterone, nmol/L | 3.9 ± 2.0 | 3.7 ± 1.9 | 3.8 ± 2.0 | 2.9 ± 0.9 |
| Free androgen index (%) | 11.6 ± 8.3b | 9.9 ± 8.0b | 10.7 ± 8.1b | 3.2 ± 2.0 |
| Androstenedione, nmol/L | 13.3 ± 6.3 | 12.6 ± 6.3 | 12.9 ± 6.3 | 11.9 ± 6.3 |
| Follicle count | 43.6 ± 15.7b | 42.8 ± 14.8b | 43.1 ± 14.7b | 15.1 ± 4.9 |
| Ovarian volume, mL | 10.3 ± 3.4b | 10.1 ± 3.3b | 10.2 ± 3.3b | 5.7 ± 1.9 |
| (B) Metabolic features | ||||
| Waist–hip ratio | 0.92 ± 0.10 | 0.91 ± 0.10 | 0.91 ± 0.10 | 0.85 ± 0.10 |
| Systolic BP, mm Hg | 118.3 ± 10.1 | 117.2 ± 10.0 | 117.7 ± 10.2 | 112.8 ± 7.9 |
| Diastolic BP, mm Hg | 75.4 ± 8.2 | 74.2 ± 8.5 | 74.5 ± 8.5 | 73.3 ± 7.1 |
| LDL, mmol/L | 3.1 ± 0.8 | 2.9 ± 0.8 | 3.0 ± 0.8 | 2.4 ± 0.7 |
| HDL, mmol/L | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.5 ± 0.4 |
| Cholesterol–HDL ratio | 4.3 ± 1.2 | 4.0 ± 1.2 | 4.1 ± 1.2 | 3.0 ± 1.0 |
| Triglyceride, mmol/L | 1.3 ± 0.8 | 1.2 ± 0.8 | 1.3 ± 0.8 | 0.8 ± 0.4 |
| C-reactive protein, mg/L | 4.5 ± 4.5 | 3.8 ± 4.3 | 4.0 ± 4.3 | 1.1 ± 1.8 |
| SHBG, nmol/L | 32.1 ± 16.5b | 42.7 ± 33.0b | 37.1 ± 26.6 | 58.2 ± 24.2 |
| Hemoglobin A1C (%) | 5.3 ± 0.5 | 5.2 ± 0.5 | 5.3 ± 0.5 | 4.9 ± 0.3 |
| Fasting insulin, pmol/L | 92.8 ± 59.3b | 85.3 ± 57.5b | 89.7 ± 57.9b | 37.5 ± 19.0 |
| Fasting glucose, mmol/L | 5.0 ± 0.7 | 5.0 ± 0.7 | 5.0 ± 0.7 | 4.7 ± 0.3 |
| HOMA-IR | 3.1 ± 2.2b | 2.8 ± 2.1b | 3.0 ± 2.2b | 1.1 ± 0.6 |
| AUC insulin | 1022.9 ± 751.1 | 946.5 ± 694.7 | 991.9 ± 714.7 | 566.9 ± 278.8 |
| AUC glucose | 12.6 ± 3.1 | 12.3 ± 3.1 | 12.3 ± 3.3 | 9.3 ± 3.6 |
Abbreviations: BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SHBG, sex hormone-binding globulin; HOMA-IR, homeostatic model assessment of insulin resistance; AUC, area under the curve.
a Values presented as mean ± standard deviation. Comparisons for metabolic features are adjusted for BMI. Integrated 2-hour insulin and glucose secretion is expressed as area under the curve values in arbitrary units.
b Significant differences compared to controls are indicated.
c A total of 89 women met the diagnosis of PCOS based on Rotterdam criteria from all 168 women screened.
d A total of 43 women met the criteria for controls from all 168 women screened.
Clinical and Metabolic Features of Women With PCOS Phenotypes
Of the 168 women screened, 59 met the criteria for Frank PCOS, 8 for Non-PCO PCOS, 12 for Ovulatory PCOS, 10 for Normoandrogenic PCOS and 43 women met the criteria for controls (ie, included 2 healthy women with confirmed male factor infertility). An assessment of diagnostic features among PCOS phenotypes and controls showed a significant group effect for BMI, menstrual cycle length, hirsutism score, FAI, follicle count, and ovarian volume (Table 2 panel A; P < .0001). Post-tests revealed that women with Frank PCOS had higher BMI and differed from controls in all diagnostic criteria for PCOS except total testosterone. By contrast, women classified as Non-PCO did not differ from controls in ovarian morphology but differed in that they had higher BMI, reported longer intervals between menses, and had higher hirsutism scores and FAI. Women with Ovulatory PCOS had BMI and menstrual cycle lengths similar to controls but had higher hirsutism scores, follicle counts, and OV. Finally, women with Normoandrogenic PCOS were similar to controls in BMI and indices of androgen excess but had longer menstrual cycles, higher follicle counts, and larger OV.
Table 2.
Comparison of Diagnostic Criteria and Metabolic Features Among Women With 4 Clinical Phenotypes of PCOS and Controls.a
| Frank (n = 59) | Non-PCO (n = 8) | Ovulatory (n = 12)b | Normoandrogenic (n = 10) | Controls (n = 43)c | |
|---|---|---|---|---|---|
| (A) Diagnostic features | |||||
| Age, years | 27.6 ± 4.4 | 28.5 ± 4.9 | 28.3 ± 4.9 | 28.3 ± 3.1 | 26.9 ± 4.0 |
| BMI, kg/m2 | 32.5 ± 8.7a | 39.0 ± 6.4a | 30.3 ± 7.0ab | 25.2 ± 7.0b | 24.8 ± 5.0b |
| Menstrual cycle length, d | 135 ± 102a | 83 ± 26abc | 39 ± 25bc | 137 ± 134ab | 30 ± 6c |
| Modified hirsutism score | 12.1 ± 6.6a | 12.9 ± 6.4a | 10.1 ± 2.4a | 1.7 ± 2.5b | 3.1 ± 3.2b |
| Total testosterone, nmol/L | 4.0 ± 2.1 | 3.4 ± 1.7 | 2.5 ± 0.9 | 3.1 ± 1.0 | 2.9 ± 0.9 |
| Free androgen index (%) | 11.2 ± 7.5a | 14.6 ± 13.2a | 5.9 ± 4.0b | 3.6 ± 3.3b | 3.2 ± 2.0b |
| Androstenedione, nmol/L | 13.6 ± 6.6 | 8.7 ± 3.1 | 12.2 ± 7.3 | 9.4 ± 1.1 | 11.9 ± 6.3 |
| Follicle count | 46.9 ± 13.3a | 19.5 ± 9.0b | 40.3 ± 7.4a | 40.5 ± 15.8a | 15.1 ± 4.9b |
| Ovarian volume, mL | 10.8 ± 3.2a | 6.3 ± 2.2bc | 9.7 ± 2.8ab | 9.1 ± 3.3ab | 5.7 ± 1.9c |
| (B) Metabolic features | |||||
| Waist–hip ratio | 0.91 ± 0.1 | 0.94 ± 0.1 | 0.82 ± 0.1 | 0.91 ± 0.2 | 0.85 ± 0.1 |
| Systolic BP, mm Hg | 117.6 ± 10.0 | 123.4 ± 10.0 | 114.3 ± 10.2 | 113.8 ± 8.7 | 112.8 ± 7.9 |
| Diastolic BP, mm Hg | 74.9 ± 8.4 | 78.3 ± 7.2 | 70.3 ± 8.8 | 72.0 ± 8.4 | 73.3 ± 7.1 |
| LDL, mmol/L | 3.1 ± 0.8 | 3.1 ± 0.7 | 2.6 ± 0.7 | 2.5 ± 0.8 | 2.4 ± 0.7 |
| HDL, mmol/L | 1.2 ± 0.3 | 1.1 ± 0.2 | 1.4 ± 0.2 | 1.3 ± 0.3 | 1.5 ± 0.4 |
| Cholesterol–HDL ratio | 4.2 ± 1.2 | 4.7 ± 1.2 | 3.2 ± 0.9 | 3.4 ± 1.0 | 3.0 ± 1.0 |
| Triglyceride, mmol/L | 1.3 ± 0.9 | 1.4 ± 0.5 | 1.0 ± 0.9 | 0.9 ± 0.8 | 0.8 ± 0.4 |
| C-reactive protein, mg/L | 4.0 ± 3.9 | 8.3 ± 6.2 | 1.5 ± 1.7 | 1.8 ± 3.7 | 1.1 ± 1.8 |
| SHBG, nmol/L | 35.7 ± 24.6 | 28.2 ± 25.5 | 56.3 ± 36.4 | 83.4 ± 46.4 | 58.2 ± 24.2 |
| Hemoglobin A1C (%) | 5.3 ± 0.6 | 5.3 ± 0.4 | 5.2 ± 0.2 | 5.1 ± 0.4 | 4.9 ± 0.3 |
| Fasting insulin, pmol/L | 87.0 ± 57.2 | 138.5 ± 59.6 | 72.3 ± 47.5 | 49.4 ± 41.6 | 37.5 ± 19.0 |
| Fasting glucose, mmol/L | 5.0 ± 0.6 | 5.4 ± 1.4 | 4.7 ± 0.3 | 5.1 ± 0.7 | 4.7 ± 0.3 |
| HOMA-IR | 2.9 ± 2.2 | 4.4 ± 2.0 | 2.2 ± 1.5 | 1.7 ± 1.6 | 1.1 ± 0.6 |
| 2-hr AUC insulin | 969.8 ± 703.7 | 1491.5 ± 1049.3 | 826.0 ± 465.7 | 548.7 ± 267.2 | 566.9 ± 278.8 |
| 2-hr AUC glucose | 12.5 ± 3.0 | 13.4 ± 3.6 | 10.6 ± 3.8 | 11.6 ± 1.3 | 9.3 ± 3.6 |
Abbreviations: BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SHBG, sex hormone-binding globulin; HOMA-IR, homeostatic model assessment of insulin resistance; AUC, area under the curve.
a Values presented as mean ± standard deviation. Comparisons for metabolic features are adjusted for BMI. Significant differences for within-row comparisons are denoted by different letters. Integrated 2-hour insulin and glucose secretion is expressed as AUC values in arbitrary units.
b A total of 12 women met the diagnosis of Ovulatory PCOS from all 168 women screened.
c A total of 43 women met the criteria for controls from all 168 women screened.
An assessment of metabolic features among PCOS phenotypes and controls showed a significant group effect for HDL, cholesterol–HDL ratio, CRP, fasting insulin, HOMA-IR, and integrated 2-hour insulin and glucose secretion following a glucose challenge (P < .0015). Post-tests showed that women with Normoandrogenic PCOS were similar in cardiovascular risk factors and insulin sensitivity compared to controls whereas women with Frank, Non-PCO, and Ovulatory phenotypes had higher fasting insulin than controls. Women with Frank and Non-PCO phenotypes also differed from controls with lower HDL and higher cholesterol–HDL ratio, CRP, HOMA-IR, and integrated 2-hour insulin and glucose secretion. They also had higher CRP and fasting insulin compared to women with Normoandrogenic PCOS. When data were adjusted for BMI, no differences were retained among PCOS phenotypes or when compared to controls (Table 2 panel B).
Features of Women With Hirsutism or PCO Morphology
Of the 168 women evaluated, 18 with hirsutism alone and 9 with PCO alone were identified. An assessment of diagnostic features among women with hirsutism alone, PCO alone, and controls showed a significant group effect for BMI, hirsutism score, follicle count, and OV (Table 3 panel A; P < .0002). Women with hirsutism alone had higher BMI and hirsutism scores compared to controls. Women with PCO alone differed from controls only in their higher follicle counts and OV. An assessment of metabolic features among women with hirsutism alone, PCO alone, and controls showed a significant group effect for SHBG, fasting insulin, and HOMA-IR (P < .0002). Post-tests indicated that women with hirsutism had lower SHBG and higher fasting insulin and HOMA-IR compared to both women with PCO alone and controls. When data were adjusted for BMI, no differences were retained among the groups (Table 3 panel B).
Table 3.
Comparison of Clinical and Metabolic Features Among Women With Hirsutism Alone, Polycystic Ovaries Alone, and Controls.a
| Hirsute (n = 18)b | Polycystic Ovaries (n = 9)c | Controls (n = 43)d | |
|---|---|---|---|
| (A) Diagnostic features | |||
| Age, years | 30.3 ± 4.5 | 28.8 ± 5.4 | 26.9 ± 4.0 |
| BMI, kg/m2 | 32.3 ± 8.0a | 24.9 ± 4.6b | 24.8 ± 5.0b |
| Menstrual cycle length, d | 30.7 ± 3.7 | 29.9 ± 3.4 | 30.0 ± 5.5 |
| Modified hirsutism score | 13.2 ± 3.2a | 5.3 ± 1.3b | 3.1 ± 3.2b |
| Total testosterone, nmol/L | 2.9 ± 1.3 | 2.9 ± 0.5 | 2.9 ± 0.9 |
| Free androgen index (%) | 6.4 ± 4.5 | 2.6 ± 1.3 | 3.2 ± 2.0 |
| Androstenedione, nmol/L | 10.8 ± 8.7 | 11.2 ± 5.2 | 11.9 ± 6.3 |
| Follicle count | 17.1 ± 5.8b | 34.6 ± 7.8a | 15.1 ± 4.9b |
| Ovarian volume, mL | 6.7 ± 2.7ab | 9.0 ± 2.7a | 5.7 ± 1.9b |
| (B) Metabolic features | |||
| Waist–hip ratio | 0.91 ± 0.10 | 0.85 ± 0.10 | 0.85 ± 0.10 |
| Systolic BP, mm Hg | 121.2 ± 9.9 | 110.1 ± 8.5 | 112.8 ± 7.9 |
| Diastolic BP, mmHg | 77.3 ± 6.2 | 72.4 ± 7.3 | 73.3 ± 7.1 |
| LDL, mmol/L | 2.8 ± 0.8 | 2.1 ± 0.5 | 2.4 ± 0.7 |
| HDL, mmol/L | 1.3 ± 0.4 | 1.5 ± 0.4 | 1.5 ± 0.4 |
| Cholesterol–HDL ratio | 3.7 ± 1.1 | 2.7 ± 0.5 | 3.0 ± 1.0 |
| Triglyceride, mmol/L | 0.9 ± 0.3 | 0.8 ± 0.3 | 0.8 ± 0.4 |
| C-reactive protein, mg/L | 3.0 ± 2.6 | 0.5 ± 0.6 | 1.1 ± 1.8 |
| SHBG, nmol/L | 40.1 ± 18.1 | 67.7 ± 29.8 | 58.2 ± 24.2 |
| Hemoglobin A1C (%) | 5.1 ± 0.3 | 5.1 ± 0.4 | 4.9 ± 0.3 |
| Fasting insulin, pmol/L | 88.4 ± 48.5 | 35.7 ± 20.7 | 37.5 ± 19.0 |
| Fasting glucose, mmol/L | 4.9 ± 0.5 | 4.8 ± 0.2 | 4.7 ± 0.3 |
| HOMA-IR | 2.9 ± 1.7 | 1.1 ± 0.7 | 1.1 ± 0.6 |
| AUC insulin | 729.8 ± 369.2 | 306.1 ± 150.3 | 566.9 ± 278.8 |
| AUC glucose | 10.6 ± 4.0 | 11.9 ± 7.0 | 9.3 ± 3.6 |
Abbreviations: BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SHBG, sex hormone-binding globulin; HOMA-IR, homeostatic model assessment of insulin resistance; AUC, area under the curve.
a Values presented as mean ± standard deviation. Comparisons for metabolic features are adjusted for BMI. Significant differences for within-row comparisons are denoted by different letters. Integrated 2-hour insulin and glucose secretion is expressed as AUC values in arbitrary units
b A total of 18 women met the diagnosis of hirsutism-alone from all 168 women screened.
c A total of 9 women met the criteria for polycystic ovaries-alone from all 168 women screened.
d A total of 43 women met the criteria for controls from all 168 women screened.
Discussion
Revised diagnostic thresholds for PCO using newer ultrasound technology and reliable methods for estimating follicle populations have been recently proposed.13,14,15 The extent to which new criteria for PCO affect the prevalence of PCOS phenotypes is yet to be reported. In this study, we evaluated the prevalence and metabolic profile of PCOS phenotypes in over 100 consecutive women self-reporting concerns over PCOS. Applying NIH, Rotterdam, and AE-PCOS Society guidelines, we found a PCOS prevalence of 53%, 70%, and 62%, respectively, consistent with others evaluating PCOS in consecutive patient or unselected populations using older or modified sonographic criteria for PCO.21–23 That the relative prevalence mirrored previous studies likely reflects the use of a consistent cutoff value for OV by all studies—which we recently cocorroborated using new imaging technology13—or that use of old criteria for PCO was valid when studies used older sonographic technology. The precise impact of technology is difficult to interpret in this context since studies did not report the age of their equipment. To truly determine the benefit of using updated ultrasound technology, future research should focus on quantifying the precise differences in sonographic end points when comparing old versus new ultrasound technology.
Despite expected variations in PCOS prevalence using the 3 international guidelines, each was effective in detecting differences in both clinical and metabolic parameters among women with PCOS and controls. However, it must be stressed that stratification by more discrete phenotypes clearly revealed that women with the most severe manifestations of PCOS primarily drove differences in clinical and metabolic profiles. This supports the conclusion that risks of chronic disease cannot be uniformly ascribed to the entire PCOS population unless using NIH criteria. An evaluation of the prevalence of PCOS phenotypes revealed that Frank, Ovulatory, Normoandrogenic, and Non-PCO subtypes were all represented in women self-reporting features of PCOS. Similar to European and Asian populations,10,24–30 Frank PCOS was the most prevalent phenotype noted in our North American cohort. We also noted that a substantial proportion of women screened were categorized as having Normoandrogenic or Ovulatory PCOS (24%), which lends further credence to the validity of these phenotypes for North American populations. In contrast to other studies reporting a prevalence between 16% and 40%,25,27–30 only 9% of the participants assessed in our study were classified by the Non-PCO phenotype. This might be explained by our use of newer technology with high-resolution capabilities that allowed for a more comprehensive assessment of ovarian morphology.
Consistent with earlier studies, metabolic aberrations were greatest for women with Frank and Non-PCO PCOS.9,10,24–26,28,30 Women with Ovulatory PCOS had features that tended to be intermediate to Frank PCOS and controls, whereas we detected no differences in the metabolic status of women with Normoandrogenic PCOS compared to controls. Metabolic disturbances were dependent on BMI, as previously shown,31,32 supporting the conclusion that dietary and lifestyle interventions in these populations are crucial for preventing progression to poorer reproductive and metabolic outcomes.33 Of note is the finding that we detected these differences in reproductive and metabolic features among groups despite reporting much higher follicle populations in women with PCOS and controls compared to earlier studies.9,25,30
Our study also explored clinical and metabolic differences in women who did not meet the diagnosis of PCOS but had isolated hirsutism or PCO. We identified 13% of women with concerns over PCOS as having idiopathic hirsutism and noted that they tended to be heavier and have poorer metabolic profiles compared to controls. Impaired insulin sensitivity in women with hirsutism alone has been documented by others.34 Although this implies a role for androgen excess in mediating insulin sensitivity, we noted a significant dependence on adiposity in the metabolic profile of this cohort. The presence of PCO alone has been documented in up to 32% of unselected healthy women with regular menstrual cycles, which places into question the specificity of PCO to PCOS.12 Using updated criteria for PCO, we noted that a much lower percentage of healthy women in the general population had isolated PCO (ie, only 7%). Some hypothesize that PCO alone constitutes the mildest form of PCOS as these women showed intermediate serum anti-Mullerian hormone levels35 and responses to a gonadotropin-releasing hormone challenge36 compared to women with Frank PCOS and controls. We noted that women with PCO alone did not differ from controls in their metabolic profile, consistent with previous reports,12,35 but in contrast to others showing subtle variations in insulin sensitivity and androgen levels.36–40 It is difficult to fully appreciate discrepancies between studies since they failed to report actual follicle counts or other sonographic end points for their cohorts.
Our study was limited by several factors. First, our study population had the potential for bias since participants were recruited based on self-reported concerns over PCOS. Therefore, our study was not designed to assess the prevalence of PCOS phenotypes at large but rather the likelihood of encountering PCOS phenotypes in women with concerns over symptoms—much like what happens in clinical practice. It would be expected that those with the most concerns over PCOS would select in for evaluation (ie, Frank PCOS). Second, our PCOS and control populations were not matched for adiposity. Because of this, we performed a correction for BMI, which, as a conservative measure, might have underestimated differences among groups. Although our study was strengthened by the inclusion of healthy control participants from the general population, we noted a tendency for lean rather than overweight women with regular menstrual cycles to present for evaluation as controls. This might have reflected the reduced likelihood of predictable menstruation in overweight/obese women or their reluctance to respond to an ad-seeking “otherwise healthy women.” Third, our comparisons among phenotypes were limited by sample size. Although an assessment of over 100 women allowed us to fulfill our primary objective of describing prevalence of PCOS in a consecutive pool of women and was sufficiently powered (at an α level of .05) to detect differences in metabolic status among women diagnosed with PCOS by NIH (92% power), Rotterdam (88% power), and AE-PCOS Society (90% power) standards, power to detect differences in metabolic parameters among PCOS phenotypes was variable and insufficient in many cases. Fourth, we studied an ethnically diverse cohort that might have influenced findings since PCOS symptoms have been reported to vary among ethnicities.41 Last, the cross-sectional design of our study only allowed for the report of associations among metabolic disturbances and PCOS phenotypes. Future longitudinal studies are needed to address potential causal mechanisms for phenotypic variation in PCOS.
In summary, use of updated criteria for PCO yielded an expected prevalence of PCOS in women self-reporting concerns over PCOS features when using any of the 3 consensus guidelines. By contrast, revised criteria for PCO reduced the prevalence of PCO in healthy women with regular menstrual cycles as well as the occurrence of Non-PCO PCOS, supporting the conclusion that PCO is a significant marker of PCOS. Metabolic abnormalities in PCOS were linked to adiposity, and the absence of metabolic disturbances in normoandrogenic PCOS supports reduced health risks in women without androgen excess. Given recent advances in imaging technology, a reconsideration of criteria for PCO morphology should be implemented when investigating long-term health outcomes in women with clinical variations in PCOS.
Acknowledgments
We are grateful to Francoise Vermeylen, Director of the Cornell Statistical Consulting Unit, for her assistance with the statistical analyses.
Footnotes
Authors’ Note: Nina M. Clark, and Amanda J. Podolski contributed equally to this manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Cornell University and fellowship awards from the Strategic Training Initiative in Research in Reproductive Health Sciences, Saskatchewan Health Research Foundation and Canadian Institutes of Health Research.
References
- 1. Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–191 [Google Scholar]
- 2. Azziz R. Diagnosis of polycystic ovarian syndrome: the Rotterdam criteria are premature. J Clin Endocrinol Metab. 2006;91(3):781–785 [DOI] [PubMed] [Google Scholar]
- 3. Lujan M, Chizen D, Pierson R. Diagnostic criteria for polycystic ovary syndrome: pitfalls and controversies. J Obstet Gynecol Canada. 2008;30(8):671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zawadzki JK, Dunaif A. Diagnostic Criteria for Polycystic Ovary Syndrome: Towards a Rational Approach. Boston: Blackwell Scientific Publications; 1992 [Google Scholar]
- 5. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Fertil Steril. 2004;81(1):19–25 [DOI] [PubMed] [Google Scholar]
- 6. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–47 [DOI] [PubMed] [Google Scholar]
- 7. Azziz R, Carmina E, Dewailly D, et al. Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An androgen excess society guideline. J Clin Endocrinol Metab. 2006;91(11):4237–4245 [DOI] [PubMed] [Google Scholar]
- 8. Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–697 [DOI] [PubMed] [Google Scholar]
- 9. Moghetti P, Tosi F, Bonin C, et al. Divergences in insulin resistance between the different phenotypes of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98(4):E628–E637 [DOI] [PubMed] [Google Scholar]
- 10. Guastella E, Longo RA, Carmina E. Clinical and endocrine characteristics of the main polycystic ovary syndrome phenotypes. Fertil Steril. 2010;94(6):2197–2201 [DOI] [PubMed] [Google Scholar]
- 11. Coulam CB, Annegers JF, Kranz JS. Chronic anovulation syndrome and associated neoplasia. Obstet Gynecol. 1983;61(4):403–407 [PubMed] [Google Scholar]
- 12. Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-rotterdam: A common, age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95(11):4965–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lujan ME, Jarrett BY, Brooks ED, et al. Updated ultrasound criteria for polycystic ovary syndrome: Reliable thresholds for elevated follicle population and ovarian volume. Hum Reprod. 2013;28(5):1361–1368 [DOI] [PubMed] [Google Scholar]
- 14. Dewailly D, Gronier H, Poncelet E, et al. Diagnosis of polycystic ovary syndrome (PCOS): Revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123–3129 [DOI] [PubMed] [Google Scholar]
- 15. Allemand MC, Tummon IS, Phy JL, Foong SC, Dumesic DA, Session DR. Diagnosis of polycystic ovaries by three-dimensional transvaginal ultrasound. Fertil Steril. 2006;85(1):214–219 [DOI] [PubMed] [Google Scholar]
- 16. Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21(11):1440–1447 [DOI] [PubMed] [Google Scholar]
- 17. Fraser IS, Critchley HOD, Munro MG, Broder M. Can we achieve international agreement on terminologies and definitions used to describe abnormalities of menstrual bleeding? Hum Reprod. 2007;22(3):635–643 [DOI] [PubMed] [Google Scholar]
- 18. Lujan ME, Brooks ED, Kepley AL, Chizen DR, Pierson RA, Peppin AK. Grid analysis improves reliability in follicle counts made by ultrasonography in women with polycystic ovary syndrome. Ultrasound Med Biol. 2010;36(5):712–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lujan M, Bloski TG, Chizen DR, Lehotay DC, Pierson RA. Digit ratios do not serve as anatomical evidence of prenatal androgen exposure in clinical phenotypes of polycystic ovary syndrome. Hum Reprod. 2010;25(1):204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Legro RS, Schlaff WD, Diamond MP, et al. Total testosterone assays in women with polycystic ovary syndrome: Precision and correlation with hirsutism. J Clin Endocrinol Metab. 2010;95(12):5305–5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amato MC, Galluzzo A, Finocchiaro S, Criscimanna A, Giordano C. The evaluation of metabolic parameters and insulin sensitivity for a more robust diagnosis of polycystic ovary syndrome. Clin Endocrinol. 2008;69(1):52–60 [DOI] [PubMed] [Google Scholar]
- 22. Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod. 2012;27(10):3067–3073 [DOI] [PubMed] [Google Scholar]
- 23. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551 [DOI] [PubMed] [Google Scholar]
- 24. Pehlivanov B, Orbetzova M. Characteristics of different phenotypes of polycystic ovary syndrome in a Bulgarian population. Gynecol Endocrinol. 2007;23(10):604–609 [DOI] [PubMed] [Google Scholar]
- 25. Diamanti-Kandarakis E, Panidis D. Unravelling the phenotypic map of polycystic ovary syndrome (PCOS): a prospective study of 634 women with PCOS. Clin Endocrinol. 2007;67(5):735–742 [DOI] [PubMed] [Google Scholar]
- 26. Guo M, Chen ZJ, Macklon NS, et al. Cardiovascular and metabolic characteristics of infertile chinese women with PCOS diagnosed according to the rotterdam consensus criteria. Reprod Biomed Online. 2010;21(4):572–580 [DOI] [PubMed] [Google Scholar]
- 27. Ma YM, Li R, Qiao J, Zhang XW, et al. Characteristics of abnormal menstrual cycle and polycystic ovary syndrome in community and hospital populations. Chin Med J (Engl). 2010;123(16):2185–2189 [PubMed] [Google Scholar]
- 28. Yilmaz M, Isaoglu U, Delibas IB, Kadanali S. Anthropometric, clinical and laboratory comparison of four phenotypes of polycystic ovary syndrome based on Rotterdam criteria. J Obstet Gynaecol Res. 2011;37(8):1020–1026 [DOI] [PubMed] [Google Scholar]
- 29. Wijeyaratne CN, Seneviratne Rde A, Dahanayake S, et al. Phenotype and metabolic profile of South Asian women with polycystic ovary syndrome (PCOS): results of a large database from a specialist endocrine clinic. Hum Reprod. 2011;26(1):202–213 [DOI] [PubMed] [Google Scholar]
- 30. Panidis D, Tziomalos K, Misichronis G, et al. Insulin resistance and endocrine characteristics of the different phenotypes of polycystic ovary syndrome: a prospective study. Hum Reprod. 2012;27(2):541–549 [DOI] [PubMed] [Google Scholar]
- 31. Escobar-Morreale HF, Villuendas G, Botella-Carretero JI, Sancho J, San Millan JL. Obesity, and not insulin resistance, is the major determinant of serum inflammatory cardiovascular risk markers in pre-menopausal women. Diabetologia. 2003;46(5):625–633 [DOI] [PubMed] [Google Scholar]
- 32. Panidis D, Tziomalos K, Papadakis E, et al. The clinical significance and primary determinants of hirsutism in patients with polycystic ovary syndrome. Eur J Endocrinol. 2013;168(6):871–877 [DOI] [PubMed] [Google Scholar]
- 33. Nybacka A, Carlstrom K, Stahle A, Nyren S, Hellstrom PM, Hirschberg AL. Randomized comparison of the influence of dietary management and/or physical exercise on ovarian function and metabolic parameters in overweight women with polycystic ovary syndrome. Fertil Steril. 2011;96(6):1508–1513 [DOI] [PubMed] [Google Scholar]
- 34. Ucak S, Basat O, Satir E, Altunas Y. Evaluation of various insulin sensitivity indices in lean idiopathic hirsutism patients. Endocr J. 2012;59(4):291–296 [DOI] [PubMed] [Google Scholar]
- 35. Catteau-Jonard S, Bancquart J, Poncelet E, Lefebvre-Maunoury C, Robin G, Dewailly D. Polycystic ovaries at ultrasound: Normal variant or silent polycystic ovary syndrome? Ultrasound Obstet Gynecol. 2012;40(2):223–229 [DOI] [PubMed] [Google Scholar]
- 36. Chang PL, Lindheim SR, Lowre C, et al. Normal ovulatory women with polycystic ovaries have hyperandrogenic pituitary-ovarian responses to gonadotropin-releasing hormone-agonist testing. J Clin Endocrinol Metab. 2000;85(3):995–1000 [DOI] [PubMed] [Google Scholar]
- 37. Carmina E, Wong L, Chang L, et al. Endocrine abnormalities in ovulatory women with polycystic ovaries on ultrasound. Hum Reprod. 1997;12(5):905–909 [DOI] [PubMed] [Google Scholar]
- 38. Adams JM, Taylor AE, Crowley WF, Hall JE. Polycystic ovarian morphology with regular ovulatory cycles: Insights into the pathophysiology of polycystic ovarian syndrome. J Clin Endocrinol Metab. 2004;89(9):4343. [DOI] [PubMed] [Google Scholar]
- 39. Norman RJ, Hague WM, Masters SC, Wang XJ. Subjects with polycystic ovaries without hyperandrogenaemia exhibit similar disturbances in insulin and lipid profiles as those with polycystic ovary syndrome. Hum Reprod. 1995;10(9):2258–2261 [DOI] [PubMed] [Google Scholar]
- 40. Cenk Sayin N, Gücer F, Balkanli-Kaplan P, Ali Yüce M, Yardim T. Insulin resistance and lipid profile in women with polycystic appearing ovaries: implications with regard to polycystic ovary syndrome. Gynecol Endocrinol. 2003;17(5):387–396 [DOI] [PubMed] [Google Scholar]
- 41. Wang S, Alvero R. Racial and ethnic differences in physiology and clinical symptoms of polycystic ovary syndrome. Semin Reprod Med. 2013;31(5):365–369 [DOI] [PubMed] [Google Scholar]