Abstract
There is now accumulating evidence that endometriosis is a disease associated with an epigenetic disorder. Genomic imprinting is an epigenetic phenomenon known to regulate DNA methylation of either maternal or paternal alleles. We hypothesize that hypermethylated endometriosis-associated genes may be enriched at imprinted gene loci. We sought to determine whether downregulated genes associated with endometriosis susceptibility are associated with chromosomal location of the known paternally and maternally expressed imprinting genes. Gene information has been gathered from National Center for Biotechnology Information database geneimprint.com. Several researchers have identified specific loci with strong DNA methylation in eutopic endometrium and ectopic lesion with endometriosis. Of the 29 hypermethylated genes in endometriosis, 19 genes were located near 45 known imprinted foci. There may be an association of the genomic location between genes specifically downregulated in endometriosis and epigenetically imprinted genes.
Keywords: endometriosis, imprinting genes, chromosomal location, hypermethylation
Introduction
Endometriosis represents a common gynecological disease, but the mechanisms contributing to the establishment of this disorder lesions still remain controversial.1 Retrograde transplantation of endometrial tissue into the pelvic cavity at the time of menstruation is believed to account for peritoneal endometriosis. Nobody explains why most women of reproductive age experience retrograde menstruation but do not develop endometriosis.2
One possibility is that eutopic endometrium in women who will develop endometriosis in later life has increased proliferative potential, which promotes establishment of endometriotic lesions, possibly through aberrant expression of specific genes in the eutopic endometrium. Epigenetic alterations have been explored in pathological conditions such as endometriosis. The previous report showed that many decidualization-related genes were downregulated in eutopic endometrium of endometriosis.3–10 The promoter region of these genes has been hypermethylated in endometriosis when compared with controls. Twenty-nine endometriosis susceptibility genes were silenced by epigenetic aberration (Table 1, left column). These genes are considered to be upregulated during the decidualization process for successful implantation and pregnancy, suggesting that dysfunctional expression of decidualization-related genes in endometrium appears critical to endometriosis.56
Table 1.
Endometriosis-Susceptibility Genes Silenced By Epigenetic Aberration (Left Column) Located on Chromosomes Where Human Imprinting Genes Are Located (Right Column).a
| Genes Specifically Downregulated in Endometriosis (Endometriosis Susceptibility Genes) | The Known Imprinted Genes Near Endometriosis-Susceptibility Genes | |||||
|---|---|---|---|---|---|---|
| Location | Gene Symbol | Reference | Location | Gene Symbol | Paternal/Maternal | Reference |
| 1p31.2 | PGE2R | 11,12 | 1p31 AS | DIRAS3 | Paternal | 13–15 |
| 2q14.2 | IL1RN | |||||
| 2q33-q35 | IHH | 16 | 2q33.3 AS | GPR1 | Paternal | 17 |
| 2q33.3 | ZDBF2 | Paternal | ||||
| 4q13-q21 | IL8 | |||||
| 5q23 | HBEGF | |||||
| 6p21.2 | CDKN1A | 6q21 | AIM1, LIN28B | Paternal | 18,19 | |
| 6p21 AS | BTNL2 | Maternal | ||||
| 6p22.3 | PRL | |||||
| 6q25.1 | ESR1 | |||||
| 7p13-p12 | IGFBP1 | 20 | 7p13 AS | GLI3 | Maternal | 21 |
| 7p15.2 | HOXA10 | 22,23 | 7p15-p14 AS | HOXA5, HOXA4, HOXA2, HOXA11, HOXA3 | Maternal | 24–26 |
| 7p15-p14 | EVX1 | Paternal | ||||
| 7p21 | IL6 | |||||
| 7p21.2 | TWIST | |||||
| 9p21 | CDKN2A | 9q21.13 | C9orf85 | Paternal | ||
| 9p21 | CDKN2B | 9q21.32 | FLJ46321 | Maternal | ||
| 9q34 | PAEP | 9q34.3 | PHPT1 | Maternal | 27–30 | |
| 9q34.3 | EGFL7 | Paternal | ||||
| 11q22-q23 | PR-B | 6 | 11q22.3 | ZC3H12C | Paternal | 31–34 |
| 11q22.3 AS | KBTBD3 | Paternal | ||||
| 12p13.1-p12 | CDKN1B | 35 | 12p12.1 AS | ABCC9 | Maternal | 36–38 |
| 12p13.31 AS | RBP5 | Maternal | ||||
| 12p13.33 | FKBP4 | 38 | 12p13.31 AS | RBP5 | Maternal | 37 |
| 13q14.1 | FOXO1 | 39 | 13q14-q21 AS | HTR2A | Maternal | |
| 13q14.2 | RB1 | Maternal | ||||
| 16q22.1 | CDH1 | 5,40 | 16q22.1 AS | ACD | Maternal | 41,42 |
| 17q11.2 | CCL8 | 17q11 | PYY2 | Paternal | 43 | |
| 19q13.1 | TGFbeta | 44 | 19q13.1 | CHST8 | Maternal | |
| 19q13.3-q13.4 | IL11 | 45 | 19q13.4 AS | PEG3, ZIM2 | Paternal | 46–49 |
| 19q13.4 AS | MZF1 | Maternal | ||||
| 19q13.4 | MIMT1, ZNF264 | Paternal | ||||
| 19q13.4 | LILRB4 | Maternal | ||||
| 20p12 | BMP2 | 50 | 20p12.1 | C20orf82, ISM1 | Paternal | 51 |
| 20q12-q13 | SRC1 | 20q13.3 | COL9A3, GNAS | Maternal | 52–55 | |
| 20q13.32 AS | GNASAS, MIR296, MIR298 | Paternal | ||||
| 20q13.32 | SANG | Paternal | ||||
| 20q13.33 | C20orf20 | Maternal | ||||
| 22q12.2 | LIF | 22q12.2 | FLJ20464 | Paternal | ||
| 22q12.3 | TIMP3 | |||||
| 20q13.1 | C/EBPbeta | |||||
| Xp11.22-p11.21 | TRO | |||||
a The imprinted gene information has been gathered from NCBI database geneimprint.com (http://www.geneimprint.com/site/genes-by-species). Left column, 29 hypermethylated genes were included as our working data set. Right column, 45 known imprinted genes that are located within or in close proximity to endometriosis susceptibility genes.
The decidualization process is characterized by terminal differentiation of endometrial stromal cells.57 Synchronization of embryonic development with the stromal decidualization is a key step for the establishment of successful implantation and pregnancy. A defective decidualization response might be associated with a reduced differentiation and a relatively increased proliferative potential. Targeted deletion of decidualization-related genes such as HOXA10 and cyclooxygenase 2 has demonstrated marked infertility.58,59 A decrease in these gene expressions in eutopic endometrium has been found in women with endometriosis-associated infertility.60 Promoter hypermethylation of eutopic endometrium is considered to be the leading mechanism for epigenetic gene regulation in women who will develop endometriosis in later life.61
Genomic imprinting, DNA methylation, and chromatin remodeling are known to regulate transcription of target genes. Genomic imprinting is an epigenetic process by which the male and female germ lines guide the allele-specific DNA methylation marking and histone modification onto specific gene regions of parental alleles.62 The majority of imprinted genes tend to be nonrandomly grouped in clusters.13 The unique chromatin packaging at certain gene promoters provides these genomic loci how and when a gene becomes accessible or inaccessible to the transcription machinery. Since, in mammals, imprinted genes are critical for normal development, growth, behavior, and numerous physiological processes, endometriosis susceptibility genes affected by the loss or duplication of the active allele can cause disease. Misregulation of the clusters of imprinted genes is implicated in several diseases including Beckwith-Wiedemann syndrome, Silver-Russell syndrome, Prader-Willi syndrome, Angelman syndrome, pseudohypoparathyroidism, transient neonatal diabetes, metabolic syndrome, and cancer.13 This phenomenon may expand the significance of the epigenome in endometriosis development. Simultaneous hypermethylation in targeted decidualization-related genes via the unique chromatin packaging may be an important mechanism producing rapid epigenetic variation in endometriosis development. Although downregulation of decidualization-related genes has been implicated in endometriosis, genomic interactions between endometriosis-associated hypermethylated genes and epigenetically imprinted genes are poorly understood.
Therefore, we hypothesize that hypermethylated endometriosis genes may be enriched at imprinted gene loci. The objective of this study was to determine an association of the genomic location between hypermethylated gene sites in endometriosis and known imprinted gene regions.
Materials and Methods
A computerized literature search was performed to identify relevant studies reported in English language. We searched MEDLINE electronic databases (http://www.ncbi.nlm.nih.gov/sites/entrez) published up to October 2013, combining the key words “endometriosis,” “maternal,” “paternal,” “imprinting,” and “programming.” Various combinations of the terms were used, depending on the database searched. The imprinted gene information has been gathered from National Center for Biotechnology Information (NCBI) database geneimprint.com (http://www.geneimprint.com/site/genes-by-species). Each gene was also linked to NCBI Entrez Gene pages (http://www.ncbi.nlm.nih.gov/sites/entrez). In addition, references in each article were searched to identify potentially missed studies.
Results
The analysis of parent-of-origin patterns of expression resulted in the identification of 83 imprinted genes in human, including 55 paternally expressed genes (PEGs) and 28 maternally expressed genes (MEGs) (http://www.geneimprint.com/site/genes-by-species). Among a total of 83 genes identified as parentally imprinted genes, 16 genes were associated with human reproduction and embryogenesis. They include 10 PEGs (DIRAS3,14–16 BMP8B,63–65 CYP1B1,66,67 ZFAT,68–70 ZFAT-AS1,68–70 IGF2,70–74 IGF2AS,75 MIMT1,76 MIR296,77,78 and MIR29877,78) and 6 MEGs (DVL1,72,79–82 FGFRL1,83 CDKN1C,84 PEG10,85 KCNQ1DN,86 and KCNQ187; Supplementary data 1). Supplementary data 1 shows the known paternally and maternally expressed imprinting genes associated with human reproduction and embryogenesis.
Some researchers have performed an integrative pathway analysis of a microarray gene expression and proteomics data sets in endometriosis.88–92 We obtained a comprehensive pathway annotation set of the endometriosis susceptibility genes specifically defined based on insufficient decidualization from knowledge-based public resources.88 Table 1 shows endometriosis susceptibility genes silenced by epigenetic aberration located on chromosomes where human imprinting genes are located. Supplementary data 2 shows the endometriosis-susceptibility genes that are located in close proximity to the known imprinting genes. In all, 29 hypermethylated genes were previously analyzed89–91 and included as our working data set. We have examined the relation between these genes and their location in the human genome that is available in the imprinting gene database (http://www.geneimprint.com/site/genes-by-species).
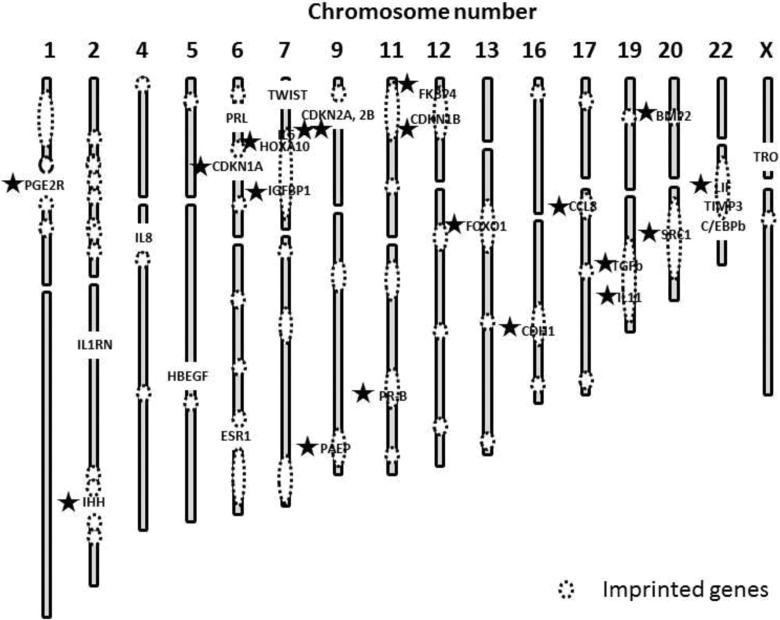
Of the 29 hypermethylated genes, we emphasized 19 pairs of interactions between endometriosis-susceptibility genes and parentally imprinted genes with potential implication. A total of 19 genes were located near 45 known imprinted foci (Figure 1).
Figure 1.
Geographical interactions between endometriosis-susceptibility genes and parentally imprinted genes of the 29 hypermethylated genes, 19 genes (⋆) were located within, or in close proximity to, 45 known imprinted foci (dot circle).
Discussion
This study revealed that among 29 decidualization-related genes specifically downregulated in endometriosis, 19 genes were located within, or in close proximity to, the 45 known imprinting gene loci. However, there has been so far virtually no data that 19 genes are located directly adjacent to these imprinted genes in a “head-to-head” orientation that regulates adjacent genes organized in a divergent fashion. This study also does not confirm functional interactions between endometriosis genes and imprinted genes.
The following 2 or 3 examples may exemplify functional interactions between genes associated with disease susceptibility and imprinted genes. First, Greig cephalopolysyndactyly syndrome (GCPS) is a rare autosomal dominant disorder, caused by genetic alterations in the GLI3 imprinting gene. Greig cephalopolysyndactyly syndrome is mainly characterized by craniofacial abnormalities and limb malformations. Wagner et al showed hemizygosity for the genes of IGFBP1 and GLI3, located on 7q13, in patients with GCPS.93 Second, a monoallelic loss of FOXO1 gene and RB1 imprinting gene, located on 13q14, was identified in some cases of cellular angiofibroma, a rare benign stromal tumor of the female genital region.94 Loss of heterozygosity or epigenetic alterations in targeted genes colocalized or near localized within certain imprinted loci have been explored in these disorders, which may appear to result from altered activity of one or more genes near the imprinted gene cluster. In future, genome-wide analysis of the location of histones and histone modifications will be analyzed by isolation and purification of DNA bound to histones and protamines for evaluating DNA methylation status of candidate genes in endometriosis.
Allele-specific DNA methylation is well studied in imprinted domains by parent-of-origin mechanism, with the maintenance of genomic imprinting memory.13 On the other hand, differential epigenetic marking or epigenetic asymmetry is found more commonly at nonimprinted loci, where the DNA methylation is dictated by the local haplotype. Epigenetic alterations in targeted genes colocalized or near localized within certain imprinted loci have been explored in pathological conditions such as male infertility. Hammoud et al reported that, unlike fertile men, infertile men had changes in the amount of H3 lysine 4 or H3 lysine 27 methylation retained at developmental transcription factors and certain imprinted genes.95 The genome-wide analysis of epigenetic markings in the sperm of infertile men demonstrates differences in composition and epigenetic markings compared with fertile men, especially at certain imprinted and developmental loci.
The gametic differentially methylated regions (DMRs) are directly inherited from the mature gametes during spermatogenesis or oogenesis at fertilization. The somatic DMRs are only acquired in postimplantation embryos during embryogenesis. The analysis of endometriosis-specific genes enriched in a set of DMRs using the mature gametes or somatic cells in patients with endometriosis may offer the opportunity to elucidate the dynamics of genomic methylation of gametic or somatic imprinting.
The fetal origin hypothesis of a wide range of adverse outcomes in later life, including adult obesity, type 2 diabetes, hypertension, and cardiovascular diseases, in persons born with fetal malnutrition has been well established.96 Maternal nutrition seems to play a key role in fetal programming of metabolic and cardiovascular risks.97 Similar to developmental origins of health and disease theory, maternal conditions, lifestyle factors, and intrauterine adverse environment during pregnancy may increase endometriosis risk in adulthood.11 Decidualization resistance resulting from the restriction of intrauterine development of fetal uterus is considered as an important cause of endometriosis in adults.12,17 Decline in decidualization-related genes in the fetus may contribute to endometriosis and infertility in her later life. Shared genetics between endometriosis and infertility could account for this association. Epigenetic imprinting can underlie the described pathomechanisms.
In conclusion, the present study found that there is an association of the genomic location between genes specifically downregulated in endometriosis and epigenetically imprinted genes. Future studies are needed to explore the functional interactions and actual distances/differences in localization between the imprinted genes and hypermethylated endometriotic genes.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by Grant-in-aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan to the Department of Obstetrics and Gynecology, Nara Medical University (H. Kobayashi).
Supplemental Material: The online supplemental data are available at http://rs.sagepub.com/supplemental
References
- 1. Acién P, Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet Gynecol. 2013;2013:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Delbandi AA, Mahmoudi M, Shervin A, et al. Eutopic and ectopic stromal cells from patients with endometriosis exhibit differential invasive, adhesive, and proliferative behavior. Fertil Steril. 2013;100(3):761–769 [DOI] [PubMed] [Google Scholar]
- 3. Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1(2):106–111 [DOI] [PubMed] [Google Scholar]
- 4. Guo SW. Epigenetics of endometriosis. Mol Hum Reprod. 2009;15(10):587–607 [DOI] [PubMed] [Google Scholar]
- 5. Monteiro JB, Colón-Díaz M, García M, et al. Endometriosis is characterized by a distinct pattern of histone 3 and histone 4 lysine modifications. Reprod Sci. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nasu K, Kawano Y, Tsukamoto Y, et al. Aberrant DNA methylation status of endometriosis: epigenetics as the pathogenesis, biomarker and therapeutic target. J Obstet Gynaecol Res. 2011;37(7):683–695 [DOI] [PubMed] [Google Scholar]
- 7. Izawa M, Taniguchi F, Terakawa N, Harada T. Epigenetic aberration of gene expression in endometriosis. Front Biosci (Elite Ed). 2013;5:900–910 [DOI] [PubMed] [Google Scholar]
- 8. Colón-Díaz M, Báez-Vega P, García M, et al. HDAC1 and HDAC2 are differentially expressed in endometriosis. Reprod Sci. 2012;19(5):483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cakmak H, Taylor HS. Implantation failure: molecular mechanisms and clinical treatment. Hum Reprod Update. 2011;17(2):242–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawano Y, Nasu K, Li H, et al. Application of the histone deacetylase inhibitors for the treatment of endometriosis: histone modifications as pathogenesis and novel therapeutic target. Hum Reprod. 2011;26(9):2486–2498 [DOI] [PubMed] [Google Scholar]
- 11. Benyshek DC. The “early life” origins of obesity-related health disorders: new discoveries regarding the intergenerational transmission of developmentally programmed traits in the global cardiometabolic health crisis. Am J Phys Anthropol. 2013;152(suppl 57):79–93 [DOI] [PubMed] [Google Scholar]
- 12. Kao LC, Germeyer A, Tulac S, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinol. 2003;144(7):2870–2881 [DOI] [PubMed] [Google Scholar]
- 13. Chen Z, Robbins KM, Wells KD, Rivera RM. Large offspring syndrome: a bovine model for the human loss-of-imprinting overgrowth syndrome Beckwith-Wiedemann. Epigenetics. 2013;8(6):591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu Z, Luo RZ, Lu Y, et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J Clin Invest. 2008;118(12):3917–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu F, Xia W, Luo RZ, et al. The human ARHI tumor suppressor gene inhibits lactation and growth in transgenic mice. Cancer Res. 2000;60(17):4913–4920 [PubMed] [Google Scholar]
- 16. Li J, Wang SJ, Sun L, Li YL. Differential expression of the anti-oncogene ARHI between patients with and without endometriosis. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31(5):796–800 [PubMed] [Google Scholar]
- 17. Tomassetti C, Meuleman C, Pexsters A, et al. Endometriosis, recurrent miscarriage and implantation failure: is there an immunological link? Reprod Biomed Online. 2006;13(1):58–64 [DOI] [PubMed] [Google Scholar]
- 18. Chuang PC, Sun HS, Chen TM, Tsai SJ. Prostaglandin E2 induces fibroblast growth factor 9 via EP3-dependent protein kinase Cdelta and Elk-1 signaling. Mol Cell Biol. 2006;26(22):8281–8292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kusakabe KT, Abe H, Kondo T, Kato K, Okada T, Otsuki Y. DNA microarray analysis in a mouse model for endometriosis and validation of candidate factors with human adenomyosis. J Reprod Immunol. 2010;85(2):149–160 [DOI] [PubMed] [Google Scholar]
- 20. Karagiannis GS, Weile J, Bader GD, Minta J. Integrative pathway dissection of molecular mechanisms of moxLDL-induced vascular smooth muscle phenotype transformation. BMC Cardiovasc Disord. 2013;13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoshimoto S, Kuo CT, Chong KK, et al. AIM1 and LINE-1 epigenetic aberrations in tumor and serum relate to melanoma progression and disease outcome. J Invest Dermatol. 2012;132(6):1689–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leinonen JT, Surakka I, Havulinna AS, et al. Association of LIN28B with adult adiposity-related traits in females. PLoS One 2012;7(11):e48785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yin X, Pavone ME, Lu Z, Wei J, Kim JJ. Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J Clin Endocrinol Metab. 2012;97(1):E35–E43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Biesecker LG. The Greig cephalopolysyndactyly syndrome. Orphanet J Rare Dis. 2008;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zanatta A, Rocha AM, Carvalho FM, et al. The role of the Hoxa10/HOXA10 gene in the etiology of endometriosis and its related infertility: a review. J Assist Reprod Genet. 2010;27(12):701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mormile R, Vittori G. Endometriosis: a history written by aberrant hoxa10 gene expression and epidermal growth factor (EGF) system polymorphism? J Reprod Infertil. 2013;14(1):46–47 [PMC free article] [PubMed] [Google Scholar]
- 27. Kalisz M, Winzi M, Bisgaard HC, Serup P. EVEN-SKIPPED HOMEOBOX 1 controls human ES cell differentiation by directly repressing GOOSECOID expression. Dev Biol. 2012;362(1):94–103 [DOI] [PubMed] [Google Scholar]
- 28. Naik S, Riordan-Eva E, Thomas NS, et al. Large de novo deletion of 7p15.1 to 7p12.1 involving the imprinted gene GRB10 associated with a complex phenotype including features of Beckwith Wiedemann syndrome. Eur J Med Genet. 2011;54(1):89–93 [DOI] [PubMed] [Google Scholar]
- 29. Powlesland RM, Charles AK, Malik KT, et al. Loss of heterozygosity at 7p in Wilms' tumour development. Br J Cancer. 2000;82(2):323–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Charpentier MS, Dorr KM, Conlon FL. Transcriptional regulation of blood vessel formation: the role of the CASZ1/Egfl7/RhoA pathway. Cell Cycle. 2013;12(14):2165–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pinte SB, Soncin F. Egfl7 promotes tumor escape from immunity. Oncoimmunol. 2012;1(3):375–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu AJ, Xia XH, DU ST, Gu JC. Clinical significance of PHPT1 protein expression in lung cancer. Chin Med J (Engl). 2010;123(22):3247–3251 [PubMed] [Google Scholar]
- 33. Xiong DH, Wang JT, Wang W, et al. Genetic determination of osteoporosis: lessons learned from a large genome-wide linkage study. Hum Biol. 2007;79(6):593–608 [DOI] [PubMed] [Google Scholar]
- 34. Liu L, Zhou Z, Huang S, et al. Zc3h12c inhibits vascular inflammation by repressing NF-κB activation and pro-inflammatory gene expression in endothelial cells. Biochem J. 2013;451(1):55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Milunsky J, DeStefano AL, Huang XL, et al. Familial paragangliomas: linkage to chromosome 11q23 and clinical implications. Am J Med Genet. 1997;72(1):66–70 [DOI] [PubMed] [Google Scholar]
- 36. Hiura H, Sugawara A, Ogawa H, et al. A tripartite paternally methylated region within the Gpr1-Zdbf2 imprinted domain on mouse chromosome 1 identified by meDIP-on-chip. Nucleic Acids Res. 2010;38(15):4929–4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu HK, Squire JA, Catzavelos CG, Weksberg R. Relaxation of imprinting of human insulin-like growth factor II gene, IGF2, in sporadic breast carcinomas. Biochem Biophys Res Commun. 1997;235(1):123–129 [DOI] [PubMed] [Google Scholar]
- 38. Camargo-Kosugi CM, da Silva ID, Sato H, et al. The V109G polymorphism in the p27 gene is associated with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2009;145(2):180–183 [DOI] [PubMed] [Google Scholar]
- 39. Czeschik JC, Voigt C, Goecke TO, et al. Wide clinical variability in conditions with coarse facial features and hypertrichosis caused by mutations in ABCC9. Am J Med Genet A. 2013;161A(2):295–300 [DOI] [PubMed] [Google Scholar]
- 40. Ho JC, Cheung ST, Poon WS, Lee YT, Ng IO, Fan ST. Down-regulation of retinol binding protein 5 is associated with aggressive tumor features in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2007;133(12):929–936 [DOI] [PubMed] [Google Scholar]
- 41. Yang H, Zhou Y, Edelshain B, Schatz F, Lockwood CJ, Taylor HS. FKBP4 is regulated by HOXA10 during decidualization and in endometriosis. Reproduction 2012;143(4):531–538 [DOI] [PubMed] [Google Scholar]
- 42. Shazand K, Baban S, Privé C, et al. FOXO1 and c-jun transcription factors mRNA are modulated in endometriosis. Mol Hum Reprod. 2004;10(12):871–877 [DOI] [PubMed] [Google Scholar]
- 43. Govatati S, Tangudu NK, Deenadayal M, Chakravarty B, Shivaji S, Bhanoori M. Association of E-cadherin single nucleotide polymorphisms with the increased risk of endometriosis in Indian women. Mol Hum Reprod. 2012;18(5):280–287 [DOI] [PubMed] [Google Scholar]
- 44. Hutz JE, Krause AS, Achermann JC, et al. IMAGe association and congenital adrenal hypoplasia: no disease-causing mutations found in the ACD gene. Mol Genet Metab. 2006;88(1):66–70 [DOI] [PubMed] [Google Scholar]
- 45. O'Connor BC, Macke EL, Keegan CE. Additive effect of TAp63 deficiency on the adrenocortical dysplasia (acd) phenotype. Mamm Genome. 2011;22(11-12):714–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. De Silva A, Bloom SR. Gut Hormones and Appetite Control: A Focus on PYY and GLP-1 as Therapeutic Targets in Obesity. Gut Liver. 2012;6(1):10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Whyte MP, Totty WG, Novack DV, Zhang X, Wenkert D, Mumm S. Camurati-Engelmann disease: unique variant featuring a novel mutation in TGFβ1 encoding transforming growth factor beta 1 and a missense change in TNFSF11 encoding RANK ligand. J Bone Miner Res. 2011;26(5):920–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mikolajczyk M, Wirstlein P, Skrzypczak J. Leukaemia inhibitory factor and interleukin 11 levels in uterine flushings of infertile patients with endometriosis. Hum Reprod. 2006;21(12):3054–3058 [DOI] [PubMed] [Google Scholar]
- 49. Jiang X, Yu Y, Yang HW, Agar NY, Frado L, Johnson MD. The imprinted gene PEG3 inhibits Wnt signaling and regulates glioma growth. J Biol Chem. 2010;285(11):8472–8480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Deng Y, Wang J, Wang G, et al. p55PIK transcriptionally activated by MZF1 promotes colorectal cancer cell proliferation. Biomed Res Int. 2013;2013:868131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Porcellini E, Carbone I, Martelli PL, et al. Haplotype of single nucleotide polymorphisms in exon 6 of the MZF-1 gene and Alzheimer's disease. J Alzheimers Dis. 2013;34(2):439–447 [DOI] [PubMed] [Google Scholar]
- 52. Flisikowski K, Venhoranta H, Nowacka-Woszuk J, et al. A novel mutation in the maternally imprinted PEG3 domain results in a loss of MIMT1 expression and causes abortions and stillbirths in cattle (Bos taurus). PLoS One. 2010;5(11):e15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kurihara I, Lee DK, Petit FG, et al. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3(6):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yuan B, Xian R, Ma J, Chen Y, Lin C, Song Y. Isthmin inhibits glioma growth through antiangiogenesis in vivo. J Neurooncol. 2012;109(2):245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tobi EW, Heijmans BT, Kremer D, et al. DNA methylation of IGF2, GNASAS, INSIGF and LEP and being born small for gestational age. Epigenetics. 2011;6(2):171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kobayashi H, Uekuri C, Shigetomi H. Towards an understanding of the molecular mechanism of endometriosis: unbalancing epithelial-stromal genetic conflict. Gynecol Endocrinol. 2014;30(1):7–15 [DOI] [PubMed] [Google Scholar]
- 57. Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25(6):445–453 [DOI] [PubMed] [Google Scholar]
- 58. Sroga JM, Gao F, Ma X, Das SK. Overexpression of cyclin D3 improves decidualization defects in Hoxa-10(-/-) mice. Endocrinol. 2012;153(11):5575–5586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang H, Ma WG, Tejada L, et al. Rescue of female infertility from the loss of cyclooxygenase-2 by compensatory up-regulation of cyclooxygenase-1 is a function of genetic makeup. J Biol Chem. 2004;279(11):10649–10658 [DOI] [PubMed] [Google Scholar]
- 60. Fambrini M, Sorbi F, Bussani C, Cioni R, Sisti G, Andersson KL. Hypermethylation of HOXA10 gene in mid-luteal endometrium from women with ovarian endometriomas. Acta Obstet Gynecol Scand. 2013;92(11):1331–1334 [DOI] [PubMed] [Google Scholar]
- 61. Kobayashi H, Iwai K, Niiro E, Morioka S, Yamada Y. Fetal programming theory: Implication for the understanding of endometriosis [published online December 27, 2013]. Hum Immunol. 2013 [DOI] [PubMed] [Google Scholar]
- 62. Swaney WT. Genomic imprinting and mammalian reproduction. Horm Behav. 2011;59(3):369–374 [DOI] [PubMed] [Google Scholar]
- 63. Wetendorf M, DeMayo FJ. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol Cell Endocrinol. 2012;357(1-2):108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mima K, Fukagawa T, Kurashige J, et al. Gene expression of bone morphogenic protein 8B in the primary site, peripheral blood and bone marrow of patients with gastric cancer. Oncol Lett. 2013;6(2):387–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Whittle AJ, Carobbio S, Martins L, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149(4):871–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kaur-Knudsen D, Bojesen SE, Nordestgaard BG. Cytochrome P450 1B1 and 2C9 genotypes and risk of ischemic vascular disease, cancer, and chronic obstructive pulmonary disease. Curr Vasc Pharmacol. 2012;10(4):512–520 [DOI] [PubMed] [Google Scholar]
- 67. Li YG, Wang X. Association of the CYP1B1 gene polymorphism with susceptibility to endometriosis. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2009;26(1):66–69 [DOI] [PubMed] [Google Scholar]
- 68. Doi K, Fujimoto T, Okamura T, et al. ZFAT plays critical roles in peripheral T cell homeostasis and its T cell receptor-mediated response. Biochem Biophys Res Commun. 2012;425(1):107–112 [DOI] [PubMed] [Google Scholar]
- 69. Yoshida Y, Tsunoda T, Takashima Y, et al. ZFAT is essential for endothelial cell assembly and the branch point formation of capillary-like structures in an angiogenesis model. Cell Mol Biol Lett. 2010;15(4):541–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Barbaux S, Gascoin-Lachambre G, Buffat C, et al. A genome-wide approach reveals novel imprinted genes expressed in the human placenta. Epigenetics. 2012;7(9):1079–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bergman D, Halje M, Nordin M, Engström W. Insulin-like growth factor 2 in development and disease: a mini-review. Gerontology. 2013;59(3):240–249 [DOI] [PubMed] [Google Scholar]
- 72. St-Pierre J, Hivert MF, Perron P, et al. IGF2 DNA methylation is a modulator of newborn's fetal growth and development. Epigenetics. 2012;7(10):1125–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rancourt RC, Harris HR, Barault L, Michels KB. The prevalence of loss of imprinting of H19 and IGF2 at birth. FASEB J. 2013;27(8):3335–3343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim H, Park JH, Ku SY, Kim SH, Choi YM, Kim JG. Association between endometriosis and polymorphisms in insulin-like growth factors (IGFs) and IGF-I receptor genes in Korean women. Eur J Obstet Gynecol Reprod Biol. 2011;156(1):87–90 [DOI] [PubMed] [Google Scholar]
- 75. Devaney JM, Hoffman EP, Gordish-Dressman H, Kearns A, Zambraski E, Clarkson PM. IGF-II gene region polymorphisms related to exertional muscle damage. J Appl Physiol. 2007;102(5):1815–1823 [DOI] [PubMed] [Google Scholar]
- 76. Flisikowski K, Venhoranta H, Nowacka-Woszuk J, et al. A novel mutation in the maternally imprinted PEG3 domain results in a loss of MIMT1 expression and causes abortions and stillbirths in cattle (Bos taurus). PLoS One. 2010;5(11):e15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Choi SY, Yun J, Lee OJ, et al. MicroRNA expression profiles in placenta with severe preeclampsia using a PNA-based microarray. Placenta. 2013;34(9):799–804 [DOI] [PubMed] [Google Scholar]
- 78. Robson JE, Eaton SA, Underhill P, Williams D, Peters J. MicroRNAs 296 and 298 are imprinted and part of the GNAS/Gnas cluster and miR-296 targets IKBKE and Tmed9. RNA. 2012;18(1):135–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dillman AR, Minor PJ, Sternberg PW. Origin and evolution of dishevelled. G3 (Bethesda). 2013;3(2):251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Elbert M, Cohen D, Müsch A. PAR1b promotes cell-cell adhesion and inhibits dishevelled-mediated transformation of Madin-Darby canine kidney cells. Mol Biol Cell 2006;17(8):3345–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ostern R, Fagerheim T, Hjellnes H, Nygård B, Mellgren SI, Nilssen Ø. Diagnostic laboratory testing for Charcot Marie Tooth disease (CMT): the spectrum of gene defects in Norwegian patients with CMT and its implications for future genetic test strategies. BMC Med Genet. 2013;14:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sonderegger S, Pollheimer J, Knöfler M. Wnt signalling in implantation, decidualisation and placental differentiation—review. Placenta. 2010;31(10):839–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kang S, Li SZ, Wang N, et al. Association between genetic polymorphisms in fibroblast growth factor (FGF)1 and FGF2 and risk of endometriosis and adenomyosis in Chinese women. Hum Reprod. 2010;25(7):1806–1811 [DOI] [PubMed] [Google Scholar]
- 84. Qian K, Chen H, Wei Y, Hu J, Zhu G. Differentiation of endometrial stromal cells in vitro: down-regulation of suppression of the cell cycle inhibitor p57 by HOXA10? Mol Hum Reprod. 2005;11(4):245–251 [DOI] [PubMed] [Google Scholar]
- 85. Dória S, Sousa M, Fernandes S, et al. Gene expression pattern of IGF2, PHLDA2, PEG10 and CDKN1C imprinted genes in spontaneous miscarriages or fetal deaths. Epigenetics. 2010;5(5):444–450 [DOI] [PubMed] [Google Scholar]
- 86. Xin Z, Soejima H, Higashimoto K, et al. A novel imprinted gene, KCNQ1DN, within the WT2 critical region of human chromosome 11p15.5 and its reduced expression in Wilms' tumors. J Biochem. 2000;128(5):847–853 [DOI] [PubMed] [Google Scholar]
- 87. Travers ME, Mackay DJ, Dekker Nitert M, et al. Insights into the molecular mechanism for type 2 diabetes susceptibility at the KCNQ1 locus from temporal changes in imprinting status in human islets. Diabetes. 2013;62(3):987–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Munro SK, Farquhar CM, Mitchell MD, Ponnampalam AP. Epigenetic regulation of endometrium during the menstrual cycle. Mol Hum Reprod. 2010;16(5):297–310 [DOI] [PubMed] [Google Scholar]
- 89. Petracco RG, Kong A, Grechukhina O, Krikun G, Taylor HS. Global gene expression profiling of proliferative phase endometrium reveals distinct functional subdivisions. Reprod Sci. 2012;19(10):1138–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Scotchie JG, Fritz MA, Mocanu M, Lessey BA, Young SL. Proteomic analysis of the luteal endometrial secretome. Reprod Sci. 2009;16(9):883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sakai N, Maruyama T, Sakurai R, et al. Involvement of histone acetylation in ovarian steroid-induced decidualization of human endometrial stromal cells. J Biol Chem. 2003;278(19):16675–16682 [DOI] [PubMed] [Google Scholar]
- 92. Uchida H, Maruyama T, Arase T, et al. Histone acetylation in reproductive organs: significance of histone deacetylase inhibitors in gene transcription. Reprod Med Biol. 2005;4(2):115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wagner K, Kroisel PM, Rosenkranz W. Localization of genes and anonymous DNA probes on the short arm of chromosome 7. Mamm Genome. 1992;3(1):39–41 [DOI] [PubMed] [Google Scholar]
- 94. Magro G, Righi A, Casorzo L, et al. Mammary and vaginal myofibroblastomas are genetically related lesions: fluorescence in situ hybridization analysis shows deletion of 13q14 region. Hum Pathol. 2012;43(11):1887–1893 [DOI] [PubMed] [Google Scholar]
- 95. Hammoud SS, Nix DA, Hammoud AO, Gibson M, Cairns BR, Carrell DT. Genome-wide analysis identifies changes in histone retention and epigenetic modifications at developmental and imprinted gene loci in the sperm of infertile men. Hum Reprod. 2011;26(9):2558–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fall CH. Fetal malnutrition and long-term outcomes. Nestle Nutr Inst Workshop Ser. 2013;74:11–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Brenseke B, Prater MR, Bahamonde J, Gutierrez JC. Current thoughts on maternal nutrition and fetal programming of the metabolic syndrome. J Pregnancy. 2013;2013:368461 [DOI] [PMC free article] [PubMed] [Google Scholar]