Abstract
Previous studies found that grape seed extract (GSE), which is rich in proanthocyanidins, could protect demineralized dentin collagen from collagenolytic activities following clinically relevant treatment. Because of proanthocyanidin’s adverse interference to resin polymerization, it was believed that GSE should be applied and then rinsed off in a separate step, which in effect increases the complexity of the bonding procedure. The present study aimed to investigate the feasibility of combining GSE treatment with phosphoric acid etching to address the issue. It is also the first attempt to formulate collagen-cross-linking dental etchants. Based on Fourier-transformed infrared spectroscopy and digestion assay, it was established that in the presence of 20% to 5% phosphoric acid, 30 sec of GSE treatment rendered demineralized dentin collagen inert to bacterial collagenase digestion. Based on this positive result, the simultaneous dentin etching and collagen protecting of GSE-containing phosphoric acid was evaluated on the premise of a 30-second etching time. According to micro-Raman spectroscopy, the formulation containing 20% phosphoric acid was found to lead to overetching. Based on scanning and transmission electronic microscopy, this same formulation exhibited unsynchronized phosphoric acid and GSE penetration. Therefore, addition of GSE did render phosphoric acid a collagen-stabilizing etchant, but the preferable phosphoric acid concentration should be <20%.
Keywords: dental acid etching, demineralized dentin matrix, cross-linking reagents, proanthocyanidins, microbial collagenase, biode gradation
Introduction
Life span of composite restorations has been long criticized to be inadequate compared with the traditional amalgam fillings (Soncini et al., 2007). Studies revealed that bonding to dentin deteriorates more readily than that to enamel in the long term (De Munck et al., 2003; Loguercio et al., 2008). The hydrolytic and enzymatic breakdown of interfacial collagen is believed to be the leading culprit for such degradation. Indeed, demineralized collagen within the adhesive-resin hybrid layer is exposed to the collagenolytic environment due to causes such as high water sorption, poor infiltration, and incomplete polymerization of adhesive resins (Wang and Spencer, 2002; Van Meerbeek et al., 2005; Malacarne et al., 2006). Furthermore, matrix metalloproteinases (MMPs), the endogenous collagenases/gelatinases, were activated by the acid-etching process (Breschi et al., 2010). Consequently, measures that enhance dentin collagen’s resistance toward collagenolytic activities have great potential to improve the longevity of dentin bonding.
Such a goal of stabilizing collagenous tissues can be achieved by cross-linking (Khor, 1997). The clinical settings of dental restoration put additional constraint on the cross-linker: it must be effective within a short treatment time. In this context, the proanthocyanidin (PA)–rich grape seed extract (GSE) emerged, highly promising to be utilized in dentin bonding. While earlier studies have established that GSE-PA is an efficient collagen cross-linker following moderate to long treatment times (Bedran-Russo et al., 2007; Castellan et al., 2010; Green et al., 2010; Liu et al., 2011), its extraordinary cross-linking efficacy within very short treatment times (<30 sec) was discovered recently (Liu et al., 2013a; Liu et al., 2013b). Moreover, GSE-PA exhibits superior inhibitory effect (compared with chlorhexidine) on such endogenous enzymes as MMP-2, MMP-8, and MMP-9 (Epasinghe et al., 2013). Furthermore, the low toxicity of GSE-PA supports its use in intraoral applications (Yamakoshi et al., 2002). Nonetheless, GSE’s use in self-etch adhesives is limited because PA readily scavenges free radicals (Quideau et al., 2011) and consequently hampers the polymerization of adhesive resins (Liu and Wang, 2013a). Because of the same reason, when incorporated in etch-and-rinse systems, GSE is best used as a primer that, unlike regular primers, needs to be rinsed off. In essence, the stabilization of dentin collagen with GSE requires more bottles and additional steps in bonding procedure, quite contrary to the current trend of dental adhesive development (Poticny, 2013). Considering that both GSE and etchant need to be removed, we wonder if we can combine them. Mechanism-wise, it is hopeful because the interaction between polyphenols and collagen is proposed to be primarily driven by the hydrophobic effect between phenyl rings of polyphenol and pyrrolidine rings of proline (Haslam, 1996). This mechanism is distinctively different from that of common chemical cross-linkers such as glutaraldehyde (GA) in that it does not involve pH-sensitive groups, so the interactions should not be compromised by an acidic environment.
In light of this, we undertook the present work to first investigate GSE’s efficacy in stabilizing dentin collagen in the presence of phosphoric acid. GSE- and GA-containing phosphoric acid solutions (latter for comparison) were prepared, and their capability of cross-linking ultrathin dentin films was evaluated by Fourier-transformed infrared (FTIR) spectroscopy and matrix-assisted laser desorption/ionization (MALDI) mass spectrometric digestion assay. Second, the performance of GSE-containing phosphoric acid formulations in simultaneous dentin etching and collagen protecting was examined on dentin slabs with micro-Raman spectroscopy and scanning and transmission electronic microscopy (SEM and TEM). The hypothesis is that (1) GSE can effectively enhance dentin collagen’s resistance to bacterial collagenase in the presence of phosphoric acid and (2) such a stabilizing effect can be manifested when GSE and phosphoric acid are simultaneously delivered to dentin surface. To the best of our knowledge, this is the first attempt to formulate a multifunctional collagen-cross-linking etchant. The results should not only verify the feasibility of mixing GSE with phosphoric acid but also provide a new perspective to the improvement of current adhesive systems.
Materials & Methods
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless stated otherwise. MegaNatural Gold GSE (Lot. 05592502-01) was donated by manufacturer (Polyphenolics, Madera, CA, USA). Three GSE-containing formulations (GSE20, GSE10, GSE5) were prepared by mixing GSE powder, ethanol, deionized water, and 85% phosphoric acid to final concentrations (weight percentage with respect to total mass) of 2% GSE, 20% ethanol, and 20%, 10%, and 5% phosphoric acid, respectively. Three GA-containing formulations (GA20, GA10, GA5) were similarly made, except the cross-linker was GA at 2.5%. Collagenase (type I, from Clostridium histolyticum, ≥125 U/mg) solution was made at 0.1% (w/v) in TESCA buffer (50-mM N-tris (hydroxymethyl)methyl-2-aminoethanesulfonic acid, 0.36-mM CaCl2, pH = 7.4). Formulations of reagents were based on previous studies (Liu and Wang, 2013b).
Dentin Film Preparation and Analyses
Sixteen noncarious human third molars were collected from young adults, with patients’ informed consent under a protocol approved by University of Missouri–Kansas City Adult Health Sciences Institutional Review Board. Extracted teeth were stored at 4°C in 0.96% (w/v) phosphate buffered saline containing 0.002% sodium azide. Six randomly selected teeth were processed into dentin films and the rest into dentin slabs, as described in the following section. For each of the 6 teeth, the occlusal portion of crown and side walls of enamel were removed by a water-cooled low-speed diamond saw (Buehler, Lake Bluff, IL, USA). The resultant dentin block was sectioned in the mesial-distal direction, with a tungsten carbide knife mounted on SM2500S microtome (Leica, Deerfield, IL, USA) into 6-µm-thick films. Fifty films were obtained from each tooth, resulting in a pool of 300 films with uniform size of approximately 5 × 5 mm. From this pool, 30 films were randomly selected and assigned to 6 groups (n = 5) and treated by the phosphoric acid formulations as follows. Each dentin film was completely demineralized with 35% phosphoric acid for 15 sec, rinsed in deionized water, and spread on a plastic coverslip. After excessive water was blotted away, a small drop of selected GA- or GSE-containing phosphoric acid was applied to cover the entire film. After 30 sec, the film was rinsed and immersed in copious deionized water for 30 min to thoroughly remove residual treatment solution.
After overnight air-drying, FTIR spectra of the films were collected at 4-cm−1 resolution and 128 scans with an FTIR spectrometer equipped with attenuated total reflectance attachment (Spectrum One, Perkin-Elmer, Waltham, MA, USA). Band area calculation was done with Spectrum software (Perkin-Elmer) following 2-point baseline correction. Furthermore, dentin films were incubated in 30 µL of collagenase at 37°C for 1 hr, and the percentage of digested film in each supernatant was determined following an established protocol (Liu and Wang, 2013b; Appendix).
Dentin Slab Preparation and Analyses
Ten molars were processed into dentin slabs as follows. After crown removal, a uniform smear layer was created on the dentin surface utilizing wet 600-grit SiC sandpaper (Buehler) for 30 sec. Further sections were made in the occlusal-apical direction at increments of 1 mm (for micro-Raman and SEM) or 0.5 mm (for TEM), followed by one cut parallel to and ~1.5 mm below the abraded surface to free the slabs. Slabs for micro-Raman and SEM were notched at the midposition from the side opposite to the abraded surface for the purpose of subsequent fracturing. A total of 54 slabs were prepared: 42 notched (18 for micro-Raman, 24 for SEM) and 12 unnotched slabs (TEM).
For micro-Raman, the abraded surfaces of slabs were etched with GSE20, GSE10, and GSE5 (n = 6) for 30 sec. After 30-min rinsing, the slabs were cryofractured in liquid nitrogen and placed under focus of a 100× water-immersion lens of a LabRam HR800 Raman spectrometer (Horiba Jobin Yvon, Edison, NJ, USA) with monochromatic He-Ne laser (operating at 632.8 nm). Spectra were acquired over the region of 500 to 1,800 cm−1, with 30-sec acquisition time and at positions corresponding to 1-µm intervals across the interfaces of water, demineralized dentin (DD), and intact dentin (ID). Each specimen was scanned 3 times at randomly selected locations, and the distance between water-DD and DD-ID interfaces in each scan was averaged to one value, representing the etching depth for the specimen.
Slabs for SEM were etched for 30 sec on the abraded surfaces with 1 of the 6 GA- or GSE-containing phosphoric acid formulations, rinsed for 30 min, and then underwent 1 hr of collagenase digestion or deionized water immersion at 37°C. Specimens for each etching-digestion combination were prepared in duplicates. Slabs for TEM were etched, rinsed, and digested in the same way as SEM specimens (also in duplicates) except that only GSE–phosphoric acid formulations were investigated. For both SEM and TEM, the subsequent specimen processing (including fracturing of SEM specimens) and observation followed a published protocol (Liu et al., 2013b; Appendix).
Results
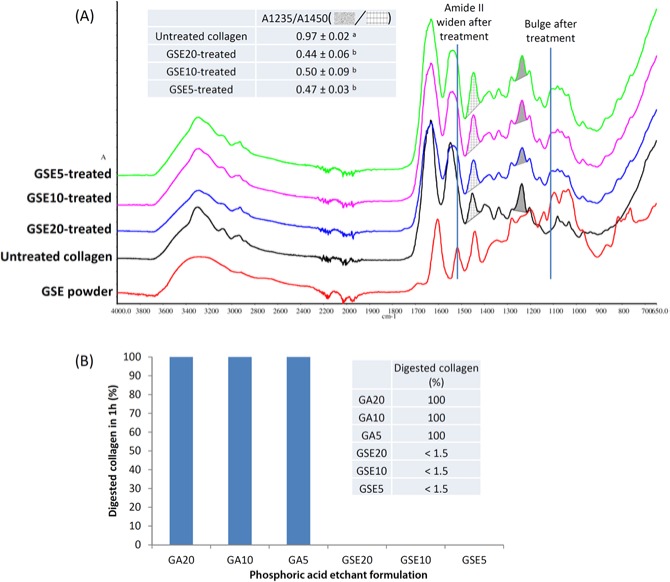
Compared with DD collagen’s FTIR spectra (Liu and Wang, 2013b), GA–phosphoric acid did not cause any perceivable change (spectra therefore not shown). In contrast, all GSE–phosphoric acid treatments resulted in pronounced alterations (Fig. 1A), including wider amide II bands (~1540 cm−1), bulge formation (~1108 cm−1), and significantly decreased ratio of amide III (shade, ~1235 cm−1) to CH2 bending (grid, ~1450 cm−1) (A1235/A1450; Fig. 1A, inset). The A1235/A1450 ratios were equivalent within the GSE–phosphoric acid–treated group regardless of phosphoric acid concentration. Figure 1B shows the resistance of treated dentin films against collagenase. Films treated with GA–phosphoric acid completely vanished, whereas those with GSE–phosphoric acid withstood one-hour digestion with the fraction of degraded collagen lower than 1.5%, the detecting limit of the MALDI–based method (Liu and Wang, 2013b).
Figure 1.
Grape seed extract (GSE)- and glutaraldehyde (GA)-containing phosphoric acid etchants’ effect on demineralized dentin collagen. (A) Fourier-transformed infrared spectra of GSE–phosphoric acid–treated demineralized dentin collagen film (blue, purple, green), natural demineralized dentin collagen (black), and GSE powder (red). Following GSE–phosphoric acid treatment, the widening of amide II and the formation of bulge are attributed to the spectral trace of GSE powder, as is the decreased amide III/CH2 bending (A1235/A1450) ratio (inset) because GSE powder’s spectrum augments collagen’s CH2 bending ( ) but contributes little to its amide III (
) but contributes little to its amide III ( ). (B) The percentage of dentin film degraded by 1 hr of bacterial collagenase digestion. Statistical analysis was performed with SPSS 21 and nominal level of significance set at 0.05. The normality of distribution and homogeneity of variances were confirmed with Shapiro-Wilk and Levene tests, respectively. Comparison of means was performed with one-way analysis of variance and Tukey post hoc test. Values with the same subscripts indicate statistical equivalence (n = 5).
). (B) The percentage of dentin film degraded by 1 hr of bacterial collagenase digestion. Statistical analysis was performed with SPSS 21 and nominal level of significance set at 0.05. The normality of distribution and homogeneity of variances were confirmed with Shapiro-Wilk and Levene tests, respectively. Comparison of means was performed with one-way analysis of variance and Tukey post hoc test. Values with the same subscripts indicate statistical equivalence (n = 5).
The 30-second etching depth of GSE-containing formulations was studied with micro-Raman. A representative optical image of scanned interface is shown in Figure 2A. As spectra collection started from water, flat low-intensity lines were recorded (black, Fig. 2B). Owing to GSE’s fluorescence, no well-defined Raman spectra could be acquired when scanning into DD, but the abruptly elevated baseline (blue) indicated the water-DD interface. Moving closer to ID, the interference of fluorescence faded, and the DD-ID interface was marked by the emergence of spectrum with prominent mineral peak at 960 cm−1 (dark green). The etching depth of different GSE–phosphoric acid formulations was shown in Fig. 2C, which is statistically equivalent to their GA-containing counterparts (Appendix).
Figure 2.
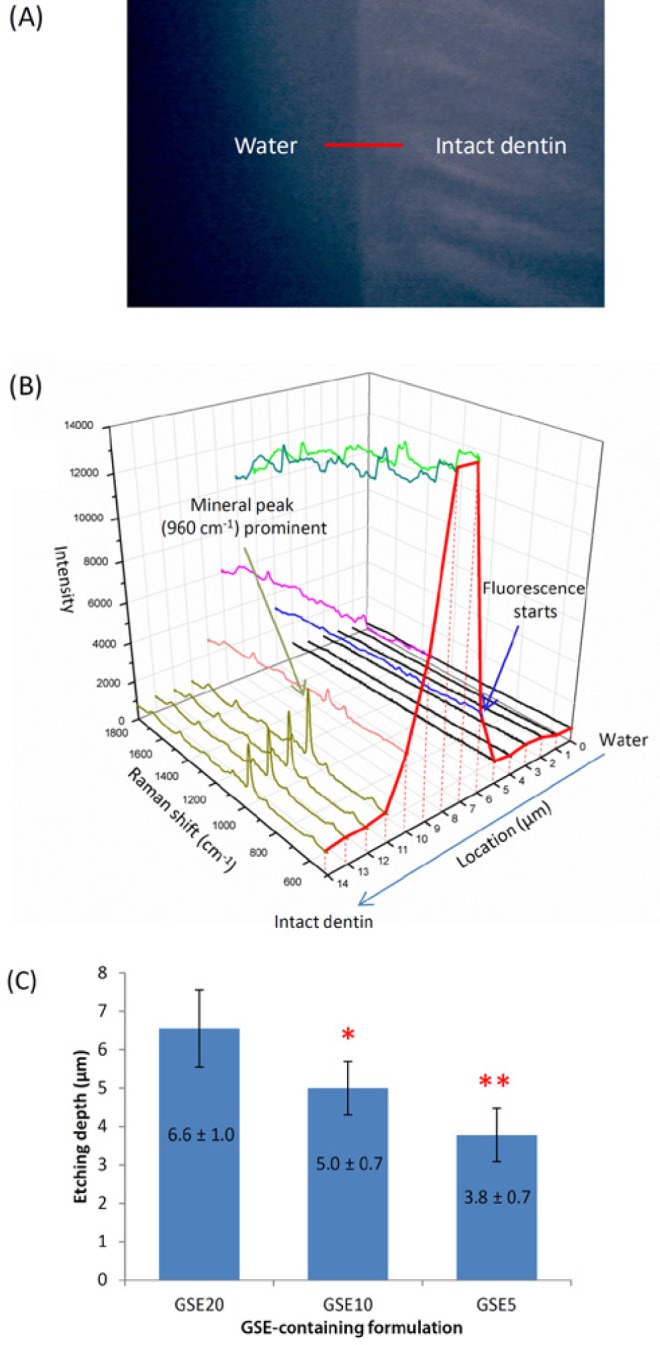
Etching depth as determined by micro-Raman spectroscopy. (A) Optical image of the fractured cross section of a representative specimen etched by GSE10 for 30 sec. (B) Raman spectra collected from this specimen. Note the change of baseline intensity (red curve) with respect to location. At 6 µm, the abrupt rise in baseline indicates the start of fluorescence (blue arrow) and, therefore, the water–demineralized dentin interface. At 11 µm, the spectrum shows prominent mineral peak (dark green arrow), indicating the demineralized dentin–intact dentin interface. (C) Thirty-second etching depth of GSE20, GSE10, and GSE5. Statistical analysis was performed with SPSS 21 and nominal level of significance set at 0.05. The normality of distribution and homogeneity of variances were confirmed with Shapiro-Wilk and Levene tests, respectively. Comparison of means was performed with 1-way analysis of variance and Tukey post hoc test. Statistically different values are indicated by asterisks (* and **) (n = 6). GSE, grape seed extract.
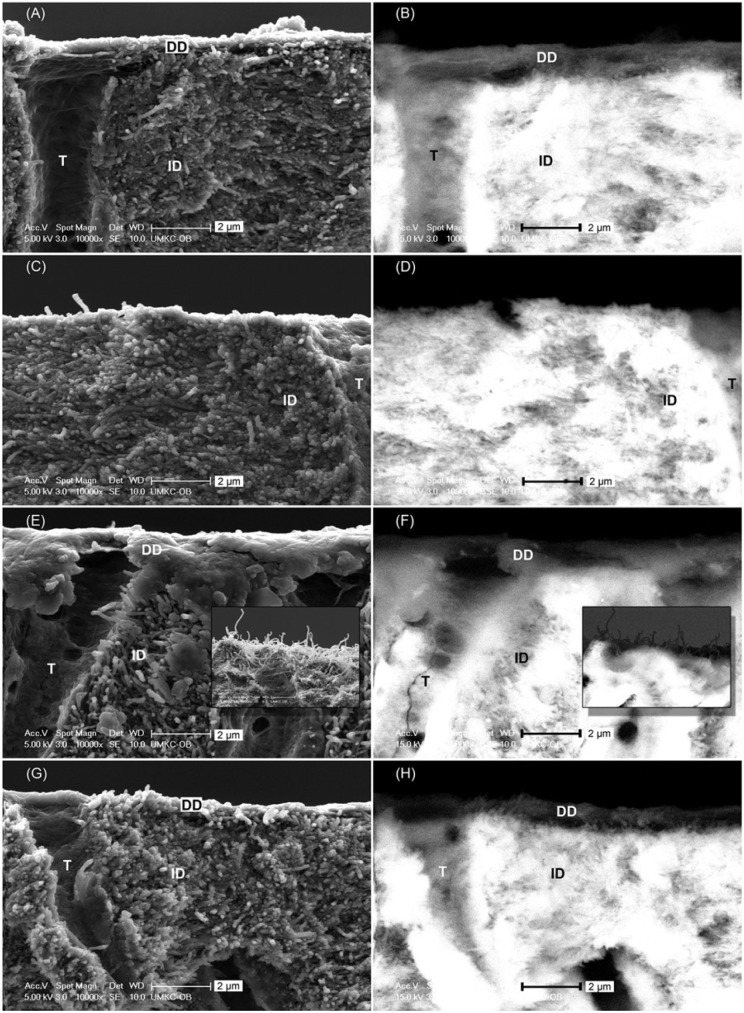
When not challenged by collagenase, the cross section of etched dentin shared similar morphologic traits regardless of constituent cross-linker—that is, distinct collagen fibrils seen under secondary electron SEM (Fig. 3A) in the DD layer characterized by dark color under backscattered electron SEM (Fig. 3B). After digestion, DD of GA–phosphoric acid–treated specimen was completely gone (Fig. 3C, 3D). In contrast, DD of GA–phosphoric acid–treated dentin was still present, but its appearance depended upon phosphoric acid concentration. The GSE20-etched specimen had a thin, featureless demineralized film draping over the edge (Fig. 3E, 3F), and the film seemed to be torn off from the complementary piece, leaving hair-like DD fibrils on it (Fig. 3E, 3F, insets). In comparison, the DD layers of GSE10- and GSE5-etched dentin were not altered by the digestion (Fig. 3G, 3H).
Figure 3.
Scanning electronic microscopy images of cross section of fractured dentin slabs under (A, C, E, G) secondary electron mode and (B, D, F, H) backscattered electron mode. (A, B) Typical morphology of etched dentin predigestion, regardless of cross-linkers. (C, D) Glutaraldehyde–phosphoric acid–etched dentin after 1 hr of digestion, showing no demineralized layer left. (E, F) GSE20-etched dentin after 1 hr of digestion, featuring a thin demineralized film draping over the edge and hair-like morphology on the complementary piece (insets). (G, H) GSE10- and GSE5-etched dentin after 1 hr of digestion, showing a morphology unchanged from predigestion. DD, demineralized dentin; GSE, grape seed extract; ID, intact dentin; T, tubule.
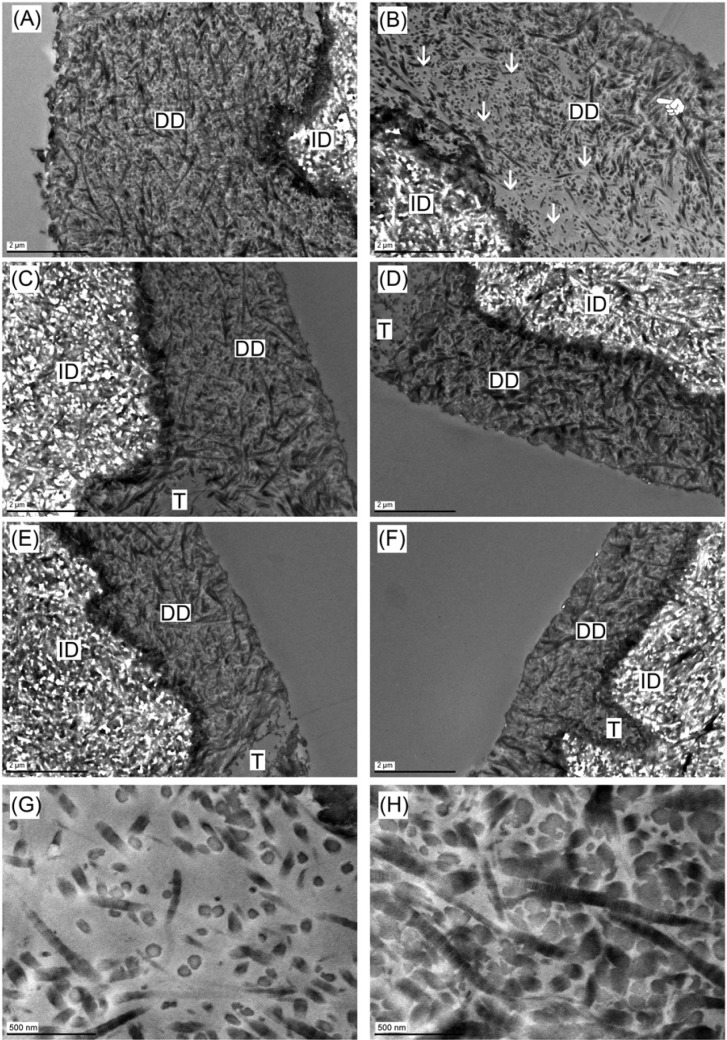
Consistent with SEM, the TEM images of undigested GSE–phosphoric acid–etched specimens (regardless of phosphoric acid concentration) displayed DD layer (Fig. 4A, 4C, 4E) in which fibrils exhibit the characteristic 67-nm banding pattern of type I collagen (Fig. 4H). After digestion, DD in GSE20-etched specimens was no longer intact; voids were evident (Fig. 4B, arrows) close to the bottom of DD layer. Collagen fibrils in the voids were much scarcer (Fig. 4G) than intact DD (Fig. 4H). In comparison, the GSE10- and GSE5-etched specimens had no voids across the entire DD layer (Fig. 4D, 4F) postdigestion.
Figure 4.
Transmission electronic microscopy images of (A) GSE20-etched dentin before 1 hr of digestion. (B) GSE20-etched dentin after 1 hr of digestion. Note the voids (arrows) close to the bottom of DD layer. In the top DD layer (finger), such voids were not seen. (C) GSE10-etched dentin without digestion. (D) GSE10-etched dentin after 1 hr of digestion. (E) GSE5-etched dentin without digestion. (F) GSE5-etched dentin after 1 hr of digestion. (G) High-magnification view of voids in Figure 4B (arrows). (H) High-magnification view of the top DD layer in Figure 4B (finger), showing the densely packed fibrils with intact banding pattern. DD, demineralized dentin; GSE, grape seed extract; ID, intact dentin; T, tubule.
Discussion
Traditional collagen cross-linkers such as the gold standard GA and “zero-length” cross-linker carbodiimide are incompatible with phosphoric acid, as they depend on deprotonated amines to form covalent bonds. At low pH such as that of phosphoric acid etchants, amines are predominantly protonated and unreactive for covalent bonding (Migneault et al., 2004; Madison and Carnali, 2013). Conversely, PAs cross-link collagen on a noncovalent basis (Haslam, 1996) and are therefore anticipated to remain active at low pH.
In agreement with our anticipation, DD collagen’s FTIR spectra were altered by GSE–phosphoric acid but not by GA–phosphoric acid (Fig. 1A). The short treatment time (30 sec) and extensive rinsing (30 min) substantiated that DD collagen interacts with and immobilizes PA in the presence of 20% to 5% phosphoric acid. Additionally, the A1235/A1450 ratio (Fig. 1A, inset) indicates that the amount of PA incorporated in collagen was not affected by the phosphoric acid concentration (Liu and Wang, 2013b). It is not clear whether these PA molecules immobilized by dentin collagen still hamper the subsequent adhesive polymerization, which should be investigated in future studies. Results of the digestion assay (Fig. 1B) matched well with the FTIR analysis. The GA–phosphoric acid–treated DD collagen, which exhibited FTIR spectra unaltered from natural DD, was completely digested in 1 hr like natural DD (Liu and Wang, 2013b). Conversely, the GSE–phosphoric acid–treated DD collagen, which had equivalent A1235/A1450 ratios, was invariably inert. Thus, the first hypothesis is accepted.
Once we established that GSE could afford potent protection of DD collagen in the presence of phosphoric acid, we moved on to evaluate the etching performance of GSE–phosphoric acid formulations. Note that the etching period in the present work was raised from the most common 15 to 30 sec considering that the GSE component may need a longer time to exert a stabilizing effect. Micro-Raman spectroscopy, a convenient tool to probe the chemical profile of dentin and hybrid layer (Wang and Spencer, 2003; Wang and Yao, 2010), unfortunately could not provide the distribution of GSE within the DD layer due to fluorescence. Nonetheless, based on the abrupt rise of baseline as indicator of the water-DD interface (Fig. 2B), the etching depth could be obtained. The thickness of DD layer (Fig. 2C) after GSE20 etching (6-7 µm) was higher than that also determined with micro-Raman (3-5 µm) but on specimens etched under usual protocols (15-sec ~37% phosphoric acid gel) (Wang and Yao, 2010). This implies that 30-sec GSE20 etching may lead to overetching, an undesired condition related to suboptimal resin infiltration (Van Meerbeek et al., 2005).
We then investigated the GSE–phosphoric acid formulations’ ability to stabilize collagen while etching dentin. SEM images revealed that GA–phosphoric acid only etched dentin (Fig. 3A, 3B) but did not increase DD collagen’s resistance to bacterial collagenase (Fig. 3C, 3D). In contrast, the GSE–phosphoric acid formulations did both (Fig. 3E-3H), corroborating the digestion assay (vide supra). Interestingly, although the GSE20-etched dentin still had DD layer visible postdigestion, its morphology obviously changed (Fig. 3E, 3F), suggesting partial degradation. Thus, the second hypothesis can be only partially accepted. To elucidate the cause of this phenomenon, the GSE–phosphoric acid–etched dentin with and without digestion was examined by TEM. As seen in Figure 4A, 4C, and 4E, the DD layers of GSE–phosphoric acid–treated specimens were featured by densely packed collagen fibrils predigestion. However, fewer collagen fibrils were visible postdigestion in the bottom portion of DD layer in GSE20-etched specimen (Fig. 4B, arrows). The voids were not spotted in the top portion of GSE20-etched DD layer or anywhere in GSE10- and GSE5-etched DD layer. It appears that the top DD layer of GSE20-etched specimen was resistant to bacterial collagenase, but the bottom portion was substantially degraded. It thus resulted in the SEM specimen of GSE20-etched dentin to have a collagen film floating on weak collagen-fibril support after digestion and before fracturing. When fractured, cracks propagated along the weak collagen-fibril support, then through the collagen film, hence producing the drape-like appearance on one piece and the hair-like appearance on the complementary piece (Fig. 3E, 3F, insets).
The suboptimal protection for collagen in GSE20-etched DD layer is obviously not due to higher phosphoric acid concentration, as the digestion assay has shown otherwise. Therefore, it may exemplify an unsynchronized penetration of phosphoric acid and GSE. Indeed, GSE is primarily composed of (epi)catechin monomers and oligomers, which are not only intrinsically more hydrophobic (indicated by involvement in hydrophobic interactions) and less mobile (higher molecular weight) than the small hydrophilic phosphoric acid but also known to self-assemble into even bigger micelles in hydroalcoholic media at acidic pH (Poncet-Legrand et al., 2003; Pianet et al., 2008). As such, GSE diffuses slower than phosphoric acid within the intratubular space, which is filled with water. In addition, the retention of collagen may have retarded the diffusion of GSE micelles in the intertubular space as well. Concomitantly, GSE20 etched too fast for GSE to catch up, which leaves the collagen fibrils at the etching front not fully protected as the top of DD layer. These SEM and TEM results, in combination with the micro-Raman study, point to an optimal phosphoric acid concentration in GSE-containing etchants to be <20%. It should be noted that the effect of using less-concentrated phosphoric acid etchants on enamel etching/bonding deserves further investigation.
In summary, we confirmed that GSE could render DD collagen inert to bacterial collagenase in the presence of 20% to 5% phosphoric acid. On the premise of longer etching time (30 sec), the preferable etchant formulation was identified to have 2% GSE and <20% phosphoric acid. Imminent future studies should include the evaluation of short- and long-term bonding strength using the GSE–phosphoric acid etchants on dental substrates, including dentin and enamel.
Supplementary Material
Acknowledgments
We thank Dr. Andrew Keightley and the Proteomics and Mass Spectrometry Facility of the School of Biological Sciences, University of Missouri–Kansas City, for the help with the matrix-assisted laser desorption/ionization mass spectroscopy.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This investigation was supported by R15-DE021023 from the National Institutes of Health, Bethesda, Maryland.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bedran-Russo AK, Pereira PN, Duarte WR, Drummond JL, Yamauchi M. (2007). Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. J Biomed Mater Res B Appl Biomater 80:268-272. [DOI] [PubMed] [Google Scholar]
- Breschi L, Martin P, Mazzoni A, Nato F, Carrilho M, Tjäderhane L, et al. (2010). Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent Mater 26:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellan CS, Pereira PN, Grande RH, Bedran-Russo AK. (2010). Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dent Mater 26:968-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Munck J, Van Meerbeek B, Yoshida Y, Inoue S, Vargas M, Suzuki K, et al. (2003). Four-year water degradation of total-etch adhesives bonded to dentin. J Dent Res 82:136-140. [DOI] [PubMed] [Google Scholar]
- Epasinghe DJ, Yiu CK, Burrow MF, Hiraishi N, Tay FR. (2013). The inhibitory effect of proanthocyanidin on soluble and collagen-bound proteases. J Dent 41:832-839. [DOI] [PubMed] [Google Scholar]
- Green B, Yao X, Ganguly A, Xu C, Dusevich V, Walker MP, et al. (2010). Grape seed proanthocyanidins increase collagen biodegradation resistance in the dentin/adhesive interface when included in an adhesive. J Dent 38:908-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam E. (1996). Natural polyphenols (vegetable tannins) as drugs: possible modes of action. J Nat Prod 59:205-215. [DOI] [PubMed] [Google Scholar]
- Khor E. (1997). Methods for the treatment of collagenous tissues for bioprostheses. Biomaterials 18:95-105. [DOI] [PubMed] [Google Scholar]
- Liu R, Fang M, Xiao Y, Li F, Yu L, Zhao S, et al. (2011). The effect of transient proanthocyanidins preconditioning on the cross-linking and mechanical properties of demineralized dentin. J Mater Sci Mater Med 22:2403-2411. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang Y. (2013a). Effect of proanthocyanidins and photo-initiators on photo-polymerization of a dental adhesive. J Dent 41:71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang Y. (2013b). Proanthocyanidins’ efficacy in stabilizing dentin collagen against enzymatic degradation: MALDI-TOF and FTIR analyses. J Dent 41:535-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen M, Yao X, Xu C, Zhang Y, Wang Y. (2013a). Enhancement in dentin collagen’s biological stability after proanthocyanidins treatment in clinically relevant time periods. Dent Mater 29:485-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Dusevich V, Wang Y. (2013b). Proanthocyanidins rapidly stabilize the demineralized dentin layer. J Dent Res 92:746-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loguercio AD, Moura SK, Pellizzaro A, Dal-Bianco K, Patzlaff RT, Grande RH, et al. (2008). Durability of enamel bonding using two-step self-etch systems on ground and unground enamel. Oper Dent 33:79-88. [DOI] [PubMed] [Google Scholar]
- Madison SA, Carnali JO. (2013). PH optimization of amidation via carbodiimides. Industrial and Engineering Chemistry Research 52:13547-13555. [Google Scholar]
- Malacarne J, Carvalho RM, de Goes MF, Svizero N, Pashley DH, Tay FR, et al. (2006). Water sorption/solubility of dental adhesive resins. Dent Mater 22:973-980. [DOI] [PubMed] [Google Scholar]
- Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC. (2004). Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 37:790-802. [DOI] [PubMed] [Google Scholar]
- Pianet I, André Y, Ducasse MA, Tarascou I, Lartigue JC, Pinaud N, et al. (2008). Modeling procyanidin self-association processes and understanding their micellar organization: a study by diffusion NMR and molecular mechanics. Langmuir 24:11027-11035. [DOI] [PubMed] [Google Scholar]
- Poncet-Legrand C, Cartalade D, Putaux JL, Cheynier V, Vernhet A. (2003). Flavan-3-ol aggregation in model ethanolic solutions: incidence of polyphenol structure, concentration, ethanol content, and ionic strength. Langmuir 19:10563-10572. [Google Scholar]
- Poticny DJ. (2013). Adhesive systems continue to evolve: a case report. Dent Today 32:79-80, 82-83. [PubMed] [Google Scholar]
- Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. (2011). Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed Engl 50:586-621. [DOI] [PubMed] [Google Scholar]
- Soncini JA, Maserejian NN, Trachtenberg F, Tavares M, Hayes C. (2007). The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth findings from the New England children’s Amalgam trial. J Am Dent Assoc 138:763-772. [DOI] [PubMed] [Google Scholar]
- Van Meerbeek B, Van Landuyt K, De Munck J, Hashimoto M, Peumans M, Lambrechts P, et al. (2005). Technique-sensitivity of contemporary adhesives. Dent Mater J 24:1-13. [DOI] [PubMed] [Google Scholar]
- Wang Y, Spencer P. (2002). Quantifying adhesive penetration in adhesive/dentin interface using confocal Raman microspectroscopy. J Biomed Mater Res 59:46-55. [DOI] [PubMed] [Google Scholar]
- Wang Y, Spencer P. (2003). Hybridization efficiency of the adhesive/dentin interface with wet bonding. J Dent Res 82:141-145. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yao X. (2010). Morphological/chemical imaging of demineralized dentin layer in its natural, wet state. Dent Mater 26:433-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakoshi J, Saito M, Kataoka S, Kikuchi M. (2002). Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem Toxicol 40:599-607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.