Abstract
Homeostasis of healthy periodontal tissues is affected by innate and adaptive immunosurveillance mechanisms in response to the normal oral flora. Recent comparisons of germ-free (GF) and normal specific-pathogen-free (SPF) mice have revealed the impact of host immunosurveillance mechanisms in response to the normal oral flora on alveolar bone height. Prior reports that alveolar bone height is significantly less in normal SPF mice compared with their age- and strain-matched GF counterparts suggest that naturally occurring alveolar bone loss is a normal component of healthy periodontal tissue homeostasis. In this report, histomorphometric analyses confirmed increased alveolar bone loss and revealed increased numbers of TRAP+ osteoclastic cells lining the alveolar bone surface in SPF compared with GF mice. Increased numbers of RANKL+ cells and IL17+ cells in the periodontium of SPF mice demonstrate possible molecular mechanisms mediating the up-regulated osteoclastogenesis and alveolar bone loss in SPF mice compared with GF mice. Increased numbers of T-lymphocytic cells and T-helper cells in the junctional epithelium of SPF mice compared with GF mice suggest that the adaptive immune response contributes to physiologic alveolar bone loss in the healthy periodontium. This GF animal model study notably begins to elucidate the impact of host immunosurveillance mechanisms in response to the normal oral flora, mediating catabolic alveolar bone homeostasis in the healthy periodontium.
Keywords: helper T-cells, RANKL protein, interleukin-17, tartrate-resistant acid phosphatase, osteoclastic bone loss, periodontium
Introduction
Recently, it has been reported that naturally occurring alveolar bone loss is significantly blunted in germ-free (GF) mice when compared with specific-pathogen-free (SPF) mice (Hajishengallis et al., 2011). Evidence that the normal oral flora were responsible for the observed alveolar bone loss in SPF mice was obtained by both co-caging experiments of GF and SPF mice as well as longitudinal measurements of natural bone loss during the first 15 wk of life (Hajishengallis et al., 2011). These observations are consistent with those of an earlier study (Brown et al., 1969) and suggest that the commensal oral flora affects alveolar bone homeostasis in the healthy periodontium. While it has been shown that host immune response mechanisms to perio-pathogenic bacteria mediate pathologic alveolar bone loss (Kornman et al., 1997; Baker, 2000; Taubman et al., 2005), host innate defense mechanisms regulating the normal oral flora and the impact on alveolar bone homeostasis during health are not clear.
Periodontal disease–associated alveolar bone loss is driven by the elevated expression of pro-inflammatory signaling factors supporting osteoclastogenesis, resulting in catabolic bone destruction (Di Benedetto et al., 2013; Ebersole et al., 2013). The host immune response to perio-pathogenic bacteria significantly contributes to alveolar bone loss (Kornman et al., 1997; Baker, 2000; Taubman et al., 2005), which is largely attributed to overexpression of receptor activator of nuclear factor-κB (RANKL) on activated T-cells (Taubman et al., 2005; Kawai et al., 2006). Prior studies of gingival tissue harvested from SPF mice vs. GF mice have revealed increased basal expression of pro-inflammatory cytokines (Dixon et al., 2004), including RANKL mRNA and interleukin-17 (IL-17) mRNA (Hajishengallis et al., 2011), which suggests that host innate defense system regulation of the commensal flora may have catabolic effects on alveolar bone homeostasis during health. Since perio-pathogenic inoculated oral flora vs. normal oral flora differs in the quantity and diversity of bacteria, it is unclear whether alveolar bone loss in health is mediated by the same mechanisms driving alveolar bone loss during periodontal disease states.
This investigation applies histomorphometric analysis of the linear distance from the cement-enamel junction (CEJ) to the alveolar bone crest (ABC) to examine alveolar bone loss in GF vs. SPF mice. We performed immunohistochemistry (IHC) labeling and staining of mouse junctional epithelial (JE) tissues to further investigate the impact of the commensal oral flora on the innate defense system of the periodontium and to begin to elucidate host immune response mechanisms having catabolic effects on alveolar bone homeostasis. SPF mice had a more severe alveolar bone loss phenotype, with increased numbers of tartrate-resistant acid phosphatase (TRAP)+ osteoclastic cells and RANKL+ cells at the alveolar bone surface, as well as significantly increased numbers of neutrophils, CD3+ T-lymphocytic cells, CD4+ T-helper cells, and IL-17+ cells in the JE tissue. Similar to the up-regulated numbers of osteoclastic and inflammatory/immune cells and elevated expression of pro-inflammatory cytokines found in the periodontium of periodontitis-afflicted mice vs. normal mice (Graves et al., 2012), this study reveals similar relative differences in SPF mice vs. GF mice. These findings imply that the host innate defense response mediating homeostasis with the commensal oral microbiota induces a low-grade basal inflammation which causes alveolar bone loss during health.
Materials & Methods
Mice
Animal procedures described in this study were approved by the institutional animal care and use committees, in compliance with established Federal and state policies. Germ-free (GF) C3H/Orl mice (Charles River Laboratories International, Wilmington, MA, USA) were maintained in isolators under sterile conditions at the Royal Veterinary College, University of London. GF mice were fed a soft chow diet. The sterility of GF animals was examined by aerobic and anaerobic culture of oral swabs and fecal pellets on non-selective media and by polymerase chain reaction (PCR) with universal 16S primers. Specific-pathogen-free (SPF) mice were maintained in individually ventilated cages at the animal care facilities of Queen Mary University of London. SPF mice were fed a standard chow diet. Original GF mice were divided, and half were raised in conventional cages to become the SPF mice; GF and SPF mice were propagated under uniform breeding conditions. The bacterial composition of the natural oral flora of the C3H/Orl SPF mice has been previously characterized (Hajishengallis et al., 2011).
Histology
Eleven- to 12-week-old GF mice and SPF mice (n = 6/gp) were euthanized, and tissues were harvested for histological processing. Upper (maxilla) and lower (mandible) jaws were dissected, and the periodontal soft tissues were not disturbed. Jaws were immediately fixed in Bouin’s solution for 24 hr, rinsed with 70% ethanol, and demineralized in acetic acid/formalin/sodium chloride solution for 2 wk. Specimens were embedded in paraffin in a bucco-lingual orientation. Serial frontal sections (5 μm) were cut parallel to the long axis of each tooth (sagittal), yielding sections for analyses from the mesial/mesio-buccal root of the second molar through the distal/disto-buccal root of the second molar. Two serial sections were mounted per charged glass slides. Sections were numbered, and slides were maintained in corresponding order. All quantitative analyses were performed by a single blinded examiner (K.I.).
Histomorphometry
Hematoxylin and eosin (H&E) staining was performed in all specimens. We carried out qualitative analysis to evaluate tissue-cell morphological differences and performed histomorphometric analysis of the linear distance from the cemento-enamel junction (CEJ) to the alveolar bone crest (ABC) to quantify alveolar bone loss. Linear measurements were performed at 12 pre-determined sites (every fifth serial section) through the mesio-distal width of the second molars, quantifying the linear distance between the CEJ and ABC, at both the buccal and lingual/palatal surfaces. Measurements were performed via a micro-grid at 200X magnification (Irie et al., 2008) with a Nikon Eclipse E400 light microscope (Nikon Corp., Tokyo, Japan). Representative images (100X) were acquired via a Spot RT camera (SPOT Imaging Solutions, Sterling Heights, MI, USA) and MetaVue software (Molecular Devices LLC, Sunnyvale, CA, USA).
Immunohistochemistry
We performed immunohistochemistry (IHC) labeling for primary antibodies in second molar sections to evaluate the expression of neutrophils, T-cell populations, and pro-inflammatory signaling factors within the JE tissue and adjacent alveolar bone surface. IHC primary antibody staining and analyses were performed in intermittent serial sections, at 12 pre-determined sites (every fifth serial section) through the mesio-distal width of the second molars. Approximately 12 sections per second molar specimen were stained for each primary antibody, which included neutrophil elastase (sc-71674; Santa Cruz Biotechnology, Santa Cruz, CA, USA), CD3 (ab5690; Abcam, Cambridge, MA, USA), CD4 (sc-71674; Santa Cruz Biotechnology), IL-17 (ab79056; Abcam), RANKL (ab9957; Abcam), or an IgG matched isotype control. No primary antibody controls were performed for each antibody of interest. Standard IHC staining protocol was performed in conjunction with Abcam IHC-P staining kits. Neutrophil elastase+ (neutrophil) cells, CD3+ (T-lymphocytic) cells, CD4+ (T-helper) cells, and IL-17+ cells were enumerated per JE tissue area. RANKL+ cells occurring at the edge of the alveolar bone surface were enumerated and reported as cells per millimeter (Sanbe et al., 2009). Representative images (200X) of the JE tissue were acquired at the buccal surface.
In addition, we carried out tartrate-resistant acid phosphatase (TRAP) staining to enumerate TRAP+ bone lining osteoclastic cells per millimeter bone perimeter at the edge of the alveolar bone surface (Sanbe et al., 2009). TRAP+ cells were detected by the azo dye method (Sigma-Aldrich Kit, St. Louis, MO, USA), and Mayer’s hematoxylin counterstaining was used for labeling cell nuclei. Representative images (200X) of the alveolar bone surface were acquired at the buccal surface.
Statistical Analysis
Initial experiments revealed no significant differences in the number of positive cells of interest per junctional epithelium tissue area between the JE of the mandibular second molar vs. the maxillary second molar, within both the GF group and the SPF group (p < .01, random mixed-model test). Therefore, enumeration of positive cells of interest per JE tissue area was performed in either maxillary second molar sections or mandibular second molar sections from each mouse. Student’s two-tailed unpaired t test and non-parametric Mann-Whitney test for comparison between the two groups were performed with SPSS statistical software (SPSS 17.0J for Windows; SPSS Japan, Tokyo, Japan). Data are presented as mean ± standard deviation, and statistical significance is p < .05.
Results
Commensal Bacteria Increase Alveolar Bone Loss
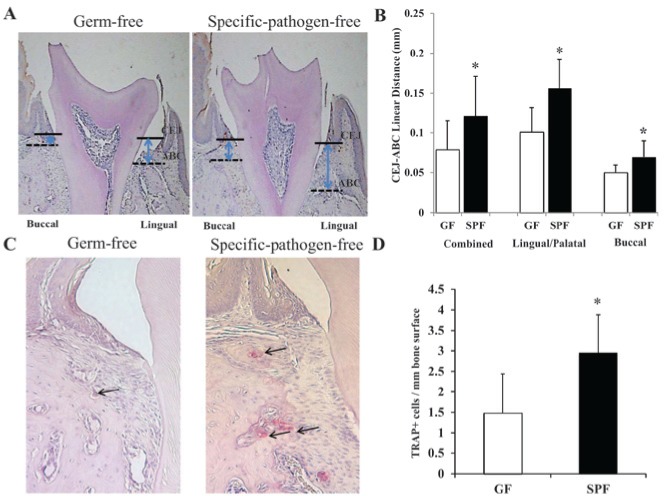
Linear measurements (Fig. 1) assessing the distance between the cemento-enamel junction (CEJ) and the alveolar bone crest (ABC) demonstrated significantly greater alveolar bone loss at second molars of SPF mice vs. GF mice. This histomorphometric analysis revealed increased alveolar bone loss in SPF mice vs. GF mice, which was more severe at the lingual/palatal surfaces (roughly 1.5X greater in SPF mice vs. GF mice) than the buccal surfaces (roughly 1.3X greater in SPF mice vs. GF mice) (Figs. 2A, 2B).
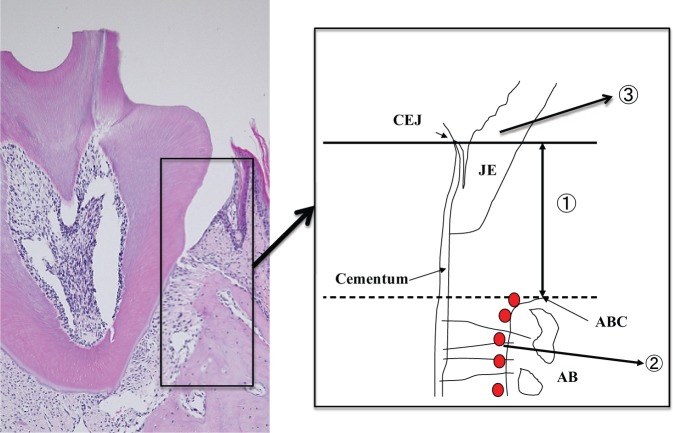
Figure 1.
Diagram of mouse periodontal tissue for histological analysis. AB, alveolar bone; ABC, alveolar bone crest; CEJ, cemento-enamel junction; JE, junctional epithelium. (1) The distances between CEJ and ABC were measured to evaluate the degree of alveolar bone resorption. (2) RANKL+ cells and TRAP+ osteoclastic cells occurring along the edge of the alveolar bone surface were counted. (3) The number of neutrophils, CD3, CD4, and IL-17-positive cells infiltrating the JE area were enumerated.
Figure 2.
Histomorphometric analysis of alveolar bone loss. (A, B) Measures of the linear distance between the CEJ and ABC and (C, D) TRAP+ osteoclastic cells per mm alveolar bone surface in GF mice vs. SPF mice (*p < .01; n = 6). Arrows in C indicate TRAP+ osteoclastic cells lining the alveolar bone surface. ABC, alveolar bone crest; CEJ, cemento-enamel junction; GF, germ-free mice; SPF, specific-pathogen-free mice.
Commensal Bacteria Increase Osteoclastic Cell Numbers Lining Alveolar Bone
Based on the exacerbated alveolar bone loss phenotype of the SPF mice vs. GF mice, we enumerated osteoclastic cells lining the alveolar bone surface. Histomorphometric analysis demonstrated increased numbers of TRAP+ osteoclastic cells per millimeter bone perimeter at the edge of the alveolar bone surface in SPF mice vs. GF mice (Figs. 2C, 2D), which correlates with the increased alveolar bone loss found in SPF mice vs. GF mice.
Commensal Bacteria Increase T-cell Numbers, IL-17, and RANKL Expression
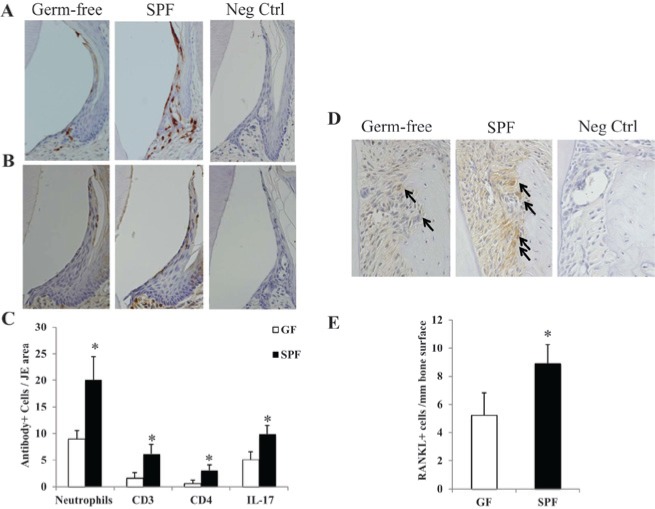
As has been reported previously (Tsukamoto et al., 2012; Zenobia et al., 2013), there were significantly more neutrophils located in the JE of SPF mice vs. GF mice (Figs. 3A, 3C). To begin to elucidate oral commensal flora-driven host immune response mechanisms having catabolic effects on alveolar bone homeostasis during health, we assessed T-cell numbers, IL-17, and RANKL expression in the periodontium of SPF mice vs. GF mice. IHC demonstrated increased CD3+ (T-lymphocytic) cell numbers, CD4+ (T-helper) cell numbers, and IL-17+ cell numbers in the JE tissue of SPF mice vs. GF mice (Figs. 3B, 3C). IHC labeling for the osteoclastogenic signaling factor RANKL revealed significantly increased RANKL+ cell numbers along the edge of the alveolar bone surface in SPF mice vs. GF mice (Figs. 3D, 3E).
Figure 3.
Immunohistochemical examination of JE tissue and adjacent alveolar bone surface. (A) Neutrophil elastase staining demonstrating neutrophil infiltration into the JE tissue. (B) IL-17 staining demonstrating IL-17+ cell infiltration into the JE tissue. (C) Enumeration of neutrophils and lymphocytic (CD3+, CD4+, IL-17+) cells per JE tissue area in GF mice vs. SPF mice (*p < .01; n = 6). (D, E) RANKL+ cells per mm alveolar bone surface in GF mice vs. SPF mice (*p < .01; n = 6). Arrows in D indicate RANKL+ cells lining the alveolar bone surface. GF, germ-free mice; JE, junctional epithelial; SPF, specific-pathogen-free mice.
Discussion
In 1969, Brown and co-authors published the first manuscript reporting increased alveolar bone loss in SPF mice compared with GF mice (Brown et al., 1969). In that early report, the loss of alveolar bone height in SPF mice was interpreted as further evidence for the contribution of bacteria to periodontitis-associated pathologic bone loss (Brown et al., 1969). More recently, a second report of alveolar bone loss in untreated SPF compared with GF mice was published in an investigation elucidating virulence mechanisms of P. gingivalis (Hajishengallis et al., 2011). Two separate and independent approaches validated that SPF mice compared with GF mice have increased alveolar bone loss, and demonstrated that commensal colonization of the mouse was necessary for observation of the decrease in alveolar bone height. In contrast to Brown and co-authors’ (1969) explanation of increased alveolar bone loss in SPF mice in the context of periodontal disease, Hajishengallis and co-authors (2011) and the present study interpreted the increased alveolar bone loss as a consequence of normal host homeostasis in health.
Notably, and not previously reported, SPF mice vs. GF mice have increased numbers of TRAP+ osteoclastic cells lining the alveolar bone surface, which correlates with the increased alveolar bone loss phenotype of the SPF mice. The increased neutrophils, CD3+ T-lymphocytic cells, CD4+ T-helper cells, and IL17+ cells in the JE tissue and increased RANKL+ cells at the alveolar bone surface of SPF mice suggest that the host immune response mechanisms mediating homeostasis with the commensal oral microbiota induce a basal low-grade inflammatory state supporting osteoclastogenesis, resulting in naturally occurring alveolar bone loss in the healthy periodontium. Advances in osteoimmunology research demonstrating that hematopoietic cells regulate osteoblastogenesis highlight the need for future investigations assessing potential alterations in bone formation in SPF vs. GF mice, which could contribute to the increased alveolar bone loss phenotype of the SPF mice.
Evaluation of findings from this investigation of the impact of commensal bacteria on healthy periodontium vs. studies assessing the impact of perio-pathogenic bacteria on diseased periodontium provides indirect evidence that host immune response mechanisms intended to maintain homeostasis with the oral flora mediate alveolar bone loss in both health and disease. Similar to increased CD3+ T-lymphocytic and CD4+ T-helper cell numbers found infiltrating periodontitis-afflicted vs. healthy gingival tissue (Celenligil et al., 1990), SPF mice vs. GF mice had significantly increased numbers of CD3+ T-lymphocytic and CD4+ T-helper cells infiltrating the JE tissue. Furthermore, the four-fold- and two-fold-increased CD3+ T-lymphocytic cell numbers and RANKL expression in the periodontal tissue of SPF mice vs. GF mice closely parallel the reported 4-fold- and 2.5-fold-elevated T-lymphocytic cell numbers and RANKL expression in periodontitis-afflicted vs. healthy gingival tissue (Kawai et al., 2006). Last, the increased IL-17+ cell numbers found in the JE of SPF mice vs. GF mice correlate with elevated IL-17 expression reported in supernatants of gingival tissue cell cultures and gingival crevicular fluid isolated from individuals with periodontitis vs. periodontally healthy individuals (Vernal et al., 2005).
The decreased CD3+ T-lymphocytic cells, CD4+ T-helper cells, and IL17+ cells in the JE tissue and decreased RANKL+ cells at the alveolar bone surface in GF mice are novel findings in the periodontium of the GF animal model. While activated T-cells are recognized as the primary cell population expressing supra-physiological levels of RANKL (Kong et al., 1999; Kotake et al., 2001; Weitzmann et al., 2001) and IL-17 (Kotake et al., 1999; Sato et al., 2006; Adamopoulos and Bowman, 2008) under inflammatory disease states resulting in pathologic bone loss, recent investigations reporting that activated neutrophils express RANKL (Poubelle et al., 2007; Chakravarti et al., 2009) and IL-17 (Hoshino et al., 2008; Li et al., 2010; Lin et al., 2011; Keijsers et al., 2013) suggest that both innate and adaptive immune cells contribute to alveolar bone loss. Additionally, stromal/osteoblastic cells are known to up-regulate RANKL expression during periodontal disease states associated with catabolic alveolar bone loss (Teitelbaum, 2000; Boyle et al., 2003). While not within the scope of this study, future investigations elucidating the RANKL and IL-17 expression fold increase within specific periodontal cell populations, and the relative contributions across cell populations, will clarify the cellular-molecular mechanisms mediating alveolar bone loss in the periodontium during health and disease.
Page and Schroeder (1982) postulated that the subgingival plaque range of effectiveness in generating alveolar bone loss is about 2.5 mm, which was driven by Garant and Cho’s (1979) theory that locally produced bone resorption factors have an effective radius of action, and by findings that the distance from the apical extension of the subgingival plaque to the alveolar crest ranged from 0.5 mm to 2.7 mm (Waerhaug, 1979). When one considers the proposed range of effectiveness of subgingival plaque in causing pathologic alveolar bone loss, the host immune response to the normal oral flora may play a role in establishing the physiologic dimensions of biologic width (Gargiulo et al., 1961). The biologic width, composed of the junctional epithelium and supra-crestal connective tissue surrounding teeth, has been reported to range from 1.5 mm to 2.7 mm in healthy periodontium (Schmidt et al., 2013). While future investigations are necessary, oral commensal flora interactions with the host immune defense system may support biologic width tissue homeostasis, providing for a protective zone of junctional epithelium and supra-crestal connective tissue in periodontal health.
While 11- to 12-week-old mice were studied for the evaluation of early alterations in alveolar bone homeostasis in the mature young adult skeleton, further investigations of growing GF mice and aging adult GF mice are needed to elucidate the impact of the normal oral flora on the development and aging of the alveolar bone. We acknowledge that findings reported here in the C3H/Orl GF mice may not be consistent across mouse strains, because genetic differences in mouse strains have implications for commensal flora composition, host immune response, and skeletal tissue physiology. Future investigations of the commensal oral flora composition, junctional epithelial immune cells, and alveolar bone homeostasis in the periodontium of different GF mouse strains will begin to reveal the role of genetics in the commensal flora interactions with the innate defense system of the periodontium and its impact on naturally occurring alveolar bone loss. The non-uniform hardness diets administered to the SPF mice vs. GF mice, a potential study design weakness, will need to be evaluated, since no known prior studies have investigated the impact of diet hardness on alveolar bone homeostasis in GF mice.
This novel investigation of the impact of the normal oral microbiome on the host immune defense system in the healthy periodontium begins to elucidate immune regulatory mechanisms having catabolic effects on alveolar bone homeostasis during health. This is the first known histomorphometric study evaluating the alveolar bone loss phenotype in GF vs. SPF mice, which was assessed previously by morphometric analyses of defleshed maxillae/mandibles. To our knowledge, the enumeration of osteoclastic cell numbers lining the alveolar bone surface performed here is the initial comparative analysis of osteoclast cell numbers in the alveolar bone of GF mice vs. SPF mice. Most notably, this is the first known investigation of periodontal tissue homeostasis in the GF animal model for quantitative assessment of T-cell (CD3+ T-lymphocytic cell and CD4+ T-helper cell) numbers and IL-17 and RANKL expression in the periodontium. Novel findings from this study suggest that the normal commensal flora stimulates the innate defense system of the periodontium, up-regulating not only the number of neutrophils but also T-cell (CD3+ T-lymphocytic cell and CD4+ T-helper cell) and IL-17 expression in the JE tissue and RANKL expression at the alveolar bone surface. Similar to increased neutrophil numbers infiltrating the JE tissue of SPF mice, we speculate that the increased T-cell (CD3+ T-lymphocytic cell and CD4+ T-helper cell) numbers and IL-17 and RANKL expression are due to the host innate defense system inflammatory surveillance mechanisms regulating colonization of the commensal oral flora. Indirect evidence from prior periodontitis disease studies suggests that the commensal oral flora induced up-regulation of T-cell (CD3+ T-lymphocytic cell and CD4+ T-helper cell) numbers and that IL-17 and RANKL expression in the normal periodontium supports osteoclast-mediated alveolar bone loss during health. Future investigations characterizing differences in host immune defense regulation of the normal oral microbiome in healthy periodontium vs. perio-pathogenic oral flora in diseased periodontium will advance our understanding of the mechanisms mediating alveolar bone loss during health vs. disease.
Footnotes
This work was supported by National Institutes of Health (NIH) grant DE018274 to Richard Darveau.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Adamopoulos IE, Bowman EP. (2008). Immune regulation of bone loss by Th17 cells. Arthritis Res Ther 10:225-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PJ. (2000). The role of immune responses in bone loss during periodontal disease. Microbes Infect 2:1181-1192. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. (2003). Osteoclast differentiation and activation. Nature 423:337-342. [DOI] [PubMed] [Google Scholar]
- Brown LR, Roth GD, Hoover D, Flanagan V, Nielsen AH, Werder AA. (1969). Alveolar bone loss in leukemic and nonleukemic mice. J Periodontol 40:725-730. [DOI] [PubMed] [Google Scholar]
- Celenligil H, Kansu E, Ruacan S, Eratalay K, Caglayan G. (1990). Immunohistological analysis of gingival lymphocytes in adult periodontitis. J Clin Periodontol 17:542-548. [PubMed] [Google Scholar]
- Chakravarti A, Raquil MA, Tessier P, Poubelle PE. (2009). Surface RANKL of Toll-like receptor 4-stimulated human neutrophils activates osteoclastic bone resorption. Blood 114:1633-1644. [DOI] [PubMed] [Google Scholar]
- Di Benedetto A, Gigante I, Colucci S, Grano M. (2013). Periodontal disease: linking the primary inflammation to bone loss. Clin Dev Immunol 2013:503754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DR, Reife RA, Cebra JJ, Darveau RP. (2004). Commensal bacteria influence innate status within gingival tissues: a pilot study. J Periodontol 75:1486-1492. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Dawson DR, 3rd, Morford LA, Peyyala R, Miller CS, Gonzaléz OA. (2013). Periodontal disease immunology: ‘double indemnity’ in protecting the host. Periodontol 2000. 62:163-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garant PR, Cho MI. (1979). Histopathogenesis of spontaneous periodontal disease in conventional rats. I. Histometric and histologic study. J Periodontal Res 14: 297-309. [DOI] [PubMed] [Google Scholar]
- Gargiulo AW, Wentz FM, Orban B. (1961). Dimensions and relations of the dentogingival junction in humans. J Periodontol 32:261-267. [Google Scholar]
- Graves DT, Kang J, Andriankaja O, Wada K, Rossa C., Jr (2012). Animal models to study host-bacteria interactions involved in periodontitis. Front Oral Biol 15:117-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, et al. (2011). Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 10:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Nagao T, Nagi-Miura N, Ohno N, Yasuhara M, Yamamoto K, et al. (2008). MPO-ANCA induces IL-17 production by activated neutrophils in vitro via classical complement pathway-dependent manner. J Autoimmun 31:79-89. [DOI] [PubMed] [Google Scholar]
- Irie K, Tomofuji T, Tamaki N, Sanbe T, Ekuni D, Azuma T, et al. (2008). Effects of ethanol consumption on periodontal inflammation in rats. J Dent Res 87:456-460. [DOI] [PubMed] [Google Scholar]
- Kawai T, Matsuyama T, Hosokawa Y, Makihara S, Seki M, Karimbux NY, et al. (2006). B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol 169:987-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijsers RR, Hendriks AG, van Erp PE, van Cranenbroek B, van de Kerkhof PC, Koenen HJ, et al. (2013). In vivo induction of cutaneous inflammation results in the accumulation of extracellular trap-forming neutrophils expressing RORγt and IL-17. J Invest Dermatol 134:1276-1284. [DOI] [PubMed] [Google Scholar]
- Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, et al. (1999). Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402:304-309. [DOI] [PubMed] [Google Scholar]
- Kornman KS, Page RC, Tonetti MS. (1997). The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000 14: 33-53. [DOI] [PubMed] [Google Scholar]
- Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. (1999). IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 103:1345-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake S, Udagawa N, Hakoda M, Mogi M, Yano K, Tsuda E, et al. (2001). Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum 44:1003-1012. [DOI] [PubMed] [Google Scholar]
- Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, et al. (2010). IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest 120:331-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. (2011). Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol 187:490-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RC, Schroeder HE. (1982). Periodontitis in man and other animals. A comparative review. Basel, Switzerland: S. Karger. [Google Scholar]
- Poubelle PE, Chakravarti A, Fernandes MJ, Doiron K, Marceau AA. (2007). Differential expression of RANK, RANK-L, and osteoprotegerin by synovial fluid neutrophils from patients with rheumatoid arthritis and by healthy human blood neutrophils. Arthritis Res Ther 9:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanbe T, Tomofuji T, Ekuni D, Azuma T, Irie K, Tamaki N, et al. (2009). Vitamin C intake inhibits serum lipid peroxidation and osteoclast differentiation on alveolar bone in rats fed on a high-cholesterol diet. Arch Oral Biol 54:235-240. [DOI] [PubMed] [Google Scholar]
- Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. (2006). Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 203:2673-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JC, Sahrmann P, Weiger R, Schmidlin PR, Walter C. (2013). Biologic width dimensions—a systematic review. J Clin Periodontol 40:493-504. [DOI] [PubMed] [Google Scholar]
- Taubman MA, Valverde P, Han X, Kawai T. (2005). Immune response: the key to bone resorption in periodontal disease. J Periodontol 76:2033-2041. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. (2000). Bone resorption by osteoclasts. Science 289:1504-1508. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Usui M, Yamamoto G, Takagi Y, Tachikawa T, Yamamoto M, et al. (2012). Role of the junctional epithelium in periodontal innate defense and homeostasis. J Periodontal Res 47: 750-757. [DOI] [PubMed] [Google Scholar]
- Vernal R, Dutzan N, Chaparro A, Puente J, Antonieta Valenzuela M, et al. (2005). Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J Clin Periodontol 32:383-389. [DOI] [PubMed] [Google Scholar]
- Waerhaug J. (1979). The angular bone defect and its relationship to trauma from occlusion and downgrowth of subgingival plaque. J Clin Periodontol 6:61-82. [DOI] [PubMed] [Google Scholar]
- Weitzmann MN, Cenci S, Rifas L, Haug J, Dipersio J, Pacifici R. (2001). T cell activation induces human osteoclast formation via receptor activator of nuclear factor kappaB ligand-dependent and -independent mechanisms. J Bone Miner Res 16:328-337. [DOI] [PubMed] [Google Scholar]
- Zenobia C, Luo XL, Hashim A, Abe T, Jin L, Chang Y, et al. (2013). Commensal bacteria-dependent select expression of CXCL2 contributes to periodontal tissue homeostasis. Cell Microbiol 15: 1419-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]