Abstract
A full understanding of the key regulators controlling periodontal development and homeostasis is necessary for the design of improved periodontal regenerative therapies. Small leucine-rich proteoglycans (SLRPs) are extracellular matrix molecules suggested to regulate collagen organization and cell signaling. Mice with double-deficiency of 2 SLRPs, fibromodulin and biglycan (dKO), acquire skeletal abnormalities, but their roles in regulating the periodontium remain undefined and were the focus of our studies. Transmission electron microscopy studies showed abnormal collagen fibrils in the periodontal ligament (PDL) and altered remodeling of alveolar bone in dKO mice. Immunohistochemistry (IHC) revealed increased staining of SLRPs (asporin, lumican, and decorin) and dentin matrix protein-1 (DMP1, a mechanosensory/osteocyte marker), while osteoblast markers, bone sialoprotein and osteopontin, remained unchanged. Disruption of homeostasis was further evidenced by increased expression of receptor-activator of nuclear factor-κB ligand (RANKL) and elevated numbers of osteoclasts, especially noted around the alveolar bone of molars (buccal side) and incisors. Polymerase chain reaction (PCR) array revealed hyperactive transforming growth factors beta/bone morphogenetic protein (TGFβ/BMP) signaling in dKO PDL tissues, which was further confirmed by elevated expression of phosphorylated Smad5 (p-Smad5) by IHC in dKO PDL. These studies highlight the importance of SLRPs in maintaining periodontal homeostasis through regulation of TGFβ/BMP signaling, matrix turnover, and collagen organization.
Keywords: extracellular matrix, periodontal ligament, proteoglycan, tooth root, mineralization, signal transduction
Introduction
Tooth function and longevity are supported by tissues of the periodontium, including alveolar bone, periodontal ligament (PDL), and cementum. The PDL is a fibrous connective tissue situated between 2 mineralized tissues, cementum and alveolar bone (Berkovitz, 1990). PDL cells promote formation of an extracellular matrix (ECM), composed mainly of collagen fibrils (primarily type I) that insert into the surrounding hard tissues to suspend the tooth. The softer PDL absorbs functional loads by acting as a cushion between the 2 mineralized tissues, alveolar bone and tooth cementum (Ho et al., 2010; Marchesan et al., 2011). When mechanically loaded, the PDL behaves as a viscoelastic gel, orchestrated by a meshwork of cell-cell and cell-matrix interactions involving locally secreted growth factors. Growth factors sequestered within the ECM, when exposed to the loaded environment, are proposed to be released and subsequently trigger downstream signaling. In turn, this affects cell differentiation and hard-tissue remodeling (Meikle, 2006). Thus, ECM molecules of the PDL may display both structural and cell-regulatory functions.
Small leucine-rich proteoglycans (SLRPs) act as regulators of ECM turnover (Chen and Birk, 2013). Class I SLRPs include decorin (Dcn), biglycan (Bgn), and asporin (Asp), which bear chondroitin sulfate or dermatan sulfate glycosaminoglycan chains. Class II SLRPs include lumican (Lum) and fibromodulin (Fmod), both of which have keratan sulfate glycosaminoglycan chains attached. It has been reported that SLRP-deficient mice acquire diminished bone mass (Xu et al., 1998), gait impairment, ectopic ossification of tendons, and severe osteoarthritis (Ameye et al., 2002), and defects of collagen fibrillogenesis in the PDL, predentin/dentin (Goldberg et al., 2006) and the temporomandibular joint (Ameye et al., 2002; Matheson et al., 2005; Bi et al., 2007; Embree et al., 2010). However, the roles of SLRPs in regulating the periodontal apparatus remain undefined.
Previous studies have suggested that SLRPs interact directly with soluble growth factors, including epidermal growth factors (Santra et al., 2002), transforming growth factors beta (TGFβ), and bone morphogenetic proteins (BMPs), to mediate differentiation and mineralization (Bi et al., 2007; Embree et al., 2010). The tissue-specific responses to TGFβ/BMP signaling may be partly dependent on the expression and abundance of other influencing factors. Here we propose that Fmod and Bgn are key players in periodontal development and homeostasis through regulating the local concentrations of TGFβ/BMP signaling. The Fmod and Bgn double-deficient (dKO) mice provide the opportunity for the role(s) of SLRPs during development and maintenance of the periodontal apparatus to be defined, and potential periodontal regenerative therapies to be informed.
Materials & Methods
Animals
Fmod and Bgn dKO mice have been previously described (Ameye et al., 2002; Bi et al., 2007). Mandibles were collected from male Bgn-/0Fmod-/- mice and age-matched wild-type (WT) counterparts at 4 (developmental stage), 8, 11 (growth stages), and 52 (early stage of senescence) wk of age (n = 3-5 for each age group).
Transmission Electron Microscopy (TEM)
Mouse PDL tissues were harvested and fixed in 2% glutaraldehyde in 0.1M cacodylate buffer (pH 7.4) at 4°C and prepared for TEM as previously described (Niethamer et al., 2012). Quantification data were obtained by Image J (NIH, Bethesda, MD, USA) analysis.
Histology
For histology, mandibles were fixed in Bouin’s solution (Electron Microscopy Sciences, Hatfield, PA, USA), demineralized in AFS (acetic acid, formaldehyde, and sodium chloride), and embedded in paraffin for microtome sectioning, as previously described (Foster, 2012). Procedures for picrosirius red stain, tartrate-resistant acid phosphatase (TRAP), and immunohistochemistry (IHC) were as previously described (Foster et al., 2013). The Appendix Fig. displays the schematic model of the buccolingual section of a mouse mandible for tissue orientation. For TRAP, a commercially available kit was used according to the manufacturer’s instructions (Clontech Laboratories, Mountain View, CA, USA). We calculated TRAP-positive cells per alveolar bone (mm) by dividing the number of TRAP-positive cells by the length of the perimeter, as described below. The perimeter of the buccal side alveolar bone was measured from the cemento-enamel junction of M1 to the bottom of the socket. The perimeter of the alveolar bone on the incisor enamel side was measured with the mesial midsection as land marker. One-way analysis of variance (ANOVA) was used for statistical analysis (α = 0.05). For IHC, biotinylated secondary antibodies and peroxidase substrate were used for antigen detection (Vector Laboratories, Burlingame, CA, USA). Primary antibodies included rabbit anti-Asp (1:200, Abcam, Cambridge, MA, USA), Dcn (1:200, Takara, Tokyo, Japan), Lum (1:50, R&D Systems, Minneapolis, MN, USA), receptor activator of nuclear factor-κB ligand (RANKL) (1:20, R&D Systems), bone sialoprotein (BSP) (1:200, kindly provided by Dr. Renny Franceschi, University of Michigan, Ann Arbor, MI, USA), osteopontin (OPN) (LF-175, 1:200, kindly provided by Dr. Larry Fisher, NIDCR/NIH, Betheda, MD, USA), DMP1 (1:600, Takara), and rabbit anti-p-Smad5 (1:100, Abcam).
X-ray Micro-computed Tomography (µCT)
Whole mandibles were scanned in a µCT system (µCT 50, Scanco Medical AG, Bassersdorf, Switzerland). Scans were performed at 70 kV, 85 µA, 300 ms integration time, and at a resolution of 10 µm. After reconstruction, the images were stored in 3D arrays. The mineralized tissues were differentially segmented by a global thresholding procedure (Muller et al., 1996), and morphometric parameters were determined by a direct 3D approach (Hildebrand et al., 1999). The average PDL width was measured for the mesial root of the first molar (M1) from the bifurcation of the roots to the apex of the root. The alveolar bone parameters were measured on the buccal side of the mesial root of M1 from the bifurcation of the roots to the end of the socket. A mid-sagittal line was drawn on the root, and the alveolar bone was measured from the edges of the lamina dura on both mesial and distal sides to the edge of bone on the buccal side. Parameters determined in the alveolar bone analysis were bone wall width, bone mineral density, and bone mineral content. A Student’s t test was performed for statistical analysis (α = 0.05).
PCR Array
Total RNA from PDL tissue of male dKO mice (n = 3) and WT mice (n = 4) at 11 wk was extracted by means of an RNeasy micro kit (Qiagen, Valencia, CA, USA). Mouse TGFβ/BMP signaling pathway PCR array (SA Biosciences, Frederick, MD, USA) was performed on the Roche LightCycler 480 II system. Results were analyzed with SA Biosciences PCR array Web-based data analysis software. A Student’s t test was performed for statistical analysis (α = 0.05).
Results
Defective Collagen Bundles and Enhanced SLRP Expression in dKO Periodontium
Gross histology showed that dKO mouse teeth and periodontal apparatus were similar to those of age-matched WT mice at all time points examined (4, 8, and 52 wk), including the absence of periodontal pocket formation or apical migration of the epithelial attachment.
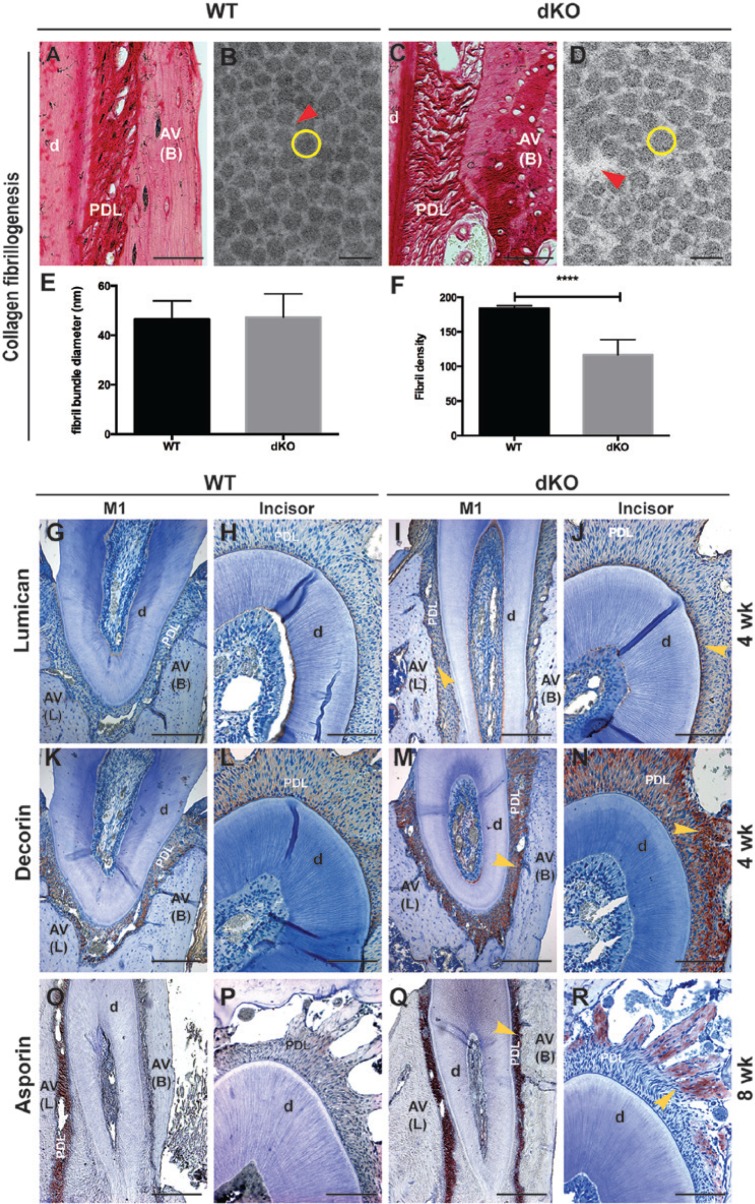
Picrosirius red staining revealed that bundles of collagen fibrils were loosely formed in the PDL, and the collagen fibrils in alveolar bone were disorganized in dKO mice at 8 wk compared with those in WT mice (Figs. 1C vs. 1A). TEM confirmed this observation (Figs. 1D vs. 1B). Quantitative analysis of TEM data showed no difference in the diameters of PDL collagen fibril bundles of dKO mice compared with those in WT mice (Fig. 1E); however, the density of PDL collagen fibril was significantly decreased by 36.5% in dKO mice vs. WT mice (Fig. 1F).
Figure 1.
Defective collagen bundles and enhanced small leucinerich proteoglycan (SLRP) expression in the periodontium of double deficient (dKO) mice. (A, C) Picrosirius red staining of the buccal side periodontal ligament (PDL) of the first molar (M1) of wild-type (WT) and dKO mice, respectively; (B, D) transmission electron microscopy (TEM) image of PDL collagen fibril bundles of WT and dKO mice, respectively (red arrows indicate spaces between fibril bundles, and yellow circles indicate a representative single fibril bundle). (E) The diameters of PDL collagen fibril bundles from dKO mice are the same as in WT mice at 8 wk. (F) The collagen fibril density is significantly decreased by 36.5% in the PDL of dKO mice compared with that of WT mice at 8 wk (****p < .0001 by a Student’s t test). (G-R) Protein expression patterns of lumican (Lum), decorin (Dcn), and asporin (Asp) in PDL of WT (G, H, K, L, O, and P) and dKO (I, J, M, N, Q, and R; yellow arrows indicate the increased expression of corresponding proteins in dKO) were evaluated by immunohistochemistry. Increased expression of Lum (G-J) and Dcn (K-N) was noted in the PDL from dKO mice at 4 wk, while Asp (O-R) was enhanced markedly in the PDL at the buccal side of M1 (Q) and in the PDL near the alveolar bone of the incisor (R) of dKO mice at 8 wk. D, dentin; AV (L), lingual side of alveolar bone; AV (B), buccal side of alveolar bone. Bar represents: 100 μm in A, C, H, J, L, N, P, and R; 100 nm in B and D; and 200 μm in G, I, K, M, O, and Q.
All SLRPs examined in this investigation (Lum, Dcn, and Asp) were expressed in mouse molar and incisor PDL, although distribution patterns varied by location and age (Figs. 1G-1R). Lum was expressed more intensely in PDL of dKO mice compared with WT mice at 4 wk, with strongest expression near cementum and alveolar bone surfaces (Figs. 1I, 1J vs. 1G, 1H). Differences were less marked at older ages, with robust expression at 8 wk and weak expression at 52 wk (data not shown). Similarly, Dcn was expressed more intensely in dKO compared with WT mice at 4 wk, especially near alveolar bone (Figs. 1M, 1N vs. 1K, 1L), while differences were reduced at 8 and 52 wk (data not shown). Asp expression was similar between dKO and WT mice at 4 wk (data not shown), and its expression was dramatically increased in dKO vs. WT mice at 8 wk, surrounding the molar and in the bone-associated PDL around the incisor (Figs. 1O, 1R vs. 1O, 1P). Asp, Lum, and Dcn were enhanced in an age- and tissue-specific manner in dKO mice, suggestive of an attempt to compensate for loss of Fmod and Bgn.
Altered PDL Cell Function and Alveolar Bone Remodeling in dKO Mice
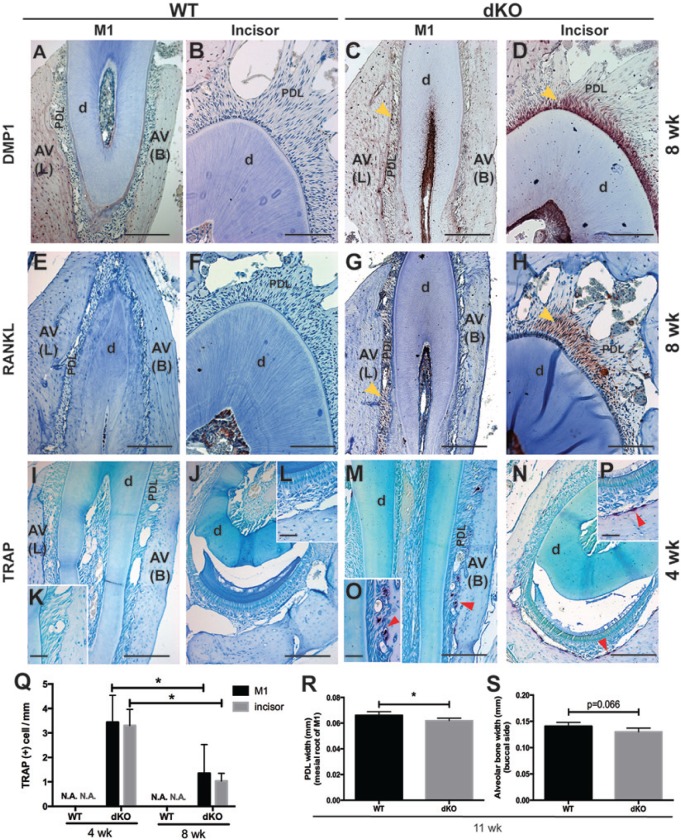
We next examined whether deletion of Bgn and Fmod affected PDL cell function, using antibodies against OPN, DMP1, and BSP, markers associated with mineralization. Analysis of IHC data revealed enhanced expression of DMP1 in the PDL of dKO mice at 8 wk, especially at the incisor-associated region, while DMP1 was not detected in the PDL of WT mice at 8 wk (Figs. 2C, 2D vs. 2A, 2B). No difference in staining was noted in OPN or BSP staining (data not shown). Published results demonstrating increased osteoclast activity in bones of Bgn-deficient mice (Chiu et al., 2012) prompted us to examine osteoclast behavior in dKO mice. Enhanced expression of osteoclast regulating factor, RANKL, was observed in the PDL of dKO mice at 8 wk, while it was not detected in the PDL of WT mice at 8 wk (Figs. 2G, 2H vs. 2E, 2F). TRAP-positive osteoclast-like cells were identified in PDL of dKO mice at 4 wk (Figs. 2M-2P) and 8 wk (data not shown), especially on the buccal and apical sides of molar alveolar bone and incisor alveolar bone, respectively, whereas WT tissues exhibited minimal TRAP staining under the same experimental conditions (Figs. 2I-2L). Quantification of TRAP-positive cells also revealed that the number of osteoclasts in dKO mice at 8 wk was significantly decreased by ~60% compared with that in dKO mice at 4 wk, indicating a decreased bone resorption rate with aging (Fig. 2Q). Furthermore, µCT quantitative analysis showed that the average width of the PDL around the mesial root of M1 was significantly decreased by ~7%, and the width of the alveolar bone wall on the buccal side of the first molar showed a trend of decreasing (p = .066) in dKO mice at 11 wk (Figs. 2R, 2S). Bone mineral density and bone mineral content did not change in dKO mice compared with WT mice at 11 wk (data not shown).
Figure 2.
Altered periodontal ligament (PDL) cell function and alveolar bone remodeling in double-deficient (dKO) mice. (A-P) Protein expression patterns of dentin matrix protein-1 (DMP1) and receptor-activator of nuclear factor-κB ligand (RANKL) in the PDL of wild-type (WT) (A, B, E, and F) and dKO (C, D, G, and H) mice were evaluated by immunohistochemistry (IHC) (yellow arrows indicate the increased expression of the corresponding proteins in dKO). Osteoclast cells were analyzed by tartrate-resistant acid phosphatase (TRAP) staining in WT (I-L) and dKO (M-P) mice. Both DMP1 and RANKL were not noted in the PDL region of WT mice. However, both DMP1 and RANKL are elevated markedly in the PDL of dKO mice at 8 wk, especially in the region around the incisor. TRAP-positive cells (indicated by red arrows in M-P) are shown in the PDL region of dKO mice at 4 wk near the buccal side of alveolar bone and near the alveolar bone of incisors, while they are not detected in WT mice at 4 wk. d, dentin; AV (L), lingual side of alveolar bone; AV (B), buccal side of alveolar bone. Bar represents 50 μm in K, L, O, and P, 100 μm in B, D, F, and H, and 200 μm in A, C, E, G, I, J, M, and N. (Q) Quantitative analysis of TRAP-positive cells per alveolar bone (mm) reveals that, in addition to the higher number of osteoclasts in dKO mice at both ages (4 and 8 wk) compared with WT mice, the number of osteoclasts in the alveolar bone around M1 or incisor is significantly decreased by ~60% with aging (from 4 to 8 wk) (*p < .05 by one-way ANOVA). (R) Micro-computed tomography (µCT) quantitative analysis shows that the average width of PDL around the mesial root of M1 is significantly decreased by ~7%. (S) The width of the alveolar bone wall on the buccal side of the first molar shows a decreasing trend (p = .066) in dKO mice at 11 wk (*p < .05 by Student’s t test).
Hyperactive TGFβ/BMP Signaling in the PDL of dKO Mice
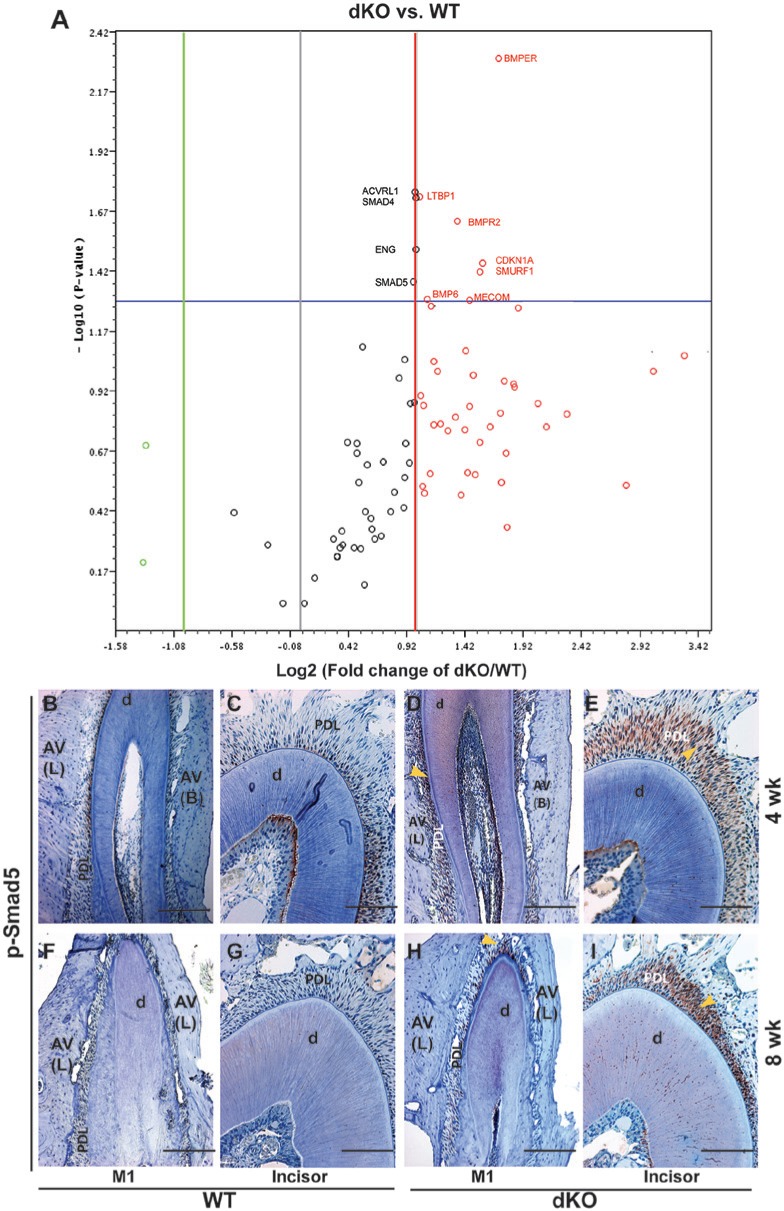
To determine whether TGFβ/BMP signaling was altered in dKO PDL, we performed a PCR expression array on RNA extracted from PDL tissue of WT and dKO mice. The focused TGFβ/BMP array included members of the TGFβ superfamily, Smads, and their target genes. Differences in gene expression of WT vs. dKO mice were assessed by volcano plots in association with statistical verification. Fig. 3A and the Table reveal trends in overall gene expression differences: 50% of the TGFβ/ BMP signaling-related genes were up-regulated (red circles) in PDL of dKO mice; and 7 of the up-regulated genes were significantly increased more than two-fold, i.e., Bmper, Cdkn1a, Smurf1, Mecom, Bmpr2, Bmp6, and Ltbp1 (see Table for the names of these genes). Importantly, Smad 4 and 5, genes encoding key regulators of the BMP signaling pathway, were significantly up-regulated (approximately two-fold). No genes were significantly down-regulated in PDL of dKO mice compared with WT mice, as assessed by the array we used. IHC staining confirmed hyperactive TGFβ/BMP signaling by demonstrating increased expression of p-Smad5 (active form) in dKO PDL at all ages assayed (52 wk not shown) (Figs. 3D, 3E, 3H, 3I vs. 3B, 3C, 3F, 3G).
Figure 3.
Increased transforming growth factors beta/bone morphogenetic protein (TGFβ/BMP) signaling in the periodontal ligament (PDL) of double-deficient (dKO) mice. (A) Differences in TGFβ/BMP signaling pathway-related genes in PDL of dKO vs. wild-type (WT) mice are shown by the volcano plot. The Y-axis shows the values of minus Log10 (p value), and the X-axis shows the values of Log2 (fold change of dKO vs. WT mice). Circles above the horizontal blue line represent genes with statistically significant changes (p < .05) compared with WT mice. The middle vertical gray line represents zero-fold change, with the green and red lines on either side of it representing two-fold change boundaries. Red circles represent up-regulated genes with two-fold change, and light green circles represent down-regulated genes with more than two-fold change. Black circles represent genes with changes less than two-fold. Eleven genes (noted on the volcano plot and also listed in the Table) are significantly (p < .05) up-regulated, with two- to three-fold changes. (B-I) Protein expression patterns (evaluated by immunohistochemitry [IHC]) of phosphorylated Smad5 (p-Smad5) of WT (B, C, F, and G) and dKO (D, E, H, and I) mice. Yellow arrows indicate the increased expression of p-Smad 5 in dKO mice at 4 wk (B-E) and 8 wk (F-I). p-Smad 5 is elevated in the PDL of dKO mice at 4 and 8 wk compared with WT mice. d, dentin; AV (L), lingual side of alveolar bone; AV (B), buccal side of alveolar bone. Bar represents 200 μm in B, D, F, and H and 100 μm in C, E, G, and I.
Table.
Significantly Increased Genes in PDL of Fmod and Bgn dKO vs. WT Mice
| Gene Symbol | Gene Name | Fold Change | p value |
|---|---|---|---|
| Bmper | BMP-binding endothelial regulator | 3.2509 | .004863 |
| Cdkn1a | Cyclin-dependent kinase inhibitor 1A (P21) | 2.9639 | .034686 |
| Smurf1 | SMAD specific E3 ubiquitin protein ligase 1 | 2.9164 | .037990 |
| Mecom | MDS1 and EVI1 complex locus | 2.7416 | .049740 |
| Bmpr2 | Bone morphogenetic protein receptor, type II (serine/threonine kinase) | 2.5418 | .023341 |
| Bmp6 | Bone morphogenetic protein 6 | 2.1324 | .049415 |
| Ltbp1 | Latent transforming growth factor beta binding protein 1 | 2.0432 | .018319 |
| Smad4 | MAD homolog 4 (Drosophila) | 1.9873 | .018548 |
| Eng | Endoglin | 1.9873 | .030399 |
| Acvrl1 | Activin A receptor, type II-like 1 | 1.9839 | .017581 |
| Smad5 | MAD homolog 5 (Drosophila) | 1.9611 | .041706 |
Discussion
In this study, we investigated the functional importance of the SLRPs, Fmod and Bgn, in periodontal development and function. Fmod and Bgn dKO mice exhibited disorganized PDL collagen fibrils, altered distribution of other SLRPs, disturbed homeostasis of hard tissue, and increased TGFβ/BMP signaling, revealing important functions for Fmod and Bgn as PDL ECM constituents and regulators of cell signaling.
SLRPs Regulate Collagen Fibrillogenesis and Periodontal Homeostasis
As collagen-binding molecules, SLRPs mediate the intermolecular cross-linking of collagen during collagen fibrillogenesis (Kalamajski and Oldberg, 2010). With the loss of Fmod and Bgn, PDL tissues exhibited more spaces between and within collagen fibril bundles, possibly because of the decreased quality and quantity of cross-links formed by SLRPs. In WT mice, Fmod and Bgn were expressed more intensely in the insertion region of the collagen fibrils into the cementum (data not shown), an observation consistent across species (Ababneh et al., 1999; Matias et al., 2003), suggesting that SLRPs play a role in PDL-cementum attachment. SLRP-mediated collagen cross-linking not only holds fibrils tighter, but also protects collagens from enzyme degradation (Geng et al., 2006). Insufficient cross-linking could lead to defects in collagen fibril stability, subsequently disturbing homeostasis of the periodontium. Compensatory accumulation of other SLRPs was noted in our and other studies of single- and double-SLRP ablation models (Svensson et al., 1999; Jepsen et al., 2002). Altered distribution of SLRPs in these models is likely related to similar functionality of SLRPs within the same class and altered expression in the event of mechanical disturbances of PDL collagen fibrils. However, each SLRP seems to have its own unique distribution and function in regulating collagen fibril organization and assembly. For example, Dcn was more uniformly expressed in PDL region, while Asp seems to be more sensitive to local changes in mechanical strain, especially when Fmod and Bgn were absent; Fmod has a higher binding affinity to ColI than Lum, with a greater role in the fusion of small fibrils into large fibers (Svensson et al., 1999). Furthermore, we found that Asp, Lum, and Dcn responded to the depletion of Fmod and Bgn in an age- and tissue-specific manner, with Lum and Dcn uniformly enhanced in the PDL at 4 wk and Asp at 8 wk, more specifically in the PDL on the buccal side and the PDL near alveolar bone around the incisor. This variation in the distribution and relative content of SLRPs marks the physical-chemical changes translated from age- and function-related differences, as discussed in studies by the Ho group (Leong et al., 2012). Overall, our finding suggests that SLRPs cannot compensate completely for the function of one another, and further investigations are needed to identify the specific structural and functional roles of SLRPs at different stages of successive construction and remodeling of the ECM.
SLRPs are expressed in the ECM of mineralized tissues and influence mineralization. Whereas DMP1 and BSP were over-expressed in odontoblasts of Fmod-deficient mice (Goldberg et al., 2006), reduced expression of osteoblast markers, including OPN, BSP, and osteocalcin, was observed in calvarial cells from Bgn-deficient mice, leading to diminished ability to mineralize in vitro (Chen et al., 2004). Over-expression of Bgn in bone marrow mesenchymal stem cells resulted in enhanced alkaline phosphatase activity and increased expression of collagen I, Runx2, and osteocalcin, indicating a positive effect of Bgn on osteogenesis (Wu et al., 2013). In contrast, Asp maintains PDL homeostasis as a negative effector of cytodifferentiation and mineralization (Kajikawa et al., 2014). In the present study, expression of DMP1 in incisor PDL was enhanced progressively within the PDL region with age in dKO vs. WT mice. DMP1 is suggested to be a mechanosensor, with increased expression in mechanically loaded osteocytes (Gluhak-Heinrich et al., 2003). It is important to note that rodent incisors are continuously growing. In addition to the mechanical forces generated by the direct usage of incisors and strains transmitted from molars during chewing, mechanical strains imposed on the continuously growing incisor may induce the surrounding periodontal complex to exhibit unique and robust expression of biomolecule markers such as DMP1, an ECM protein reported to act as a mechanosensor (Gluhak-Heinrich et al., 2003). Further studies targeted at determining the specific cells expressing DMP-1 within the dKO PDL region (e.g., by in situ hybridization) may provide additional insights into the function of DMP1.
In addition to increased apoptosis of bone marrow stromal cells and osteoblast differentiation defects (Chen et al., 2002), TRAP-positive cells have been observed in Bgn-deficient mice, corresponding to increased alveolar bone resorption (Chiu et al., 2012). We report that Fmod and Bgn dKO mice feature increased RANKL expression in the PDL, potentially due to the altered forces on blood vessels within the PDL of dKO vs. WT mice (Robling and Turner, 2009). This observation is supported by the predominance of TRAP-positive cells on the compression side of the alveolar bone in dKO mice. Additionally, dKO mice have narrower width of the PDL region and buccal side alveolar bone around M1, although the overall bone quality of dKO mice is similar to that of WT mice. We hypothesize that increased bone resorption in Fmod and Bgn dKO mice resulted from disorganized and mechanically dysfunctional collagen fibrils, leading to increased remodeling of mineralized tissue at sites of compression.
Fmod and Bgn Regulate TGFβ/BMP Signaling in the PDL
Interactions between TGFβ/BMP and ECM constituents are critical for modulating TGFβ/BMP activity and function. SLRP family members have been shown to modify TGFβ/BMP bio-availability and/or function by binding to and sequestering TGFβ/BMPs within the ECM (Schaefer and Schaefer, 2010). Fmod, Bgn, Asp, and Dcn are capable of binding to members of the TGFβ superfamily and thereby regulate their activity (Hildebrand et al., 1994; Cabello-Verrugio and Brandan, 2007; Kajikawa et al., 2014). Additionally, Bgn enhances the binding of BMP4 to its inhibitory effectors, effectively reducing BMP4 signaling during the early embryonic development of xenopus (Moreno et al., 2005). However, Bgn-deficient osteoblasts exhibited decreased sensitivity to BMP4, due to reduced binding of BMP4 (Chen et al., 2004). A tissue-specific regulation of TGFβ/BMP signaling by SLRPs may be indirect, via local variations in other modifiers of the signaling pathway. For example, in myoblasts, the interaction of Dcn with lipoprotein-receptor-related protein 1 (LRP-1) modulates TGFβ activity (Cabello-Verrugio and Brandan, 2007). Additionally, the Asp variant (D14-PLAP-1) has stronger affinity to BMP2 to repress its activity compared with other variants (Kajikawa et al., 2014) in PDL cells. Analysis of our data suggests that the absence of Fmod and Bgn in the periodontal region activates TGFβ/BMP signaling, and the increased expression of Asp acts as negative feedback to correct the hyperactive BMP within the ECM. The dysregulated genes uncovered with the PCR array may act as secondary effectors to modulate the homeostasis of the periodontium. Further studies are needed to address their specific functions during the process of periodontal development and homeostasis.
In conclusion, we observed that deletion of Fmod and Bgn results in defective collagen fibrillogenesis, altered PDL cell function and alveolar bone remodeling, and hyperactive TGFβ/BMP signaling. These results support a strong role for Fmod and Bgn in regulating the TGFβ/BMP signaling pathway during periodontal development and in the maintenance of homeostasis. Although further research is needed, these novel findings provide insights for consider in the design of new periodontal regenerative therapies.
Supplementary Material
Acknowledgments
The authors thank Drs. Min Ao, Brendan Lopez, and Kanako Nagatomo for their contributions to this research.
Footnotes
This research was supported by the Intramural Research Programs of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (MJS) and the National Institute of Dental and Craniofacial Research (NIDCR) (MFY) (Bethesda, MD, USA).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Ababneh KT, Hall RC, Embery G. (1999). The proteoglycans of human cementum: immunohistochemical localization in healthy, periodontally involved and ageing teeth. J Periodontal Res 34:87-96. [DOI] [PubMed] [Google Scholar]
- Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, Young MF. (2002). Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J 16:673-680. [DOI] [PubMed] [Google Scholar]
- Berkovitz BK. (1990). The structure of the periodontal ligament: an update. Eur J Orthod 12:51-76. [DOI] [PubMed] [Google Scholar]
- Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, et al. (2007). Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 13:1219-1227. [DOI] [PubMed] [Google Scholar]
- Cabello-Verrugio C, Brandan E. (2007). A novel modulatory mechanism of transforming growth factor-beta signaling through decorin and LRP-1. J Biol Chem 282:18842-18850. [DOI] [PubMed] [Google Scholar]
- Chen S, Birk DE. (2013). The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J 280:2120-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XD, Shi S, Xu T, Robey PG, Young MF. (2002). Age-related osteoporosis in biglycan-deficient mice is related to defects in bone marrow stromal cells. J Bone Miner Res 17:331-340. [DOI] [PubMed] [Google Scholar]
- Chen XD, Fisher LW, Robey PG, Young MF. (2004). The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. FASEB J 18:948-958. [DOI] [PubMed] [Google Scholar]
- Chiu R, Li W, Herber RP, Marshall SJ, Young M, Ho SP. (2012). Effects of biglycan on physico-chemical properties of ligament-mineralized tissue attachment sites. Arch Oral Biol 57:177-187. [DOI] [PubMed] [Google Scholar]
- Embree MC, Kilts TM, Ono M, Inkson CA, Syed-Picard F, Karsdal MA, et al. (2010). Biglycan and fibromodulin have essential roles in regulating chondrogenesis and extracellular matrix turnover in temporomandibular joint osteoarthritis. Am J Pathol 176:812-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BL. (2012). Methods for studying tooth root cementum by light microscopy. Int J Oral Sci 4:119-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BL, Soenjaya Y, Nociti FH, Holm E, Zerfas PM, Wimer HF, et al. (2013). Deficiency in acellular cementum and periodontal attachment in BSP null mice. J Dent Res 92:166-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, McQuillan D, Roughley PJ. (2006). SLRP interaction can protect collagen fibrils from cleavage by collagenases. Matrix Biol 25:484-491. [DOI] [PubMed] [Google Scholar]
- Gluhak-Heinrich J, Ye L, Bonewald LF, Feng JQ, MacDougall M, Harris SE, et al. (2003). Mechanical loading stimulates dentin matrix protein 1 (DMP1) expression in osteocytes in vivo. J Bone Miner Res 18:807-817. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Septier D, Oldberg A, Young MF, Ameye LG. (2006). Fibromodulin-deficient mice display impaired collagen fibrillogenesis in predentin as well as altered dentin mineralization and enamel formation. J Histochem Cytochem 54:525-537. [DOI] [PubMed] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, et al. (1994). Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J 302(Pt 2):527-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand T, Laib A, Muller R, Dequeker J, Ruegsegger P. (1999). Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res 14:1167-1174. [DOI] [PubMed] [Google Scholar]
- Ho SP, Kurylo MP, Fong TK, Lee SS, Wagner HD, Ryder MI, et al. (2010). The biomechanical characteristics of the bone-periodontal ligament-cementum complex. Biomaterials 31:6635-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen KJ, Wu F, Peragallo JH, Paul J, Roberts L, Ezura Y, et al. (2002). A syndrome of joint laxity and impaired tendon integrity in lumican- and fibromodulin-deficient mice. J Biol Chem 277:35532-35540. [DOI] [PubMed] [Google Scholar]
- Kajikawa T, Yamada S, Tauchi T, Awata T, Yamaba S, Fujihara C, et al. (2014). Inhibitory effects of PLAP-1/asporin on periodontal ligament cells. J Dent Res 93:400-405. [DOI] [PubMed] [Google Scholar]
- Kalamajski S, Oldberg A. (2010). The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol 29:248-253. [DOI] [PubMed] [Google Scholar]
- Leong NL, Hurng JM, Djomehri SI, Gansky SA, Ryder MI, Ho SP. (2012). Age-related adaptation of bone-PDL-tooth complex: Rattus-Norvegicus as a model system. PloS One 7:e35980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesan JT, Scanlon CS, Soehren S, Matsuo M, Kapila YL. (2011). Implications of cultured periodontal ligament cells for the clinical and experimental setting: a review. Arch Oral Biol 56:933-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson S, Larjava H, Hakkinen L. (2005). Distinctive localization and function for lumican, fibromodulin and decorin to regulate collagen fibril organization in periodontal tissues. J Periodontal Res 40:312-324. [DOI] [PubMed] [Google Scholar]
- Matias MA, Li H, Young WG, Bartold PM. (2003). Immunohistochemical localization of fibromodulin in the periodontium during cementogenesis and root formation in the rat molar. J Periodontal Res 38:502-507. [DOI] [PubMed] [Google Scholar]
- Meikle MC. (2006). The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur J Orthod 28:221-240. [DOI] [PubMed] [Google Scholar]
- Moreno M, Munoz R, Aroca F, Labarca M, Brandan E, Larrain J. (2005). Biglycan is a new extracellular component of the Chordin-BMP4 signaling pathway. EMBO J 24:1397-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller R, Koller B, Hildebrand T, Laib A, Gianolini S, Ruegsegger P. (1996). Resolution dependency of microstructural properties of cancellous bone based on three-dimensional mu-tomography. Technol Health Care 4:113-119. [PubMed] [Google Scholar]
- Niethamer TK, Yardeni T, Leoyklang P, Ciccone C, Astiz-Martinez A, Jacobs K, et al. (2012). Oral monosaccharide therapies to reverse renal and muscle hyposialylation in a mouse model of GNE myopathy. Mol Genet Metab 107:748-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robling AG, Turner CH. (2009). Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr 19:319-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra M, Reed CC, Iozzo RV. (2002). Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping but distinct from the EGF-binding epitope. J Biol Chem 277:35671-35681. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Schaefer RM. (2010). Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res 339:237-246. [DOI] [PubMed] [Google Scholar]
- Svensson L, Aszodi A, Reinholt FP, Fassler R, Heinegard D, Oldberg A. (1999). Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem 274:9636-9647. [DOI] [PubMed] [Google Scholar]
- Wu B, Ma X, Zhu D, Liu Y, Sun Z, Liu S, et al. (2013). Lentiviral delivery of biglycan promotes proliferation and increases osteogenic potential of bone marrow-derived mesenchymal stem cells in vitro. J Mol Histol 44:423-431. [DOI] [PubMed] [Google Scholar]
- Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S, et al. (1998). Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet 20:78-82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.