Abstract
The cyclic adenosine 3′,5′-monophosphate signalling pathway is now recognised to transduce signals in a compartmentalised manner such that individual stimuli only engage a subset of the pathway components that are physically constrained within defined subcellular locales, thus resulting in a precise functional outcome. As we are starting to appreciate the complexity of the spatial organisation and of the temporal regulation of this pathway, it is becoming clear that disruption of local signalling may lead to pathology and that local manipulation of cAMP signals may offer alternative approaches to treat disease.
Introduction
Adenosine 3′,5′-monophosphate (cAMP) acts as the intracellular message for numerous hormones and neurotransmitters and regulates a large variety of cellular functions and biological processes, including gene transcription, cell metabolism, proliferation, development, as well as more specialised functions depending on the specific cell type. In its simpler formulation, the cAMP signalling pathway involves a hormone (the ‘first’ messenger) that binds and activates a specific G protein-coupled receptor (GPCR) and in turn activates adenylyl cyclases (ACs) to synthesise cAMP. The intracellular (or ‘second’) messenger cAMP then binds to a limited number of intracellular effectors, most notably to protein kinase A (PKA), which in turn is activated and phosphorylates downstream targets. However, as it was realised very early on after its definition[1], the proposed model of a linear pathway involving extracellular stimulus, transmembrane receptor, cAMP synthesis and PKA activation, is too simplistic[2]. Most cells express at the plasma membrane multiple GPCR that signal via generation of cAMP and each individual cell contains a variety of components that can be phosphorylated by PKA. Yet, to attain coordinated behaviour of the organism cells must respond accurately to individual extracellular cues and this was envisaged to require a higher degree of sophistication[2]. Recent research has revealed that cAMP-mediated signalling relies on an intricate network of multiple signalling pathways within which a tight spatial control of signal propagation allows for the signal to be transduced along defined branches of the network, depending on the specific extracellular stimulus[3]. Two properties of the system critically contribute to such spatial control: the molecular components of the system are present in a multiplicity of isoforms or variants, each exhibiting unique regulatory mechanisms, and such components are largely confined to defined subcellular locations. As we are starting to unravel the structural basis of compartmentalisation, it appears that hormone and neurotransmitter signalling needs re-evaluation on the basis of a model where signal propagation is locally regulated. This new perspective is leading to novel mechanistic insight into pathophysiological mechanisms and is suggesting original avenues for therapeutic intervention. In this review, recent findings highlighting the importance of local control of cAMP signalling in health and disease are discussed and how this knowledge may be used for therapy is illustrated.
Diversity and confinement
A key feature of the cAMP signalling pathway is the high degree of diversity and unique regulatory mechanisms of its multiple components. There are several hundred GPCRs, a large subset of which signals through either Gαs or Gαi and thus either induce or block cAMP synthesis by ACs. Gβγ subunits of the heterotrimeric G proteins, which regulate the activity of some AC isoforms, are also present in multiple types, with at least 5 Gβ subunits and at least 11 Gγ subunits[4]. There are ten isoforms of AC, each displaying a unique combination of regulatory mechanisms[5], with multiple isoforms often being expressed in the same cell. While AC1-9 are plasma membrane-associated enzymes and generate cAMP in response to extracellular stimuli, AC10 is localized intracellularly, is insensitive to G-proteins and is regulated by Ca2+ and bicarbonate, thus acting as a metabolic sensor[6]. AC10 is particularly intriguing because, given its intracellular localization, represents a potential local source of cAMP different from the plasma membrane and therefore apt to selectively initiate signalling events that occur deep inside the cell. Although AC10 was originally described in spermatozoa, its distribution appears to extend to other cell types, albeit expression levels may be low, and a number of reports have recently suggested that, for example, a nuclear AC10 may have a role in the phosphorylation cAMP response element-binding protein CREB[7] whereas a subset of AC10 localized to mitochondria may regulate oxidative phosphorylation[8•]. Another mechanism that may allow for selective activation of PKA subsets away from the plasma membrane is suggested by recent studies showing persistent GPCR signalling to AC and generation of cAMP after receptor internalisation into endosomes. Interestingly, intracellular signalling by GPCRs appears to have distinctive features compared to signalling through the same receptors at the plasma-membrane, showing unique selectivity for ligands[9•], different kinetics of the cAMP signal generated[10•,11], unique coupling to downstream signalling pathways[12] and unique modalities of signal inactivation[11], with some evidence suggesting that signalling through intracellular GPCRs may lead to specific functional outcomes[9•,10•].
The amplitude and duration of the cAMP signal depends on the activity of the cAMP-degrading phosphodiesterases (PDEs). Eight different families of PDEs are responsible for cAMP degradation (PDE1, 2, 3, 4, 7, 8, 10, 11). Each of these families may include multiple genes and a number of splice variants, thus enormously increasing the number of isoforms expressed. Most individual cells express multiple PDE variants with each isoform showing a unique combination of subcellular localisation and regulatory mechanisms[13]. By locally degrading cAMP these enzymes shape local cAMP signals[14] with individual PDE isoforms being functionally coupled to specific GPCRs to degrade cAMP selectively in response to a given stimulus[15].
The main effector of cAMP, PKA, is a tetrameric complex including two regulatory (R) and two catalytic (C) subunits, with three genes encoding for C (Cα, Cβ and Cγ) and four genes encoding for R (RIα, RIβ, RIIα, RIIβ). Depending on the type of R subunit present (RI or RII), PKA holoenzymes are classified as type I and type II, which show different sensitivity to cAMP activation and different subcellular localisation. Spatial confinement of PKA to organelles and subcellular structures is mediated by A kinase anchoring proteins (AKAPs) a large and diverse family of scaffolding proteins with more than 50 members identified so far[16]. AKAPs anchor PKA in proximity of its targets, thus allowing for their preferential phosphorylation [17]. Through binding of other components of the cAMP signalling pathways, including ACs[18], PDEs[19] and phosphatases[20], AKAPs organise signalling complexes where the signal is generated, modulated and relayed to the appropriate target. PKA can inhibit some AC isoforms and can activate some PDEs. Thus, the presence within the same complex of AC, PKA and PDEs results in feed-back loops whereby an initial rise in cAMP activates PKA which in turn can inhibit further cAMP synthesis (via inhibition of the AC) and potentiate cAMP degradation (via activation of PDEs), producing a local pulse of cAMP[21].
Compartmentalised cAMP signalling
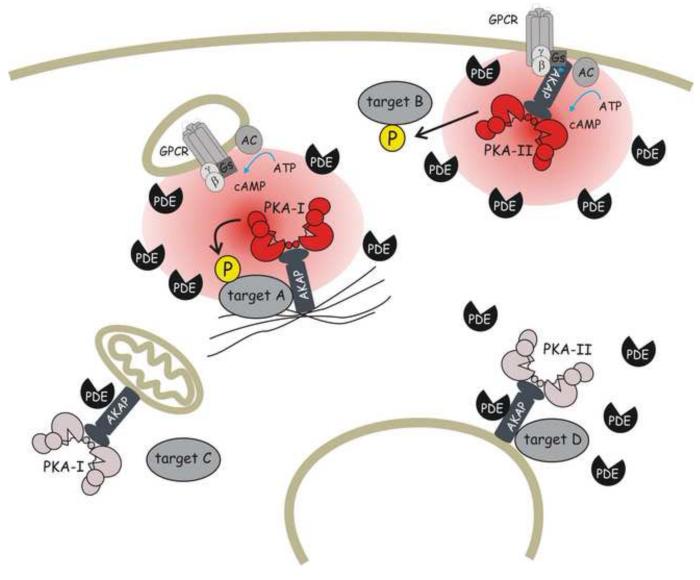
How does the cell make sense of such multiplicity of components and correctly relay individual stimuli to achieve the appropriate functional outcome? Compartmentalisation, which is the spatial confinement of multiple elements of the cAMP signalling pathway, appears to be the answer. Spatial control not only involves the protein components of the pathway but also cAMP itself (Fig 1), as directly demonstrated initially in cardiac myocytes[22].
Fig 1. Compartmentalised cAMP signalling.
The schematic shows two distinct cAMP pools (illustrated by a red shaded oval area) generated by an AC anchored at the plasma membrane and activated by a GPCR exposed to the extracellular environment and an AC associated to an internalised GPCR on the cytoplasmic face of an endosome. PDEs, by degrading cAMP, limit its diffusion outside a spatially confined microdomain and contribute to define the boundaries of the cAMP pools. The two pools of cAMP activate distinct subsets of PKA anchored to different AKAPs. It is interesting to note that PKA-I and PKA-II have different sensitivity to cAMP activation, thus providing a further opportunity for signal discrimination. Activation of an individual subset of PKA results in the selective phosphorylation of the target that is coupled with the specific microdomain. PKA subsets localised outside the cAMP pools do not sense an increase in cAMP concentration and therefore are not activated.
GPCR = G protein-coupled receptor
AC = Adenylyl cyclase
PKA-I, PKA-II = Isoforms I and II of protein Kinase A
AKAP = A Kinase Anchoring Protein
PDE = Phosphodiesterase
P = phosphate group
In heart cells, cAMP regulates the inotropic, chronotropic and lusitropic responses to catecholamine stimulation. Early studies had shown that whereas activation of β-adrenergic receptors increases strength of contraction and affects metabolic enzymes, no such effects are elicited by activation of the prostaglandin receptor, even when these two stimuli generate a similar increase in intracellular cAMP concentration [23]. How different hormones acting via cAMP could elicit such different functional outcomes remained a puzzling question for many years. Recent work using real time detection of cAMP signals in intact cells[24] showed that the β-AR and the prostaglandin receptor generate spatially distinct pools of cAMP which in turn preferentially activate different subsets of AKAP-anchored PKA enzymes, leading to the phosphorylation of different downstream targets[25••]. Thus catecholamines, but not prostaglandin, raise the phosphorylation level of key components of the excitation-contraction machinery and increase myocyte contractility via generation of a spatially confined pool of cAMP that selectively affects these targets[25••].
The diversity of the pathway components discussed above, coupled with a complex interconnectivity of the cAMP pathway with other signalling pathways[26], provides the basis for a sophisticated local control of cAMP signals. One example is the modulation of cAMP by cGMP. cGMP is generated by NO-mediated activation of soluble guanylyl cyclases (sGC) or activation of plasma membrane-bound, particulate guanylyl cyclases (pGC) by natriuretic peptides. cGMP can modulate cAMP levels by regulating the activity of cAMP-degrading PDEs. cGMP is a potent activator of PDE2 [27] and at the same time cGMP acts as a competitive inhibitor of PDE3[28]. Through such regulatory mechanisms, stimuli that elevate cGMP may either attenuate or amplify cAMP signals[29]. Cardiac myocytes express both PDE2 and PDE3 and in these cells cGMP signals have opposing effects on different local pools of cAMP with different effects on myocyte contractility[30••]. The compartment-specific effect of cGMP depends on the different subcellular localisation of PDE2 and PDE3 and on the specific stimulus (NO or natriuretic peptides) that generates cGMP[30••]. As there is evidence that also cGMP, like cAMP, is compartmentalised[31, 30••], a local generation of cGMP is likely to affect PDEs only within a restricted microdomain. It is interesting to consider that the activity of constitutively expressed isoforms of nitric oxide synthetase (eNOS and nNOS) are Ca2+-dependent and that Ca2+ signalling is also well known to be compartmentalised, thereby providing a mechanism for local generation of NO, activation of sGC and cGMP production. cGMP levels are in turn regulated by cGMP-degrading PDEs (such as PDE5) that can be spatially confined[32] and subject to their specific regulatory mechanisms. Clearly, the integrated effects of these complex signalling networks are vast and spatial confinement of signalling events allows for a specific branch of the pathway to be appropriately activated, with other branches not being involved.
Temporal control of local signalling
Signal propagation is not only regulated in space but the signal at specific sites can also be uniquely regulated in time. One interesting example is cAMP signalling in pancreatic islet β-cells. The cAMP/PKA pathway acts as an important amplifier of glucose-induced insulin secretion via generation of Ca2+ signals and promotion of exocytosis[33]. β-cells express more that fifteen different GPCRs that signal either via an increase or a decrease of intracellular cAMP levels[34]. Apart from the incretin hormones GLP1 and GIP, which serve to augment insulin secretion following entry of food to the gut, a number of other ligands, including lipids and a variety of peptides and biogenic amines, can either activate or inhibit cAMP synthesis in β-cells. The level of such ligands may change independently of food intake and could potentially interfere with appropriate levels of insulin secretion after a meal. In addition, GLP1-stimulated cAMP is also involved in regulating pancreatic β-cell differentiation, growth and survival through PKA-mediated phosphorylation and enhanced nuclear translocation of CREB[35]. Increase in β-cell mass, a mechanism thought to counterbalance insulin resistance in peripheral tissues and to protect against hyperglycemia and development of diabetes type 2[36], is unlikely to occur regularly as a consequence of release of incretins. Thus β-cells must discriminate and integrate different cAMP signals and appear to do so by operating a tight spatial and temporal control of cAMP levels. The importance of local control of cAMP signalling is evidenced by the fact that β-cells express several AKAPs and disruption of PKA anchoring to AKAPs affects GLP1-induced insulin secretion[37]. In addition, real time imaging of cAMP combined with detection of Ca2+ signals showed that pulsatile secretion of insulin in response to GLP1 is sustained by Ca2+ oscillations that are tightly coupled to cAMP oscillations [38]. Brief cAMP transients were found to be sufficient to trigger Ca2+ signals whereas only prolonged elevation of [cAMP] led to PKA catalytic subunit translocation to the nucleus[38], thus providing a mechanism whereby short-lived cAMP signals can activate cytoplasmic events, such as ion-channel activity or exocytosis, whereas long-lasting cAMP signals are required for regulation of nuclear transcription factor activity. Interestingly, in the insulin-secreting cell line MIN6, the oscillatory circuit involving cAMP and Ca2+ was found to depend on oscillations of PKA activity, with the level of PKA activation modulating the frequency of the oscillations[39•]. The possibility that different information may be encoded by differences in oscillation frequency combined with the subcellular localisation of PKA via anchoring to AKAPs potentially affords for powerful diversification of downstream signalling.
Compartmentalisation and disease
The physiological relevance of compartmentalised cAMP/PKA signalling is documented by numerous studies using a variety of experimental approaches, including knock out models targeting spatially confined components of the pathway[40]. There is evidence that mutations or genetic polymorphism involving a number of these components are responsible for or associated with a variety of pathological conditions including long QT syndrome[41], predisposition to cardiac dysfunction[42], familial breast cancer[43] and schizophrenia[44]. In addition, disruption of compartmentalised signalling may arise from more complex and subtle changes in the intracellular environment that may affect anchoring and/or appropriate local signalling. These may include changes in phosphorylation[45], protein stability or other post-translational modifications[46] involving pathway components, or changes affecting other signalling pathways that impact of cAMP signalling[47]. In support of the view that disruption of compartmentalised cAMP signalling may lead to pathology, reduced anchoring of PKA to AKAPs[48], disruption of β-adrenergic receptor localisation[49••], and reorganisation of multiple protein complexes involved in cAMP signalling[50•] have been shown in cardiac myocytes from failing hearts, suggesting that hampered compartmentalisation may underpin the aberrant cAMP response typical of heart failure.
One particularly exciting aspect of cAMP/PKA compartmentalised signalling is that this model allows us to think in terms ‘compartmentalised treatment’. Although GPCRs are a favourite target for drug development, recent advances in receptor pharmacology have revealed that the regulation of receptors and ligand-receptors interactions are more complex than previously thought and that the specific nature of the signal transduced depends on the nature of the ligand and on the dynamically changing intracellular environment[51], with important implications for the development of specific drugs with minimal side effects. In this context, targeting the signalling pathway distally from the receptor and closer to specific intracellular effectors via selective modulation of cAMP signals at specific intracellular locations may represent a valid alternative approach. Preliminary evidence suggests that compartment-specific manipulation of cAMP signalling may be achievable.
One example involves the enzyme PDE4D5, one of over 20 distinct PDE4 isoforms that interacts with a number of proteins and, as a consequence of being part of multiple macromolecular complexes, regulates different cellular functions[52]. Drugs that inhibit PDE4 enzymes cannot discriminate among the different PDE4 isoforms and for this reason their therapeutic use is limited by serious side effects. However, the poor selectivity of conventional inhibitors can be overcome by selective displacement of PDE4D5 from individual macromolecular complexes using competing peptides. In one study, displacement of PDE4D5 from a PDE4D5/RAK1/FAK complex using a peptide that selectively disrupts the interaction of PDE4D5 with RAK1 was shown to result in a dramatic reduction of cell polarisation and invasive phenotype in a cancer cell model, whereas selective displacement of PDE4D5 from a different complex, PDE4D5/β-arrestin, had no effect on this phenotype[53••]. Interestingly, the functional consequence of displacing PDE4D5 from β-arrestin is different and results in increased PKA-dependent phosphorylation of the β2-adrenegic receptor[54]. In another study, selective disruption of PDE4D5 interaction with the small heat shock protein Hsp20 was shown to counteract the hypertrophic growth of cardiac myocytes, an effect thought to be mediated by local increase of cAMP and PKA-mediated phosphorylation of Hsp20[55•]. Thus, selective displacement of PDE4D5 from specific subcellular sites appears to result in local increase in cAMP levels and activation of a unique downstream response.
Conclusions
Compartmentalisation is now largely accepted as the mechanism that allows individual extracellular cues that signal via cAMP to mediate specific cellular events. However, most of the particulars of how the cAMP/PKA signalling network components structure themselves within the three-dimensional matrix of the cell remain to be elucidated. The challenge ahead is to unravel the details and build a topographical map of cAMP signalling where a link is established between individual GPCRs, the confined pools of cAMP that result from their activation, the identity of the PDEs that regulate each cAMP pool and their unique regulation, the effector(s) that each cAMP pool activates and the downstream targets that are affected by such activation. We can expect that different cell types will show a unique topography of the network and that such topography will differ in normal and pathological conditions. With this information at hand, it will then be possible to identify domains where local manipulation of cAMP signals may affect accurately a specific function and to investigate whether such local manipulation may be a valid therapeutic approach.
Highlights.
cAMP/PKA signalling is tightly controlled in space and time
Local control of cAMP/PKA signals relies on spatial confinements of the pathway components
Altered cAMP/PKA compartmentalisation may lead to disease
Local manipulation of cAMP signals may offer an alternative therapeutic approach
Acknowledgements
The work described in this paper was supported by the Fondation Leducq (O6 CVD 02), the British Heart Foundation (PG/07/091/23698) and the NSF-NIH CRCNS program (NIH R01 AA18060).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sutherland EW, Rall TW. Formation of adenosine-3,5-phosphate (cyclic adenylate) and its relation to the action of several neurohormones or hormones. Acta Endocrinol Suppl (Copenh) 1960;34(Suppl 50):171–174. doi: 10.1530/acta.0.xxxivs171. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg SF, Brunton LL. Compartmentation of G protein-coupled signaling pathways in cardiac myocytes. Annu Rev Pharmacol Toxicol. 2001;41:751–773. doi: 10.1146/annurev.pharmtox.41.1.751. [DOI] [PubMed] [Google Scholar]
- 3.Zaccolo M. cAMP signal transduction in the heart: understanding spatial control for the development of novel therapeutic strategies. Br J Pharmacol. 2009;158:50–60. doi: 10.1111/j.1476-5381.2009.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermans E. Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol Ther. 2003;99:25–44. doi: 10.1016/s0163-7258(03)00051-2. [DOI] [PubMed] [Google Scholar]
- 5.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 6.Tresguerres M, Levin LR, Buck J. Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int. 2011;79:1277–1288. doi: 10.1038/ki.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zippin JH, Farrell J, Huron D, Kamenetsky M, Hess KC, Fischman DA, Levin LR, Buck J. Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. J Cell Biol. 2004;164:527–534. doi: 10.1083/jcb.200311119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •8.Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9:265–276. doi: 10.1016/j.cmet.2009.01.012. This paper presents evidence in support of generation of cAMP within mitochondria by soluble adenylyl cyclase in response to metabolically generated carbon dioxide.
- •9.Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5:734–742. doi: 10.1038/nchembio.206. In this paper the authors provide an explanation for the puzzling observation that the parathyroid hormone receptor 1 mediates different functions depending on which ligand, parathyrod hormone (PTH) or parathirod hormone-related peptide (PTHrP), it binds. Here they show that PTHrP action is restricted to the plasma membrane whereas PTH can signal via activation of internalised receptors.
- •10.Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse MJ. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009;7:e1000172. doi: 10.1371/journal.pbio.1000172. This report describes for the first time that thyroid-stimulating hormone receptor (TSHR) continues to stimulate cAMP production after receptor internalisation, generating downstream responses that are different from those triggered by TSHR expressed at the cell surface.
- 11.Feinstein TN, Wehbi VL, Ardura JA, Wheeler DS, Ferrandon S, Gardella TJ, Vilardaga JP. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat Chem Biol. 2011;7:278–284. doi: 10.1038/nchembio.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol. 2009;5:428–434. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- 13.Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev. 2011;91:651–690. doi: 10.1152/physrev.00030.2010. [DOI] [PubMed] [Google Scholar]
- 14.Zaccolo M. Phosphodiesterases and compartmentalized cAMP signalling in the heart. Eur J Cell Biol. 2006;85:693–697. doi: 10.1016/j.ejcb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Mongillo M, McSorley T, Evellin S, Sood A, Lissandron V, Terrin A, Huston E, Hannawacker A, Lohse MJ, Pozzan T, et al. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ Res. 2004;95:67–75. doi: 10.1161/01.RES.0000134629.84732.11. [DOI] [PubMed] [Google Scholar]
- 16.Beene DL, Scott JD. A-kinase anchoring proteins take shape. Curr Opin Cell Biol. 2007;19:192–198. doi: 10.1016/j.ceb.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lygren B, Carlson CR, Santamaria K, Lissandron V, McSorley T, Litzenberg J, Lorenz D, Wiesner B, Rosenthal W, Zaccolo M, et al. AKAP complex regulates Ca2+ reuptake into heart sarcoplasmic reticulum. EMBO Rep. 2007;8:1061–1067. doi: 10.1038/sj.embor.7401081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dessauer CW. Adenylyl cyclase--A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol Pharmacol. 2009;76:935–941. doi: 10.1124/mol.109.059345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stangherlin A, Stangherlin A, Zaccolo M. Local Termination of cAMP signals: the Role of AKAP-Anchored Phosphodiesterases. J Cardiovasc Pharmacol. 2011 doi: 10.1097/FJC.0b013e3182214f2b. [DOI] [PubMed] [Google Scholar]
- 20.Redden JM, Dodge-Kafka KL. AKAP Phosphatase Complexes in The Heart. J Cardiovasc Pharmacol. 2011 doi: 10.1097/FJC.0b013e31821e5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, Wong W, Hoshi N, Langeberg LK, Cooper DM, Dessauer CW, et al. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol Cell. 2006;23:925–931. doi: 10.1016/j.molcel.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 23.Keely SL. Prostaglandin E1 activation of heart cAMP-dependent protein kinase: apparent dissociation of protein kinase activation from increases in phosphorylase activity and contractile force. Mol Pharmacol. 1979;15:235–245. [PubMed] [Google Scholar]
- 24.Zaccolo M, De Giorgi F, Cho CY, Feng L, Knapp T, Negulescu PA, Taylor SS, Tsien RY, Pozzan T. A genetically encoded, fluorescent indicator for cyclic AMP in living cells. Nat Cell Biol. 2000;2:25–29. doi: 10.1038/71345. [DOI] [PubMed] [Google Scholar]
- ••25.Di Benedetto G, Zoccarato A, Lissandron V, Terrin A, Li X, Houslay MD, Baillie GS, Zaccolo M. Protein kinase A type I and type II define distinct intracellular signaling compartments. Circ Res. 2008;103:836–844. doi: 10.1161/CIRCRESAHA.108.174813. This paper demonstrates that the different action of catecholamines and prostaglandin on heart contractility relies on compartmentalised increase of cAMP, selective activation of different anchored PKA subsets and distinct phosphorylation of downstream targets.
- 26.Fimia GM, Sassone-Corsi P. Cyclic AMP signalling. Journal of Cell Science. 2001;114:1971–1972. doi: 10.1242/jcs.114.11.1971. [DOI] [PubMed] [Google Scholar]
- 27.Martinez SE, Beavo JA, Hol WG. GAF Domains: Two-Billion-Year-Old Molecular Switches that Bind Cyclic Nucleotides. Mol Intervent. 2002;2:317–323. doi: 10.1124/mi.2.5.317. [DOI] [PubMed] [Google Scholar]
- 28.Shakur Y, Holst LS, Landstrom TR, Movsesian M, Degerman E, Manganiello V. Regulation and function of the cyclic nucleotide phosphodiesterase (PDE3) gene family. Prog Nucleic Acid Res Mol Biol. 2001;66:241–277. doi: 10.1016/s0079-6603(00)66031-2. [DOI] [PubMed] [Google Scholar]
- 29.Zaccolo M, Movsesian MA. cAMP and cGMP signaling cross-talk: role of phosphodiesterases and implications for cardiac pathophysiology. Circ Res. 2007;100:1569–1578. doi: 10.1161/CIRCRESAHA.106.144501. [DOI] [PubMed] [Google Scholar]
- ••30.Stangherlin A, Gesellchen F, Zoccarato A, Terrin A, Fields LA, Berrera M, Surdo NC, Craig MA, Smith G, Hamilton G, et al. cGMP Signals Modulate cAMP Levels in a Compartment-Specific Manner to Regulate Catecholamine-Dependent Signaling in Cardiac Myocytes. Circ Res. 2011 doi: 10.1161/CIRCRESAHA.110.230698. Using Fluorescence Resonance Energy Transfer imaging and reporters targeted to different subcellular compartments the authors show that local changes in cGMP can either increase or decrease cAMP levels, depending on the specific cGMP-generating stimulus and on the specific cAMP pool.
- 31.Castro LR, Verde I, Cooper DM, Fischmeister R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation. 2006;113:2221–2228. doi: 10.1161/CIRCULATIONAHA.105.599241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kass DA, Champion HC, Beavo JA. Phosphodiesterase type 5: expanding roles in cardiovascular regulation. Circ Res. 2007;101:1084–1095. doi: 10.1161/CIRCRESAHA.107.162511. [DOI] [PubMed] [Google Scholar]
- 33.Braun M, Ramracheya R, Johnson PR, Rorsman P. Exocytotic properties of human pancreatic beta-cells. Ann N Y Acad Sci. 2009;1152:187–193. doi: 10.1111/j.1749-6632.2008.03992.x. [DOI] [PubMed] [Google Scholar]
- 34.Ahren B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8:369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- 35.Kim SJ, Nian C, Widenmaier S, McIntosh CH. Glucose-dependent insulinotropic polypeptide-mediated up-regulation of beta-cell antiapoptotic Bcl-2 gene expression is coordinated by cyclic AMP (cAMP) response element binding protein (CREB) and cAMP-responsive CREB coactivator 2. Mol Cell Biol. 2008;28:1644–1656. doi: 10.1128/MCB.00325-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahren B. Type 2 diabetes, insulin secretion and beta-cell mass. Curr Mol Med. 2005;5:275–286. doi: 10.2174/1566524053766004. [DOI] [PubMed] [Google Scholar]
- 37.Lester LB, Langeberg LK, Scott JD. Anchoring of protein kinase A facilitates hormone-mediated insulin secretion. Proc Natl Acad Sci U S A. 1997;94:14942–14947. doi: 10.1073/pnas.94.26.14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dyachok O, Isakov Y, Sagetorp J, Tengholm A. Oscillations of cyclic AMP in hormone-stimulated insulin-secreting beta-cells. Nature. 2006;439:349–352. doi: 10.1038/nature04410. [DOI] [PubMed] [Google Scholar]
- •39.Ni Q, Ganesan A, Aye-Han NN, Gao X, Allen MD, Levchenko A, Zhang J. Signaling diversity of PKA achieved via a Ca2+-cAMP-PKA oscillatory circuit. Nat Chem Biol. 2011;7:34–40. doi: 10.1038/nchembio.478. This paper reports that in insulin-secreting MIN6 β-cells a highly integrated oscillatory circuit is present involving PKA, Ca2+ and cAMP, which are found to oscillate in a synchronous manner. PKA appears to have a key role in the oscillatory circuit as it dictates the circuit oscillation frequency.
- 40.Skroblin P, Grossmann S, Schafer G, Rosenthal W, Klussmann E. Mechanisms of protein kinase A anchoring. Int Rev Cell Mol Biol. 2010;283:235–330. doi: 10.1016/S1937-6448(10)83005-9. [DOI] [PubMed] [Google Scholar]
- 41.Chen L, Marquardt ML, Tester DJ, Sampson KJ, Ackerman MJ, Kass RS. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci U S A. 2007;104:20990–20995. doi: 10.1073/pnas.0710527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kammerer S, Burns-Hamuro LL, Ma Y, Hamon SC, Canaves JM, Shi MM, Nelson MR, Sing CF, Cantor CR, Taylor SS, et al. Amino acid variant in the kinase binding domain of dual-specific A kinase-anchoring protein 2: a disease susceptibility polymorphism. Proc Natl Acad Sci U S A. 2003;100:4066–4071. doi: 10.1073/pnas.2628028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wirtenberger M, Schmutzhard J, Hemminki K, Meindl A, Sutter C, Schmutzler RK, Wappenschmidt B, Kiechle M, Arnold N, Weber BH, et al. The functional genetic variant Ile646Val located in the kinase binding domain of the A-kinase anchoring protein 10 is associated with familial breast cancer. Carcinogenesis. 2007;28:423–426. doi: 10.1093/carcin/bgl164. [DOI] [PubMed] [Google Scholar]
- 44.Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, Malloy MP, Chubb JE, Huston E, Baillie GS, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- 45.Manni S, Mauban JH, Ward CW, Bond M. Phosphorylation of the cAMP-dependent protein kinase (PKA) regulatory subunit modulates PKA-AKAP interaction, substrate phosphorylation, and calcium signaling in cardiac cells. J Biol Chem. 2008;283:24145–24154. doi: 10.1074/jbc.M802278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Vadrevu S, Dunlop A, Day J, Advant N, Troeger J, Klussmann E, Jaffrey E, Hay RT, Adams DR, et al. Selective SUMO modification of cAMP-specific phosphodiesterase-4D5 (PDE4D5) regulates the functional consequences of phosphorylation by PKA and ERK. Biochem J. 2010;428:55–65. doi: 10.1042/BJ20091672. [DOI] [PubMed] [Google Scholar]
- 47.Gold MG, Stengel F, Nygren PJ, Weisbrod CR, Bruce JE, Robinson CV, Barford D, Scott JD. Architecture and dynamics of an A-kinase anchoring protein 79 (AKAP79) signaling complex. Proc Natl Acad Sci U S A. 2011;108:6426–6431. doi: 10.1073/pnas.1014400108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zakhary DR, Moravec CS, Bond M. Regulation of PKA binding to AKAPs in the heart: alterations in human heart failure. Circulation. 2000;101:1459–1464. doi: 10.1161/01.cir.101.12.1459. [DOI] [PubMed] [Google Scholar]
- ••49.Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327:1653–1657. doi: 10.1126/science.1185988. An elegant approach is used in this paper which combines nanoscale live-cell scanning ion conductance and FRET imaging to show that in a rat model of heart failure the β2-adrenergic receptors are redistributed from the trensverse tubules to the cell crests, resulting in disrupted compartmentalisation of cAMP signals.
- •50.Aye TT, Soni S, van Veen TA, van der Heyden MA, Cappadona S, Varro A, de Weger RA, de Jonge N, Vos MA, Heck AJ, et al. Reorganized PKA-AKAP associations in the failing human heart. J Mol Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2011.06.003. Using a chemical proteomic approach the authors provide evidence that the association profile of PKA with several AKAPs is largely altered in human failing hearts.
- 51.Millar RP, Newton CL. The year in G protein-coupled receptor research. Mol Endocrinol. 2010;24:261–274. doi: 10.1210/me.2009-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35:91–100. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- ••53.Serrels B, Sandilands E, Serrels A, Baillie G, Houslay MD, Brunton VG, Canel M, Machesky LM, Anderson KI, Frame MC. A complex between FAK, RACK1, and PDE4D5 controls spreading initiation and cancer cell polarity. Curr Biol. 2010;20:1086–1092. doi: 10.1016/j.cub.2010.04.042. This paper reports that FAK/RACK1/PDE4D5 is a ‘direction-sensing’ complex that acts to recruit components of the cAMP pathway to the leading edge of polarising cells and that selective displacement of PDE4D5 from this complex dramatically affects polarity and the invasive phenotype in a breast cancer cell model.
- 54.Smith KJ, Baillie GS, Hyde EI, Li X, Houslay TM, McCahill A, Dunlop AJ, Bolger GB, Klussmann E, Adams DR, et al. 1H NMR structural and functional characterisation of a cAMP-specific phosphodiesterase-4D5 (PDE4D5) N-terminal region peptide that disrupts PDE4D5 interaction with the signalling scaffold proteins, beta-arrestin and RACK1. Cell Signal. 2007;19:2612–2624. doi: 10.1016/j.cellsig.2007.08.015. [DOI] [PubMed] [Google Scholar]
- •55.Sin YY, Edwards HV, Li X, Day JP, Christian F, Dunlop AJ, Adams DR, Zaccolo M, Houslay MD, Baillie GS. Disruption of the cyclic AMP phosphodiesterase-4 (PDE4)-HSP20 complex attenuates the beta-agonist induced hypertrophic response in cardiac myocytes. J Mol Cell Cardiol. 2011;50:872–883. doi: 10.1016/j.yjmcc.2011.02.006. This paper shows that disruption of the complex Hsp20/PDE4D5 using a competing peptide is sufficient to locally increase cAMP and to induce PKA-mediated phosphorylation of Hsp20, leading to protection against the hypertrophic response induced in cardiac myocytes by catecholamine stimulation.