Abstract
Although Alzheimer’s Disease (AD) is the most common neurodegenerative disease, the etiology of AD is not well understood. In some cases, genetic factors explain AD risk, but a high percentage of late-onset AD is unexplained. The fact that AD is associated with a number of physical and systemic manifestations suggests that AD is a multifactorial disease that affects both the CNS and periphery. Interestingly, a common feature of many systemic processes linked to AD is involvement in energy metabolism. The goals of this review are to 1) explore the evidence that peripheral processes contribute to AD risk, 2) explore ways that AD modulates whole-body changes, and 3) discuss the role of genetics, mitochondria, and vascular mechanisms as underlying factors that could mediate both central and peripheral manifestations of AD. Despite efforts to strictly define AD as a homogeneous CNS disease, there may be no single etiologic pathway leading to the syndrome of AD dementia. Rather, the neurodegenerative process may involve some degree of baseline genetic risk that is modified by external risk factors. Continued research into the diverse but related processes linked to AD risk is necessary for successful development of disease –modifying therapies.
1. Introduction
Alzheimer’s disease (AD) is the most common form of dementia, affecting nearly 10% of individuals over the age of 65 and nearly 50% of those over the age of 85.[1] Increasing longevity in the population combined with the high incidence of AD in older adults will only exacerbate the societal and economic impact of AD in coming years. The neuropathological hallmarks of AD include amyloid plaques and neurofibrillary tangles which are present on microscopic examination of the brain. These neuropathological changes are accompanied by accelerated atrophy in the brain’s gray matter cortex, reflecting loss of neurons, in areas such as the hippocampus and parietal lobes. Ultimately, both gray and white matter abnormalities are observed.[2] The earliest clinical features of AD include short term memory impairment and executive dysfunction corresponding to neurodegeneration in areas that mediate these functions.[3]
AD is classically viewed as a primary neurodegenerative process. In its terminal phases, however, it is well-known that AD patients have physical decline and thus the AD process quite clearly is associated with systemic manifestations that extend beyond the CNS. This physical decline is undoubtedly driven to some extent by the progressive functional and behavioral decline associated with the CNS degeneration.[4] On the other hand, physical decline is observable to varying degrees in the earliest stages of the disease, prior to the presence of significant functional and behavioral decline that clearly underlies some of the physical manifestations seen late in the disease.[5]
The presence of physical or systemic manifestations of AD early in the disease, or even before the onset of clinically recognizable symptoms, suggests that physical decline may not simply represent a secondary result of the CNS pathological process. In fact, studies have long suggested that abnormalities in metabolic and biochemical processes described in AD brains are also present in peripheral cells such as skin fibroblasts derived from AD patients.[6, 7] Individuals with AD also have mitochondrial dysfunction evident in both the CNS and periphery [8] (for example, lymphocytes, [9]) suggesting that pathological processes may co-exist in both brain and non-neural tissues. There remains uncertainty regarding the causal relationship between these variables. To what extent the systemic changes represent an effect of a CNS process, contribute causally to the CNS disease, (i.e., reverse causation) or reflect a biological process that is present in both body and brain remains unclear.
This review sets out to examine these systemic manifestations of AD through the lens of three hypotheses asserting different cause and effect relationships: 1) systemic processes drive CNS dysfunction (for instance, as risk factors for brain dysfunction and AD), 2) AD brain processes drive systemic manifestations (i.e., downstream effects), and 3) a common underlying biological process is present both peripherally and in the CNS suggesting a systemic etiological process. We will also review the concept that AD is perhaps a multifactorial disease that affects both CNS and systemic processes.
2. Alzheimer’s disease and whole body changes: the chicken or the egg?
Association studies clearly demonstrate that patients with AD have a number of systemic (i.e., non-CNS) manifestations that accompany the CNS dysfunction that defines AD. Risk factor studies suggest that the neurodegenerative process may be instigated or exacerbated to some degree by peripheral processes. On the other hand, some of the peripheral manifestations may be the downstream result of AD processes, mediated by dysfunctional CNS control of peripheral processes or through behavior changes (i.e., reduced physical activity, forgetting to eat, etc.) that result in systemic manifestations. The primary goal of this review is to summarize evidence in favor of these possibilities and examine a third possibility that a systemic underlying factor may be common to both CNS and peripheral dysfunction associated with the clinical AD syndrome.
2.1 Systemic Processes Contribute to AD
A large number of apparent risk and protective factors have been identified that appear to influence an individual’s long term risk of developing AD. Some risk components are likely genetic in nature, although additional factors likely involve processes that originate outside of the CNS, such as diabetes, obesity, and physical inactivity. The precise mechanisms of their influence on AD risk likely include broad systemic effects that presumably transfer to the brain and either influence the initiation of disease processes or exacerbate the CNS dysfunction underlying the disease. Here, we review evidence that systemic factors may contribute to the initiation or exacerbation of AD.
2.1.1. Physical Activity
Physical exercise results in broad physiologic adaptations including improvements in cardiovascular fitness, vascular health, metabolic profile (reduced body fat, increased insulin sensitivity) and body composition (increased lean mass and bone density).[10] Increasing evidence suggests that exercise not only improves general health but positively impacts brain health through a number of potential mechanisms. Increased physical activity decreases AD risk [11] and has been postulated to have a trophic effect on the brain, particularly the hippocampus. For instance, exercise is associated with increased brain-derived neurotrophic factor (BDNF) [12] and other important neurochemicals, [13] supporting a role of exercise in brain growth and survival. Exercise appears to stimulate neurogenesis[14] as evidenced by increased counts of new neurons in adult animals on an exercise regimen. Broadly, exercise may modulate vascular risk factors (atherosclerosis,[15] heart disease,[16] stroke,[17] diabetes [18–23]) that place an individual at risk for dementia, vascular dementia, and AD. More specifically, studies have shown increased inflammation in AD,[24] and exercise decreases systemic inflammatory markers.[25]
Numerous clinical studies suggest a relationship between physical activity and risk of dementia in late life. Cross-sectional studies suggest physical activity is positively associated with cognition, particularly executive and visuospatial function.[26–30]. Multiple longitudinal studies report a relationship between self-reported exercise and cognitive decline, [31–36] and overall physical activity in midlife or later life is associated with a reduced risk of developing AD in late-life.[37, 38] These longitudinal studies suggest that systemic benefits of physical activity may modulate positive cognitive outcomes. This line of thought is further supported by results from intervention studies that have shown improvements in cognitive outcomes following exercise. [39–43] We and others have shown that physical activity and fitness levels are associated with larger brain volume.[42, 44, 45] This relationship may be mediated by exercise effects on neurotrophic factors such as BDNF. Serum levels of BDNF are positively correlated with hippocampal volume[46] and exercise acutely increases hippocampal levels of BDNF in animals [47] while blocking BDNF function ameliorates exercise-induced improvement in cognitive function.[48]
Most of the data supporting a link between exercise and brain health comes from studies of aerobic exercise – walking is the most common form of physical activity for older adults – and little data exists on the role of resistance exercise (i.e., weight lifting) in promoting brain health. There is evidence that resistance exercise may be important in preventing age- and AD-related cognitive decline. For instance, several small studies have found that resistance training is associated with modest cognitive benefits in those with [49] and without cognitive impairment.[43] Association studies suggest that reduced muscle strength is a risk factor for developing AD [50] and that lower strength is associated with greater cognitive decline.[51] These association studies however, cannot assess the cause and effect relationship of muscle strength with cognitive decline and it remains unclear if the declines in muscle strength causally influence AD processes or are a consequence of the early disease process. In fact, AD is associated with measurable changes in body composition, including lean mass and bone density, [52–54] suggesting that body composition changes are an early systemic manifestation of AD rather than factors that exacerbate or initiate the disease process. This will be reviewed in more detail below. Nevertheless, there is strong evidence that the physiologic adaptations (increased cardiorespiratory fitness, metabolic profile, increased muscle mass) from exercise and physical activity may result in beneficial brain effects that result in a lower risk of AD and dementia.
2.1.2. Type 2 Diabetes
Another peripheral process linked to risk and progression of AD is impaired glucose metabolism. Numerous epidemiologic studies have shown that diabetes and insulin resistance are strong risk factors for cognitive decline and AD, [55–60] and we and others have shown that impaired glucose metabolism is associated with increased progression from mild cognitive impairment to AD. [61, 62] Moreover, clinical studies using FDG-PET have demonstrated that decreased glucose metabolism occurs very early in AD brain and is predictive of disease diagnosis.[63, 64]
Several potential mechanisms may link insulin resistance and brain function. Insulin can cross the blood brain barrier, [65] where it likely modulates several processes including neurotransmission[66–68] cell survival, [69] and amyloid trafficking. [70] Insulin signaling deficits are present in AD brain post-mortem, [71, 72] although the temporal relationship between peripheral insulin resistance and CNS insulin resistance is uncertain. Elevated peripheral insulin, which precedes and often accompanies diabetes, is associated with an increased risk for dementia, [73] although high insulin levels may actually be protective in AD by compensating for impaired insulin signaling, known to occur in cognitively-impaired individuals. [74, 75] Finally, increased glycated hemoglobin (HbA1c), impaired fasting glucose, impaired glucose tolerance, and homeostatic model assessment of insulin resistance (HOMA-IR) have all been associated with impaired memory performance or longitudinal cognitive decline in nondemented adults.[76–79]
Although it is well-accepted that diabetes increases AD risk, the relationship between insulin resistance and amyloid pathology is somewhat more controversial. As mentioned, insulin can increase amyloid efflux from the cell, [70] and insulin and amyloid compete for receptor binding and are even degraded by a common enzyme.[80, 81] However, using advanced imaging techniques, one very recent study has indicated no relationship between insulin resistance and amyloid during life, [82] and supports previous work that showed diabetic individuals did not have more brain amyloid at autopsy compared to controls.[83] It is thus possible that individuals with metabolic impairment are simply more vulnerable to the effects of amyloid aggregation than healthy individuals, or perhaps that aggregation of a protein other than amyloid-beta affects these individuals. In fact, autopsy data indicate that amylin, a peptide produced in the pancreas and co-secreted with insulin, aggregates in the brain of individuals with both AD and vascular dementia independent of amyloid-beta deposition.[84] It has also been shown in animal models that the receptor for amylin may modulate amyloid-beta’s effects on long-term potentiation.[85] However, further studies are needed to determine whether amylin plays a role in cognitive decline and AD.
2.1.3. Obesity and lipid metabolism
Obesity is an important risk factor for dementia and AD. The relationship between obesity and dementia risk seems to peak at mid-life [86, 87] and in a meta-analysis was shown to occur independently of diabetes diagnosis.[88] Higher BMI in midlife is associated with structural brain changes [89, 90] and increased risk of cognitive decline and AD in late life. [91, 92] Thus, it is possible that dysregulation of systemic metabolic processes related to obesity and lipid metabolism may also affect late life AD risk.
The relationship between midlife obesity and AD risk may be modulated indirectly through vascular mechanisms (discussed below). However, a more direct potential link between obesity and cognitive decline involves lipids. Dyslipidemia occurs frequently in obese individuals,[93] and is characterized by increased levels of low density lipoprotein (LDL). In culture, oxidized LDL is associated with increased formation of “lipid rafts,” [94, 95] which are groups of molecules that change the fluidity of the plasma membrane and are integral to cell signaling. Interestingly, the processing of amyloid precursor protein to form amyloid-beta depends upon dynamic interactions with these microdomains [96]. In fact, recent work has shown that palmitoylation of APP increases amyloid processing through targeting of APP to lipid rafts.[97]
Lipid raft formation directly affects recruitment of signaling proteins and receptors to particular regions of the membrane. Cholesterol, sphingomyelin, and ceramide are important components of lipid rafts, and reduced sphingomyelin and increased ceramide levels have been observed in AD plasma [98]. Very recently, subjects in the middle and highest tertiles of ceramide at baseline had a 10 and 7.6-fold increased risk of AD, respectively.[99] However, another study indicated that ceramide only predicted cognitive decline and neuronal loss in subjects with MCI. [100] Thus, the effect of disease stage on the relationship between ceramide and cognitive function is not well understood. Ceramide has been shown to increase amyloid-beta generation,[101] Apolipoprotein (APOE) binding,[102] and APOE secretion, [103] and sphingomyelinase (which generates ceramide) may be involved in cell death.[104, 105] Studies have also shown that ceramide can play a role in insulin resistance and mitochondrial dysfunction (reviewed in [106]), making this molecule an intriguing player in multiple systemic processes that have been linked to AD.
Although a number of studies suggest that obesity is a risk factor for dementia and AD, this risk effect appears to be modified, and perhaps reversed, by age. Studies in older adults, rather than middle aged adults, suggest that a higher BMI may be associated with a lower risk of cognitive decline, dementia, and AD. [107–109] As discussed below in more detail, these observations suggest that obesity may influence long term risk of AD but that the early or preclinical stages of AD may be associated with weight loss.
2.1.4. Inflammation and Cytokines
A number of studies suggest inflammatory processes may play a role in AD. Although increased markers of inflammation are observed in AD brain postmortem, [110] these likely reflect local neurodegenerative processes occurring in brain. Thus, studies have examined if plasma biomarkers can predict cognitive decline and AD risk. Tumor necrosis factor alpha (TNF-α) has been associated with cognitive decline, [111] and both TNF-α and another inflammatory molecule, IL-1β, have been associated with increased AD risk. [112] Inflammatory processes are actually a common link between many AD risk factors discussed in this section: TNF-α, for instance, has been shown to be increased in T2D and obesity, and decreased with exercise,[113] although not all studies are consistent. Interestingly, a very recent large study that analyzed data from 3 independent cohorts found that only 4 plasma analytes (APOE, B-type natriuretic peptide, C-reactive protein, and pancreatic polypeptide) were linked with MCI or AD diagnosis.[114] One of these analytes, C-reactive protein, is known to be particularly responsive to inflammatory processes.
Although some studies have linked peripheral inflammatory markers to AD, clinical trials to reduce inflammation have produced contentious findings. For instance, in cross-sectional and population-based cohort studies, use of anti-inflammatory drugs is associated with reduced AD risk.[115–117] However, clinical trials have failed to show that anti-inflammatory therapy prevents AD [118] or improves cognitive function in either AD subjects or individuals with family history of AD. [119, 120] Safety concerns were often noted, and one trial was halted.[121] In summary, although peripheral inflammation has been observed in AD, the degree to which inflammation drives brain changes is unclear and at the present time there is little clinical evidence that inflammation is an efficacious target for AD prevention or treatment.
2.2. AD Drives Systemic Changes
It is well known that the brain mediates a variety of peripheral processes through mechanisms such as the autonomic nervous system and motor circuits. For instance, the CNS modulates bone health through autonomic output from the hypothalamus,[122] a central regulator of a number of peripheral metabolic processes. Additionally, as AD brain dysfunction progresses the associated functional declines result in reduced levels of physical activity which may in turn result in body composition changes (i.e. increased fat mass and reductions in lean mass and bone density). Thus, CNS processes can influence peripheral processes both directly (i.e. autonomic output) or indirectly through behavioral and functional changes (i.e., reduced physical activity, forgetting to eat). This section reviews evidence of peripheral effects that may be mediated or modulated by the AD neurodegenerative process.
2.2.1. Body Composition
As discussed, obesity is a clear risk factor, particularly in midlife, for the future development of AD. However, there is substantial evidence that changes in body composition, such as weight loss, occur in the earliest or even preclinical stages of AD. These findings suggest that as early CNS manifestations of AD occur in the brain (i.e., plaques and tangles) there are co-occurring systemic changes associated with the onset of disease.
As noted above, while higher body mass index (BMI) is associated with increased dementia risk, [91, 92] this risk appears to attenuate or reverse in older adults where higher BMI is associated with a lower risk of cognitive decline, dementia, and AD. [107–109] In fact, studies using sensitive measures of body composition suggest that changes in lean mass (i.e. muscle mass) and bone density may be among the earliest manifestations of the AD clinical syndrome. We have found that individuals in the early stages of AD have reduced lean mass [52] and lower bone density [53] than nondemented controls. Bone density has been correlated specifically with measures of hypothalamic atrophy in AD, while reductions in lean mass and bone density were both associated with greater whole brain atrophy and cognitive decline. [54] Reductions in lean mass may be attributed in part to functional impairment in individuals with AD, which can result in feeding difficulties, especially in subjects with aged caregivers, [4] although our studies included individuals in the earliest stages of AD when these types of difficulties are not overtly present.
Although functional impairments occurring in later stages of AD may contribute to the observed decrease in body weight, other research suggests that weight loss occurs before significant functional decline has begun. For instance, weight loss has been shown to occur prior to development of AD.[5] In a prospective study that followed following elderly individuals for 20 years, individuals who were diagnosed with AD at follow-up had lost more weight since baseline than individuals with normal cognition. In this case, weight loss occurred prior to AD diagnosis, indicating that this effect was likely not due to functional or behavioral changes impacting nutrition.[123] Lower BMI is associated with faster cognitive decline over one year in individuals with MCI,[107] consistent with studies suggesting that weight loss in elderly individuals may be an early systemic manifestation of the AD process. [124–128] An inverse relationship has been observed between BMI and AD biomarkers in both normal and cognitively-impaired subjects, with the relationship most strongly evident in individuals with MCI.[129] MCI is a heterogeneous pathological state, suggesting that individuals with MCI who are normal or low weight (BMI 18.5–25 kg/m2) are more likely to have amyloid-based cognitive impairment compared to those who are overweight (BMI > 25 kg/m2).[129]
Interestingly, a relationship between BMI and AD neuropathology has also been observed in cognitively-normal elderly subjects. We and others have shown that neuropathological changes of AD found at autopsy are associated with low and declining body mass index (BMI).[129, 130] Given that elevated BMI at midlife is a risk factor for AD, it is possible that low BMI in late life is somehow a consequence of the disease process. However, this relationship may also suggest that there are multiple etiologies leading to AD. For instance, it is possible that individuals with elevated BMI may exhibit more vascular-related pathology and lower amyloid neuropathology for a given level of cognitive function, while subjects with lower BMI may be more apt to exhibit more classic AD neuropathology in the absence of vascular disease.
2.2.2. Physical function and fitness
Motor slowing has been observed in subjects with early AD,[131] indicating that motor dysfunction may be a very early manifestation of disease. Cognitively-impaired subjects have been shown to exhibit greater decline in strength and performance on tests of physical function compared to controls,[132] and it is reported that cognitive decline predicts decline in upper muscle strength.[133] Along these lines, we have found that AD subjects also exhibit reduced VO2peak (independent of dementia severity or physical function decline), and this reduction is correlated with brain atrophy. [44, 45] Similarly, our imaging studies suggest that decreased aerobic fitness is associated with hippocampal atrophy in early AD.[134] It is possible that declining functional capacity, especially in later stages of the disease, has a detrimental effect on physical fitness in these individuals. However, mild decreases in VO2peak have been observed by us even in the earliest clinical stages of the disease prior to the emergence of clinically significant physical function impairment. This suggests that motor dysfunction may not simply be the result of progressive CNS dysfunction but an early systemic manifestation of the disease.
2.3. Common Peripheral and CNS Etiological Processes
Data from the prior sections suggests that systemic processes can influence AD risk while AD-related CNS dysfunction can influence whole-body health. In this section, we will review evidence suggesting that abnormal physiologic processes may underlie both whole body and brain health. For instance, mitochondrial dysfunction is one underlying factor that precedes both CNS neuropathological manifestations and peripheral metabolic dysfunction often observed in AD. Thus, a common underlying etiological process may mediate some of the apparent co-occurring decline in both the body and the brain that is associated with the clinical syndrome of AD.
2.3.1. Genetic factors
Genetic factors are likely to play a common underlying role in peripheral and central dysfunction associated with AD, although the functional actions of many of the risk genes for AD are not fully known. The most commonly identified gene that increases sporadic AD risk in roughly 40% of individuals is Apolipoprotein ε4 (APOE ε4). APOE is involved in lipid transport and cholesterol metabolism within the cell.[135] Interestingly, differences in regional white and gray matter are detectable in APOE ε4 carriers from infancy.[136] Although the precise mechanisms are still not well understood, we have previously discussed evidence that aberrant lipid metabolism may play a role in AD risk. We and others have found that the APOE ε4 allele is associated with decreased cognition, gray matter volume in memory areas (the hippocampus), white matter tract integrity, and increased magnetic resonance imaging markers for cardiovascular disease [137–139]. Furthermore, decreases in cerebral glucose metabolism are a known biomarker for AD, [140] and cognitively-normal, middle-aged APOE ε4 carriers have AD-like changes in cerebral glucose metabolism, [141, 142] with a possible gene-dose effect. [143]
A handful of studies using functional magnetic resonance imaging of the default mode network (DMN) have also shown differential oxygen uptake in the brain at rest in young APOE ε4 carriers, indicating differences in brain metabolic function early in life. [144–146] Some have argued that default mode network changes may more closely represent actual brain oxygen consumption [147, 148] and thus mitochondrial function. In fact, cytochrome oxidase activity, a marker of mitochondrial bioenergetics, has been measured directly in the brains of young adult APOE ε4 carriers. These individuals exhibited mitochondrial dysfunction decades before they would likely have any cognitive symptoms.[149] This indicates a potential underlying factor for the effects of genotype and mitochondrial bioenergetics, which will be discussed later. Moreover, genetics may even affect the responsiveness of individuals to interventions that aim to reduce AD risk: the finding that higher leisure-time physical activity decreased AD risk in late life was strongest in individuals who were APOE ε4-positive. [150] However, recent studies that have examined insulin resistance in APOE ε4 carriers have not shown any effect of genotype, [151, 152] suggesting that the relationship between insulin resistance and AD may be through a separate mechanism.
Although APOE ε4 is by far the most widely-recognized genetic risk factor of late-onset AD, other genes have also been linked to AD. To date, 660 candidate genes for AD risk have been identified, although results are inconsistent between studies. The development of genome-wide association studies (GWAS) has greatly improved AD genetic knowledge.[153] GWAS with fewer than 1000 case or control subjects have implicated novel AD risk single nucleotide polymorphisms (SNPs) in GOLPH2, GAB2, and PCDH11X genes[154–157]. More powerful, higher number case and control GWAS have identified or replicated novel AD risk SNPs in BIN1, CLU, CR1 and PICALM genes, [158–165] and a recent meta-analysis of GWAS identified a total of 20 genetic susceptibility loci (11 new genes, in addition to 9 previously-identified risk genes).[157]. However, interpretation of GWAS findings to reveal disease-relevant biological mechanisms remains a challenge because the genetic architecture of AD is incomplete [166]. Even the most robust genetic associations appear to explain only a small portion of the disease burden in the population, with the majority of the heritable component of the disease unexplained.
2.3.1. Mitochondrial Dysfunction
There is a large body of evidence that mitochondrial dysfunction, and perhaps energy failure, plays a central role in AD pathophysiology.[167, 168] Mitochondrial deficits in AD are not isolated to neurons, but occur systemically. [8, 169] Mitochondrial dysfunction likely contributes to insulin resistance [170] and has been shown to occur in pre-diabetic animal models. [171] Very recently, mitochondrial dysfunction at the molecular level was shown to lie upstream of pancreatic beta-cell death and was linked to development of diabetes in mice.[172] Moreover, muscle contraction, as occurs during exercise, has been linked to improvement in mitochondrial energy metabolism and to normalization of insulin signaling.[173] It is possible that mitochondrial dysfunction is one underlying factor that precedes both CNS neuropathological symptoms and contributes to peripheral metabolic dysfunction often observed in AD.
Family history studies also support a role for mitochondrial dysfunction in AD. In contrast to nuclear DNA, mitochondrial DNA is inherited maternally, and both specific mitochondrial haplotypes and maternal family history are linked to AD-related structural, cognitive, CSF, and metabolic biomarkers. [174–177] These findings of increased AD-related change in maternal lines of AD suggest that transmission of risk is preferentially found in maternal inheritance. This provides indirect evidence that mitochondrial function is related to manifestation of AD symptoms. Perhaps more intriguing than genetic risk, however, is that mitochondrial dysfunction accumulates throughout the aging process.[178–180] This suggests a role for both inherited and acquired mitochondrial dysfunction in modulating AD risk. In AD patients, overt markers of mitochondrial dysfunction have been consistently observed. For instance, activity of cytochrome c oxidase, an enzyme in the electron transport chain essential for energy production, is decreased in the brains of AD patients [169, 181–185] and in adult children with a maternal family history of AD.[186] Furthermore, mitochondrial DNA isolated from AD brain exhibits a loss of integrity, such as increased number of deletions and mutations.[187–189]
Interestingly, alterations in the association of mitochondria with other cellular compartments are also observed in AD and may further contribute to mitochondrial dysfunction. For instance, mitochondria are normally associated with the endoplasmic reticulum (ER) through physical ER connections called mitochondria associated membranes (MAMs). These structures play an important role in communication between the mitochondria and ER, linking them structurally and functionally. These membrane areas have the characteristics of previously discussed lipid rafts, and the activity of enzymes linked to AD, such as γ-secretase, is heavily enriched in MAM regions. [190, 191] MAMs are important for regulating processes such as phospholipid synthesis and calcium levels [192, 193] and may provide a potential link between processes such as apoptosis and synchronization of energy production and energy use via calcium signaling. [190, 193, 194] The function of MAMs is altered in AD, with consequences ranging from mitochondrial dysfunction to altered APP processing.[195] Loss of DNA integrity, dysregulated calcium homeostastis, and a failure to adequately match energy supply and demand due to downregulation of key enzymes may all contribute to mitochondrial damage. This evidence provides an additional potential link between mitochondrial function, lipid metabolism, and AD.
It is known that overproduction of reactive oxygen species (ROS) and subsequent oxidative stress plays a key role in mitochondrial dysfunction. [196] Interestingly, mitochondria themselves are the primary source of ROS production in the cell. [197] Under normal conditions, ROS serve important signaling functions,[198] but damaged mitochondria can overproduce ROS and increase cellular oxidative stress.[197] ROS generation is increased in MCI, [199] and oxidative stress can mediate AD pathophysiology through increased production and secretion of amyloid beta (Aβ). [200, 201] In fact, mitochondrial dysfunction may play a role in the downstream protein aggregation that serves as the neuropathological hallmark of AD. Aβ deposition initially occurs within the neuron, [202] and Aβ trafficking into mitochondria precedes plaque formation. [203] Cell culture studies have shown that functional mitochondria are necessary for Aβ to induce cellular toxicity.[204] Although the temporal relationship between impaired mitochondrial function and amyloid neuropathology in humans is not well understood, studies in transgenic mice suggest that mitochondrial dysfunction occurs prior to Aβ plaque formation. [205] Furthermore, Aβ is postulated to impair mitochondrial protein import, [206] and complex IV dysregulation in triple transgenic AD mice is dependent on Aβ,[207] providing multiple mechanisms for impaired mitochondrial function. These effects may trigger a vicious cycle where damaged mitochondria can generate Aβ and Aβ can then enter mitochondria and exacerbate damage. Interestingly, amyloid is imported into mitochondria by translocase of the outer membrane (TOM) machinery, [208] and TOMM40, which encodes the channel protein subunit of the translocase of the outer mitochondrial membrane (TOMM) complex [209] is in high linkage disequilibrium with APOE.[210] This makes it difficult to discern which gene actually contributes to AD risk.
2.3.2. Vascular mechanisms
The strongest modifiable risk factors for AD are also risk factors for vascular disease, including sedentary behavior, glucose intolerance, and obesity.[211, 212] Vascular pathologies are increasingly recognized as having an important role in late-life dementias given recent observations of the heterogeneity of associated dementia pathologies.[213] Although most studies attempt to describe vascular and AD pathologies as discrete syndromes, the clinical and neuropathological boundaries frequently overlap. Vascular-related injury often coexists with AD neuropathology at autopsy,[214] and the presence of cerebrovascular disease lowers the burden of AD neuropathological changes associated with a given level of cognitive impairment. [215, 216] Many individuals clinically diagnosed with AD actually exhibit a “mixed dementia” with both AD neuropathological changes and abnormalities indicative of vascular damage. [217, 218]
Mechanistically, vascular mechanisms may compromise cognitive function and contribute to AD in several ways. For instance, cardiovascular risk factors are linked to white matter lesions in elderly subjects.[219] White matter lesions are prevalent in both aging and AD,[216] and we have shown that cardiorespiratory fitness correlates with longitudinal brain atrophy in AD.[134] Moreover, many conditions comorbid with vascular disease, such as hypertension and diabetes, have been linked to dementia. High blood pressure has been observed in subjects with both AD and vascular dementia,[220] and high blood pressure during midlife is associated with increased late life AD risk.[221] The link between AD and diabetes has been previously discussed, but it is interesting that pancreatic dysfunction is linked to high levels of amylin, which aggregates in a manner similar to amyloid-beta and cause pancreatic beta-cell cytotoxicity.[222] Amylin also aggregates in the brain in both vascular dementia and AD, linking peripheral metabolic dysfunction with vascular disease and cognitive decline.[84] Both AD and vascular dementia have also been linked to decreased regional blood flow.[223] Decreased blood flow to the brain reduces the critical supply of glucose and oxygen to the brain that is necessary to sustain proper neuronal metabolism. Finally, vascular disease may be linked to impaired energy metabolism through mitochondrial function. In cardiac microvascular endothelial cells, high glucose induces apoptosis through induction of FoxO3a, [224] a transcription factor known to regulate mitochondrial gene expression and production of reactive oxygen species.[225] A similar mechanism may also hold true for brain endothelial cells.
Thus, there may be multiple paths to AD dementia, with genetic, mitochondrial, and vascular influences. It is intriguing that diabetics, for instance, have a higher risk for AD diagnosis and yet lower amyloid pathology postmortem.[217] There is thus evidence that vascular risk factors may lower the threshold for additional damage necessary to cause clinical expression of AD symptoms.
3. Additional considerations
Despite efforts to strictly define AD as a homogeneous CNS disease, there may be no single etiologic pathway leading to the syndrome of AD dementia. An individual’s baseline risk is likely determined by inherited nuclear and mitochondrially-encoded genes. Environmental factors, such as midlife obesity, insulin resistance, and inflammatory processes, likely modify this baseline risk. The contributions of genetic vs. environmental factors certainly vary from individual to individual in complex ways. Because environmental “risk modifiers” often interact (for instance, midlife obesity can lead to insulin resistance), environmental factors may play a more central role in the development of dementia in some individuals. These individuals may have lower levels of traditional AD neuropathological burden (i.e. amyloid plaques and neurofibrillary tangles) compared to genetically predisposed individuals who generally have higher levels of AD neuropathology. Vascular mechanisms and processes influencing cellular stress may influence the emergence and progression of the clinical manifestation of the dementia syndrome. Continued research into the diverse but related processes linked to AD risk is necessary, as individuals with genetic risk (and potentially more amyloid pathology) may benefit more from amyloid clearance drugs, whereas individuals who exhibit more vascular-related pathology may preferentially benefit from lifestyle interventions such as diet and exercise.
Limitations and forward progress
Although promising, there are limitations to many studies discussed in this article. Studies that are observational or cross-sectional, for instance, cannot be used to establish causality – this requires well-designed clinical trials. Because AD has a notably long asymptomatic time course, and many of the classical outcome measures (such as neuropsychometric tests) are not sensitive to the earliest signs of the disease, design of randomized clinical trials can be difficult and expensive. However, there has also been exciting progress in the field, notably in the development of advanced imaging techniques. These techniques can be used to determine individuals at risk for AD prior to cognitive impairment. For instance, ligands that can be used to visualize both Aβ and Tau using PET imaging in vivo will allow future randomized clinical trials to investigate lifestyle interventions, such as exercise, or pharmaceutical interventions that may affect brain metabolism, such as intranasal insulin, as potential modifiers of AD neuropathology in the preclinical stages of the disease.
4. Conclusions
Here we present evidence that 1) peripheral processes contribute to AD risk, 2) AD influences whole-body changes, and 3) common processes may modulate both peripheral and CNS manifestations of AD dementia. It is our view that AD is a multifactorial disease that affects both CNS and systemic processes. It is possible that there is no single “smoking gun” that will explain the etiology of AD, but that several interconnected processes, many of which are directly related to energy metabolism, together determine the cognitive trajectory of individuals that are initially at more or less risk from genetic factors.
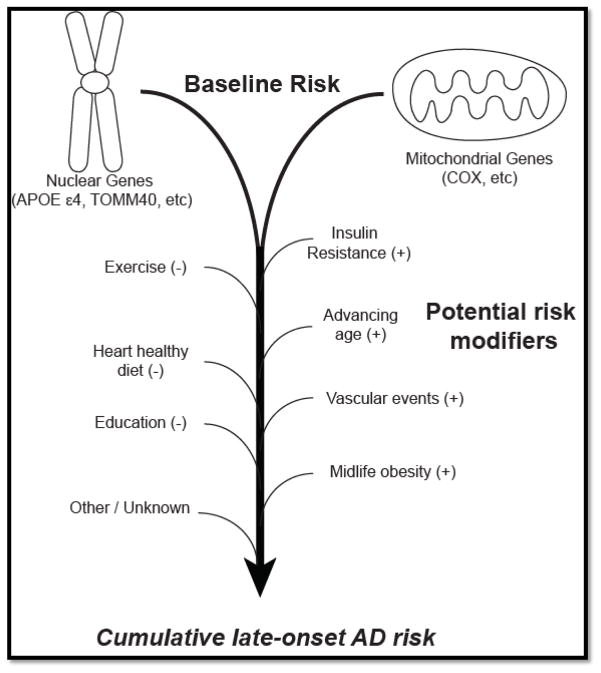
Figure 1. Schematic of potential risk factors for AD.
We propose that a combination of nuclear and mitochondrially-encoded genes determine one’s baseline risk for AD. This baseline risk can be modified – either increased (+) or decreased (−) by a wide variety of environmental factors, (diet, exercise) socioeconomic factors (i.e. education) and inevitably by the aging process.
Highlights.
Several diseases and risk factors linked to AD risk involve systemic metabolic dysfunction.
The cause and effect relationship between these factors remains imprecisely defined.
Common factors affecting both CNS and systemic processes contribute to AD
AD likely represents a heterogenous disease with both central and peripheral manifestations
Footnotes
Disclosures: The authors report no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jill K. Morris, Email: jmorris2@kumc.edu.
Robyn A. Honea, Email: rhonea@kumc.edu.
Eric D. Vidoni, Email: evidoni@kumc.edu.
Russell H. Swerdlow, Email: rswerdlow@kumc.edu.
Jeffrey M. Burns, Email: jburns2@kumc.edu.
References
- 1.Thies W, Bleiler L, Alzheimer’s A. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9(2):208–45. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Agosta F, Pievani M, Sala S, et al. White matter damage in Alzheimer disease and its relationship to gray matter atrophy. Radiology. 2011;258(3):853–63. doi: 10.1148/radiol.10101284. [DOI] [PubMed] [Google Scholar]
- 3.Backman L, Jones S, Berger AK, et al. Multiple cognitive deficits during the transition to Alzheimer’s disease. J Intern Med. 2004;256(3):195–204. doi: 10.1111/j.1365-2796.2004.01386.x. [DOI] [PubMed] [Google Scholar]
- 4.Riviere S, Gillette-Guyonnet S, Andrieu S, et al. Cognitive function and caregiver burden: predictive factors for eating behaviour disorders in Alzheimer’s disease. Int J Geriatr Psychiatry. 2002;17(10):950–5. doi: 10.1002/gps.724. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DK, Wilkins CH, Morris JC. Accelerated Weight Loss May Precede Diagnosis in Alzheimer Disease. Arch Neurol. 2006;63(9):1312–1317. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 6.Etcheberrigaray R, Ibarreta D. Ionic channels and second messenger alterations in Alzheimer’s disease. Relevance of studies in nonneuronal cells. Rev Neurol. 2001;33(8):740–9. [PubMed] [Google Scholar]
- 7.Bruel A, Cherqui G, Columelli S, et al. Reduced protein kinase C activity in sporadic Alzheimer’s disease fibroblasts. Neurosci Lett. 1991;133(1):89–92. doi: 10.1016/0304-3940(91)90064-z. [DOI] [PubMed] [Google Scholar]
- 8.Swerdlow RHK, SJ Mitochondria in Alzheimer’s Disease. Int Rev Neurobiol. 2002;53:341–385. doi: 10.1016/s0074-7742(02)53013-0. [DOI] [PubMed] [Google Scholar]
- 9.Leuner K, Schulz K, Schutt T, et al. Peripheral mitochondrial dysfunction in Alzheimer’s disease: focus on lymphocytes. Mol Neurobiol. 2012;46(1):194–204. doi: 10.1007/s12035-012-8300-y. [DOI] [PubMed] [Google Scholar]
- 10.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435–45. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 11.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302(6):627–37. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neeper SA, Gomezpinilla F, Choi J, et al. Exercise and Brain Neurotrophins. Nature. 1995;373(6510):109–109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 13.Churchill JD, Galvez R, Colcombe S, et al. Exercise, experience and the aging brain*1. Neurobiology of Aging. 2002;23(5):941–955. doi: 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 14.van Praag H, Christie BR, Sejnowski TJ, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakka TA, Laukkanen JA, Rauramaa R, et al. Cardiorespiratory Fitness and the Progression of Carotid Atherosclerosis in Middle-Aged Men. Annals of Internal Medicine. 2001;134(1):12–20. doi: 10.7326/0003-4819-134-1-200101020-00008. [DOI] [PubMed] [Google Scholar]
- 16.Blair SN, Kampert JB, Kohl HW, III, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA: The Journal of the American Medical Association. 1996;276(3):205–210. [PubMed] [Google Scholar]
- 17.Kurl S, Laukkanen JA, Rauramaa R, et al. Cardiorespiratory Fitness and the Risk for Stroke in Men. Archives of Internal Medicine. 2003;163(14):1682–1688. doi: 10.1001/archinte.163.14.1682. [DOI] [PubMed] [Google Scholar]
- 18.Seals DR, Hagberg JM, Hurley BF, et al. Effects of endurance training on glucose tolerance and plasma lipid levels in older men and women. JAMA: The Journal of the American Medical Association. 1984;252(5):645–649. [PubMed] [Google Scholar]
- 19.Hughes VA, Fiatarone MA, Fielding RA, et al. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol. 1993;264(6 Pt 1):E855–E862. doi: 10.1152/ajpendo.1993.264.6.E855. [DOI] [PubMed] [Google Scholar]
- 20.Kirwan JP, Kohrt WM, Wojta DM, et al. Endurance exercise training reduces glucose-stimulated insulin levels in 60- to 70-year-old men and women. J Gerontol. 1993;48(3):M84–M90. doi: 10.1093/geronj/48.3.m84. [DOI] [PubMed] [Google Scholar]
- 21.Cox JH, Cortright RN, Dohm GL, et al. Effect of aging on response to exercise training in humans: skeletal muscle GLUT-4 and insulin sensitivity. Journal of Applied Physiology. 1999;86(6):2019–2025. doi: 10.1152/jappl.1999.86.6.2019. [DOI] [PubMed] [Google Scholar]
- 22.Kahn SE, V, Larson G, Beard JC, et al. Effect of exercise on insulin action, glucose tolerance, and insulin secretion in aging. Am J Physiol. 1990;258(6 Pt 1):E937–E943. doi: 10.1152/ajpendo.1990.258.6.E937. [DOI] [PubMed] [Google Scholar]
- 23.Houmard JA, Tyndall GL, Midyette JB, et al. Effect of reduced training and training cessation on insulin action and muscle GLUT-4. Journal of Applied Physiology. 1996;81(3):1162–1168. doi: 10.1152/jappl.1996.81.3.1162. [DOI] [PubMed] [Google Scholar]
- 24.Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012;2(1):a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology. 2002;13(5):561–8. doi: 10.1097/00001648-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Dustman RE, Emmerson RY, Ruhling RO, et al. Age and fitness effects on EEG, ERPs, visual sensitivity, and cognition. Neurobiol Aging. 1990;11(3):193–200. doi: 10.1016/0197-4580(90)90545-b. [DOI] [PubMed] [Google Scholar]
- 27.van Boxtel MP, Paas FG, Houx PJ, et al. Aerobic capacity and cognitive performance in a cross-sectional aging study. Med Sci Sports Exerc. 1997;29(10):1357–1365. doi: 10.1097/00005768-199710000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Shay KA, Roth DL. Association Between Aerobic Fitness and Visuospatial Performance in Healthy Older Adults. Psychology and aging. 1992;7(1):15–24. doi: 10.1037//0882-7974.7.1.15. [DOI] [PubMed] [Google Scholar]
- 29.Hillman CH, Motl RW, Pontifex MB, et al. Physical activity and cognitive function in a cross-section of younger and older community-dwelling individuals. Health Psychol. 2006;25(6):678–87. doi: 10.1037/0278-6133.25.6.678. [DOI] [PubMed] [Google Scholar]
- 30.Bixby WR, Spalding TW, Haufler AJ, et al. The unique relation of physical activity to executive function in older men and women. Med Sci Sports Exerc. 2007;39(8):1408–16. doi: 10.1249/mss.0b013e31806ad708. [DOI] [PubMed] [Google Scholar]
- 31.Laurin D, Verreault R, Lindsay J, et al. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 32.Yaffe K, Barnes D, Nevitt M, et al. A Prospective Study of Physical Activity and Cognitive Decline in Elderly Women: Women Who Walk. Archives of Internal Medicine. 2001;161(14):1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 33.Pignatti F, Rozzini R, Trabucchi M, et al. Physical Activity and Cognitive Decline in Elderly Persons. Archives of Internal Medicine. 2002;162(3):361–362. doi: 10.1001/archinte.162.3.361. [DOI] [PubMed] [Google Scholar]
- 34.Albert MS, Jones K, Savage CR, et al. Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychol Aging. 1995;10(4):578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- 35.Weuve J, Kang JH, Manson JE, et al. Physical Activity, Including Walking, and Cognitive Function in Older Women. JAMA: The Journal of the American Medical Association. 2004;292(12):1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 36.Larson EB, Wang L, Bowen JD, et al. Exercise Is Associated with Reduced Risk for Incident Dementia among Persons 65 Years of Age and Older. Ann Intern Med. 2006;144(2):73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 37.Friedland RP, Fritsch T, Smyth KA, et al. Patients with Alzheimer’s disease have reduced activities in midlife compared with healthy control-group members. Proceedings of the National Academy of Sciences. 2001;98(6):3440–3445. doi: 10.1073/pnas.061002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buchman AS, Boyle PA, Yu L, et al. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78(17):1323–9. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–37. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 40.Kramer AF, Hahn S, Cohen NJ, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–9. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 41.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 42.Erickson KI, Raji CA, Lopez OL, et al. Physical activity predicts gray matter volume in late adulthood: The Cardiovascular Health Study. Neurology. 2010;75(16):1415–22. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu-Ambrose T, Nagamatsu LS, Graf P, et al. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170(2):170–8. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honea RA, Thomas GP, Harsha A, et al. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23(3):188–97. doi: 10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burns JM, Cronk BB, Anderson HS, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71(3):210–6. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang AM, Jen CJ, Chen HF, et al. Compulsive exercise acutely upregulates rat hippocampal brain-derived neurotrophic factor. J Neural Transm. 2006;113(7):803–11. doi: 10.1007/s00702-005-0359-4. [DOI] [PubMed] [Google Scholar]
- 48.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 49.Nagamatsu LS, Handy TC, Hsu CL, et al. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med. 2012;172(8):666–8. doi: 10.1001/archinternmed.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buchman AS, Wilson RS, Boyle PA, et al. Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology. 2007;29(1–2):66–73. doi: 10.1159/000109498. [DOI] [PubMed] [Google Scholar]
- 51.Boyle PA, Buchman AS, Wilson RS, et al. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol. 2009;66(11):1339–44. doi: 10.1001/archneurol.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burns JM, Johnson DK, Watts A, et al. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch Neurol. 2010;67(4):428–33. doi: 10.1001/archneurol.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loskutova N, Honea RA, Vidoni ED, et al. Bone density and brain atrophy in early Alzheimer’s disease. J Alzheimers Dis. 2009;18(4):777–85. doi: 10.3233/JAD-2009-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loskutova N, Honea RA, Brooks WM, et al. Reduced limbic and hypothalamic volumes correlate with bone density in early Alzheimer’s disease. J Alzheimers Dis. 2010;20(1):313–22. doi: 10.3233/JAD-2010-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janson J, Laedtke T, Parisi JE, et al. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53(2):474–81. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 56.Arvanitakis Z, Wilson RS, Bienias JL, et al. Diabetes Mellitus and Risk of Alzheimer Disease and Decline in Cognitive Function. Archives of Neurology. 2004;61(5):661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 57.Luchsinger JA, Reitz C, Patel B, et al. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007;64(4):570–5. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 58.Ott A, Stolk RP, van Harskamp F, et al. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53(9):1937–42. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 59.Peila R, Rodriguez BL, Launer LJ. Type 2 Diabetes, APOE Gene, and the Risk for Dementia and Related Pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51(4):1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 60.Xu W, Qiu C, Gatz M, et al. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes. 2009;58(1):71–7. doi: 10.2337/db08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Velayudhan L, Poppe M, Archer N, et al. Risk of developing dementia in people with diabetes and mild cognitive impairment. Br J Psychiatry. 2010;196(1):36–40. doi: 10.1192/bjp.bp.109.067942. [DOI] [PubMed] [Google Scholar]
- 62.Morris JK, Vidoni ED, Honea RA, et al. Impaired glycemia increases disease progression in mild cognitive impairment. Neurobiol Aging. 2014;35(3):585–9. doi: 10.1016/j.neurobiolaging.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32(4):486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 64.Mosconi L, Tsui WH, De Santi S, et al. Reduced hippocampal metabolism in MCI and AD: Automated FDG-PET image analysis. Neurology. 2005;64(11):1860–1867. doi: 10.1212/01.WNL.0000163856.13524.08. [DOI] [PubMed] [Google Scholar]
- 65.Banks WA. The source of cerebral insulin. European Journal of Pharmacology. 2004;490(1–3):5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 66.Skeberdis VA, Lan J, Zheng X, et al. Insulin promotes rapid delivery of N-methyl-D- aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci U S A. 2001;98(6):3561–6. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin Z, Jin Y, Kumar-Mendu S, et al. Insulin reduces neuronal excitability by turning on GABA(A) channels that generate tonic current. PLoS One. 2011;6(1):e16188. doi: 10.1371/journal.pone.0016188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wan Q, Xiong ZG, Man HY, et al. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature. 1997;388(6643):686–90. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- 69.van der Heide LP, Ramakers GMJ, Smidt MP. Insulin signaling in the central nervous system: Learning to survive. Progress in Neurobiology. 2006;79(4):205–221. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Gasparini L, Gouras GK, Wang R, et al. Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling. J Neurosci. 2001;21(8):2561–70. doi: 10.1523/JNEUROSCI.21-08-02561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? Journal of Alzheimer’s Disease. 2005;7(1):63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 72.Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012 doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luchsinger JA, Tang MX, Shea S, et al. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63(7):1187–92. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 74.Burns JM, Donnelly JE, Anderson HS, et al. Peripheral insulin and brain structure in early Alzheimer disease. Neurology. 2007;69(11):1094–1104. doi: 10.1212/01.wnl.0000276952.91704.af. [DOI] [PubMed] [Google Scholar]
- 75.Burns JM, Honea RA, Vidoni ED, et al. Insulin is differentially related to cognitive decline and atrophy in Alzheimer’s disease and aging. Biochim Biophys Acta. 2012;1822(3):333–9. doi: 10.1016/j.bbadis.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greenwood CE, Kaplan RJ, Hebblethwaite S, et al. Carbohydrate-Induced Memory Impairment in Adults With Type 2 Diabetes. Diabetes Care. 2003;26(7):1961–1966. doi: 10.2337/diacare.26.7.1961. [DOI] [PubMed] [Google Scholar]
- 77.Ravona-Springer R, Moshier E, Schmeidler J, et al. Changes in glycemic control are associated with changes in cognition in non-diabetic elderly. J Alzheimers Dis. 2012;30(2):299–309. doi: 10.3233/JAD-2012-120106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vanhanen M, Koivisto K, Kuusisto J, et al. Cognitive function in an elderly population with persistent impaired glucose tolerance. Diabetes Care. 1998;21(3):398–402. doi: 10.2337/diacare.21.3.398. [DOI] [PubMed] [Google Scholar]
- 79.Benedict C, Brooks SJ, Kullberg J, et al. Impaired insulin sensitivity as indexed by the HOMA score is associated with deficits in verbal fluency and temporal lobe gray matter volume in the elderly. Diabetes Care. 2012;35(3):488–94. doi: 10.2337/dc11-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xie L, Helmerhorst E, Taddei K, et al. Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. J Neurosci. 2002;22(10):RC221. doi: 10.1523/JNEUROSCI.22-10-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farris W, Mansourian S, Chang Y, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid á-protein, and the á-amyloid precursor protein intracellular domain in vivo. PNAS. 2003;100(7):4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thambisetty M, Metter EJ, Yang A, et al. Glucose Intolerance, Insulin Resistance, and Pathological Features of Alzheimer Disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol. 2013 doi: 10.1001/jamaneurol.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Janson J, Laedtke T, Parisi JE, et al. Increased Risk of Type 2 Diabetes in Alzheimer Disease. Diabetes. 2004;53(2):474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 84.Jackson K, Barisone GA, Diaz E, et al. Amylin deposition in the brain: A second amyloid in Alzheimer disease? Ann Neurol. 2013 doi: 10.1002/ana.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kimura R, MacTavish D, Yang J, et al. Beta amyloid-induced depression of hippocampal long-term potentiation is mediated through the amylin receptor. J Neurosci. 2012;32(48):17401–6. doi: 10.1523/JNEUROSCI.3028-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66(3):336–42. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tolppanen AM, Ngandu T, Kareholt I, et al. Midlife and late-life body mass index and late-life dementia: results from a prospective population-based cohort. J Alzheimers Dis. 2014;38(1):201–9. doi: 10.3233/JAD-130698. [DOI] [PubMed] [Google Scholar]
- 88.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67(6):505–12. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 89.Gazdzinski S, Kornak J, Weiner MW, et al. Body mass index and magnetic resonance markers of brain integrity in adults. Ann Neurol. 2008;63(5):652–7. doi: 10.1002/ana.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gustafson D, Lissner L, Bengtsson C, et al. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63(10):1876–1881. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- 91.Whitmer RA, Gunderson EP, Barrett-Connor E, et al. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330(7504):1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gustafson D, Rothenberg E, Blennow K, et al. An 18-Year Follow-up of Overweight and Risk of Alzheimer Disease. Archives of Internal Medicine. 2003;163(13):1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 93.Despres JP. Dyslipidaemia and obesity. Baillieres Clin Endocrinol Metab. 1994;8(3):629–60. doi: 10.1016/s0950-351x(05)80289-7. [DOI] [PubMed] [Google Scholar]
- 94.Dias HKI, Mistry J, Tarzyluck M, Hill EJ, Bennett SJ, Polidori MC, Lip GYH, Griffiths HR. Oxidised LDL-lipids alter redox ratio, lipid raft formation and increase amyloid beta production by SHSY-5Y cells. Experimental Gerontology. 2013;48(7):688. [Google Scholar]
- 95.Grandl M, Bared SM, Liebisch G, et al. E-LDL and Ox-LDL differentially regulate ceramide and cholesterol raft microdomains in human Macrophages. Cytometry A. 2006;69(3):189–91. doi: 10.1002/cyto.a.20232. [DOI] [PubMed] [Google Scholar]
- 96.Ehehalt R, Keller P, Haass C, et al. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160(1):113–23. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhattacharyya R, Barren C, Kovacs DM. Palmitoylation of amyloid precursor protein regulates amyloidogenic processing in lipid rafts. J Neurosci. 2013;33(27):11169–83. doi: 10.1523/JNEUROSCI.4704-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Han X, Rozen S, Boyle SH, et al. Metabolomics in early Alzheimer’s disease: identification of altered plasma sphingolipidome using shotgun lipidomics. PLoS One. 2011;6(7):e21643. doi: 10.1371/journal.pone.0021643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mielke MM, V, Bandaru V, Haughey NJ, et al. Serum ceramides increase the risk of Alzheimer disease: The Women’s Health and Aging Study II. Neurology. 2012;79(7):633–41. doi: 10.1212/WNL.0b013e318264e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mielke MM, Haughey NJ, Ratnam Bandaru VV, et al. Plasma ceramides are altered in mild cognitive impairment and predict cognitive decline and hippocampal volume loss. Alzheimers Dement. 2010;6(5):378–85. doi: 10.1016/j.jalz.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Puglielli L, Ellis BC, Saunders AJ, et al. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. J Biol Chem. 2003;278(22):19777–83. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- 102.Morita SY, Nakano M, Sakurai A, et al. Formation of ceramide-enriched domains in lipid particles enhances the binding of apolipoprotein E. FEBS Lett. 2005;579(7):1759–64. doi: 10.1016/j.febslet.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 103.Lucic D, Huang ZH, Gu D, et al. Cellular sphingolipids regulate macrophage apolipoprotein E secretion. Biochemistry. 2007;46(39):11196–204. doi: 10.1021/bi701106v. [DOI] [PubMed] [Google Scholar]
- 104.Kilkus J, Goswami R, Testai FD, et al. Ceramide in rafts (detergent-insoluble fraction) mediates cell death in neurotumor cell lines. J Neurosci Res. 2003;72(1):65–75. doi: 10.1002/jnr.10549. [DOI] [PubMed] [Google Scholar]
- 105.Luberto C, Hassler DF, Signorelli P, et al. Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. J Biol Chem. 2002;277(43):41128–39. doi: 10.1074/jbc.M206747200. [DOI] [PubMed] [Google Scholar]
- 106.Schmitz-Peiffer C. Targeting ceramide synthesis to reverse insulin resistance. Diabetes. 2010;59(10):2351–3. doi: 10.2337/db10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cronk BB, Johnson DK, Burns JM. Body mass index and cognitive decline in mild cognitive impairment. Alzheimer Dis Assoc Disord. 2010;24(2):126–30. doi: 10.1097/WAD.0b013e3181a6bf3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Atti AR, Palmer K, Volpato S, et al. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen Project. J Am Geriatr Soc. 2008;56(1):111–6. doi: 10.1111/j.1532-5415.2007.01458.x. [DOI] [PubMed] [Google Scholar]
- 109.Nourhashemi F, Deschamps V, Larrieu S, et al. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60(1):117–9. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 110.Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Holmes C, Cunningham C, Zotova E, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73(10):768–74. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tan ZS, Beiser AS, Vasan RS, et al. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007;68(22):1902–8. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 113.Zinman B, Hanley AJ, Harris SB, et al. Circulating tumor necrosis factor-alpha concentrations in a native Canadian population with high rates of type 2 diabetes mellitus. J Clin Endocrinol Metab. 1999;84(1):272–8. doi: 10.1210/jcem.84.1.5405. [DOI] [PubMed] [Google Scholar]
- 114.Hu WT, Holtzman DM, Fagan AM, et al. Plasma multianalyte profiling in mild cognitive impairment and Alzheimer disease. Neurology. 2012;79(9):897–905. doi: 10.1212/WNL.0b013e318266fa70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cote S, Carmichael PH, Verreault R, et al. Nonsteroidal anti-inflammatory drug use and the risk of cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 2012;8(3):219–26. doi: 10.1016/j.jalz.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 116.Andersen K, Launer LJ, Ott A, et al. Do nonsteroidal anti-inflammatory drugs decrease the risk for Alzheimer’s disease? Neurology. 1995;45:1441–1445. doi: 10.1212/wnl.45.8.1441. [DOI] [PubMed] [Google Scholar]
- 117.in t’ Veld BA, Ruitenberg A, Hofman A, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med. 2001;345(21):1515–21. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 118.Lyketsos CG, Breitner JC, Green RC, et al. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68(21):1800–8. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- 119.Aisen PS, Schafer K, Grundman M, et al. Results of a multicenter trial of rofecoxib and naproxen in Alzheimer’s disease. Neurobiology of Aging. 2003;23(1S):s429. [Google Scholar]
- 120.Martin BK, Szekely C, Brandt J, et al. Cognitive function over time in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65(7):896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meinert CL, McCaffrey LD, Breitner JC. Alzheimer’s Disease Anti-inflammatory Prevention Trial: design, methods, and baseline results. Alzheimers Dement. 2009;5(2):93–104. doi: 10.1016/j.jalz.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13(7):791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 123.Barrett-Connor E, Edelstein SL, Corey-Bloom J, et al. Weight loss precedes dementia in community-dwelling older adults. J Am Geriatr Soc. 1996;44(10):1147–52. doi: 10.1111/j.1532-5415.1996.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 124.Buchman AS, Wilson RS, Bienias JL, et al. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65(6):892–7. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 125.Stewart R, Masaki K, Xue QL, et al. A 32-Year Prospective Study of Change in Body Weight and Incident Dementia: The Honolulu-Asia Aging Study. Archives of Neurology. 2005;62(1):55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 126.BarrettConnor E, Edelstein SL, CoreyBloom J, et al. Weight loss precedes dementia in community-dwelling older adults. Journal of the American Geriatrics Society. 1996;44(10):1147–1152. doi: 10.1111/j.1532-5415.1996.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 127.White H, Pieper C, Schmader K, et al. Weight change in Alzheimer’s disease. J Am Geriatr Soc. 1996;44(3):265–272. doi: 10.1111/j.1532-5415.1996.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 128.Knopman DS, Edland SD, Cha RH, et al. Incident dementia in women is preceded by weight loss by at least a decade. 2007:739–746. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 129.Vidoni ED, Townley RA, Honea RA, et al. Alzheimer disease biomarkers are associated with body mass index. Neurology. 2011;77(21):1913–20. doi: 10.1212/WNL.0b013e318238eec1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Buchman AS, Schneider JA, Wilson RS, et al. Body mass index in older persons is associated with Alzheimer disease pathology. Neurology. 2006;67(11):1949–54. doi: 10.1212/01.wnl.0000247046.90574.0f. [DOI] [PubMed] [Google Scholar]
- 131.Goldman WP, Baty JD, Buckles VD, et al. Motor dysfunction in mildly demented AD individuals without extrapyramidal signs. Neurology. 1999;53(5):956. doi: 10.1212/wnl.53.5.956. [DOI] [PubMed] [Google Scholar]
- 132.Auyeung TW, Kwok T, Lee J, et al. Functional decline in cognitive impairment--the relationship between physical and cognitive function. Neuroepidemiology. 2008;31(3):167–73. doi: 10.1159/000154929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rogers SD, Jarrot SE. Cognitive impairment and effects on upper body strength of adults with dementia. J Aging Phys Act. 2008;16(1):61–8. [PubMed] [Google Scholar]
- 134.Vidoni ED, Honea RA, Billinger SA, et al. Cardiorespiratory fitness is associated with atrophy in Alzheimer’s and aging over 2 years. Neurobiol Aging. 2012;33(8):1624–32. doi: 10.1016/j.neurobiolaging.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–37. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 136.Dean DC, 3rd, Jerskey BA, Chen K, et al. Brain Differences in Infants at Differential Genetic Risk for Late-Onset Alzheimer Disease: A Cross-sectional Imaging Study. JAMA Neurol. 2013 doi: 10.1001/jamaneurol.2013.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Honea RA, Vidoni E, Harsha A, et al. Impact of APOE on the Healthy Aging Brain: A Voxel-Based MRI and DTI Study. J Alzheimers Dis. 2009;18(3):553–64. doi: 10.3233/JAD-2009-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Reiman EM. Linking brain imaging and genomics in the study of Alzheimer’s disease and aging. Ann N Y Acad Sci. 2007;1097:94–113. doi: 10.1196/annals.1379.011. [DOI] [PubMed] [Google Scholar]
- 139.Schilling S, DeStefano AL, Sachdev PS, et al. APOE genotype and MRI markers of cerebrovascular disease: systematic review and meta-analysis. Neurology. 2013;81(3):292–300. doi: 10.1212/WNL.0b013e31829bfda4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Villain N, Desgranges B, Viader F, et al. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer’s disease. J Neurosci. 2008;28(24):6174–81. doi: 10.1523/JNEUROSCI.1392-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical Evidence of Alzheimer’s Disease in Persons Homozygous for the {epsilon}4 Allele for Apolipoprotein E. The New England Journal of Medicine. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 142.Reiman EM, Caselli RJ, Chen K, et al. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98(6):3334–9. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Reiman EM, Chen K, Alexander GE, et al. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci U S A. 2005;102(23):8299–302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dennis NA, Browndyke JN, Stokes J, et al. Temporal lobe functional activity and connectivity in young adult APOE varepsilon4 carriers. Alzheimers Dement. 2010;6(4):303–11. doi: 10.1016/j.jalz.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Filbey FM, Slack KJ, Sunderland TP, et al. Functional magnetic resonance imaging and magnetoencephalography differences associated with APOEepsilon4 in young healthy adults. Neuroreport. 2006;17(15):1585–90. doi: 10.1097/01.wnr.0000234745.27571.d1. [DOI] [PubMed] [Google Scholar]
- 146.Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106(17):7209–14. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, Structural, and Functional Characterization of Alzheimer’s Disease: Evidence for a Relationship between Default Activity, Amyloid, and Memory. J Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Valla J, Yaari R, Wolf AB, et al. Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE epsilon4 allele, the major late-onset Alzheimer’s susceptibility gene. J Alzheimers Dis. 2010;22(1):307–13. doi: 10.3233/JAD-2010-100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rovio S, Kareholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705–11. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 151.Morris JK, Vidoni ED, Honea RA, Burns JM. Impaired glycemia increases disease progression in mild cognitive impairment. Neurobiol Aging. 2013 doi: 10.1016/j.neurobiolaging.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ragogna F, Lattuada G, Ruotolo G, et al. Lack of association of apoE epsilon4 allele with insulin resistance. Acta Diabetol. 2012;49(1):25–32. doi: 10.1007/s00592-011-0255-3. [DOI] [PubMed] [Google Scholar]
- 153.Lambert JC, Amouyel P. Genetics of Alzheimer’s disease: new evidences for an old hypothesis? Curr Opin Genet Dev. 2011;21(3):295–301. doi: 10.1016/j.gde.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 154.Li H, Wetten S, Li L, et al. Candidate single-nucleotide polymorphisms from a genomewide association study of Alzheimer disease. Arch Neurol. 2008;65(1):45–53. doi: 10.1001/archneurol.2007.3. [DOI] [PubMed] [Google Scholar]
- 155.Reiman EM, Webster JA, Myers AJ, et al. GAB2 alleles modify Alzheimer’s risk in APOE epsilon4 carriers. Neuron. 2007;54(5):713–20. doi: 10.1016/j.neuron.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Carrasquillo MM, Zou F, Pankratz VS, et al. Genetic variation in PCDH11X is associated with susceptibility to late-onset Alzheimer’s disease. Nat Genet. 2009;41(2):192–8. doi: 10.1038/ng.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013 doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Harold D, Abraham R, Hollingworth P, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41(10):1088–93. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lambert JC, Heath S, Even G, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41(10):1094–9. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 160.Carrasquillo MM, Belbin O, Hunter TA, et al. Replication of CLU, CR1, and PICALM associations with alzheimer disease. Arch Neurol. 2010;67(8):961–4. doi: 10.1001/archneurol.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jun G, Naj AC, Beecham GW, et al. Meta-analysis confirms CR1, CLU, and PICALM as alzheimer disease risk loci and reveals interactions with APOE genotypes. Arch Neurol. 2010;67(12):1473–84. doi: 10.1001/archneurol.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hollingworth P, Harold D, Sims R, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43(5):429–35. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Naj AC, Jun G, Beecham GW, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43(5):436–41. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hu X, Pickering E, Liu YC, et al. Meta-analysis for genome-wide association study identifies multiple variants at the BIN1 locus associated with late-onset Alzheimer’s disease. PLoS One. 2011;6(2):e16616. doi: 10.1371/journal.pone.0016616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Seshadri S, Fitzpatrick AL, Ikram MA, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303(18):1832–40. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gandhi S, Wood NW. Genome-wide association studies: the key to unlocking neurodegeneration? Nature Neuroscience. 2010;13(7):789–794. doi: 10.1038/nn.2584. [DOI] [PubMed] [Google Scholar]
- 167.Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010;20(Suppl 2):S265–79. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pathak D, Berthet A, Nakamura K. Energy failure: does it contribute to neurodegeneration? Ann Neurol. 2013;74(4):506–16. doi: 10.1002/ana.24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Parker WD, Jr, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology. 1990;40(8):1302–3. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- 170.Martins AR, Nachbar RT, Gorjao R, et al. Mechanisms underlying skeletal muscle insulin resistance induced by fatty acids: importance of the mitochondrial function. Lipids Health Dis. 2012;11:30. doi: 10.1186/1476-511X-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gupte AA, Bomhoff GL, Swerdlow RH, et al. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes. 2009;58(3):567–78. doi: 10.2337/db08-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Supale S, Thorel F, Merkwirth C, et al. Loss of prohibitin induces mitochondrial damages altering beta-cell function and survival and is responsible for gradual diabetes development. Diabetes. 2013;62(10):3488–99. doi: 10.2337/db13-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Thyfault JP, Cree MG, Zheng D, et al. Contraction of insulin-resistant muscle normalizes insulin action in association with increased mitochondrial activity and fatty acid catabolism. Am J Physiol Cell Physiol. 2007;292(2):C729–39. doi: 10.1152/ajpcell.00311.2006. [DOI] [PubMed] [Google Scholar]
- 174.Ridge PG, Koop A, Maxwell TJ, et al. Mitochondrial haplotypes associated with biomarkers for Alzheimer’s disease. PLoS One. 2013;8(9):e74158. doi: 10.1371/journal.pone.0074158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Honea RA, Swerdlow RH, Vidoni ED, et al. Progressive regional atrophy in normal adults with a maternal history of Alzheimer disease. Neurology. 2011;76(9):822–829. doi: 10.1212/WNL.0b013e31820e7b74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mosconi L, Brys M, Switalski R, et al. Maternal family history of Alzheimer’s disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci U S A. 2007;104(48):19067–72. doi: 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Honea RA, Vidoni ED, Swerdlow RH, et al. Maternal family history is associated with Alzheimer’s disease biomarkers. J Alzheimers Dis. 2012;31(3):659–68. doi: 10.3233/JAD-2012-120676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: Progress and perspectives. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbadis.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292(2):C670–86. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- 180.Barrientos A, Casademont J, Cardellach F, et al. Reduced steady-state levels of mitochondrial RNA and increased mitochondrial DNA amount in human brain with aging. Brain Res Mol Brain Res. 1997;52(2):284–9. doi: 10.1016/s0169-328x(97)00278-7. [DOI] [PubMed] [Google Scholar]
- 181.Kish SJ, Bergeron C, Rajput A, et al. Brain cytochrome oxidase in Alzheimer’s disease. J Neurochem. 1992;59(2):776–9. doi: 10.1111/j.1471-4159.1992.tb09439.x. [DOI] [PubMed] [Google Scholar]
- 182.Mutisya EM, Bowling AC, Beal MF. Cortical cytochrome oxidase activity is reduced in Alzheimer’s disease. J Neurochem. 1994;63(6):2179–84. doi: 10.1046/j.1471-4159.1994.63062179.x. [DOI] [PubMed] [Google Scholar]
- 183.Maurer I, Zierz S, Moller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging. 2000;21(3):455–62. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 184.Long J, He P, Shen Y, et al. New evidence of mitochondria dysfunction in the female Alzheimer’s disease brain: deficiency of estrogen receptor-beta. J Alzheimers Dis. 2012;30(3):545–58. doi: 10.3233/JAD-2012-120283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Valla J, Berndt JD, Gonzalez-Lima F. Energy hypometabolism in posterior cingulate cortex of Alzheimer’s patients: superficial laminar cytochrome oxidase associated with disease duration. J Neurosci. 2001;21(13):4923–30. doi: 10.1523/JNEUROSCI.21-13-04923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mosconi L, de Leon M, Murray J, et al. Reduced mitochondria cytochrome oxidase activity in adult children of mothers with Alzheimer’s disease. J Alzheimers Dis. 2011;27(3):483–90. doi: 10.3233/JAD-2011-110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hamblet NS, Castora FJ. Elevated levels of the Kearns-Sayre syndrome mitochondrial DNA deletion in temporal cortex of Alzheimer’s patients. Mutat Res. 1997;379(2):253–62. doi: 10.1016/s0027-5107(97)00158-9. [DOI] [PubMed] [Google Scholar]
- 188.de la Monte SM, Luong T, Neely TR, et al. Mitochondrial DNA damage as a mechanism of cell loss in Alzheimer’s disease. Lab Invest. 2000;80(8):1323–35. doi: 10.1038/labinvest.3780140. [DOI] [PubMed] [Google Scholar]
- 189.Coskun PE, Beal MF, Wallace DC. Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci U S A. 2004;101(29):10726–31. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hayashi T, Rizzuto R, Hajnoczky G, et al. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19(2):81–8. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Schon EA, Area-Gomez E. Mitochondria-associated ER membranes in Alzheimer disease. Mol Cell Neurosci. 2013;55:26–36. doi: 10.1016/j.mcn.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 192.Shiao YJ, Lupo G, Vance JE. Evidence that phosphatidylserine is imported into mitochondria via a mitochondria-associated membrane and that the majority of mitochondrial phosphatidylethanolamine is derived from decarboxylation of phosphatidylserine. J Biol Chem. 1995;270(19):11190–8. doi: 10.1074/jbc.270.19.11190. [DOI] [PubMed] [Google Scholar]
- 193.Pinton P, Giorgi C, Siviero R, et al. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27(50):6407–18. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Csordas G, Renken C, Varnai P, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174(7):915–21. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Area-Gomez E, Del Carmen Lara Castillo M, Tambini MD, et al. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 2012;31(21):4106–23. doi: 10.1038/emboj.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rocha M, Hernandez-Mijares A, Garcia-Malpartida K, et al. Mitochondria-targeted antioxidant peptides. Curr Pharm Des. 2010;16(28):3124–31. doi: 10.2174/138161210793292519. [DOI] [PubMed] [Google Scholar]
- 197.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(Pt 2):335–44. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Palmer HJ, Paulson KE. Reactive oxygen species and antioxidants in signal transduction and gene expression. Nutr Rev. 1997;55(10):353–61. doi: 10.1111/j.1753-4887.1997.tb01561.x. [DOI] [PubMed] [Google Scholar]
- 199.Bruce-Keller AJ, Gupta S, Parrino TE, et al. NOX activity is increased in mild cognitive impairment. Antioxid Redox Signal. 2010;12(12):1371–82. doi: 10.1089/ars.2009.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Leuner K, Schutt T, Kurz C, et al. Mitochondrion-Derived Reactive Oxygen Species Lead to Enhanced Amyloid Beta Formation. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2011.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Recuero M, Munoz T, Aldudo J, et al. A free radical-generating system regulates APP metabolism/processing. FEBS Lett. 2010;584(22):4611–8. doi: 10.1016/j.febslet.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 202.Wirths O, Multhaup G, Czech C, et al. Intraneuronal Abeta accumulation precedes plaque formation in beta-amyloid precursor protein and presenilin-1 double-transgenic mice. Neurosci Lett. 2001;306(1–2):116–20. doi: 10.1016/s0304-3940(01)01876-6. [DOI] [PubMed] [Google Scholar]
- 203.Caspersen C, Wang N, Yao J, et al. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J. 2005;19(14):2040–1. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 204.Cardoso SM, Santos S, Swerdlow RH, et al. Functional mitochondria are required for amyloid beta-mediated neurotoxicity. FASEB J. 2001;15(8):1439–41. doi: 10.1096/fj.00-0561fje. [DOI] [PubMed] [Google Scholar]
- 205.Yao J, Irwin RW, Zhao L, et al. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106(34):14670–5. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pagani L, Eckert A. Amyloid-Beta interaction with mitochondria. Int J Alzheimers Dis. 2011;2011:925050. doi: 10.4061/2011/925050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rhein V, Song X, Wiesner A, et al. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc Natl Acad Sci U S A. 2009;106(47):20057–62. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hansson Petersen CA, Alikhani N, Behbahani H, et al. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci U S A. 2008;105(35):13145–50. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Humphries AD, I, Streimann C, Stojanovski D, et al. Dissection of the mitochondrial import and assembly pathway for human Tom40. J Biol Chem. 2005;280(12):11535–43. doi: 10.1074/jbc.M413816200. [DOI] [PubMed] [Google Scholar]
- 210.Yu CE, Seltman H, Peskind ER, et al. Comprehensive analysis of APOE and selected proximate markers for late-onset Alzheimer’s disease: patterns of linkage disequilibrium and disease/marker association. Genomics. 2007;89(6):655–65. doi: 10.1016/j.ygeno.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–94. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 212.Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289(14):1799–804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 213.Langa KM, Foster NL, Larson EB. Mixed Dementia: Emerging Concepts and Therapeutic Implications. JAMA: The Journal of the American Medical Association. 2004;292(23):2901–2908. doi: 10.1001/jama.292.23.2901. [DOI] [PubMed] [Google Scholar]
- 214.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 215.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease. Journal of the American Medical Association. 1997;277:813–817. [PubMed] [Google Scholar]
- 216.Burns JM, Church JA, Johnson DK, et al. White Matter Lesions Are Prevalent but Differentially Related With Cognition in Aging and Early Alzheimer Disease. Arch Neurol. 2005;62(12):1870–1876. doi: 10.1001/archneur.62.12.1870. [DOI] [PubMed] [Google Scholar]
- 217.Ahtiluoto S, Polvikoski T, Peltonen M, et al. Diabetes, Alzheimer disease, and vascular dementia. Neurology. 2010;75(13):1195–1202. doi: 10.1212/WNL.0b013e3181f4d7f8. [DOI] [PubMed] [Google Scholar]
- 218.Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: how to move forward? Neurology. 2009;72(4):368–74. doi: 10.1212/01.wnl.0000341271.90478.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Breteler MMB, Van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: The Rotterdam study. Neurology. 1994;44:1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 220.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347(9009):1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 221.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322(7300):1447–51. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lorenzo A, Razzaboni B, Weir GC, et al. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368(6473):756–60. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 223.Schuff N, Matsumoto S, Kmiecik J, et al. Cerebral blood flow in ischemic vascular dementia and Alzheimer’s disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement. 2009;5(6):454–62. doi: 10.1016/j.jalz.2009.04.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Peng C, Ma J, Gao X, et al. High Glucose Induced Oxidative Stress and Apoptosis in Cardiac Microvascular Endothelial Cells Are Regulated by FoxO3a. PLoS One. 2013;8(11):e79739. doi: 10.1371/journal.pone.0079739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ferber EC, Peck B, Delpuech O, et al. FOXO3a regulates reactive oxygen metabolism by inhibiting mitochondrial gene expression. Cell Death Differ. 2012;19(6):968–79. doi: 10.1038/cdd.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]